Introduction
Pancreatic cancer remains a fatal disease with a
5-year survival rate <5% (1).
Chemotherapy regimens often fail to improve the outcome of
pancreatic cancer patients. Only 20% of pancreatic cancer patients
are eligible for surgical resection, which currently remains the
only potentially curative therapy (2). The low survival rate of patients with
pancreatic cancer points toward an increased need for novel
therapeutics, early detection and chemoprevention strategies.
Aspirin (ASP), the traditional non-steroidal
anti-inflammatory drug (NSAID), has emerged as a viable
chemopreventive agent against various types of cancer (3). ASP is reported to be capable of
suppressing pancreatic cancers growth in vitro and in
vivo(4). Clinical studies
associated with the use of ASP for pancreatic cancer
chemoprevention have met with mixed results thus far (5). Given these conflicting reports on the
use of ASP in pancreatic cancer but, simultaneously realizing the
proven benefits as a chemopreventive agent in cancer, it reaffirms
the need for further study of this drug in pancreatic cancer.
Curcumin (CUR) is a diferuloylmethane derived from turmeric
(Curcuma longa) and a pharmacologically safe agent. CUR has
recently received considerable attention due to its pronounced
anti-inflammatory, anti-oxidative and anti-carcinogenic activities
(6,7). Sulforaphane (SFN) is a naturally
occurring isothiocyanate, which is unique to cruciferous vegetables
such as broccoli, cauliflower and cabbage (8). The ultimate chemopreventive effects of
SFN involve multiple mechanisms, include apoptosis-inducing
properties (9) and induction of
cell cycle arrest.
Nuclear factor-κB (NF-κB) controls different
biological processes, such as inflammation, cell cycle and
apoptosis, and is a key antiapoptotic transcription factor in
pancreatic ductal adenocarcinoma (10). NF-κB activation has been reported in
pancreatic cancer cells, animal models of pancreatic cancer, and in
human pancreatic tissue. It has been reported that
mitogen-activated protein kinases (MAPK) participate in diverse
cellular functions such as cell proliferation, cell differentiation
and cell death (11). There are
three major MAPK family subgroups: extracellular signal-regulated
kinase 1/2 (ERK1/2), c-Jun N-terminal of stress-activated protein
kinases 1/2 (JNK1/2) and the p38 protein kinases. Previous studies
have demonstrated dual role of ERK1/2. The transient activation of
ERK1/2 plays a pivotal role in cell proliferation and that
sustained ERK1/2 activation induces cell cycle arrest and
differentiation (12,13).
Recent literature has demonstrated that rather than
administering single agents, there is increasing interest in the
use of combinations of chemopreventive agents. This approach has
provided means of obtaining increased efficacy by targeting
multiple signaling pathways and also minimized toxicity (13,14).
However, combination therapy studies specifically directed towards
pancreatic cancer prevention are still in its infancy.
To date, no group has investigated the combined
effects of low dose of aspirin (ASP), curcumin (CUR) and
sulforaphane (SFN) on pancreatic cancer. Thus, the objectives of
our study were to examine the molecular mechanism of combined
effect of low doses of ASP, CUR and SFN (ACS) in the induction of
apoptosis and anti-proliferative effects in MIA PaCa-2 and Panc-1
cells.
Materials and methods
Cell lines and cell culture
Human pancreatic cancer cell lines MIA PaCa-2 and
Panc-1 were obtained from American Type Culture Collection
(Manassas, VA, USA). Cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), 1% penicillin-streptomycin at 37°C in a 5% CO2
humidified environment.
Reagents and antibodies
ASP, CUR and SFN were purchased from LKT
Laboratories (St. Paul, MN, USA). The chemical inhibitor U0126 was
purchased from Cell Signaling Technology (Beverly, MA). Antibodies
against Phospho-ERK1/2 (Thr202/Tyr204), total ERK1/2, P-p38 MAPK
(Thr180/Tyr182), P-Akt (Ser474), total AKT, P-c-jun (Ser73), P-p53
(Ser15), cleaved caspase-3 (Asp175), cleaved PARP (Asp214), P-IκBα
(Ser32/36) and β-actin were purchased from Cell Signaling
Technology.
Cell viability assay
The cell viability assay was performed according to
the manual included with the Promega CellTitre 96 Aqueous MTS
reagent (Madison, WI, USA). Briefly, 5×103 cells were
seeded in 96-well plates. Test compounds ASP, CUR and SFN alone and
in combination ACS were added for a period of 72 h. On the last day
of the incubation period, 20% MTS and 1% of phenazine methosulfate
(PMS) were added to culture medium and incubated for 3 h at 37°C
and measured at 490 nm. All the assays were performed in
triplicates.
Cell colony formation assay
The 1×104 cells were seeded into the
six-well plates in triplicate per data point. After 24 h of
seeding, cells were treated with ASP, CUR and SFN alone and in
combinations ACS. Two weeks after treatment, cells were fixed and
stained with 0.5% crystal violet (Sigma) in methanol for 5 min.
Then, colonies consisting of 50 or more cells were counted. The
percentage cell survival was calculated (plating efficiency of
non-treated cultures = 1).
Flow cytometric analysis for
apoptosis
The detection was performed according to Annexin
V-fluorescein isothiocyanate (FITC) Vybrant Apoptosis assay kit #3
(Invitrogen, Grand Island, NY, USA). MIA PaCa-2 and Panc-1 cells
(~3×105) were seeded in six-well plates and treated with
ASP, CUR and SFN alone and in combination ACS for 48 h. The cell
suspension of 1×105 cells was then subjected to 5 μl of
FITC Annexin V and 1 μl of the 100 μg/ml PI followed by incubation
in the dark for 15 min. The samples were analyzed using Beckman
Coulter Cytomics FC500 (Brea, CA, USA).
NF-κB activation assay
The DNA-binding activity of NF-κB in pancreatic
cancer cells was quantified by ELISA, using the TransAM NF-κB p50
transcription factor assay kit (Active Motif, Carlsbad, CA, USA).
Briefly, 10 μg of total protein was incubated in 96-well plates
coated with immobilized oligonucleotide for the p50 subunit. NF-κB
binding to the target oligonucleotide was detected by incubation
with primary antibody specific for the activated form of p50
(Active Motif), visualized and quantified at 490 nm.
Western blot analysis
MIA PaCa-2 and Panc-1 cells were treated with ASP,
CUR and SFN alone and in combination for 4, 8, 24 and 48 h. Cells
were lysed in RIPA buffer and were fractionated onto SDS-PAGE gels
and then transferred to nitrocellulose membranes. The membranes
were blocked with 2% bovine serum albumin (BSA) in Tris-buffered
saline (TBS)-Tween-20 and probed with primary antibodies (1:1000
dilution) followed by horseradish peroxidase (HRP)-labeled
secondary antibodies (1:5000 dilutions). The blots were probed with
the Super Signal West Pico Chemiluminescent substrate (Thermo
Scientific, Pittsburgh, PA, USA) to visualize the immunoreactive
bands.
Statistical analysis
Results were expressed as mean ± SEM. A one-way
ANOVA followed by Dunnett’s multiple comparison test post
hoc analysis using Graph pad prism software (La Jolla, CA, USA)
was performed to analyze and compare the results. A P-value of
≤0.05% was considered significant.
Results
Combination of ASP, CUR and SFN shows a
synergistic effect on the reduction of cell viability
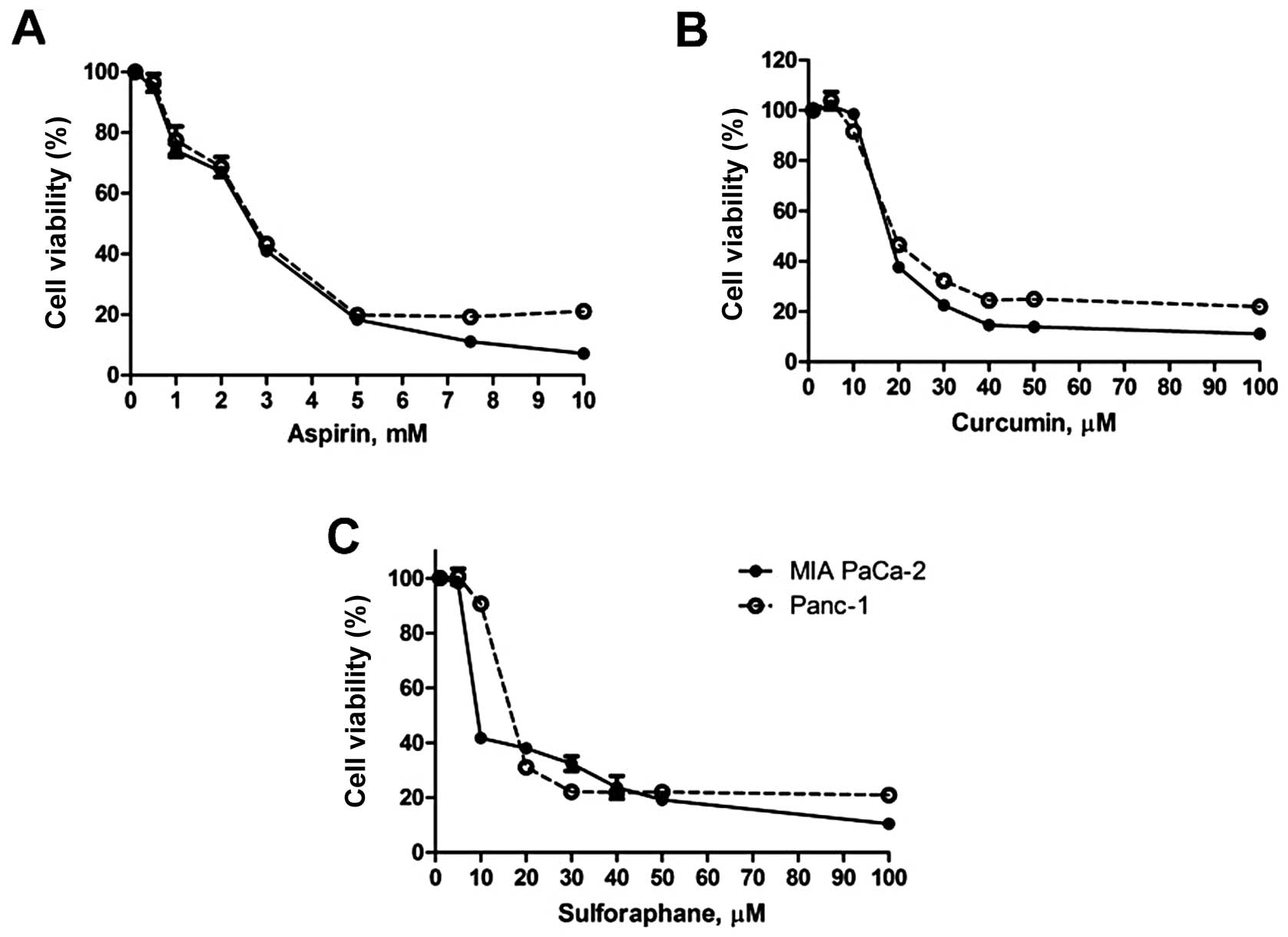
In order to evaluate the effects of ASP, CUR and SFN
on pancreatic cancer cells, we treated MIA PaCa-2 and Panc-1 with
various concentrations of the ACS treatment for 72 h, and measured
cell growth by MTS assay. A dose-dependent reduction of the growth
of MIA PaCa-2 and Panc-1 cells (Fig.
1A-C) was observed. In case of MIA PaCa-2, the IC50
concentrations for ASP, CUR and SFN observed were 2.6 mM, 19.6 μM
and 10.7 μM, respectively. Similarly, in Panc-1 cells, the
IC50 values for ASP, CUR and SFN were calculated to be
2.4 mM, 19.6 μM and 16.0 μM, respectively. Next, to examine the
effect of combined regimen on cell proliferation, MIA PaCa-2 and
Panc-1 cells were treated with ineffective concentrations of ASP (1
mM), CUR (10 μM) and SFN (5 μM) for 72 h.
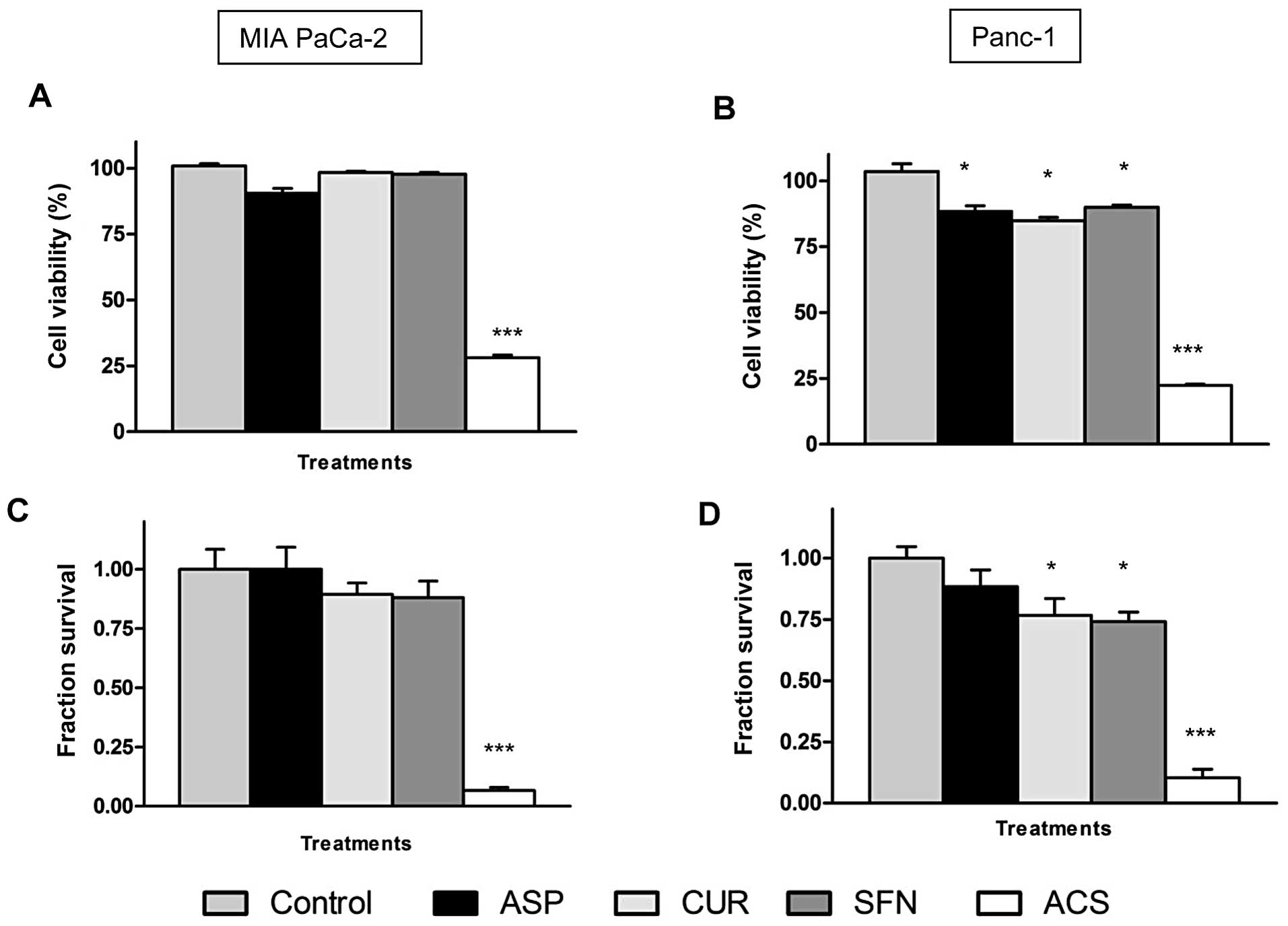
As shown in Fig. 2A,
individually, the chemopreventive agents did not show significant
change in cell viability at these concentrations, thereby
demonstrating an ineffective profile. However, when used in
combination at same concentrations, ACS showed a significant
synergistic effect with a reduction in cell viability of MIA PaCa-2
cells by as much as ~70% (P<0.001). Fig. 2B demonstrated similar results with
Panc-1 cells, where combinations of ACS showed a remarkable
decrease of ~75% (P<0.001) in cell viability. Notably, when only
dual combination studies of ASP with either CUR or SFN were
conducted at the same concentration, there was no significant
decrease in cell viability, with only ~20% decrease being observed
from dual combinations (data not shown). Thus, the triple
combination of ACS administered at low concentrations showed a
significant reduction in cell viability.
To evaluate long-term efficacy of ACS on cell
survival, a clonogenic assay was performed. The survival fraction
of the control group was set at 1 (representing 100% cell survival)
and the cell survival fraction was calculated based on individual
and combination treatment. Quantitatively, an evaluation of cell
survival on MIA PaCa-2 cells showed survival fractions of 0.92
(ASP), 0.89 (CUR) and 0.88 (SFN), whereas ACS combination showed
significant decrease in the survival fraction of 0.06 (P<0.001)
(Fig. 2C). Similar results were
observed in case of Panc-1 (Fig.
2D) with low survival fractions of cells.
ACS induces significant apoptosis in
pancreatic cancer cells
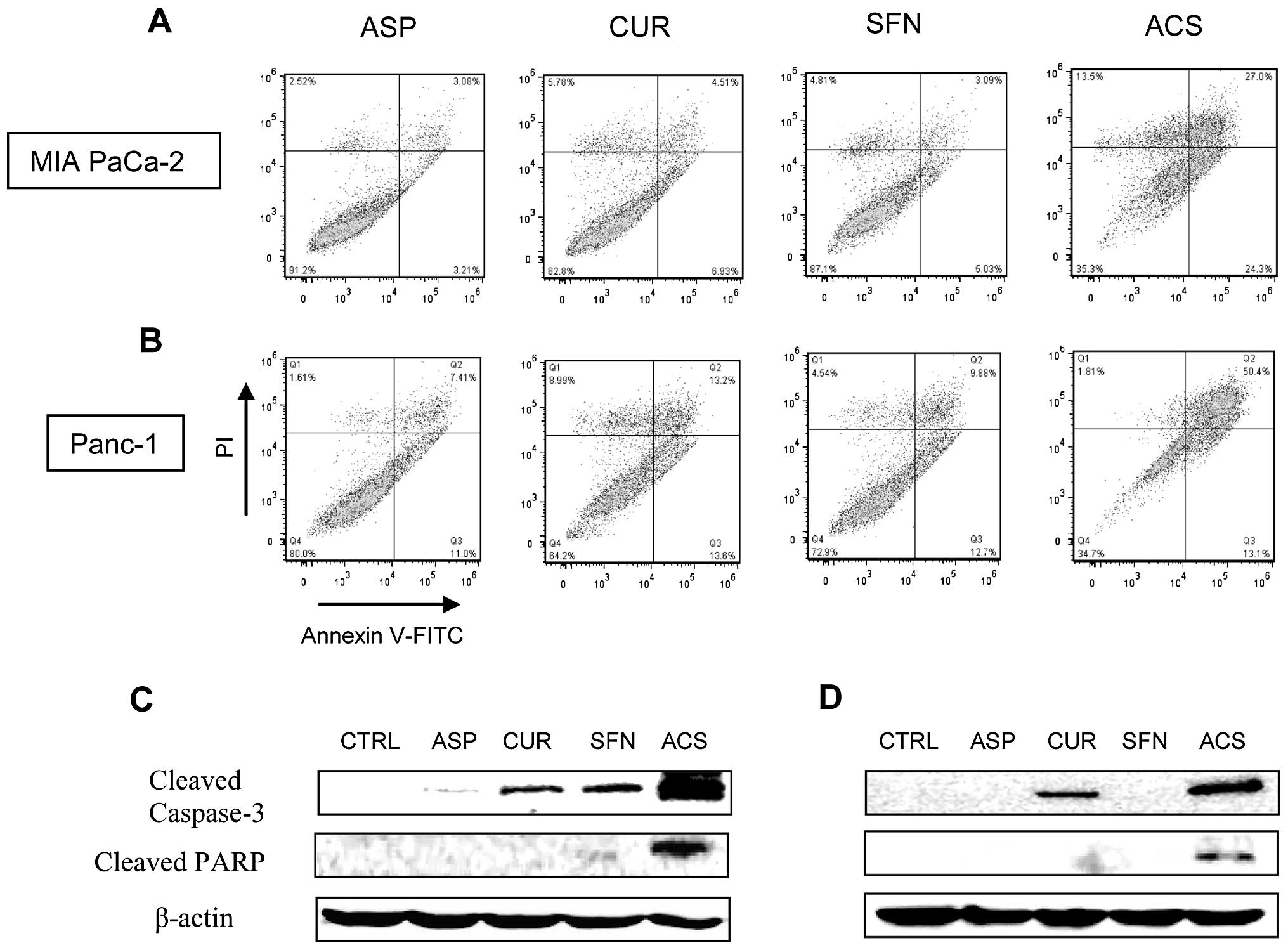
The induction of apoptosis was measured by flow
cytometry for all individual drugs and their combinations on MIA
PaCa-2 and Panc-1 cells. Individual concentrations of ASP, CUR and
SFN showed minimal apoptotic cells. In case of MIA PaCa-2 cells
(Fig. 3A), ASP, CUR and SFN showed
approximately 8, 17 and 13% cell death, respectively. However, when
mixed in combinations, the ACS combinations demonstrated ~51% cell
death (P<0.01). In case of Panc-1 cells (Fig. 3B), ACS combination showed ~63% of
apoptotic cells (P<0.01). Overall, our studies confirmed that
ACS combinations were extremely effective in inducing apoptosis of
cancer cells. Since caspase activity contributes to the overall
apoptotic morphology by cleavage of various cellular substrates, we
examined the effect of ACS treatment on caspase-3 activation and
proteolytic cleavage of PARP using western blotting. As shown in
Fig. 3C and D, there were marked
increase in the levels of cleaved caspase-3 and cleaved PARP in ACS
treatment compared with individual drug alone.
ACS inhibits NF-κB activity in pancreatic
cancer cells
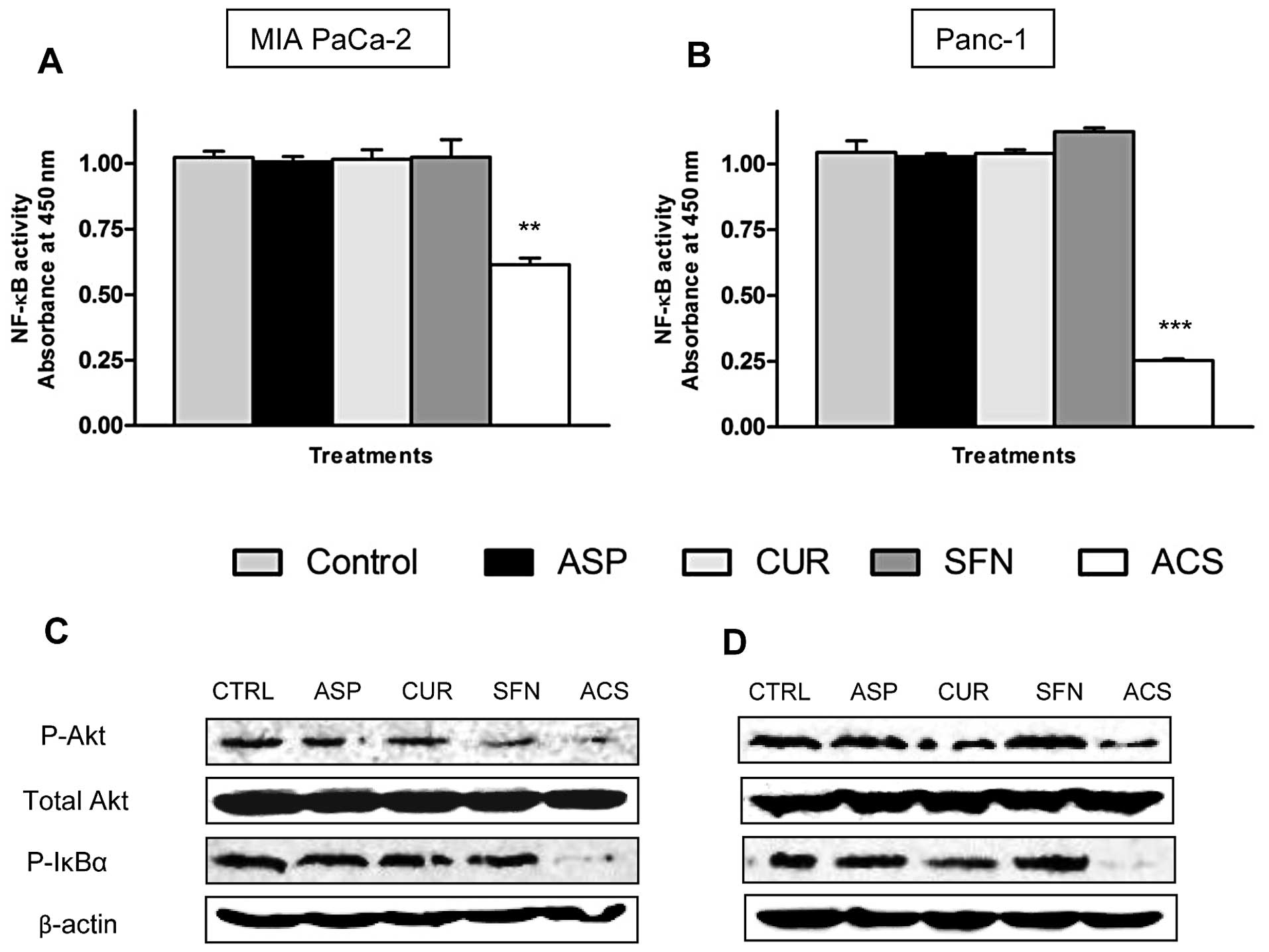
To gain further insight into the mechanism
associated with the combination ACS, the DNA binding activity of
p50 subunit of the NF-κB complex was evaluated. As shown in
Fig. 4A, ~45% (P<0.01) decrease
in the p50 binding activity was observed in MIA PaCa-2 cells in the
presence of the ACS combination, whereas ~75% (P<0.001) decrease
in NF-κB activity occurred in Panc-1 cells (Fig. 4B). These results were confirmed by
checking levels of phosphorylated IκBα by Western blotting. As
shown in Fig. 4C and D, MIA PaCa-2
cells and Panc-1 cells showed constitutive levels of P-IκBα,
whereas treatment with ACS reduced the expression of P-IκBα.
Moreover, Akt has been reported to be linked to the activation of
IκBα and NF-κB (15). Thus, studies
were conducted to examine if combination ACS inhibits
phosphorylation of IκBα through inhibition of Akt activation. As
presented in Fig. 4C and D, Akt was
constitutively active in MIA PaCa-2 and Panc-1 cells respectively,
and combination ACS inhibited phosphorylation of Akt, whereas
levels of total Akt remained the same.
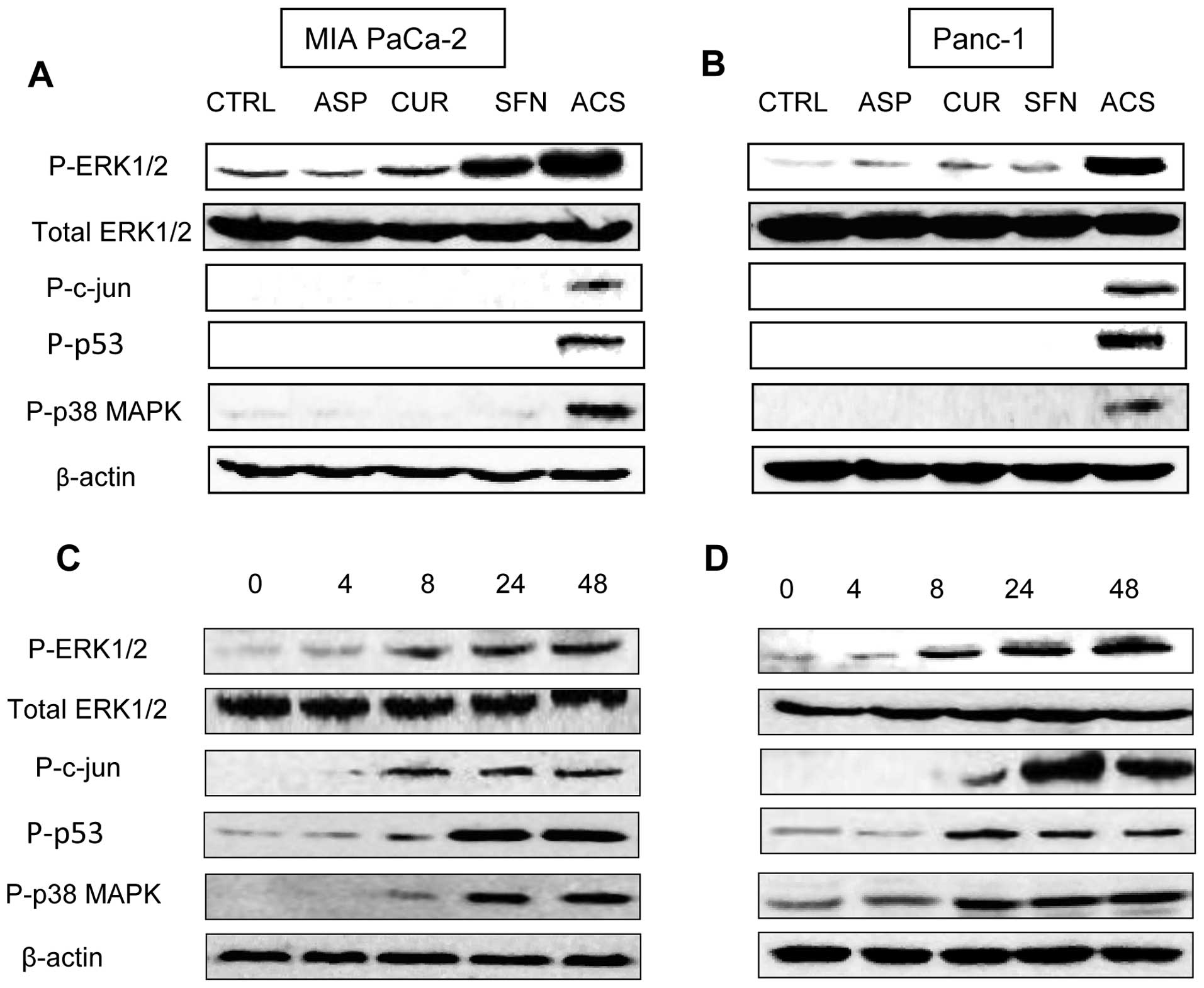
ACS induces ERK1/2 activation in
pancreatic cancer cells
The ASP, CUR and SFN are reported to modulate
ERK-MEK pathway (16–18), hence we wanted to determine whether
the ERK-MEK pathway is involved in mediating growth inhibition by
combination ACS, MIA PaCa-2 and Panc-1 protein lysates were
analyzed by western blot analysis. We observed that incubation of
MIA PaCa-2 and Panc-1 cells with combination ACS produced higher
phosphorylation of ERK1/2 compared with the control and individual
drugs (Fig. 5A and B). We also
observed increased in phosphorylation of c-jun, p53 and p38 MAPK
proteins. Next, we investigated the activation of ERK1/2 at
different time intervals for 4, 8, 24 and 48 h. The expression of
phospho-ERK1/2 was profoundly increased after 8 h of ACS treatment,
and the ERK activation was persistent for 48 h after ACS treatment.
Total ERK1/2 activity remained unchanged during all of these
conditions (Fig. 5C and D).
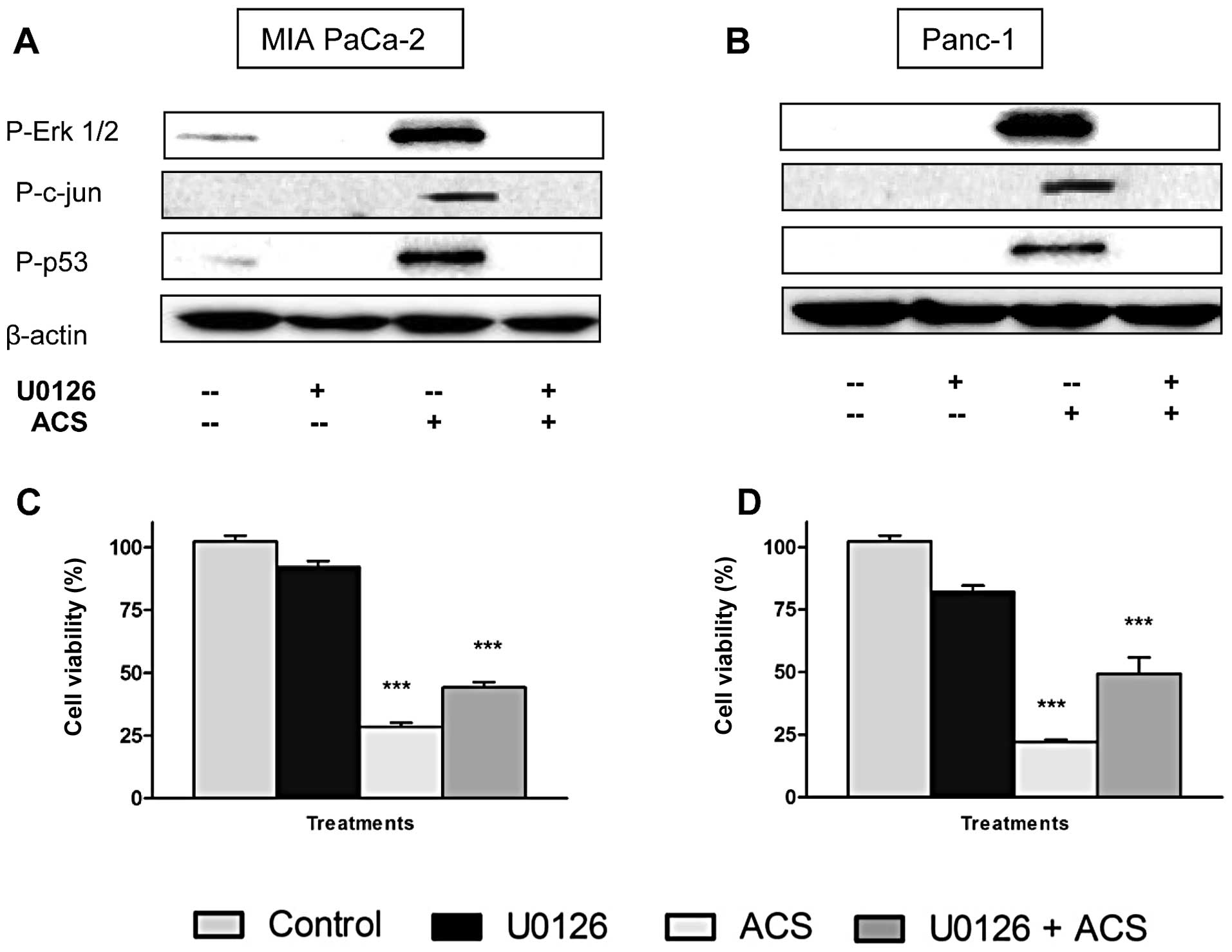
Involvement of ERK1/2 activation in
pancreatic cancer cell viability
In order to verify the involvement of ERK1/2
activation in ACS-induced apoptosis, we used MEK1/2 inhibitor,
U0126, and analyzed by western blot and cell viability assay. The
U0126 blocks MEK1/2 phosphorylation and subsequent activation of
ERK1/2. MIA PaCa-2 and Panc-1 cells were exposed to combination ACS
for 48 h after pretreatment with U0126 (10 μM) for 45 min. As shown
in Fig. 6A, ACS-induced
phosphorylation of ERK1/2, c-jun and p53 were dramatically blocked
by U0126 pretreatment in MIA PaCa-2 cells. There was no effect of
U0126 on the amount of total ERK1/2 (Fig. 6A). Similar results were observed in
Panc-1 cells (Fig. 6B). These
studies demonstrated that ACS activation of ERK1/2 was dependent on
MEK1/2.
As U0126 was able to inhibit ERK1/2 phosphorylation
induced by ACS, we next investigated whether the U0126 could
attenuate ACS-induced reduction in cell viability. As shown in
Fig. 6C and D, U0126 alone did not
alter the cell proliferation of MIA PaCa-2 and Panc-1 cells, but
pretreatment with U0126 partially attenuated ACS induced reduction
in cell viability.
Discussion
Pancreatic cancer ranks the fourth in mortality from
cancer in the United States with ~37,000 deaths each year. Early
diagnosis of this disease is difficult because it develops without
any early symptoms. Survival of patients with pancreatic cancer is
<5% over 5 years which makes this disease of great concern
(1). Therapeutic outcomes with
pancreatic cancer are not useful for patients especially upon a
late diagnosis thus strategies to prevent this disease from
occurring have become an important area of research.
Our research is focused on combination treatment
using ACS to study its effects against pancreatic cancer cells MIA
PaCa2 and Panc-1. Low concentration of single agent has largely
been demonstrated to be ineffective, hence the hypothesis that two
or more agents when delivered at low concentrations together, may
exhibit an additive or synergistic effect against the cancer cells.
This can be attributed to the multi-factorial nature of
carcinogenesis wherein cancer occurs as a result of multiple
cellular changes during a prolonged time period. The cell
proliferation studies demonstrated that low concentration of ASP,
CUR and SFN when used alone did not reduce the cell viability,
however, when combined together at same low concentration, a
significant synergistic reduction in cell viability in MIA PaCa-2
and Panc-1 cells were observed (P<0.001). Many investigators
have reported ASP, CUR and SFN alone to be effective against
pancreatic cancer; however they have used significantly higher drug
concentrations than used in this study. Concentrations of ASP (2–5
mM) and CUR (20–50 μM) have been reported in the literature to be
active against pancreatic cancer cells (7,16,17,19,20).
From our studies, we report a 2–5-fold reduction in doses with ASP
(1 mM) and CUR (10 μM). Similarly, 20–40 μM of SFN is reported to
be active against various cancers, whereas our dose of 5 μM
concentration in combination with ASP and CUR reduced the
concentration by 4–8 times (18,21,22).
Subsequent studies using apoptosis and colony formation assays
confirmed these observations and further strengthen the hypothesis
of synergistic effect using combinatorial regimens.
Akt plays critical roles in mammalian cell survival
signaling and has been shown to be activated in various cancers
(10). Activated Akt promotes cell
survival by activating the NF-κB signaling pathway (15,23)
and by inhibiting apoptosis through inactivation of several
pro-apoptotic factors including Bad, Forkhead transcription factors
and caspase-9 (24). The Akt kinase
has also been considered an attractive target for cancer prevention
and treatment. Several studies also suggest that curcumin has
molecular targets within the Akt signaling pathways, and the
inhibition of Akt activity may facilitate inhibition of
proliferation and induction of apoptosis (25–27).
Bava et al reported that curcumin downregulated
Taxol-induced phosphorylation of Akt, which interacts with NF-κB,
suggesting that enhanced anti-tumor activity by curcumin is through
the inactivation of P-Akt and NF-κB pathways (28). In addition, SFN has been reported to
induce apoptosis in pancreatic cancer cells by inhibiting caspase-3
and P-Akt (21,29,30).
In concordance with these reports, we also demonstrate that the ACS
combination downregulates activity of P-Akt and NF-κB.
Our study also presents a plausible mechanism by
which ACS combination can induce apoptosis in MIA PaCa-2 and Panc-1
cells, through activation of the ERK1/2 signaling system. It is
important to note that there are different mechanisms of ERK
activation, such as induction by growth factors could be rapid
(occurring within minutes) and transient, which leads to cell
proliferation and survival (31).
However, persistent or sustained ERK1/2 activation that last >12
h is involved in cell differentiation and death (32). The ACS combination initiates ERK1/2
induction at 8 h, and the activity remains highly elevated through
the remaining time period examined (48 h). Moreover, ERK1/2 pathway
is partially responsible for ACS-induced apoptosis, as the
suppression of proliferation is partially abrogated by inhibitor of
the MEK/ERK pathway (U0126). This finding further emphasized the
importance of ERK1/2 activation and its activity in ACS induced
apoptosis of MIA PaCa-2 and Panc-1 cells. Our results support the
pro-apoptotic role of ERK1/2 during ACS treatment and are in
agreement with previous studies, which demonstrate that ERK
activation is required for cisplatin-induced apoptosis in HeLa and
A549 cells (33). Our results also
add to the growing evidence about the involvement of sustained
activation of ERK1/2 in regulating the apoptosis. Thus, these
results provide a rationale that the low-dose ACS combination could
be developed as a potential treatment against human pancreatic
cancer.
Acknowledgements
This work was supported by National Institutes of
Health (1R03CA153812-01A1; S.P.).
References
|
1
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
2
|
Yeo TP, Hruban RH, Leach SD, et al:
Pancreatic cancer. Curr Probl Cancer. 26:176–275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Husain SS, Szabo IL and Tamawski AS: NSAID
inhibition of GI cancer growth: clinical implications and molecular
mechanisms of action. Am J Gastroenterol. 97:542–553. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stan SD, Singh SV and Brand RE:
Chemoprevention strategies for pancreatic cancer. Nat Rev
Gastroenterol Hepatol. 7:347–356. 2010.PubMed/NCBI
|
|
5
|
Jacobs EJ, Connell CJ, Rodriguez C, Patel
AV, Calle EE and Thun MJ: Aspirin use and pancreatic cancer
mortality in a large United States cohort. J Natl Cancer Inst.
96:524–528. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuo ML, Huang TS and Lin JK: Curcumin, an
antioxidant and anti-tumor promoter, induces apoptosis in human
leukemia cells. Biochim Biophys Acta. 1317:95–100. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shishodia S, Amin HM, Lai R and Aggarwal
BB: Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB
activation, induces G1/S arrest, suppresses proliferation, and
induces apoptosis in mantle cell lymphoma. Biochem Pharmacol.
70:700–713. 2005. View Article : Google Scholar
|
|
8
|
Matusheski NV, Juvik JA and Jeffery EH:
Heating decreases epithiospecifier protein activity and increases
sulforaphane formation in broccoli. Phytochemistry. 65:1273–1281.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim BR, Hu R, Keum YS, et al: Effects of
glutathione on antioxidant response element-mediated gene
expression and apoptosis elicited by sulforaphane. Cancer Res.
63:7520–7525. 2003.PubMed/NCBI
|
|
10
|
Chang F, Lee JT, Navolanic PM, et al:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: a target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pearson G, Robinson F, Beers Gibson T, et
al: Mitogen-activated protein (MAP) kinase pathways: regulation and
physiological functions. Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
12
|
Adachi T, Kar S, Wang M and Carr BI:
Transient and sustained ERK phosphorylation and nuclear
translocation in growth control. J Cell Physiol. 192:151–159. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chaudhary A, Sutaria D, Huang Y, Wang J
and Prabhu S: Chemoprevention of colon cancer in a rat
carcinogenesis model using a novel nanotechnology-based combined
treatment system. Cancer Prev Res. 4:1655–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Narayanan BA, Narayanan NK, Desai D,
Pittman B and Reddy BS: Effects of a combination of docosahexaenoic
acid and 1,4-phenylene bis(methylene) selenocyanate on
cyclooxygenase 2, inducible nitric oxide synthase and beta-catenin
pathways in colon cancer cells. Carcinogenesis. 25:2443–2449. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Im SR and Jang YJ: Aspirin enhances
TRAIL-induced apoptosis via regulation of ERK1/2 activation in
human cervical cancer cells. Biochem Biophys Res Commun. 424:65–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aoki H, Takada Y, Kondo S, Sawaya R,
Aggarwal BB and Kondo Y: Evidence that curcumin suppresses the
growth of malignant gliomas in vitro and in vivo through induction
of autophagy: role of Akt and extracellular signal-regulated kinase
signaling pathways. Mol Pharmacol. 72:29–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jakubíková J, Sedlák J, Mithen R and Bao
Y: Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane-
and erucin-induced phase II enzymes and MRP2 transcription, G2/M
arrest and cell death in Caco-2 cells. Biochem Pharmacol.
69:1543–1552. 2005.PubMed/NCBI
|
|
19
|
Ou YQ, Zhu W, Li Y, et al: Aspirin
inhibits proliferation of gemcitabine-resistant human pancreatic
cancer cells and augments gemcitabine-induced cytotoxicity. Acta
Pharmacol Sin. 31:73–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JH, Xu C, Keum YS, Reddy B, Conney A
and Kong AN: Inhibition of EGFR signaling in human prostate cancer
PC-3 cells by combination treatment with beta-phenylethyl
isothiocyanate and curcumin. Carcinogenesis. 27:475–482. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roy SK, Srivastava RK and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pham NA, Jacobberger JW, Schimmer AD, Cao
P, Gronda M and Hedley DW: The dietary isothiocyanate sulforaphane
targets pathways of apoptosis, cell cycle arrest, and oxidative
stress in human pancreatic cancer cells and inhibits tumor growth
in severe combined immunodeficient mice. Mol Cancer Ther.
3:1239–1248. 2004.
|
|
23
|
Romashkova JA and Makarov SS: NF-kappaB is
a target of AKT in anti-apoptotic PDGF signalling. Nature.
401:86–90. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cardone MH, Roy N, Stennicke HR, et al:
Regulation of cell death protease caspase-9 by phosphorylation.
Science. 282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu S, Shen G, Khor TO, Kim JH and Kong AN:
Curcumin inhibits Akt/mammalian target of rapamycin signaling
through protein phosphatase-dependent mechanism. Mol Cancer Ther.
7:2609–2620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnson SM, Gulhati P, Arrieta I, et al:
Curcumin inhibits proliferation of colorectal carcinoma by
modulating Akt/mTOR signaling. Anticancer Res. 29:3185–3190.
2009.PubMed/NCBI
|
|
27
|
Chaudhary LR and Hruska KA: Inhibition of
cell survival signal protein kinase B/Akt by curcumin in human
prostate cancer cells. J Cell Biochem. 89:1–5. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bava SV, Puliappadamba VT, Deepti A, Nair
A, Karunagaran D and Anto RJ: Sensitization of taxol-induced
apoptosis by curcumin involves down-regulation of nuclear
factor-kappaB and the serine/threonine kinase Akt and is
independent of tubulin polymerization. J Biol Chem. 280:6301–6308.
2005. View Article : Google Scholar
|
|
29
|
Shankar S, Ganapathy S and Srivastava RK:
Sulforaphane enhances the therapeutic potential of TRAIL in
prostate cancer orthotopic model through regulation of apoptosis,
metastasis, and angiogenesis. Clin Cancer Res. 14:6855–6866. 2008.
View Article : Google Scholar
|
|
30
|
Chaudhuri D, Orsulic S and Ashok BT:
Antiproliferative activity of sulforaphane in Akt-overexpressing
ovarian cancer cells. Mol Cancer Ther. 6:334–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marshall CJ: Specificity of receptor
tyrosine kinase signaling: transient versus sustained extracellular
signal-regulated kinase activation. Cell. 80:179–185. 1995.
View Article : Google Scholar
|
|
32
|
Chen JR, Plotkin LI, Aguirre JI, et al:
Transient versus sustained phosphorylation and nuclear accumulation
of ERKs underlie anti-versus pro-apoptotic effects of estrogens. J
Biol Chem. 280:4632–4638. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Liu X, Bhalla K, et al: Prevention
of apoptosis by Bcl-2: release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|