Introduction
Prostate cancer is the most commonly diagnosed
cancer in males worldwide (1).
Unlike other malignant tumors, prostate cancer is a very slow
growing tumor and may not cause pathognomonic signs or problems for
years (2,3). For this reason, prostate cancer
patients are diagnosed after symptoms of illness worsen
substantially. The late detection of prostate cancer can lead to
its wide spread to delicate organs such as the epithelium, spinal
cord and brain; therefore, early suppression of prostate cancer
progression is mandatory to prevent the development of degenerative
symptoms (3).
To date, human prostate cell lines, such as PC-3,
DU-145, and LNCaP, derived from bone, brain and lymph node
metastases, are the most commonly used cell culture models of
prostate cancer and have been used in almost all prostate cancer
trials (4–7). However, it is particularly difficult
to duplicate the genetic makeup or stimulate the bioactive behavior
of primary tumors within these cell lines (8). Therefore, primary prostate tumor cell
lines are urgently required to examine pre-clinical and early
molecular prostate cancer lesions.
RC-58T/hTER is a well-characterized human cancer
cell line derived from a primary tumor of a prostate cancer patient
(9). Early passage RC-58T cells
were transduced through infection with a retrovirus vector
expressing human telomerase reverse transcriptase (hTER), and the
original phenotypes of the primary cells were maintained with
prostate-specific markers. Among these cell lines, the
RC-58T/h/SA#4 cell line derived from soft agar was isolated and
further characterized phenotypically and genetically (9–11).
The roots of Platycodon grandiflorum A. De
Candolle (P. grandiflorum) have been used both as a food
material and a traditional oriental medicine in Eastern Asia
(12). P. grandiflorum
contains various chemical compounds, including lignoceric acid,
cerotic acid, n-octacosanoic acid and α-monopalmitin, and
triterpenoid saponins constitute its main bioactive component
(13). These saponins are present
in P. grandiflorum at approximately 1–4% and have a variety
of medical applications (14–18).
The extraction of crude saponins from P. grandiflorum
generally involves the use of butanol as a solvent. However, since
they are severely toxic to humans, crude saponins obtained by
butanol extraction are not suitable for medical purposes or as
health supplements (19). In this
study, we aimed to separate the non-toxic and edible saponins by
using a previously reported method that involves the use of Diaion
HP-20 resin (20).
Saponins extracted from P. grandiflorum (SPG)
have pharmacological activities, including anti-oxidative (21) and immunomodulatory activities
(22). They can also ameliorate
lipid metabolism of the liver in rats with hypercholesterolemia
(23) and increase hemolytic
activity and humoral immune responses in ICR mice (24). Previous studies have reported that
saponins inhibit HT-1080 human fibrosarcoma cell invasion and
matrix metalloproteinase (MMP) activity (25), induce apoptosis in MCF-7 human
breast cancer cells (26), and
suppress acrolein-induced MUC5AC expression by inhibiting the
activation of NF-κB in A549 lung cancer cells (27). Although there are several reports on
the chemical and biological characteristics of SPG, there have been
no significant findings regarding its effect on primary prostate
cancer cells.
Therefore, in the present study, we aimed to
investigate the cytotoxicity and potential mechanisms of SPG
obtained by using Diaion HP-20 resin in RC-58T/h/SA#4 primary human
prostate cancer cells.
Materials and methods
Preparation of SPG
The dried roots of P. grandiflorum (500 g)
were pulverized and extracted with 80% EtOH 5 times. After the
powder particles had settled down, the clear yellow supernatant was
filtered with a 0.22-μm pore size polytetrafluoroethylene (PTFE)
filter, and concentrated by vacuum evaporation. The 80% EtOH
extract was concentrated in a vacuum at 40°C to produce a residue
(101.6 g), which was fractionated on Diaion HP-20 and eluted with
H2O, 20% EtOH, and 100% EtOH, yielding 3 fractions.
High performance thin layer
chromatography (HPTLC) and liquid chromatography-mass spectrometry
(LC-MS)/MS analysis
Chromatography was performed on silica gel 60F254
HPTLC plates (20×10 cm; 0.25-mm layer thickness). The chromatograms
were evaluated by Camag densitometry (Camag model-3 TLC scanner
equipped with Camag CATS 4 software). The slit was set 15×8 mm and
data acquisition and processing were performed using CAT windows
software. Samples (10 μl) were applied to layers at 8-mm wide
bands, positioned 10 mm from the bottom of the plate, using a Camag
Linomat IV automated TLC applicator with nitrogen flow providing
delivery from the syringe at a speed of 10 μl/sec, maintained for
all analyses. TLC plate development was performed using a Camag
Automated Multiple Development system. The solvent front was
allowed to rise to a height of 14 cm. TLC analyses were performed
at room temperature. A mixture of butanol:ethyl acetate:distilled
water (50:10:40) was used as the mobile phase; after development,
the layers were dried and the components were visualized under UV
light.
Cell culture and proliferation
The RC-58T/h/SA#4 (primary prostate cancer cells;
androgen-positive cells) cells and RWPE-1 (human prostate
epithelial cells) cells were obtained from the Center for Prostate
Disease Research (CPDR). The cells were cultured in DMEM
supplemented with 10% fetal bovine serum (FBS), penicillin (100
IU/ml), and streptomycin (100 μg/ml) (Gibco BRL, Life Technologies,
Grand Island, NY, USA) in an incubator containing a humidified
atmosphere of 5% CO2 at 37°C.
Cell proliferation was determined by sulforhodamine
B (SRB; Sigma, St. Louis, MO, USA) assay. The cancer cells were
seeded at a concentration of 1×105 cells/well in 24-well
tissue culture plates and incubated with various concentrations of
SPG for 24 h. Following treatment, the medium was aspirated and 10%
trichloro-acetic acid was added. After a 1-h incubation at 4°C, the
plate was washed 5 times with DW-R10 dry wash resin and air-dried.
The cells were stained with 0.4% (w/v) SRB at room temperature for
1 h and then washed 5 times using 1% acetic acid. Bound SRB was
solubilized with 10 mM Tris, and the absorbance was measured at 540
nm using a microplate reader (Molecular Devices, Inc., Sunnyvale,
CA, USA).
Cell cycle analysis for sub-G1
population
Cells were seeded at a density of 1×106
cells per well in 6-well plates and cultured for 24 h in DMEM.
After culturing, the cells were treated with the indicated
concentrations of SPG for 24 h. The cells were then collected and
fixed in ice-cold 70% ethanol in medium and stored at 4°C
overnight. After resuspension, the cells were washed and incubated
with 1 μl of RNase (1 mg/ml) (Sigma), 20 μl of propidium iodide
(PI; 1 mg/ml) (Sigma), and 500 ml of PBS at 37°C for 30 min. After
staining, flow cytometry was used to analyze the sub-G1 DNA
content.
Detection of morphological apoptosis
Morphological changes characteristic of apoptosis
were assessed by fluorescence microscopy using bis-benzimide
(Hoechst 33258) staining. Briefly, the cells were seeded in 6-well
plates at a density of 1×106 cells per well, followed by
treatment with SPG for 24 h. After harvesting, the cells were
washed twice with PBS and then stained with 200 μl of bis-benzimide
(5 μg/ml) for 10 min at room temperature. Subsequently, 10 μl of
this suspension were placed on a glass slide and covered with a
cover slip. The cells were examined under a fluorescence microscope
(Olympus Optical Co. Ltd., Japan) to determine nuclei fragmentation
and chromatin condensation.
Analysis of DNA fragmentation
The cells were seeded at a density of
1×107 cells in a 100-mm dish and cultured for 24 h in
DMEM. After culturing, the cells were treated with the indicated
concentrations of SPG for 24 h, followed by centrifugation. The
pellets were lysed by lysis buffer (10 mM Tris-HCl, pH 7.5, 10 mM
EDTA, pH 8.0, 0.5% Triton X-100, 20% SDS, and 10 mg/ml of
proteinase K) and then centrifuged. After extraction with
phenol:chloroform:isoamyl alcohol (25:24:1), DNA was precipitated
with 2 vol of cold absolute ethanol. The resulting pellets were
incubated with TE buffer (10 mM Tris-HCl, pH 7.4, 1 mM EDTA, pH
8.0) and RNase (2 mg/ml) for 1 h at 37°C. Separation by
electrophoresis was then performed on 2% agarose containing
ethidium bromide. The resulting DNA bands were examined using a UV
Transilluminator Imaging System.
Assay for caspase activity
Cells were seeded at a density of 5×105
cells/well and then cultured for 24 h in DMEM. The cells were then
pre-incubated with z-VAD-fmk for 2 h, followed by treatment with
the indicated concentrations of SPG for 24 h. For growth inhibition
analysis and measurement of sub-G1 DNA content, the cells were
collected and fixed in ice-cold 70% ethanol in medium, followed by
storage at 4°C overnight. After resuspension, the cells were washed
and incubated with 1 μl of RNase (1 mg/ml) (Sigma), 20 μl of PI (1
mg/ml), and 500 ml of PBS at 37°C for 30 min. After staining, flow
cytometry was carried out to analyze the sub-G1 DNA content.
Assay for caspase inhibitor activity
The cells were seeded at a densities of
1×105 cells per well in a 24-well plate and
1×106 cells per well in a 6-well plate, then cultured
for 24 h in DMEM. The cells were pre-incubated with z-VAD-fmk for 2
h and then treated with the indicated concentrations of SPG for 24
h. The cells were collected and counted using trypan blue dye
(Gibco BRL), and were then fixed in ice-cold 70% ethanol in medium,
and then stored at 4°C overnight. After resuspension, the cells
were washed and incubated with 1 μl of RNase (1 mg/ml) (Sigma), 20
μl of PI (1 mg/ml) (Sigma), and 500 ml of PBS at 37°C for 30 min.
After staining, flow cytometry was used to analyze the sub-G1 DNA
content.
Western blot analysis
The cells were seeded at a density of
1×107 cells in a 100-mm dish, and then cultured for 24 h
in DMEM. After culturing, the cells were treated with the indicated
concentrations of SPG for 24 h, followed by centrifugation. The
resulting pellets were lysed by lysis buffer (50 mM Tris-HCl, 150
mM NaCl, 1 mM EDTA, 50 mM NaF, 30 mM
Na4P2O7, 1 mM PMSF and 2 μg/ml of
aprotinin) for 30 min on ice. The protein content of the
supernatant was measured using the BCA protein kit (Pierce,
Rockford, IL, USA). The protein samples were then loaded at 10 μg
of protein/lane and then separated by 12% SDS-PAGE at 100 V of
constant voltage/slab for 1.5 h. Following electrophoresis, the
proteins were transferred onto nitrocellulose membranes. After
blocking with 2.5 and 5% bovine serum albumin (BSA) for 1 h at
37°C, the membranes were incubated with primary antibody [anti-Bid,
anti-Bax, anti-Bcl-2, anti-poly(ADP-ribose) polymerase (PARP) and
anti-apoptosis-inducing factor (AIF)] at 4°C overnight. Finally,
the membranes were treated with horseradish peroxidase-coupled
secondary antibodies for 1 h at 4°C. The membranes were then washed
with T-TBS after each antibody binding reaction. Detection of each
protein was performed using an ECL kit (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA).
AIF translocation
RC-58T/h/SA#4 cells were seeded in 6-well plates at
seeding densities of 1×106 cells per well, followed by
treatment with SPG for 24 h. After harvesting, the cells were
washed twice with PBS and then blocked with blocking buffer (2% BSA
in T-TBS) for 1 h. The cells were incubated with AIF primary
antibody overnight at 4°C, followed by anti-rabbit secondary
antibody for 1 h. AIF translocation was analyzed under a
fluorescence microscope (Olympus Optical Co., Ltd.).
Statistical analysis
The data were analyzed using the Student's t-test to
evaluate significant differences. Levels of *p<0.05
and **p<0.01 were considered to indicate
statistically significant differences.
Results
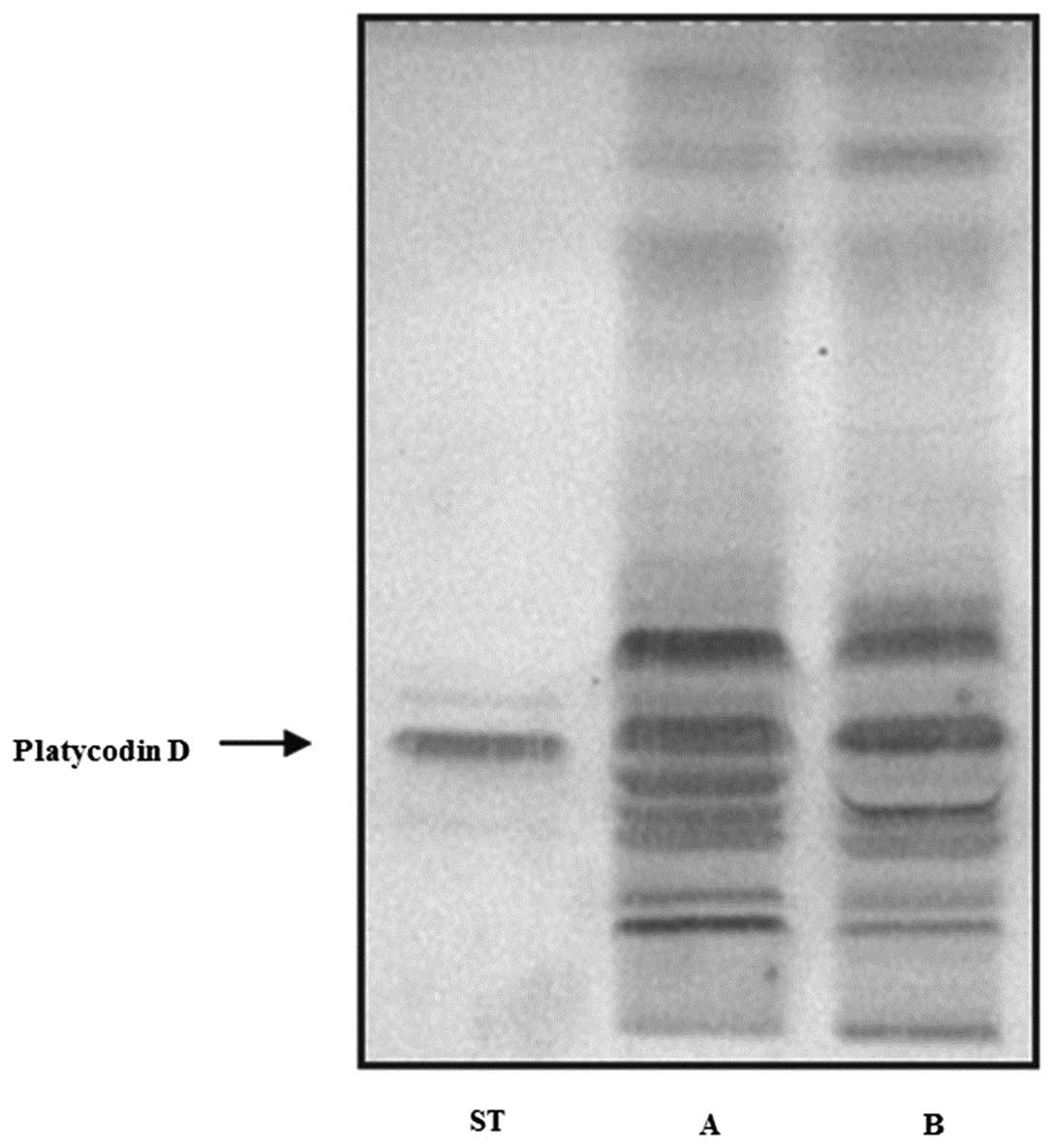
Contents of platycodin D were determined
by HPTLC and LC-MS/MS analysis
Platycodin D is a major constituent of triterpene
saponin in platycodin saponins (28). We initially confirmed the platycodin
D contents of SPG using HPTLC and LC-MS/MS. TLC comparison of
saponin fractions prepared by Diaion HP-20 and the butanol method
showed similar patterns, and they contained platycodin D (Fig. 1). In addition, saponin fractions
prepared by the Diaion HP-20 adsorption method showed a higher
content of platycodin D (25.1 mg/g) compared to those prepared by
the butanol method (14.1 mg/g) (Table
I). These results suggested that SPG prepared by the Diaion
HP-20 adsorption method was purer compared to the saponins prepared
by the butanol method.
 | Table IPlatycodin D contents in crude
saponins prepared by LC/MS/MS. |
Table I
Platycodin D contents in crude
saponins prepared by LC/MS/MS.
| Method | Platycodin D
(mg/g) |
|---|
| Diaion HP-20
method | 25.1±1.54 |
| Butanol method | 14.1±1.78 |
SPG inhibits RC-58T/h/SA#4 human prostate
cancer cell growth
We investigated the IC50 value of SPG
against human prostate cancer cells (RC-58T/h/SA#4, PC-3 and
LNCap.FGC) and normal cells (RWPE-1). These cells were treated with
10, 30, 50 and 80 μg/ml SPG for 24, 48 and 72 h. As shown in
Table II, SPG treatment decreased
the IC50 value in RC-58T/h/SA#4 cells in a
time-dependent manner. Although the IC50 value of SPG in
the RC-58T/h/SA#4 cells was higher than that in the PC-3 and
LNCap.FGC cells, the RC-58T/h/SA#4 cells were found to be more
sensitive to SPG as compared to RWPE-1 human prostate epithelial
cells.
 | Table IIIC50 values of saponins
isolated from Platycodon grandiflorum A. De Candolle in the
4 cell lines. |
Table II
IC50 values of saponins
isolated from Platycodon grandiflorum A. De Candolle in the
4 cell lines.
| IC50
(μg/ml) |
|---|
|
|
|---|
| Cell line | 24 h | 48 h | 72 h |
|---|
| RWPE-1 | 62.81±4.23 | 58.07±3.46 | 45.25±6.03 |
| RC-58T/h/SA#4 | 49.86±3.63 | 44.61±1.01 | 37.04±0.96 |
| LNCap.FGC | 38.33±1.23 | 37.56±0.97 | 33.58±2.11 |
| PC-3 | 48.33±0.97 | 37.3±0.38 | 28.84±0.38 |
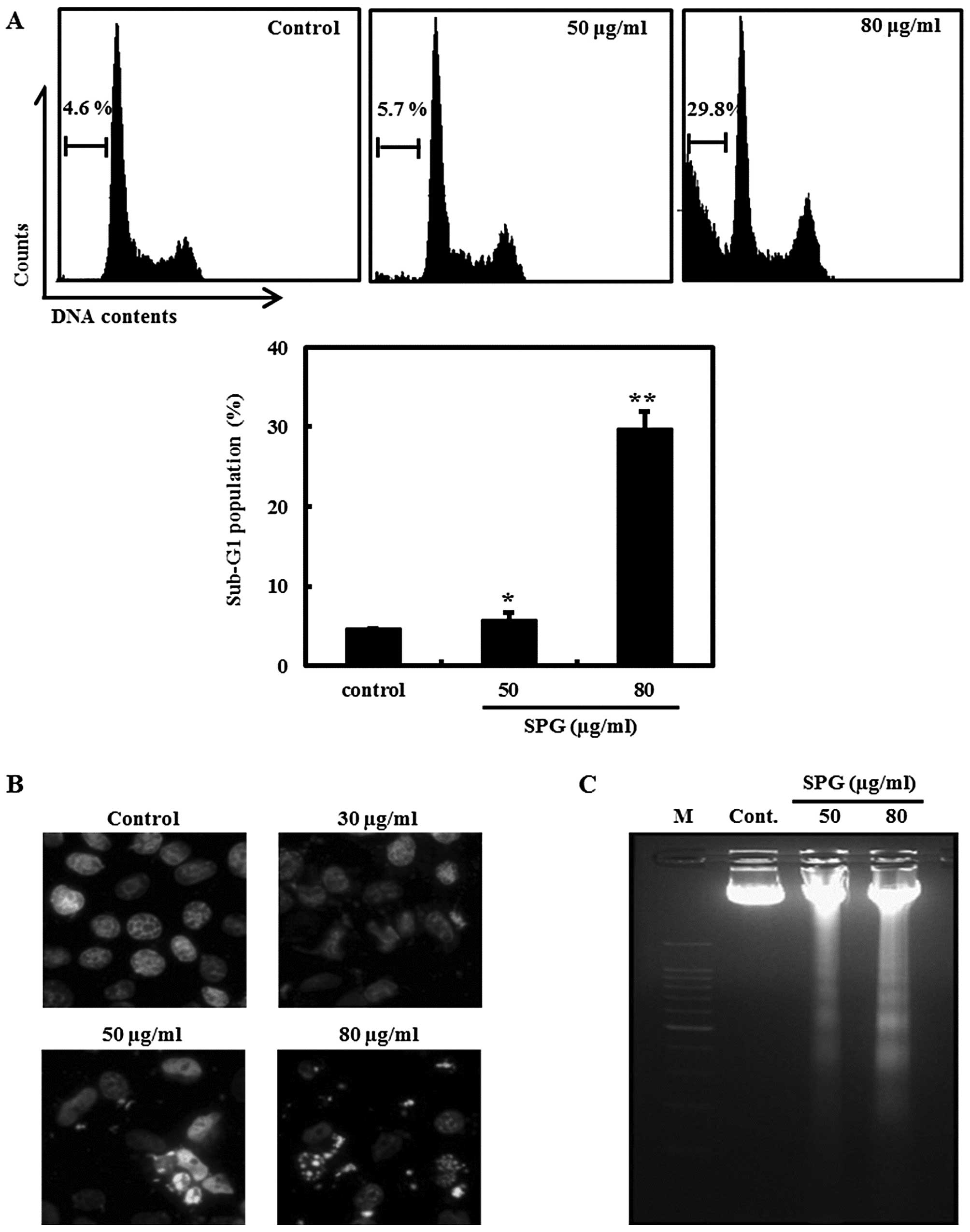
SPG leads to apoptosis in RC-58T/h/SA#4
cells
In order to determine whether SPG induces apoptosis
in RC-58T/h/SA#4 cells, we performed flow cytometry, Hoechst
staining and a DNA fragmentation assay. As shown in Fig. 2A, the proportion of the sub-G1 peak
was negligible for the control RC-58T/h/SA#4 cells not treated with
SPG, whereas the exposure of RC-58T/h/SA#4 cells for 24 h to 50 and
80 μg/ml SPG resulted in dose-dependent accumulation of cells in
the sub-G1 phase.
Induction of apoptosis by SPG was further examined
using Hoechst staining and DNA fragmentation assay. After SPG
treatment for 24 h, the morphological characteristics of apoptosis
were observed in the RC-58T/h/SA#4 cells treated with 50 and 80
μg/ml SPG, while the untreated cells did not show these
characteristics (Fig. 2B). DNA
fragmentation, another distinct feature of apoptosis, was observed
in the RC-58T/h/SA#4 cells after treatment with SPG at
concentrations of 50 and 80 μg/ml, while the untreated cells did
not show the ladder formation (Fig.
2C). These results indicate that SPG induces apoptosis in
RC-58T/h/SA#4 cells.
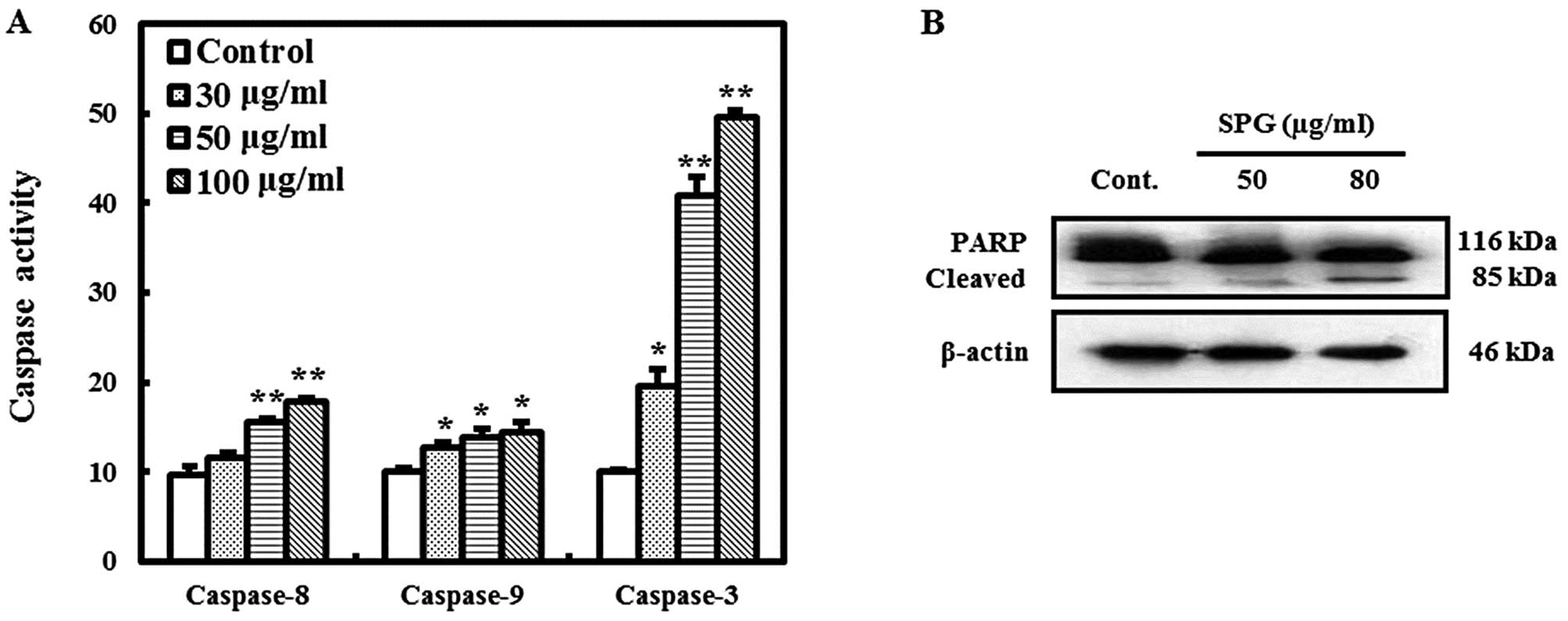
SPG induces apoptosis through caspase
activity
To confirm the importance of caspase activation in
SPG-induced apoptosis, the activities of initiator caspases
(caspase-8 and −9) and the effector caspase (caspase-3) were
investigated. As shown in Fig. 3A,
SPG markedly induced the activities of caspase-8, −9, and −3 at
concentrations of 30, 50, and 80 μg/ml in the RC-58T/h/SA#4 cells.
Furthermore, PARP, a family of proteins involved in DNA repair and
apoptosis, was identified by its predicted cleavage product of 89
kDa following SPG treatment in the RC-58T/h/SA#4 cells, but not in
the untreated cells (Fig. 3B).
These data indicate that SPG-induced apoptosis occurs through
caspase activation.
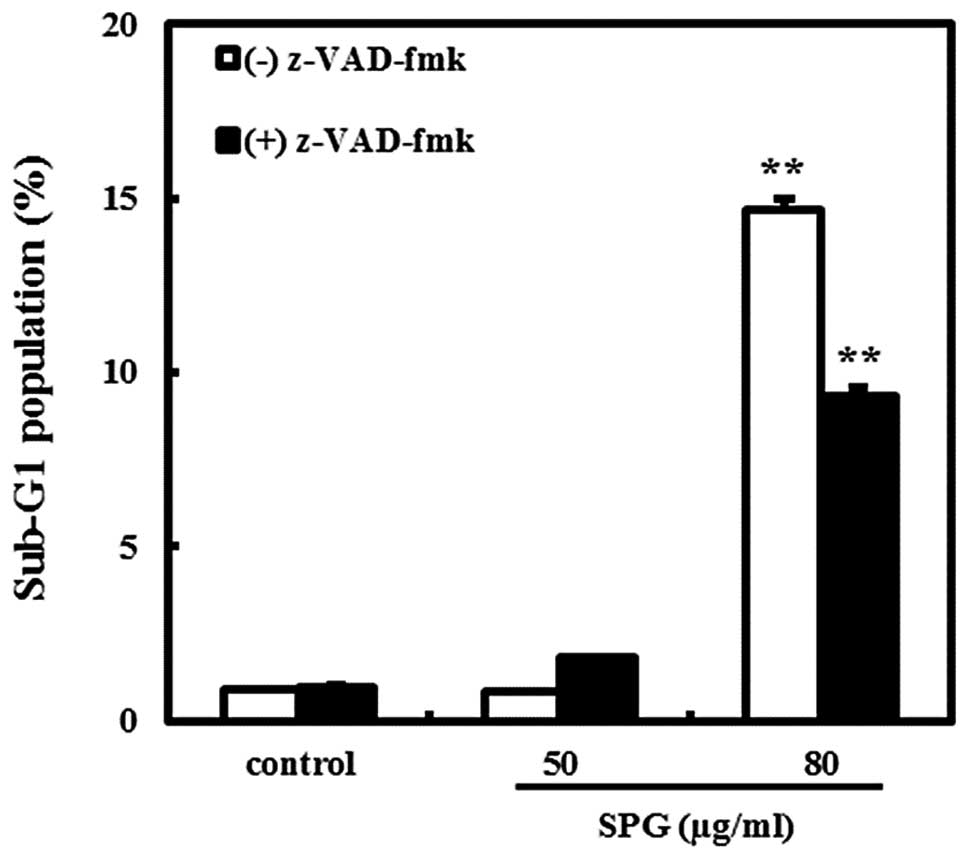
Caspase inhibitor reduces SPG-induced
apoptosis
In order to elucidate whether the apoptosis induced
by SPG is involved in the activation of caspases, we used a
universal caspase inhibitor, z-VAD-fmk. As shown in Fig. 4, the percentage of cells in the
sub-G1 phase was almost 15% at an SPG concentration of 80 μg/ml in
the absence of z-VAD-fmk, whereas approximately 10% of cells were
in the sub-G1 phase in the presence of z-VAD-fmk (10 μM). These
data indicate that the activation of caspases is involved in
SPG-induced apoptosis in RC-58T/h/SA#4 cells.
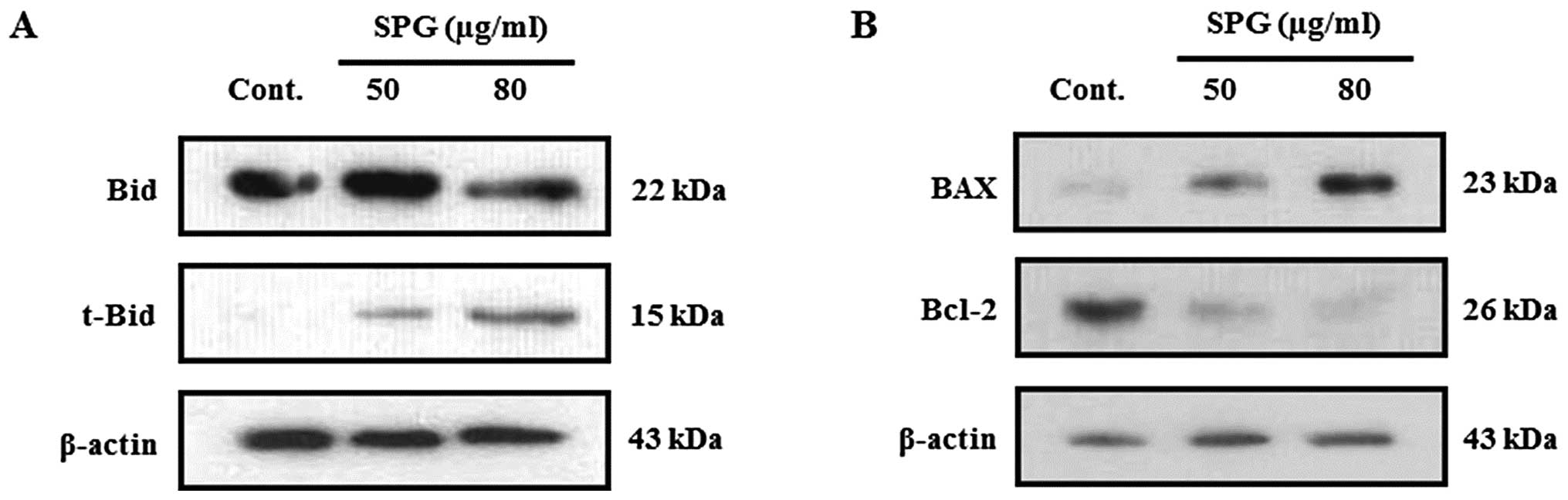
SPG induces apoptosis through activation
of the mitochondrial pathway
Caspases, particularly caspase-8, are thought to
regulate the expression of proteins involved in the mitochondrial
pathway, such as Bid, Bcl-2 and Bax. Therefore, we determined
whether Bid, a pro-apoptotic Bcl-2 family member, plays a role in
mitochondrial damage caused by SPG. As shown in Fig. 5A, full-size Bid (22 kDa) was cleaved
to yield a 15-kDa fragment (tBid) following the treatment of cells
with SPG. In addition, SPG treatment resulted in the upregulation
of Bax and the downregulation of Bcl-2 expression (Fig. 5B). These data indicate that
apoptosis induced by SPG is involved in the expression of
mitochondrial-related proteins, indicating that SPG induces
apoptosis via mitochondrial pathways.
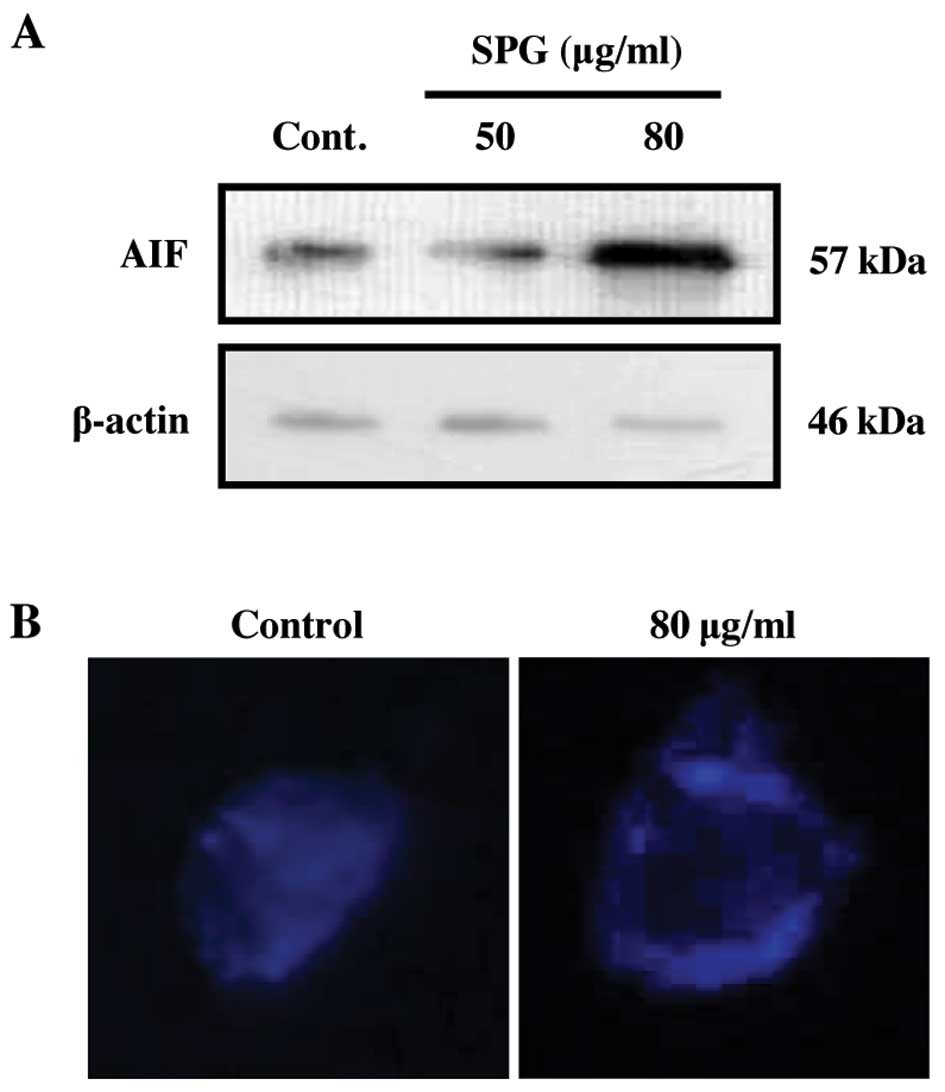
SPG stimulates release of AIF from the
mitochondria
Mitochondrial pathways and the Bcl-2 family are
thought to induce the expression of AIF upon treatment with SPG
(29,30). Thus, we examined whether SPG can
modulate the level of AIF in RC-58T/h/SA#4 prostate cancer cells.
As shown in Fig. 6A, AIF levels
gradually increased following SPG treatment. In addition, the
transfer of AIF from the mitochondria to the nucleus was detected
by immunostaining (Fig. 6B). These
results suggest that AIF translocation into the nucleus is required
for SPG-induced apoptosis in RC-58T/h/SA#4 cells.
Discussion
Representative Saponins extracted from P.
grandiflorum (SPG) have been identified as platycodin D,
platycodin D2, platycodin D3, platycoside A, platycoside E,
deapioplatycoside D and polygalacin D2 (31). Using these saponins, a number of
previous studies have shown apoptosis induction in metastatic
prostate cancer cell lines (32–35).
However, the anti-cancer activity in RC-58T/h/SA#4 primary prostate
cancer cells has not yet been investigated. In this study, to our
knowledge, we are the first to report the growth-inhibitory effect
and apoptosis mechanism of SPG in primary human prostate cancer
cells (RC-58T/h/SA#4).
Apoptotic cell death is a genetically programmed
mechanism that allows cells to commit suicide. Apoptosis is
characterized by cytoplasmic shrinkage, nuclear condensation and
DNA fragmentation. It is a cell-intrinsic, programmed suicide
mechanism that results in the controlled breakdown of the cell into
apoptotic bodies (36,37). Previous studies have reported that
triterpenoid saponins have a cytotoxic effect on tumor cells,
including prostate cancer cells (33). The present study demonstrates that
SPG is an efficient growth inhibitor in RC-58T/h/SA#4 cells.
Treatment with SPG decreased the proliferation of RC-58T/h/SA#4
cells, which were found to be more sensitive to SPG than RWPE-1
human prostate epithelial cells. RC-58T/h/SA#4 cells treated with
SPG had clearly increased sub-G1 populations and DNA fragmentation
patterns. The presence of condensed nuclei and apoptotic bodies was
confirmed by Hoechst 33258 staining in the SPG-treated
RC-58T/h/SA#4 cells. Taken together, these data indicate that SPG
inhibits RC-58T/h/SA#4 prostate cancer cell proliferation and
induces apoptosis in a dose- and time-dependent manner.
Our results are in agreement with those from
previous studies describing the antiproliferative and
apoptosis-inducing effects of platycodin D on colon cancer HT-29
cells (30), immortalized
keratinocytes (5), and breast
cancer MCF-7 cells (26).
Platycodin D is the major constituent of SPG. Upon performing TLC
for a comparison of the saponins extracted from P.
grandiflorum, we detected platycodin D, which was quantified by
performing LC-MS/MS; we observed 3.01% platycodin D. Our results
suggest that platycodin D may be the majorly active saponin in the
SPG-induced apoptosis of RC-58T/h/SA#4 cells. However, future
studies are required to determine which saponin compounds of SPG
are involved in its antiproliferative effect on RC-58T/h/SA#4
cancer cells.
During the process of apoptosis, caspases, a family
of cysteine-dependent proteases, are essential for the activation
of cell death in response to various stimuli (38). Previous studies on apoptosis
signaling mechanisms mainly support the hypothesis of the existence
of caspase-dependent pathways leading to cell death: an extrinsic
(death receptor) pathway leads to the activation of caspase-8 or
−10, which then activates downstream effector caspases capable of
PARP cleavage, such as caspase-3 and −7; and an intrinsic
(mitochondria) pathway for apoptosis is triggered by various
cellular stress stimuli (39,40).
In this pathway, mitochondria release apoptotic proteins including
cytochrome c, which activates caspase-9, and is regulated by
the Bcl-2 family (41–44). In this study, we demonstrated that
SPG induced PARP cleavage and an increase in the number of
apoptotic cells promoted by the activation of caspase-8, −9, and
−3. Furthermore, treatment with a pan-caspase inhibitor (z-VAD-fmk)
markedly blocked the apoptotic cell death in SPG-treated
RC-58T/h/SA#4 cells. These results clearly indicate that
SPG-induced apoptosis is associated with caspase activation, which
involves the degradation of the 116-kDa PARP into 85-kDa
fragments.
The mitochondrial pathway for apoptotic cell death
is governed by members of the Bcl-2 family. These proteins mediate
mitochondrial outer-membrane permeabilization and act as
pro-apoptotic (Bid, Bad, Bim and Puma) or anti-apoptotic (Bcl-2,
Bcl-xL, Bcl-W and Bcl-B) regulators (45). Bid, a family that links the Bcl-2
family members and caspases is a pro-apoptotic Bcl-2 family
containing only the BH3 domain (46). Several studies have demonstrated
that Bid is cleaved by caspase-8 following death-receptor
stimulation (45). We found that
the expression of full-length Bid decreased following SPG
treatment, and this led to the cleavage of Bid to tBid. Our results
also showed that SPG inhibited Bcl-2 expression and increased Bax
expression, leading to the decrease in the Bcl-2/Bax ratio. The
Bcl-2/Bax ratio is one of the critical factors determining whether
the cell will undergo apoptosis (47). Taken together, these data suggest
that SPG-induced apoptosis leads to the downregulation of Bcl-2
expression and the upregulation of Bax expression, which induces
outer mitochondrial membrane permeabilization and the loss of
mitochondrial potential in the apoptotic death of RC-58T/h/SA#4
cells.
Previous studies have demonstrated that apoptosis
occurs through either a caspase-dependent or -independent pathway
in prostate cancer cells (48,49).
AIF, a hallmark of caspase-independent apoptosis, is translocated
from the mitochondrial intermembrane space to the cytosol, as well
as to the nucleus during apoptosis (50). Our data show that SPG treatment
induces AIF release from the mitochondria, which supports the
contention that SPG induces caspase-independent apoptosis.
Therefore, the results from the present study suggest that SPG
induces at least 2 different signaling pathways in RC-58T/h/SA#4
cell death.
In conclusion, to our knowledge, our study is the
first to demonstrate the effectiveness of SPG as an AIF in primary
human prostate cancer cells (RC-58T/h/SA#4). We confirmed that the
apoptotic mechanisms of SPG are mediated by caspase-dependent and
-independent pathways in RC-58T/h/SA#4 cells. These findings
uncover a basic mechanism for the anticancer properties of SPG and
suggest that SPG can be used in pre-clinical strategies, such as
chemoprevention, chemotherapy and cytotoxin therapy for the
treatment of early prostate cancer.
Acknowledgements
The authors wish to thank Dr Johng S. Rhim of the
Center for Prostate Disease Research (CPDR) for providing the
RC-58T/h/SA#4 primary human prostate cancer cells and RWPE-1 human
prostate epithelial cells.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics. CA Cancer J Clin. 55:74–108. 2002.
View Article : Google Scholar
|
|
2
|
Pavone-Macaluso M, Carruba G and
Castagnetta L: Steroid receptors in prostate cancer tissues and
cells: pathophysiology, problems in methodology, clinical value and
controversial questions. Arch Esp Urol. 47:189–201. 1994.
|
|
3
|
Lee DH, Szczepanski M and Lee YJ: Role of
Bax in quercetin-induced apoptosis in human prostate cancer cells.
Biochem Pharmacol. 75:2345–2355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horoszewicz JS, Leong SS, Kawinski E, Karr
JP, Rosenthal H, Chu TM, Mirand EA and Murphy GP: LNCaP model of
human prostatic carcinoma. Cancer Res. 43:1809–1818. 1983.
|
|
5
|
Nagle RB, Ahmann FR, McDaniel KM, Paquin
ML, Clark VA and Celniker A: Cytokeratin characterization of human
prostatic carcinoma and its derived cell lines. Cancer Res.
47:281–286. 1987.PubMed/NCBI
|
|
6
|
Mickey DD, Stone KR, Wunderli H, Mickey
GH, Vollmer RT and Paulson DF: Heterotransplantation of a human
prostatic adenocarcinoma cell line in nude mice. Cancer Res.
37:4049–4058. 1977.PubMed/NCBI
|
|
7
|
Kaighn ME, Narayan KS, Ohnuki Y, Lechner
JF and Jones LW: Establishment and characterization of a human
prostatic carcinoma cell line (PC-3). Invest Urol. 17:16–23.
1979.PubMed/NCBI
|
|
8
|
Choi SR, Lee JH, Kim JY, Park KW, Jeong
IY, Shim KH, Lee MK and Seo KI: Decursin from Angelicagigas
Nakai induces apoptosis in RC-58T/h/SA#4 primary human prostate
cancer cells via a mitochondria-related caspase pathway. Food Chem
Toxicol. 49:2517–2523. 2011.PubMed/NCBI
|
|
9
|
Yasunaga Y, Nakamura K, Ko D, Srivastava
S, Moul JW, Sesterhenn IA, McLeod DG and Rhim JS: A novel human
cancer culture model for the study of prostate cancer. Oncogene.
20:8036–8041. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu Y, Li H, Miki J, Kim KH, Furusato B,
Sesterhenn IA, Chu WS, McLeod DG, Srivastava S, Ewing CM, Isaacs WB
and Rhim JS: Phenotypic characterization of telomerase-immortalized
primary non-malignant and malignant tumor-derived human prostate
epithelial cell lines. Exp Cell Res. 312:831–843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu Y, Kim KH, KO D, Nakamura K, Yasunaga
Y, Moul JW, Srivastava S, Arnstein P and Rhim JS: A
telomerase-immortalized primary human prostate cancer clonal cell
line with neoplastic phenotypes. Int J Oncol. 25:1057–1064.
2004.PubMed/NCBI
|
|
12
|
Sung NJ, Lee SJ, Shin JH, Lee IS and Chung
YC: Effects of Platycodon grandiflorum extract on blood
glucose and lipid composition in alloxan induced hyperglycemic
rats. J Korean Soc Food Sci Nutr. 25:986–992. 1996.
|
|
13
|
Fu W, Dou DQ, Shimizu N, Takeda T, Pei YH
and Chen YJ: Studies on the chemical constituents from the roots of
Platycodon grandiflorum. J Nat Med. 60:68–72. 2005.
View Article : Google Scholar
|
|
14
|
Ishii H, Tori K, Tozyo T and Yoshimura Y:
Saponin from roots of Platycodon grandiflorum Part 2
Isolation and structure of new triterpene glycosides. J Chem Soc
Perkin Trans. 1:662–668. 1984.
|
|
15
|
Nikaido T, Koike K, Mitsunaga K and Saeki
T: Two new triterpenoid saponin from Platycodon
grandiflorum. Chem Pharm Bull. 47:903–904. 1999. View Article : Google Scholar
|
|
16
|
Saeki T, Koike K and Nikaido T: A
Comparative study on commercial, botanical gardens and wild samples
of the roots of Platycodon grandiflorum. Planta Med.
65:428–431. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozaki Y: Studies on antiinflammatory
effect of Japanese Oriental medicines (kampo medicines) used to
treat inflammatory diseases. Biol Pharm Bull. 18:559–562. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JY and Lee SJ: Surface activities of
ginseng saponins and their interactions with biomolecules. Korean J
Ginseng Sci. 19:122–126. 1995.
|
|
19
|
Park KU, Wee JJ, Kim JY, Jeong CH, Kang
KS, Cho YS and Seo KI: Anticancer and immuno-activities of edible
crude saponin from soybean cake. J Korean Soc Food Sci Nutr.
34:1509–1513. 2005. View Article : Google Scholar
|
|
20
|
Kwak YS, Kyung JS, Kim SK and Wee JJ: An
isolation of crude saponin from red-ginseng efflux by Diaion HP-20
resin adsorption method. J Korean Soc Food Sci Nutr. 30:1–5.
2001.
|
|
21
|
Kim CH, Jung BY, Jung SK, Lee CH, Lee HS,
Kim BH and Kim SK: Evaluation of antioxidant activity of
Platycodon grandiflorum. J Environ Toxicol. 25:85–94.
2010.
|
|
22
|
Han SB, Park SH, Lee KH, Lee CW, Lee SH,
Kim HC, Kim YS, Lee HS and Kim HM: Polysaccharide isolated from the
radix of Platycodon grandiflorum selectively activates B
cells and macrophages but not T cells. Int Immunophamacol.
1:1969–1978. 2001.
|
|
23
|
Kim HS, Kim GJ and Kim HS: Effect of the
feeding Platycodon grandiflorum on lipid components of liver
and liver function in hypercholesterolemia rats. Korean J Food
Nutr. 11:312–318. 1998.
|
|
24
|
Xie Y, Deng W, Sun H and Li D: Platycodin
D2 is a potential less hemolytic saponin adjuvant eliciting Th1 and
Th2 immune responses. Int Immunopharmacol. 8:1143–1150. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee KJ, Hwang SJ, Choi JH and Jeong HG:
Saponins derived from the roots of Platycodon grandiflorum
inhibit HT-1080 cell invasion and MMPs activities: Regulation of
NF-κB activation via ROS signal pathway. Cancer Lett. 268:233–243.
2008.
|
|
26
|
Yu JS and Kim AK: Platycodin D induces
apoptosis in MCF-7 human breast cancer cells. J Med Food.
13:298–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi JH, Hwang YP, Han ES, Kim HG, Park
BH, Lee HS, Park BK, Lee YC, Chung YC and Jeong HG: Inhibition of
acrolein-stimulated MUC5AC expression by Platycodon
grandiflorum root-derived saponin in A549 cells. Food Chem
Toxicol. 49:2157–2166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shin DY, Kim GY, Li W, Choi BT, Kim ND,
Kang HS and Choi YH: Implication of intracellular ROS formation,
caspase-3 activation and Egr-1 induction in platycodon D-induced
apoptosis of U937 human leukemia cells. Biomed Phamacother.
63:86–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye R, Zhang X, Kong X, Han J, Yang Q,
Zhang Y, Chen Y, Li P, Liu J, Shi M, Xiong L and Zhao G:
Ginsenoside Rd attenuates mitochondrial dysfunction and sequential
apoptosis after transient focal ischemia. Neuroscience.
178:169–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim JY, Park KW, Moon KD, Lee MK, Choi JA,
Yee ST, Shim KH and Seo KI: Induction of apoptosis in HT-29 colon
cancer cells by crude saponin from Platycodi radix. Food
Chem Toxicol. 46:3753–3758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun H, Chen L, Wang J, Wang K and Zhou J:
Structure-function relationship of the saponins from the roots of
Platycodon grandiflorum for hemolytic and adjuvant activity.
Int Immunopharmacol. 11:2047–2056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chan PK: Acylation with diangeloyl groups
at C21-22 positions in triterpenoid saponins is essential for
cytotoxcity towards tumor cells. Biochem Pharmacol. 73:341–350.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee YH, Kim YJ, Kim HI, Cho HY and Yoon
HG: Potent HAT inhibitory effect of aqueous extract from Bellflower
(Platycodon grandiflorum) roots on androgen
receptor-mediated transcriptional regulation. Food Sci Biotechnol.
16:457–462. 2007.
|
|
34
|
Xie Z, Huang H, Zhao Y, Shi H, Wangc S,
Wang TTY, Chen P and Yu L: Chemical composition and
anti-proliferative and anti-inflammatory effects of the leaf and
whole-plant samples of diploid and tetraploid Gynostemma
pentaphyllum (Thunb.). Makino Food Chem. 132:125–133. 2012.
View Article : Google Scholar
|
|
35
|
Kim YS, Kim JS, Choi SU, Kim JS, Lee HS,
Roh SH, Jeong YC, Kim YK and Ryu SY: Isolation of a new saponin and
cytotoxic effect of saponins from the root of Platycodon
grandiflorum on human tumor cell lines. Planta Med. 71:566–568.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duperez L, Wirawan E, Vanden Berqhe T and
Vandenabeele P: Major cell death pathways at a glance. Microbes
Infect. 11:1050–1062. 2009. View Article : Google Scholar
|
|
37
|
Park DS, Stefanis L and Greene LA:
Ordering the multiple pathways of apoptosis. Trends Cardiovasc Med.
7:294–301. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Harada H and Grant S: Apoptosis
regulators. Rev Clin Exp Hematol. 7:117–138. 2003.
|
|
39
|
Kischkel FC, Hellbardt S, Behrmann I,
Germer M, Pawlita M, Krammer PH and Peter ME:
Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a
death-inducing signaling complex (DISC) with the receptor. EMBO J.
14:5579–5588. 1995.PubMed/NCBI
|
|
40
|
Medema JP, Scaffidi C, Kischkel FC,
Shevchenko A, Mann M, Krammer PH and Peter ME: FLICE is activated
by association with the CD95 death-inducing signaling complex
(DISC). EMBO J. 16:2794–2804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kroemer G, Zamzami N and Susin SA:
Mitochondrial control of apoptosis. Immunol Today. 18:44–51. 1997.
View Article : Google Scholar
|
|
42
|
Zou H, Henzel WJ, Liu X, Lutschg A and
Wang X: Apaf-1, a human protein homologous to C. elegans CED-4
participates in cytochrome c-dependent activation of caspase-3.
Cell. 90:405–413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: a
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kantari C and Walczak H: Caspase-8 and
bid: caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gross A, Yin XM, Wang K, Wei MC, Jockel J,
Milliman C, Erdjument-Bromage H, Tempst P and Korsmeyer SJ: Caspase
cleaved BID targets mitochondria and is required for cytochrome c
release, while BCL-XL prevents this release but not tumor necrosis
factor-R1/Fas death. J Biol Chem. 274:1156–1163. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jin S and Dai CL: Attenuation of
reperfusion-induced hepatocyte apoptosis is associated with
reversed bcl-2/bax ratio in hemi-hepatic artery-preserved portal
occlusion. J Surg Res. 174:298–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang M, Liu H, Tian Z, Griffith BN, Ji M
and Li QQ: Gossypol induces apoptosis in human PC-3 prostate cancer
cells by modulating caspase-dependent and caspase-independent cell
death pathways. Life Sci. 80:767–774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim SY, Park KW, Kim JY, Jeong IY, Byun
MW, Park JE, Yee ST, Kim KH, Rhim JS, Yamada K and Seo KI:
Thiosulfinates from Allium tuberosum L. induce apoptosis via
caspase-dependent and -independent pathways in PC-3 human prostate
cancer cells. Bioorg Med Chem Lett. 18:199–204. 2008.PubMed/NCBI
|
|
50
|
Cande C, Cohen I, Daugas E, Ravaqnan L,
Larochette N, Zamzami N and Kroemer G: Apoptosis-inducing factor
(AIF): a novel caspase-independent death effector released from
mitochondria. Biochimie. 84:215–222. 2002. View Article : Google Scholar : PubMed/NCBI
|