Introduction
Oxidative stress is thought to play a critical role
in various pathological conditions such as stroke, Parkinson’s
disease, Alzheimer’s disease, AIDS and cancer, and has been
demonstrated to be related with apoptosis. Hydrogen peroxide
(H2O2), which is a type of reactive oxygen
species (ROS), is often used to induce apoptosis and cell injury
(1,2). One important pathway of
H2O2-induced apoptosis appears to involve the
mitochondrial pathway (3,4), while the ion channel protein was found
to be involved in the regulation of apoptosis following intensive
research of the mitochondrial pathway (5,6).
Recently, members of the chloride intracellular
channel (CLIC) protein family have been implicated in apoptosis.
CLIC4 is a 28.6-kDa protein corresponding to a cDNA cloned from rat
brain and also known in the mouse as mtCLIC. CLIC is a novel
p64-related protein family with seven members (p64, CLIC1-5 and
parchorin) that are localized in various cellular compartments and
expressed in multiple tissue types (7,8).
Unlike most membrane channels, CLIC proteins are present in cells
both as an integral membrane protein form and as soluble protein
forms, suggesting that CLICs may have non-channel functions
(9,10). CLIC4 is a newly identified effector
of apoptosis which is capable of altering mitochondrial function,
leading to caspase activation and cell death (11–13).
However, reduction of CLIC4 proteins by antisense expression
enhances TNFα-mediated apoptosis independent of p53 (14). It was also reported that CLIC4
translocates to the nucleus in cells following induction of
apoptosis by a variety of stress inducers, and nuclear-targeted
CLIC4 is strongly pro-apoptotic even when the mitochondrial
apoptotic pathway is inhibited by the genetic deletion of Apaf1
(15). These data imply that the
role of CLIC4 may vary depending on cell type, subcellular
localization and intensity of stress. Based on these research
findings we hypothesed that CLIC4 may play an important role in the
apoptotic response in H2O2-induced C6 cell
death.
As there are no specific inhibitors for chloride
channels available at the present time, we used the DNA
vector-based RNAi approach for establishing a long-term siRNA
strategy to block CLIC4 expression in glioma C6 cells. We found
that H2O2 induced the mitochondrial apoptosis
pathway and increased expression of CLIC4 in glioma C6 cells. To
further determine whether CLIC4 is associated with the
mitochondrial pathway that causes
H2O2-induced C6 cell death, MMP and
expression levels of Bcl-2, Bax, cleaved caspase-3 were also
examined after treatment with different concentrations of
H2O2 in mock and siRNA groups. Western blot
assay showed that the modulating effect of CLIC4 during
H2O2-induced glioma cell injury was not
correlated with the Bcl-2 family. We also found that reduction in
expression of CLIC4 increased its translocation in C6 cells treated
with H2O2.
Materials and methods
Construction of siRNA expression
vectors
pSilencer™ neo3.1-H1 siRNA and pSilencer™
neo3.1-H1-scramble siRNA (Ambion Inc., Austin, TX, USA) were used
for DNA vector-based siRNA synthesis under the control of the H1
promoter in vitro. Three CLIC4 mRNA-targeted hairpin siRNAs
were designed from different positions of the rat glioma cell
mtCLIC sequence (GenBank™ accession number EF397567). The specific
siRNA sequences for mtCLIC are shown in Table I. These oligonucleotides contain a
sense strand of 19 or 20 nucleotides followed by a short spacer
(TTCAAGAGA), the antisense strand, and five terminators
(Biotechnic, Inc., Shanghai, China). A scrambled siRNA (Ambion)
which did not match any rat sequences in a BLAST search was used as
a negative control. After annealing, the double-stranded
complementary oligonucleotides were cloned into the BamHI
and HindIII sites of the pSilencer 3.1-H1 plasmids, followed
by amplification in E. coli and DNA sequencing.
 | Table IsiRNA primer sequences for CLIC4. |
Table I
siRNA primer sequences for CLIC4.
| Gene | Primers | 5′→3′ sequence |
|---|
| CLIC4-siRNA-1 | Forward |
GATCCGCCTTCAACAGCGAAGTCAATTCAAGAGATTGACTTCGCTGTTGAAGGTTTTTTGGAAA |
| Reverse |
AGCTTTTCCAAAAAACCTTCAACAGCGAAGTCAATCTCTTGAATTGACTTCGCTGTTGAAGGCG |
| CLIC4-siRNA-2 | Forward |
GATCCGAACAGCATGGAGGACATCTTCAAGAGAGATGTCCTCCATGCTGTTCTTTTTTGGAAA |
| Reverse |
AGCTTTTCCAAAAAAGAACAGCATGGAGGACATCTCTCTTGAAGATGTCCTCCATGCTGTTCG |
| CLIC4-siRNA-3 | Forward |
GATCCGGCTCAAGACCAGAGGCTAATTTCAAGAGAATTAGCCTCTGGTCTTGAGTTTTTTGGAAA |
| Reverse |
AGCTTTTCCAAAAAACTCAAGACCAGAGGCTAATTCTCTTGAAATTAGCCTCTGGTCTTGAGCCG |
Cell culture and transfection
The rat glioma C6 cell line was obtained from the
Cell Bank of the Chinese Academy of Sciences (CACAS) (Shanghai,
China) and cultured in monolayers in plastic flasks in Iscove’s
modified Dulbecco’s medium (IMDM) supplemented with 10% fetal
bovine serum (FBS) and antibiotics (100 kU/l penicillin and 100
mg/l streptomycin) in a 95% air, 5% CO2 incubator at
37°C. For cell transfection, Lipofectamine 2000 (Invitrogen) was
used for transfecting the plasmids following the manufacturer’s
instructions. The resulting recombinant pSilencer 3.1-H1 constructs
were then introduced into the C6 cells, and the expression of CLIC4
mRNA and protein was examined by RT-PCR and western blotting,
respectively. Cells were cultured for another 24 h after
transfection and used for experiments.
Reverse transcription-PCR
Total cellular RNA was prepared with TRIzol
(Invitrogen) and was reverse-transcribed with Moloney murine
leukemia virus (M-MLV) reverse transcriptase (Promega) according to
the manufacturer’s instructions. The PCR reaction was performed
following standard procedures. The primers used for PCR
amplification are shown in Table
II. Typical PCR parameters were: 94°C for 3 min, followed by 30
cycles of 94°C for 30 sec, 55–60°C for 30 sec, 72°C for 45 sec,
followed by a final extension step at 72°C for 10 min. After
amplification, the products were resolved by electrophoresis on
1.0% agarose gel, stained with ethidium bromide and photographed
under ultraviolet light. The amplification of the PCR products was
monitored to assure that the PCR was within the range of
linearity.
 | Table IIPrimer sequences and conditions used
for semi-quantitative RT-PCR analysis. |
Table II
Primer sequences and conditions used
for semi-quantitative RT-PCR analysis.
| Gene | Primers | 5′→3′ sequence | Amplicon size
(bp) | Annealing
temperature (°C) |
|---|
| CLIC4 | Forward |
AGCAGAAGCAGCAGCAG | 762 | 57 |
| Reverse |
ATACCTTGTCTATCCTTGATCCTA | | |
| Bcl-2 | Forward |
AACACCAGAATCAAGTGTTCG | 447 | 58 |
| Reverse |
TCAGGTGGACCACAGGTGGC | | |
| Bax | Forward |
AGGGTTTCATCCAGGATCGAGC | 468 | 58 |
| Reverse |
AGGCGGTGAGGACTCCAGCC | | |
| GAPDH | Forward |
GGGTGATGCTGGTGCTGAGTATGT | 617 | 58 |
| Reverse |
AAGAATGGGTGTTGCTGTTGAAGTC | | |
Western blotting
Anti-C terminus CLIC4 antibodies were generated as
previously described (14) and were
kindly provided by Professor Stuart H. Yuspa of the National
Institutes of Health, Bethesda, MD. The affinity-purified anti-C
terminus CLIC4 antibodies are mono-specific for CLIC4 and were used
in all experiments, including immunocytochemical and western blot
analysis. Bcl-2 (sc-492), Bax (sc-7480), cleaved caspase-3
(sc-22171), cytochrome c (sc-13156), β-actin (sc-47778), and
lamin A/C (sc-6215) antibodies were purchased from Santa Cruz
Biotechnology. SDS-PAGE and western blotting were performed using
standard techniques (16). Briefly,
cells were harvested 72 h after transfection and lysed on ice in 50
mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1%
Na3VO4, 0.1% SDS and 10 μg/ml each of
aprotinin, pepstatin and leupeptin (Sigma). Following
centrifugation at 12,000 × g for 5 min, equivalent amounts of
proteins (30 μg) were subjected to SDS-PAGE using 10–12%
polyacrylamide gels and transferred onto nitrocellulose membranes
(Whattman). The membranes were blocked with 5% bovine serum albumin
(BSA) in PBS for 1 h and then incubated overnight at 4°C with
primary antibodies against CLIC4, Bcl-2, Bax, cleaved caspase-3 and
β-actin. After extensive washing, a horseradish peroxidase-coupled
anti-rabbit (or anti-mouse) antibody (Pierce) diluted in PBS
containing 0.1% Tween-20 was added for 1 h. Protein loading was
analyzed by the immunodetection of β-actin antibody and binding was
detected by DAB stain (Sigma).
Cell viability assay
The viability of the C6 cells was determined by MTT
assays (17). Briefly, C6 cells
were seeded in 96-well plates at a density of 5×103
cells/well and incubated. The cells were then exposed to medium
containing H2O2 in different concentrations
(0, 62.5, 125, 250, 500, 750 and 1,000 μM), or treated with 250,
500 and 750 μM H2O2 and/or transfected with
the pSH1Si-scrambled plasmid or pSH1Si-CLIC4 plasmid-2. Each
treatment was repeated in 5 wells. The cells were incubated for 20
h in a 95% air, 5% CO2 incubator at 37°C. Then 10 μl of
MTT reagent (5 mg/ml in PBS; Sigma, USA) was added to each well and
incubated for another 4 h. The supernatants were discarded from the
wells, and 150 μl of DMSO was added and mixed thoroughly to
dissolve the dark blue crystals of formazan. Absorbance was
recorded at a wavelength of 570 nm. Results are expressed as the
percentage of MTT reduction, assuming that the absorbance of the
control cells was 100%.
Immunocytochemistry
The C6 cells were plated on coverslips and incubated
overnight, transfected with pSilencer neo3.1-H1-CLIC4 siRNA or
pSilencer neo3.1-H1-scrambled siRNA or pSilencer empty vector and
then treated with 250, 500 and 750 μM H2O2
for 24 h. The cells were then fixed in 4% paraformaldehyde/PBS for
30 min and extensively washed with PBS. Fixed cells were
permeabilized with 1% Triton X-100 for 10 min, washed with PBS, the
endogenous peroxidase activity was quenched after incubation in
methanol containing 3% hydrogen peroxide for 10 min, and then
blocked for 1 h in 5% bovine serum albumin/PBS. The cells were then
incubated simultaneously with anti-C terminus CLIC4 antibodies at
room temperature. As a negative control, rabbit immunoglobulins
were used to replace the primary antibody. Goat anti-rabbit IgG
conjugated with horseradish peroxidase was used as a second
antibody. Immunohistochemical staining was carried out manually at
room temperature, using an avidin-biotin-peroxidase complex method.
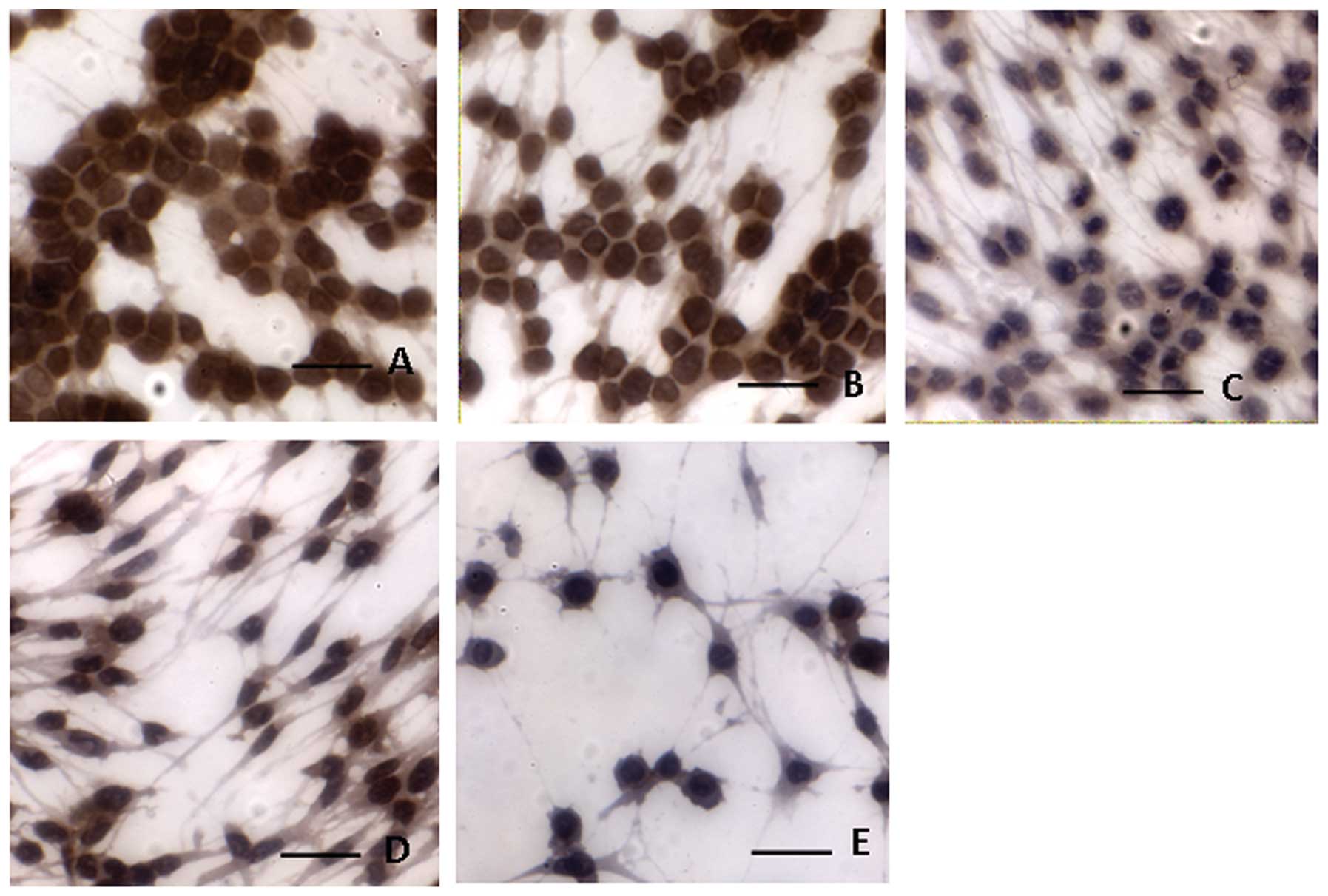
The criteria for immunohistochemical assay results are as follows:
cells exhibiting brown particle staining in the nucleus or
cytoplasm were considered positive.
Nuclear protein extraction
Nuclear extracts were prepared as previously
described with some modifications (17). Briefly, ~107 cells were
homogenized in 4 ml of cold solution A (0.6% NP-40, 150 mM NaCl, 10
mM HEPES, pH 7.9, 1 mM EDTA, 0.5 mM PMSF) using a homogenizer for
10 strokes. The cell suspension was incubated on ice for 5 min,
then centrifuged at 4°C for 10 min at 3,000 × g. The supernatant
was collected and stored at −70°C for western blot analysis of
cytosolic proteins. The pellets were resuspended in 200 μl cold
solution B (25% glycerol, 420 mM NaCl, 20 mM HEPES, pH 7.9, 1.2 mM
MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 5 mg/ml
pepstain, 5 mg/ml leupetin, 5 mg/ml aprotinin), incubated for 30
min on ice and centrifuged at 4°C for 30 min at 20,000 × g. The
supernatants containing nuclear proteins were collected, aliquoted
and stored at −70°C. The protein concentration was determined using
the Bio-Rad protein reagent.
Measurement of apoptosis by flow
cytometry
C6 cells were transfected with pSH1Si-scramble
plasmid or pSH1Si-CLIC4 plasmid-2 and then treated with 250, 500
and 750 μM H2O2 for 24 h. For quantification
of apoptosis, culture supernatants were collected and washed two
times with PBS and fixed with 70% ethanol, centrifuged and adjusted
to a concentration of 1×106 cells/ml. Incubation with 50
μg/ml propidium iodide (Sigma, USA) was carried out in the dark at
4°C for 30 min. In the DNA histogram, the amplitude of the sub-G1
DNA peak represents the number of apoptotic cells.
Mitochondrial membrane potential
measurement
The mitochondrial membrane potential (MMP) was
measured using the fluorescent probe Rhodamine 123 as previously
described (18,19). C6 cells transfected with
pSH1Si-scramble plasmid or pSH1Si-CLIC4 plasmid-2 were then treated
with 250, 500 and 750 μM H2O2 for 24 h,
respectively, followed by incubation with 10 μM Rhodamine 123. The
samples were then analyzed by a FACScan flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA). Ten thousand
cells/sample were analyzed and the mean fluorescence intensity
(MFI) in the positive cells was taken as an index of the level of
MMP.
Statistical analysis
All experiments were repeated at least three times
with different batches of cell preparations. Data are represented
as the means ± SD. Statistical analysis was carried out with the
SPSS 11.0 program using ANOVA. Results with values of P<0.05
were considered to be statistically significant.
Results
CLIC4 protein expression is upregulated
during H2O2-induced C6 cell injury
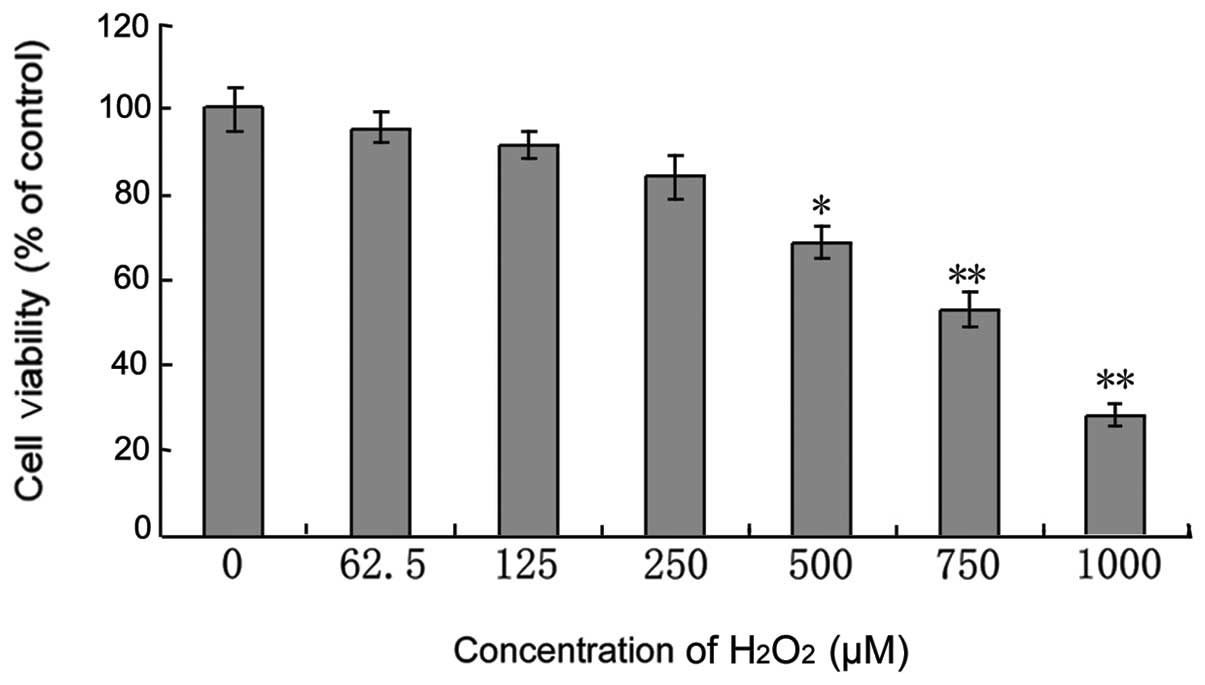
C6 cells treated with different concentrations of
H2O2 for 24 h exhibited attenuated cell
viability in a dose-dependent manner (Fig. 1). Following treatment with 250, 500
and 750 μM H2O2, the cell viability was
82.8±4.78, 65.68±4.22 and 52.83±3.63%, respectively.
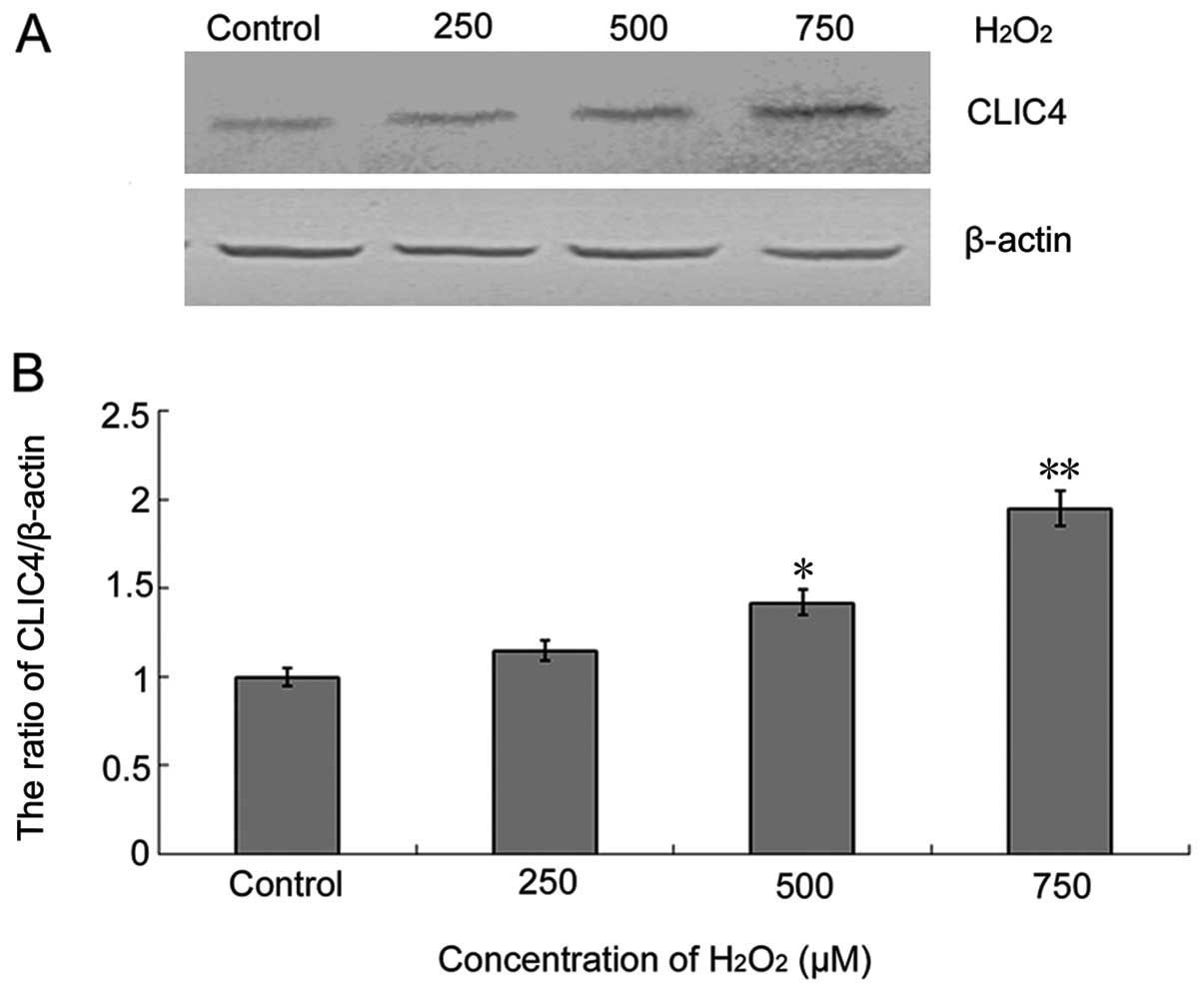
H2O2 also induced upregulation of CLIC4
protein expression in a dose-dependent manner (Fig. 2), suggesting that CLIC4 was involved
in the H2O2-induced C6 cell injury
process.
H2O2 induces C6
cell apoptosis through the mitochondrial pathway
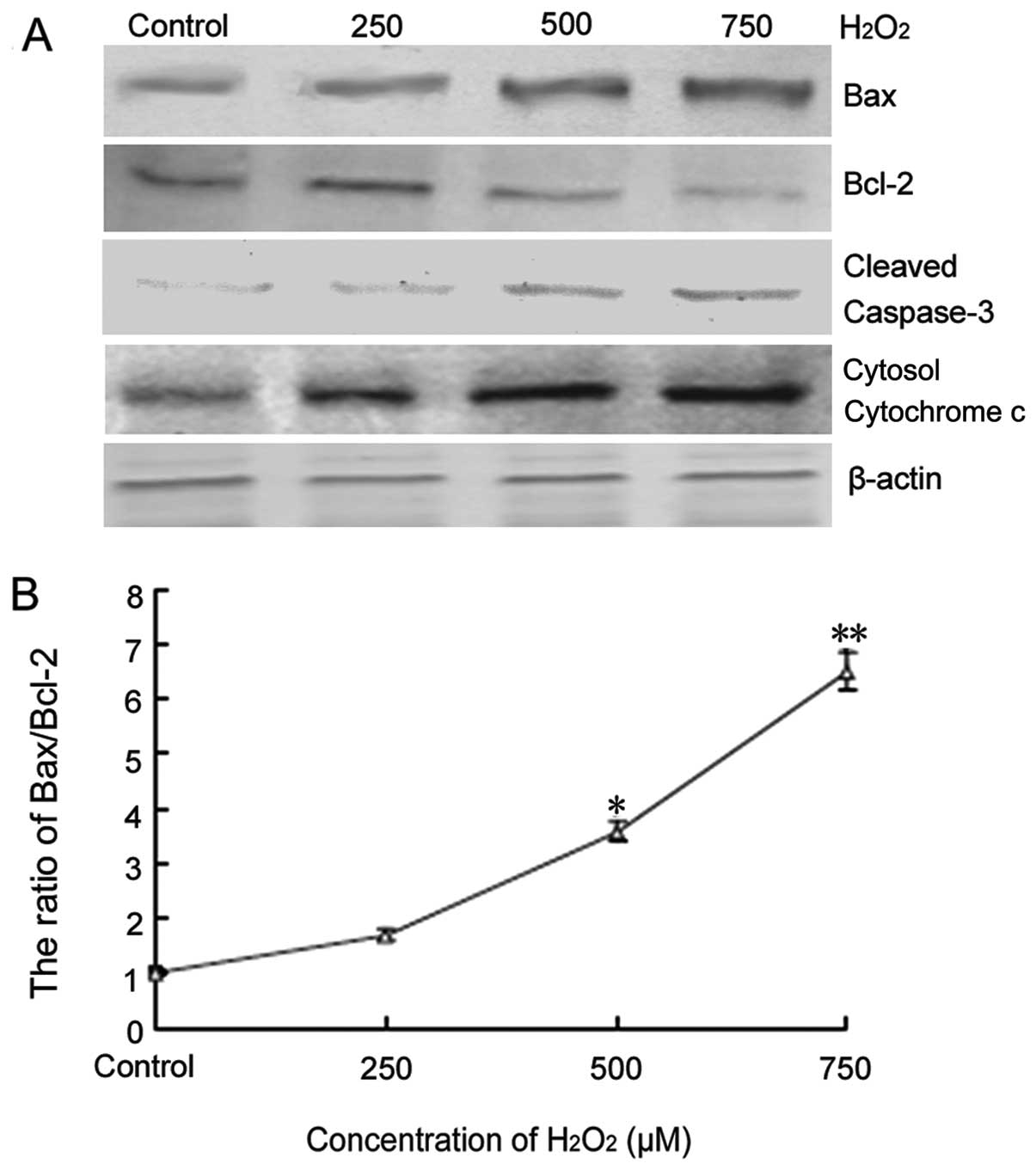
Several genes related to apoptosis were also
examined during H2O2-induced C6 cell injury.
Western blot analysis demonstrated that Bax, cytosol cytochrome
c and cleaved caspase-3 protein expression was upregulated,
whereas Bcl-2 protein expression was downregulated in a
dose-dependent manner (Fig. 3A).
The ratio of Bax/Bcl-2 was therefore increased (Fig. 3B). These results indicated that
mitochondrial apoptosis pathways were involved in
H2O2-induced C6 cell apoptosis.
CLIC4-RNAi promotes apoptosis during
H2O2-induced C6 cell injury
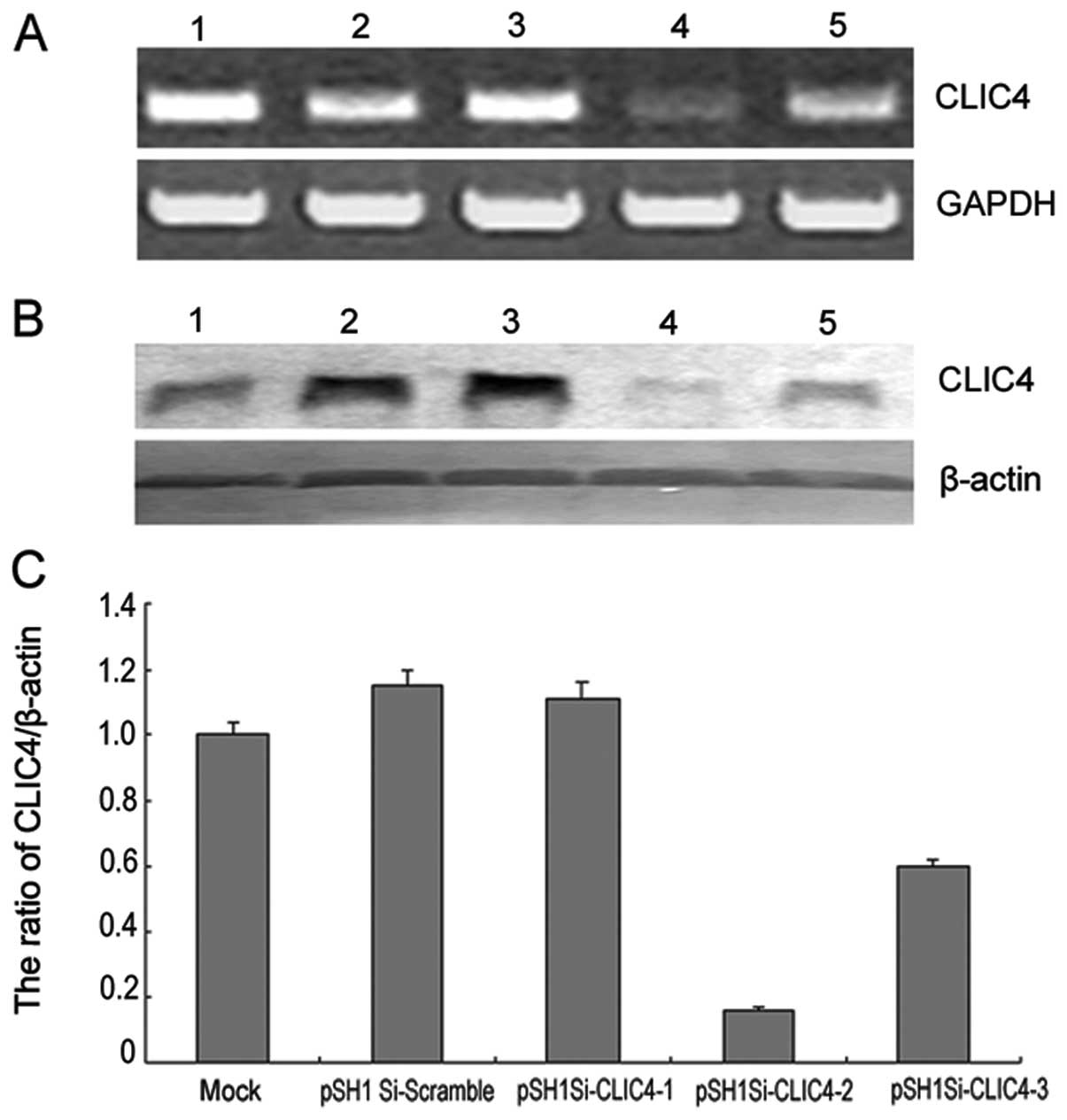
To evaluate the function of CLIC4 in
H2O2-induced C6 cell injury, C6 cells were
transfected with three different pSH1Si-CLIC4 plasmids. The
transfection efficiency was estimated by RT-PCR and western blot
analysis. Compared with the mock group or pSH1Si-scramble plasmid
group, the CLIC4 mRNA and protein expression in the pSH1Si-CLIC4
plasmid-2 group was significantly decreased (Fig. 4). Therefore, the pSH1Si-CLIC4
plasmid-2 was chosen as a candidate for further experiments. First,
the effect of CLIC4-RNAi on cell viability in C6 cells treated with
different concentrations of H2O2 was measured
by MTT assay. The cell viability was decreased in the mock group,
pSH1Si-scramble group and pSH1Si-CLIC4 group in a dose-dependent
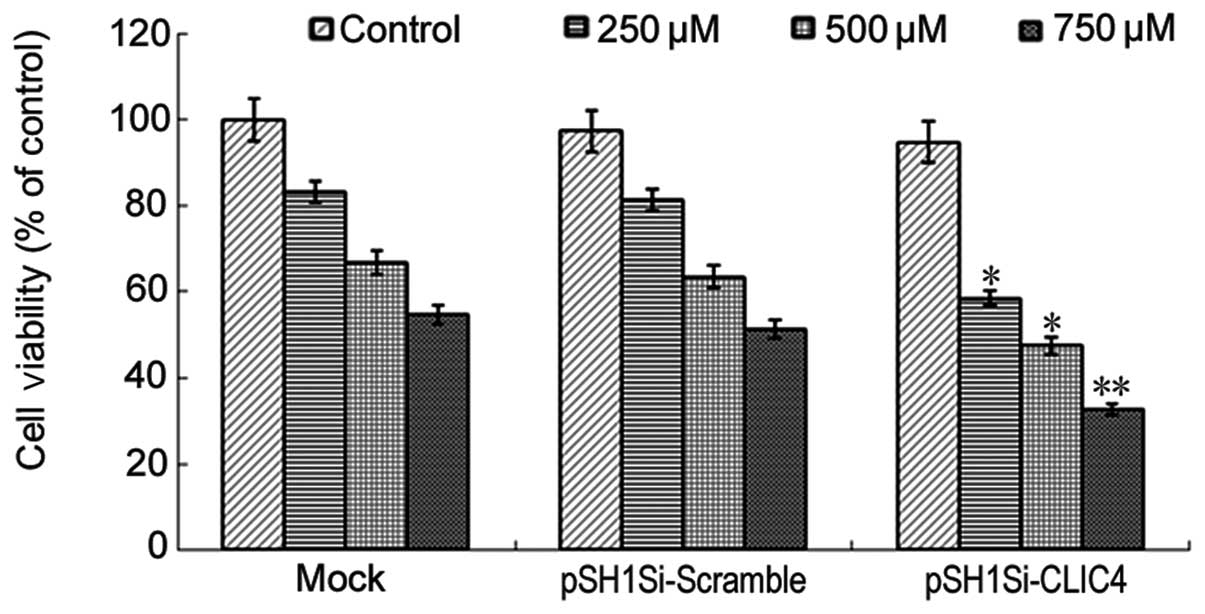
manner after 24 h (Fig. 5).
Compared with the mock group or pSH1Si-scramble plasmid group, the
cell viability in the pSH1Si-CLIC4 plasmid group was significantly
decreased (P<0.05), indicating that CLIC4-RNAi promotes C6 cell
death induced by H2O2.
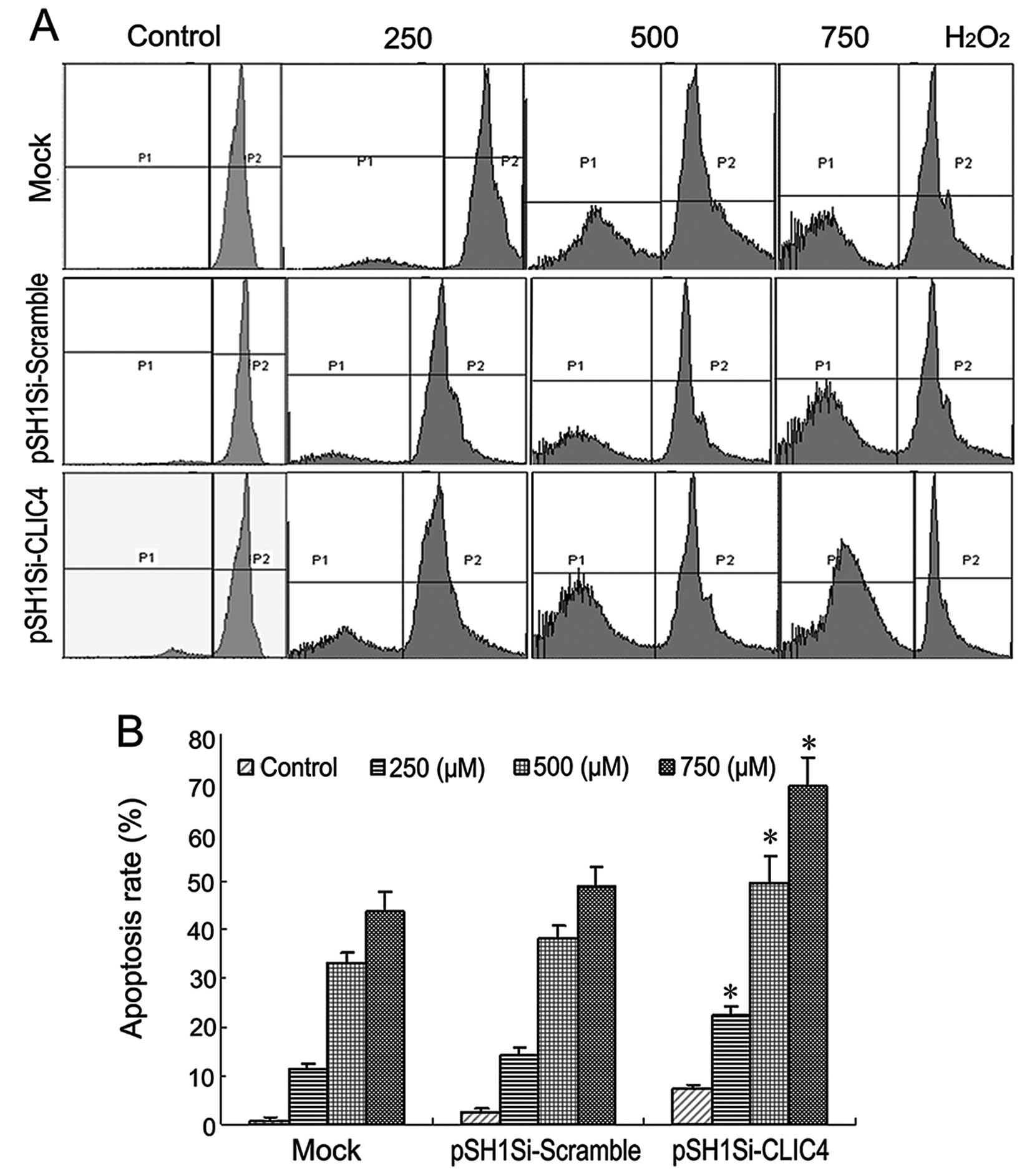
Next, we evaluated the effects of CLIC4-RNAi on the
apoptosis rate in C6 cells treated with different concentrations of
H2O2. As shown in Fig. 6, the apoptosis rate was increased in
the mock group, pSH1Si-scramble group and pSH1Si-CLIC4 group in a
dose-dependent manner. Compared with the mock group or
pSH1Si-scramble plasmid group, the apoptosis rate in the
pSH1Si-CLIC4 plasmid group was significantly increased (P<0.05).
The C6 cells transfected with the pSH1Si-CLIC4 plasmid exposed to
750 μM H2O2 exhibited an increase of 69.3% in
the apoptosis rate (P<0.05). These results suggest that
suppression of CLIC4 expression by RNA interference enhanced cell
apoptosis in H2O2-treated C6 cells.
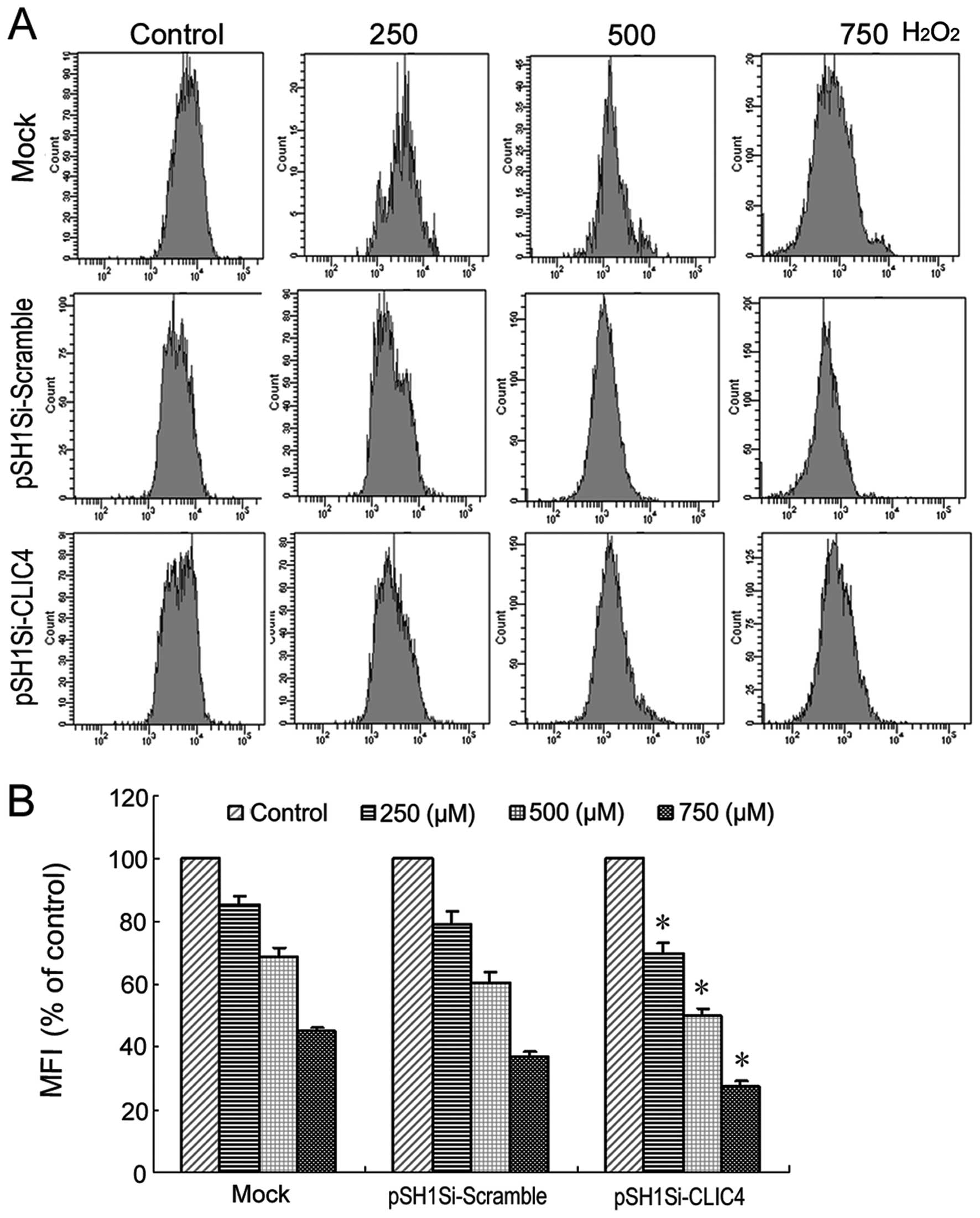
Dissipation of MMP is a critical event in the early
stages of apoptosis. FACS was used to measure the mean
mitochondrial membrane potential as determined by Rhodamine 123.
Treatment with 250, 500 and 750 μM H2O2
induced decreases in MMP that reached 85.5, 68.9 and 45.1% of the
control levels, respectively (P<0.05, P<0.01, P<0.01).
There was no difference between the mock group and the
pSH1Si-scramble plasmid group in regards to MMP. Compared with the
mock group or pSH1Si-scramble plasmid group, a significant decrease
in MMP that reached 69.8, 49.9 and 27.7% of control levels was
observed in the CLIC4 siRNA-transfected cells after
H2O2 exposure (P<0.05, P<0.05,
P<0.05) (Fig. 7). This data
clearly showed that H2O2 caused mitochondrial
dysfunction, characterized by decreased MMP which may subsequently
decrease cellular viability leading to cell death.
Apoptotic cell death is also known to be regulated
by Bax and Bcl-2 proteins. The fate of cells is thought to be
determined by the balance between pro-apoptotic and anti-apoptotic
genes. mRNA and protein expression of pro-apoptotic gene Bax was
upregulated and mRNA and protein expression of the anti-apoptotic
gene Bcl-2 was downregulated in a dose-dependent manner in the mock
group and pSH1Si-scramble plasmid group. Similar results were also
found in the pSH1Si-CLIC4 plasmid group (Fig. 8A and B). The Bax/Bcl-2 ratio is the
determination of the cell fate. As shown in Fig. 8C, the ratio of Bax/Bcl-2 in each
group was increased in a dose-dependent manner, but no differences
were noted among the mock, pSH1Si-scramble and pSH1Si-CLIC4 groups.
These results suggest that CLIC4-RNAi promotes apoptosis induced by
H2O2, but Bax/Bcl-2 is not involved in this
process.
CLIC4-RNAi enhances CLIC4 nuclear
translocation during H2O2-induced C6 cell
apoptosis
To further investigate the effect of CLIC4-RNAi on
apoptosis, we examined the levels of CLIC4 protein by
immunocytochemistry. As shown in Fig.
9, the number of C6 cells decreased in the pSH1Si-CLIC4 group
in a dose-dependent manner. Compared to the mock group and the
pSH1Si-scramble group, CLIC4 translocation to the nucleus in the
pSH1Si-CLIC4 group increased in a dose-dependent manner (Fig. 9).
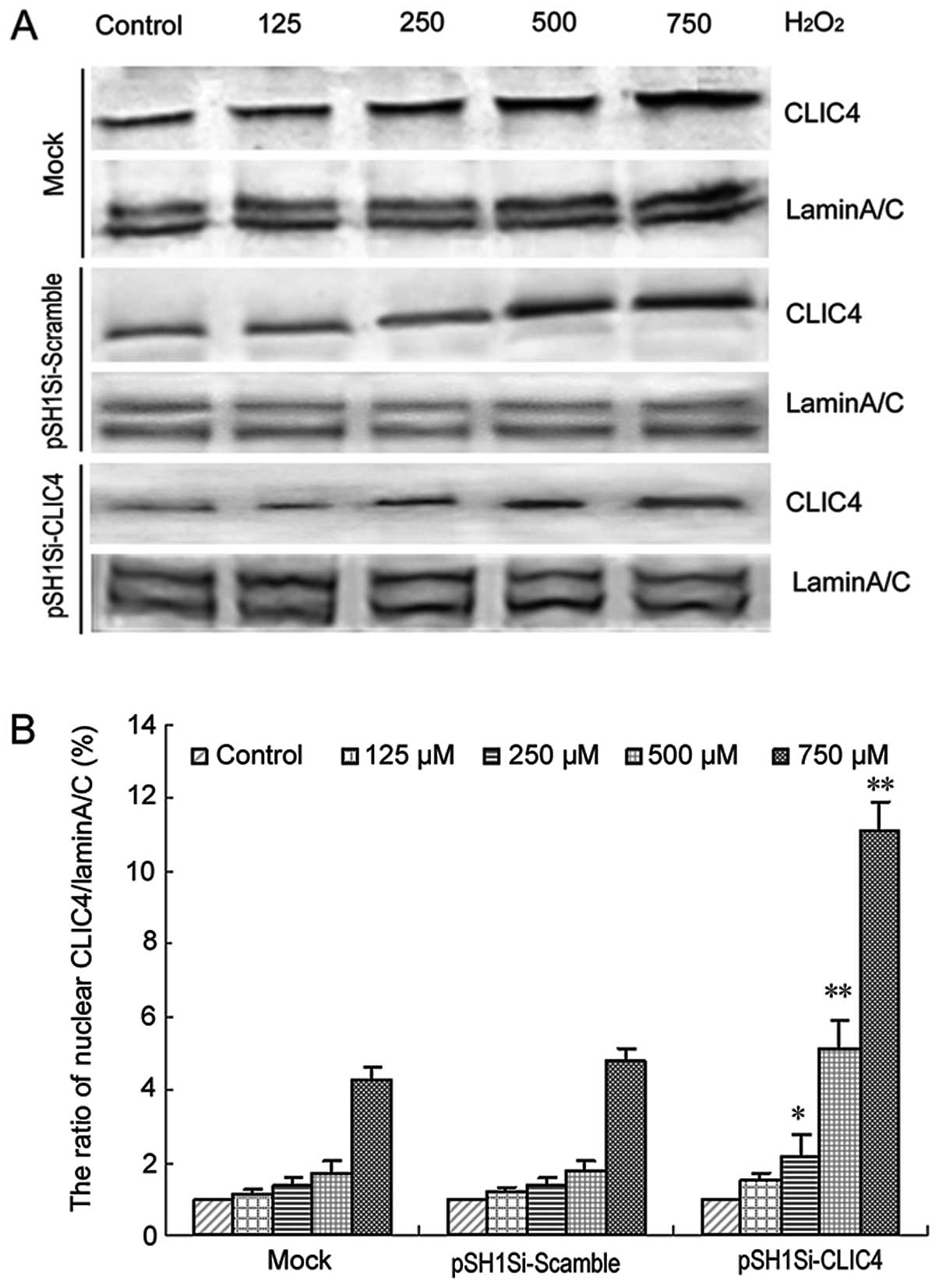
The levels of CLIC4 protein in the nucleus after
H2O2 treatment were examined in the mock
cells and cells transfected with the pSH1Si-scramble or
pSH1Si-CLIC4 plasmids. The results showed that the nuclear CLIC4
protein expression was upregulated in both the mock group and the
pSH1Si-scramble group in a dose-dependent manner (Fig. 10). Although the CLIC4 protein
expression was inhibited by RNAi in the pSH1Si-CLIC4 group, nuclear
CLIC4 protein was still increased in a dose-dependent manner in
response to increased concentration of H2O2
(Fig. 10). Compared to the
baseline, the relative fold increases in nuclear CLIC4 protein
levels in the pSH1Si-CLIC4 group were much more than those in the
mock or the pSH1Si-scramble groups. These results indicated that
CLIC4 nuclear translocation plays an important role during
H2O2-induced C6 cell apoptosis.
Discussion
Apoptosis is a tightly regulated form of cell death,
also known as programmed cell death (PCD), which is an inherent
cellular response for effective cellular disposal against
developmental and environmental insults. It is an important process
during normal development and is also involved in various diseases
such as cancer, viral infections and nerve degeneration. Apoptosis
can be induced by diverse stimuli. Common signaling mediators,
including reactive oxygen species (ROS), may damage DNA, lipids,
proteins and other macromolecules (20,21).
As a type of ROS, hydrogen peroxide (H2O2)
produces cytotoxicity (22). Thus,
H2O2 has been extensively used to duplicate
the cell model of oxidative damage and apoptosis (23).
CLIC4/mtCLIC (referred to here as CLIC4) is one of
the seven-member family of chloride intracellular channels and its
biological functions have been thoroughly studied. c-Myc and p53
binding sites have been found in the CLIC4 promoter and CLIC4 is a
direct response gene for both c-Myc and p53 (12,13).
The expression of CLIC4 transcripts is also regulated by the tumor
necrosis factor (TNF)α (11). CLIC4
is upregulated in response to p53-, TNFα- or c-Myc-mediated
apoptosis which is characterized by changes in the intrinsic
mitochondrial apoptotic pathway (11–13).
In the present study, we demonstrated that
H2O2 induces C6 cell apoptosis in a
dose-dependent manner and this apoptosis is associated with
upregulation of cleaved caspase-3 protein expression and cytochrome
c and an increased ratio of Bax/Bcl-2. We also demonstrated
that H2O2 induced decreases in MMP along with
an increased apoptosis rate. This suggests activation of the
mitochondrial apoptosis pathway in
H2O2-induced C6 cell apoptosis, consistent
with previous reports (3,4). Notably, the CLIC4 protein expression
was also upregulated during H2O2-induced
apoptosis in C6 cells, indicating CLIC4 was involved in
H2O2-induced C6 cell apoptosis.
In the present study, we identified the cDNA
nucleotide and deduced amino acid sequences of CLIC4 in SD rat
glioma cells (GenBank accession nunber EF397567). For the first
time according to the best of our knowledge, we successfully
constructed a recombinant siRNA expression plasmid which
significantly inhibits CLIC4 gene expression. Since there was an
upregulation of CLIC4 detected after H2O2
treatment in C6 cells, we considered that downregulation of CLIC4
would protect C6 cells against H2O2-induced
apoptosis. In contrast, suppression of CLIC4 expression by RNA
interference enhanced H2O2-induced C6 cell
apoptosis. The enhancement of cell apoptosis by suppression of
CLIC4 expression suggests the importance of CLIC4 in
H2O2-induced cell apoptosis, while the result
that CLIC4 protein expression was upregulated in cells treated with
H2O2 further confirmed the importance of
CLIC4 in cell apoptosis induced by H2O2. The
same results were also found to occur in TNFα-mediated apoptosis
(11,12).
The mitochondrion is a sensitive target for
oxidative damage under certain pathological conditions, e.g
exposure to H2O2. Accumulating evidence
suggests a central role of the mitochondrion in cellular apoptosis
(24,25). Dissipation of MMP is a critical
event in the early stages of apoptosis (26). The localization to the mitochondrial
inner membrane and the putative pore-forming and ion transport
activities of CLIC4 are involved in mitochondrial dysfunction and
apoptosis (8). It was reported that
CLIC4 levels were elevated in mitochondria isolated from
mtDNA-depleted cells, and specific siRNA for CLIC4 reduced MMP
measured in mtDNA-depleted L929 cells (27). Another report showed that
overexpression of CLIC4 reduced MMP, resulting in the release of
cytochrome c into the cytoplasm, activation of caspases, and
induction of apoptosis (12). In
the present study, we showed that suppression of CLIC4 expression
by RNA interference promoted a decreases in MMP along with an
increased apoptosis rate. This suggests that the MMP can be
regulated by the level of CLIC4 expression and CLIC4 is involved in
H2O2-induced C6 cell apoptosis.
The Bcl-2 family proteins play critical roles in the
control of apoptosis. The stoichiometry of pro-apoptotic and
anti-apoptotic Bcl-2 family members decides the fate of the cell.
Pro-apoptotic proteins such as Bax by translocation from the
cytosol to the mitochondria induce cytochrome c release,
whereas Bcl-2 exerts its anti-apoptotic activity, at least in part
by preventing Bax redistribution to mitochondria (28,29).
Our experiments demonstrated that H2O2
exposure caused an increase in the Bax/Bcl-2 ratio and cleaved
caspase-3 expression in a dose-dependent manner and a simultaneous
increase in CLIC4 expression in C6 cells. It was reported that
overexpression of CLIC4 induces apoptosis, and CLIC4 cooperates
with Bax in the induction of cell death (12). Thus, it appears that CLIC4 is
important in cell apoptosis induced by H2O2.
However, suppression of CLIC4 expression by RNA interference did
not increase the ratio of Bax/Bcl-2, but did increased the
apoptosis rate during H2O2-induced C6 cell
apoptosis. These results suggest that CLIC4-RNAi promotes apoptosis
induced by H2O2, but Bax/Bcl-2 is not
involved in this process. It was also reported that antisense CLIC4
did not prevent apoptosis induced by Bax, suggesting that CLIC4 and
Bax function through independent pathways (12). Other molecules in addition to the
Bcl-2 family, which are associated with CLIC4, may be involved in
H2O2-induced C6 cell apoptosis.
It was reported that nuclear translocation of CLIC4
was detected in cells undergoing p53-mediated apoptosis. When CLIC4
is targeted directly to the nucleus, apoptosis is accelerated and
proceeds in the absence of mitochondrial-dependent caspase
activation (15). To further
investigate the effect of CLIC4-RNAi on apoptosis, we examined the
levels of CLIC4 in the nucleus after H2O2
treatment. We demonstrated that H2O2 caused
nuclear translocation of CLIC4 in a dose-dependent manner.
Suppression of CLIC4 expression by RNA interference enhanced the
upregulation of CLIC4 in the nucleus and increased the apoptosis in
H2O2-treated C6 cells. This, therefore,
suggests that the translocation of CLIC4 to the nucleus occurs in
H2O2-induced C6 cell apoptosis. The nuclear
localization signal (NLS) on the C terminus of CLIC4 appears to
play a crucial role in nuclear translocation (15). CLIC4 translocation to the nucleus
may participate in the alteration of pH and chloride ion content
that could be involved in apoptotic events in the nucleus, such as
activation of nucleases and DNA fragmentation (30). The translocation of CLIC4 to the
cortical actin cytoskeleton and its association with AKAP350 at the
centrosome and midbody may be important for regulating the cell
cycle (31). Inhibiting the
expression of CLIC4 was found to trigger both mitochondrial
apoptosis involved in Bax/Bcl-2 and cytochrome c release
under starvation and endoplasmic reticulum stress-induced apoptosis
(32). These results suggest a role
for CLIC4 in apoptosis independent of ion channel regulation.
Together, this data show that H2O2-induced C6
cell apoptosis is associated with the redistribution of CLIC4 in
several cellular compartments.
In summary, we demonstrated that suppression of
CLIC4 expression by RNA interference enhanced the apoptotic
activity of H2O2. Dissipation of MMP and
nuclear translocation of CLIC4 were involved in the activation of
apoptosis induced by H2O2. These data suggest
that the CLIC4 protein plays an important role in the regulation of
oxidative stress and apoptosis, yet further studies are needed to
confirm these findings.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 30570687 and 81272876),
the Jilin Provincial Research Foundation for Basic Research, China
(nos. 201015200, 200705373 and 200505139) and the ‘211 Project’ of
Jilin University. We greatly appreciate Professor Stuart H. Yuspa
of the National Institutes of Health in Bethesda, MD for his
generous contribution of the antibodies against CLIC4.
References
|
1
|
Castillo SS, Levy M, Thaikoottathil JV and
Goldkorn T: Reactive nitrogen and oxygen species activate different
sphingomyelinases to induce apoptosis in airway epithelial cells.
Exp Cell Res. 313:2680–2686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Son JH, Yoo HH and Kim DH: Activation of
de novo synthetic pathway of ceramides is responsible for the
initiation of hydrogen peroxide-induced apoptosis in HL-60 cells. J
Toxicol Environ Health A. 70:1310–1318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh M, Sharma H and Singh N: Hydrogen
peroxide induces apoptosis in HeLa cells through mitochondrial
pathway. Mitochondrion. 7:367–373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh M and Singh N: Induction of
apoptosis by hydrogen peroxide in HPV 16-positive human cervical
cancer cells: involvement of mitochondrial pathway. Mol Cell
Biochem. 310:57–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian Y, Du YH, Tang YB, Lv XF, Liu J, Zhou
JG and Guan YY: ClC-3 chloride channel prevents apoptosis induced
by hydrogen peroxide in basilar artery smooth muscle cells through
mitochondria dependent pathway. Apoptosis. 16:468–477. 2011.
View Article : Google Scholar
|
|
6
|
Ryu SY, Peixoto PM, Teijido O, Dejean LM
and Kinnally KW: Role of mitochondrial ion channels in cell death.
Biofactors. 36:255–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jentsch TJ, Friedrich T, Schriever A and
Yamada H: The CLC chloride channel family. Pflugers Arch.
437:783–795. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashley RH: Challenging accepted ion
channel biology: p64 and the CLIC family of putative intracellular
anion channel proteins. Mol Membr Biol. 20:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harrop SJ, DeMaere MZ, Fairlie WD, et al:
Crystal structure of a soluble form of the intracellular chloride
ion channel CLIC1 (NCC27) at 1.4-A resolution. J Biol Chem.
276:44993–5000. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Littler DR, Harrop SJ, Brown LJ, et al:
Comparison of vertebrate and invertebrate CLIC proteins: the
crystal structures of Caenorhabditis elegans EXC-4 and
Drosophila melanogaster DmCLIC. Proteins. 71:364–378. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fernández-Salas E, Sagar M, Cheng C, Yuspa
SH and Weinberg WC: p53 and tumor necrosis factor alpha regulate
the expression of a mitochondrial chloride channel protein. J Biol
Chem. 274:36488–36497. 1999.PubMed/NCBI
|
|
12
|
Fernández-Salas E, Suh KS, Speransky VV,
et al: mtCLIC/CLIC4 an organellular chloride channel protein is
increased by DNA damage and participates in the apoptotic response
to p53. Mol Cell Biol. 22:3610–3620. 2002.PubMed/NCBI
|
|
13
|
Shiio Y, Suh KS, Lee H, Yuspa SH, Eisenman
RN and Aebersold R: Quantitative proteomic analysis of myc-induced
apoptosis: a direct role for Myc induction of the mitochondrial
chloride ion channel mtCLIC/CLIC4. J Biol Chem. 281:2750–2756.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suh KS, Mutoh M, Gerdes M, et al:
Antisense suppression of the chloride intracellular channel family
induces apoptosis enhances tumor necrosis factor α-induced
apoptosis and inhibits tumor growth. Cancer Res. 65:562–571.
2005.PubMed/NCBI
|
|
15
|
Suh KS, Mutoh M, Nagashima K, et al: The
organellular chloride channel protein CLIC4/mtCLIC translocates to
the nucleus in response to cellular stress and accelerates
apoptosis. J Biol Chem. 279:4632–4641. 2004.PubMed/NCBI
|
|
16
|
Konturek PC, Burnat G, Rau T, Hahn EG and
Konturek S: Effect of adiponectin and ghrelin on apoptosis of
Barrett adenocarcinoma cell line. Digest Dis Sci. 53:597–605. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elingold I, Isollabella MP, Casanova MB,
et al: Mitochondrial toxicity and antioxidant activity of a
prenylated flavonoid isolated from Dalea elegans. Chem Biol
Interact. 171:294–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buchet K and Godinot C: Functional
F1-ATPase essential in maintaining growth and membrane potential of
human mitochondrial DNA-depleted rho degrees cells. J Biol Chem.
273:22983–22989. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park KS, Nam KJ, Kim JW, et al: Depletion
of mitochondrial DNA alters glucose metabolism in SK-Hep1 cells. Am
J Physiol Endocrinol Metab. 280:E1007–E1014. 2001.PubMed/NCBI
|
|
20
|
Huang SX, Partridge MA, Ghandhi SA,
Davidson MM, Amundson SA and Hei TK: Mitochondria-derived reactive
intermediate species mediate asbestos-induced genotoxicity and
oxidative stress-responsive signaling pathways. Environ Health
Perspect. 120:840–847. 2012. View Article : Google Scholar
|
|
21
|
Peng X and Gandhi V: ROS-activated
anticancer prodrugs: a new strategy for tumor-specific damage. Ther
Deliv. 3:823–833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitamura Y, Ota T, Matsuoka Y, et al:
Hydrogen peroxide-induced apoptosis mediated by p53 protein in
glial cells. Glia. 25:154–164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Kong X, Kang J, Su J, Li Y, Zhong
J and Sun L: Oxidative stress induces parallel autophagy and
mitochondria dysfunction in human glioma U251 cells. Toxicol Sci.
110:376–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shih CM, Ko WC, Wu JS, et al: Mediating of
caspase-independent apoptosis by cadmium through the
mitochondria-ROS pathway in MRC-5 fibroblasts. J Cell Biochem.
91:384–397. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kakkar P and Singh BK: Mitochondria: a hub
of redox activities and cellular distress control. Mol Cell Biol.
305:235–253. 2007.PubMed/NCBI
|
|
27
|
Arnould T, Mercy L, Houbion A, et al:
mtCLIC is up-regulated and maintains a mitochondrial membrane
potential in mtDNA-depleted L929 cells. FASEB J. 17:2145–2147.
2003.PubMed/NCBI
|
|
28
|
Wong WW and Puthalakath H: Bcl-2 family
proteins: the sentinels of the mitochondrial apoptosis pathway.
IUBMB Life. 60:390–397. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murphy KM, Ranganathan V, Farnsworth ML,
Kavallaris M and Lock RB: Bcl-2 inhibits Bax translocation from
cytosol to mitochondria during drug-induced apoptosis of human
tumor cells. Cell Death Differ. 7:102–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pervaiz S and Clément MV: A permissive
apoptotic environment: function of a decrease in intracellular
superoxide anion and cytosolic acidification. Biochem Biophys Res
Commun. 290:1145–1150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berryman MA and Goldenring JR: CLIC4 is
enriched at cell-cell junctions and colocalizes with AKAP350 at the
centrosome and midbody of cultured mammalian cells. Cell Motil
Cytoskeleton. 56:159–172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhong J, Kong X, Zhang H, et al:
Inhibition of CLIC4 enhances autophagy and triggers mitochondrial
and ER stress-induced apoptosis in human glioma U251 cells under
starvation. PLoS One. 7:e393782012. View Article : Google Scholar
|