Introduction
Radioiodine plays a key role in the diagnosis and
treatment of differentiated thyroid cancers, and is used to
diagnose recurrent and metastatic disease and to treat
differentiated thyroid cancers (1–3). The
success of radioiodine for the treatment of differentiated thyroid
cancers lies in the iodine-concentrating ability of the cancer,
whereas most non-iodine-concentrating thyroid cancers result in
treatment failure. However, if the iodine-concentrating function of
thyroid cancers is restored, then radioiodine therapy would become
feasible (4,5).
Sodium iodide symporter (NIS) is an integral
membrane glycoprotein that mediates the active transport of iodine
into thyroid follicular cells, the first step of thyroid hormone
synthesis (6–8). The ability of the thyroid to
concentrate iodine via NIS, provides the bases for thyroid
diagnostic scintigraphic imaging using radioiodine and radioiodine
therapy in hyperthyroidism and thyroid cancer.
Since NIS was cloned and characterized in 1996
(6,7), a number of studies have been conducted
on NIS (9–13). At present, NIS is considered a novel
therapeutic gene (4,14), since it offers a way of restoring
the therapeutic effect of 131I in anaplastic and poorly
differentiated thyroid carcinomas (15,16).
Thus, coupling the delivery of the NIS gene into tumor cells by
131I administration may open new avenues of radionuclide
gene therapy. However, the majority of previous studies have used
ex vivo NIS gene transfer to produce engineered cancer cell
lines stably expressing NIS (11–14).
In such a condition of constitutive NIS expression, there is no
restriction of radioiodine application with regard to the timing.
However, such situations do not apply to gene therapy in clinic, in
which the expression of NIS is limited in terms of time and place
since the NIS gene should be exogenously delivered to the target
tumor. Radioiodine application should be matched with NIS
expression accordingly. In this regard, we believe that the in
vivo transfection of NIS gene is a more reasonable means of
emphasizing the application of radioiodine gene therapy in clinical
practice.
The aim of this study was to investigate the optimal
timing of radioiodine therapy during adenovirus-mediated human
sodium iodide symporter (hNIS) gene transfer into anaplastic
thyroid cancer (ARO) cells.
Materials and methods
Cell lines
The human ARO cell line was obtained from the
American Type Culture Collection (ATCC; Rockville, MD, USA). ARO
cells were grown in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS), 100 IU/ml penicillin and 100 μg/ml streptomycin
at 37°C in 5% CO2, and when 40–80% confluent, they were
transfected with recombinant adenoviral vector.
Human embryonic kidney (HEK) 293 cells were also
obtained from ATCC, and adenovirus-transformed HEK 293 cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL,
Grand Island, NY, USA), containing 10% FBS, 2 mM L-glutamine, 100
IU/ml penicillin and 100 μg/ml streptomycin.
Cloning of a recombinant adenoviral
vector for hNIS gene transfer
Recombinant adenoviral vector encoding hNIS
(rAd-hNIS) was produced using a homologous recombination reaction.
hNIS cDNA was kindly provided by Dr Sissy M. Jhiang of the Ohio
State University (7) and was cloned
using the AdEasy™ system (Qbiogene, Montréal, Canada), which
contains a green fluorescent protein (GFP) gene, and uses a
homologous recombination of a shuttle vector and a backbone
bacteria plasmid. Briefly, the hNIS cDNA gene was first cloned into
a shuttle vector, pAdTrack-CMV, and the resultant plasmid was
linearized by digestion with restriction endonuclease PmeI
and subsequently cotransformed into E. coli. BJ5183
recombinants containing the adenoviral backbone plasmid, pAdEasy-1,
were selected for kanamycin resistance, and recombination was
confirmed by restriction endonuclease analysis. The linearized
recombinant plasmid was then transfected into an adenovirus
packaging cell line, HEK 293. Recombinant adenovirus-producing foci
were easily confirmed using fluorescence microscopy following HEK
293 transfection by observing GFP expression (17).
Adenovirus-mediated hNIS gene transfer in
ARO cells in vitro
ARO cells (2×105) were added to each well
in 24-well plates, and then incubated for 24 h in 0.5 ml RPMI
media. The cells in each well were then transfected with rAd-hNIS
at multiplicities of infection (MOIs) of 0, 2.5, 5 or 10.
Forty-eight hours after transfection, 0.1 μCi (3.7 MBq) of
125I in 10 μM of cold iodine was applied to each well
and incubated for 10, 30, 60, 90 or 120 min in quadruplicate. To
perform inhibition assays, we inhibited hNIS activity by adding 50
M potassium perchlorate to a separate 10 MOI quadruplicate.
Following incubation, wells were washed with cold Hank’s balanced
salt solution (HBSS) and radioactivities were counted using a
γ-counter. A protein assay was also performed to calculate iodine
uptake (pmol) per mg of protein in each well. The values quoted
were the means of experiments performed in quadruplicate.
Adenovirus-mediated hNIS gene transfer in
ARO cell xenografts in vivo
Three-week-old male BALB/c nude mice (n=12) were
obtained from the Charles River Laboratories (Yokohama, Japan). The
experiments were approved by our Institutional Animal Research
Committee. Levothyroxine sodium (50–100 μg/kg/day) was supplemented
in drinking water to block thyroid 131I uptake.
Fifteen days after 2×106 ARO cells were
subcutaneously injected in 200 μl of sterile phosphate-buffered
saline (PBS) into both thighs of 12 nude mice (when ARO cell
xenografts had reached 8–10 mm in diameter), and 1.5×108
plaque-forming units (pfu) of rAd-hNIS in 50 μl PBS was injected
into the ARO cell xenografts in the right thighs (n=12) (T, treated
tumor). The same amount of normal saline was injected into ARO cell
xenografts in the left thighs (NT, non-treated tumor). rAd-hNIS and
normal saline were injected into 4 sites within each xenograft
using 30-gauge insulin syringes.
Scintigraphic 131I images of
adenovirus-mediated hNIS gene transformed ARO cell xenografts
Two, 3, 4 or 6 days following intratumoral injection
of rAd-hNIS, 131I images were captured using a γ-camera
(Sigma 410 Radioisotope Camera, Ohio-Nuclear, Inc., Solon, OH, USA)
equipped with a pinhole collimator. Nude mice were anesthetized
with an intraperitoneal (i.p.) injection of 53 mg/kg ketamine and
12 mg/kg xylazine and placed under the collimator in a prone
position. Sixty minutes after an i.p. injection of 5.5 MBq of
131I, 5-min static images of the 12 mice (3 mice per
day) were captured. Treated/non-treated (T/NT) count ratios were
calculated at 60 min post-131I injection for each mouse.
ARO xenografts were excised in all the cases after images were
captured and preserved at −70°C until the following experiment.
Detection of hNIS mRNA expression in ARO
cell xenografts by real-time polymerase chain reaction
(RT-PCR)
The following primer pairs were used to detect hNIS
mRNA using RT-PCR: 5′-GCT AAG TGG CTT CTG GGT TG-3′ (hNIS gene
sense primer); 5′-GTA AGC ACA GGC CAG GAA AA-3′ (hNIS gene
antisense primer). These hNIS gene primer pairs corresponded to the
coding regions 941–960 and 1300–1319 and yielded a product of 379
bp for the hNIS gene. For comparison purposes, RT-PCR for the
housekeeping gene glyceraldehydes-3-phosphate dehydrogenase (GAPDH)
was also performed using the primer pairs: 5′-ACC AGG GCT GCT TTT
AAC TCT-3′ (GAPDH gene sense primer); 5′-GAG TCC TTC CAC GAT ACC
AAA G-3′ (GAPDH gene antisense primer). The GAPDH gene primer pairs
corresponded to the coding regions 130–150 and 576–597 and yielded
a 468-bp product. Total RNA was isolated using RNeasy Midi kits
(Qiagen Inc., Valencia, CA, USA) and a rotor-stator homogenizer.
RT-PCR was performed over 27 amplification cycles using a GeneAmp®
PCR System 9700 (Applied Biosystem, Inc., Foster City, CA). The PCR
hNIS gene and GAPDH gene fragments were analyzed by 1.5%
agarose/ethidium bromide gel electrophoresis.
Immunohistochemical staining of excised
ARO cell xenografts
Immunohistochemical staining of paraffin-embedded
tissue sections derived from ARO cell xenografts was performed
using rabbit anti-rat thyroid iodide transporter IgG (TIT11-A,
Alpha Diagnostic International, San Antonio, TX, USA). Tissue
sections were deparaffinized by three passages in xylene, subjected
to a graded series of ethanol washes (100, 95 and 90% ethanol
solutions) and then washed in distilled water. Endogenous
peroxidase activity was blocked by incubation in 3%
H2O2/methanol for 10 min and sections were
washed in Tris-buffered saline and Tween-20 (TBS Tween-20: pH
7.4±0.05, Tris 0.005 M, NaCl 0.15 M, Tween-20 0.05%). To expose
antigens, slides were heated in 0.01 M citrate buffer for 12 min.
After cooling to room temperature for 20 min, slides were incubated
using primary antibodies (TIT11-A diluted to 1:100) for 60 min, and
washed with TBS Tween-20, anti-rabbit secondary antibody
(EnVision™+, K 4003 HRP, Rabbit, DakoCytomation Inc., Glostrup,
Denmark) for 40 min. 3,3′-Diaminobenzidine was used as the
chromogen. Slides were counterstained with Mayer’s hematoxylin and
observed under a light microscope.
Results
In vitro iodine uptake analysis of
adenovirus-mediated hNIS gene-transfected ARO cells
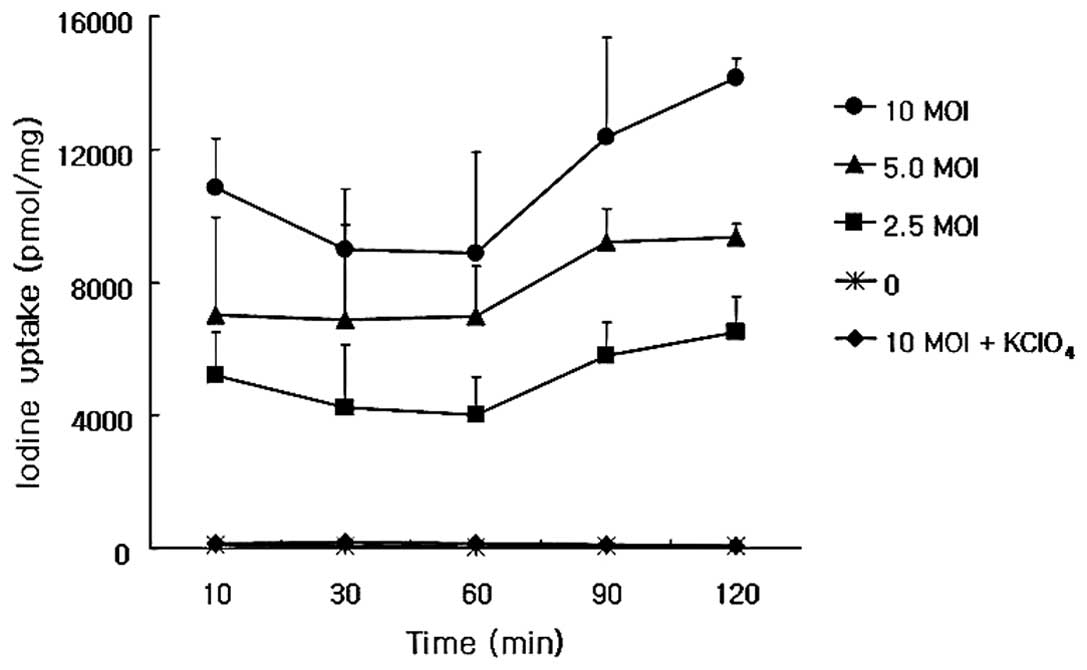
The iodine uptake of adenovirus-mediated hNIS
gene-transfected ARO cells increased for 120 min at viral titers of
2.5, 5.0 and 10 MOIs, and was completely inhibited when potassium
perchlorate was administered (Fig.
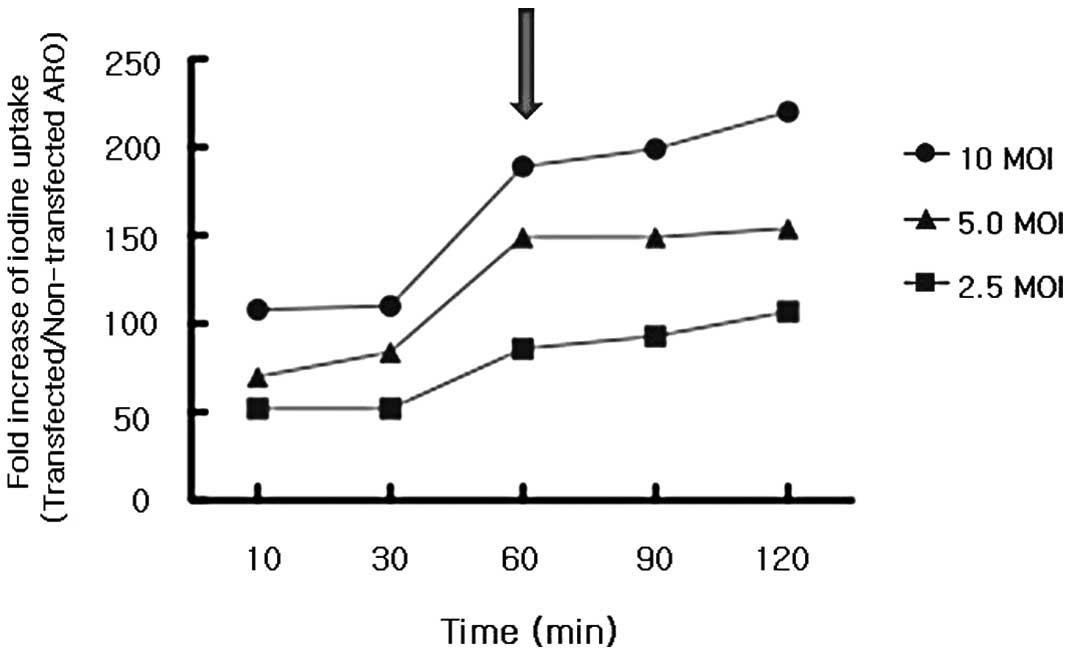
1). The fold-increase of iodine uptake by ARO cells transfected
with the rAd-hNIS versus non-transfected ARO cells also increased
for 120 min, but there was a leap forward ~60 min post-iodine
application (Fig. 2). Thus, after
the radioiodine administration, the 60 min time point was selected
for scintigraphic imaging studies.
Scintigraphic 131I images of
adenovirus-mediated hNIS gene-transfected ARO cell xenografts
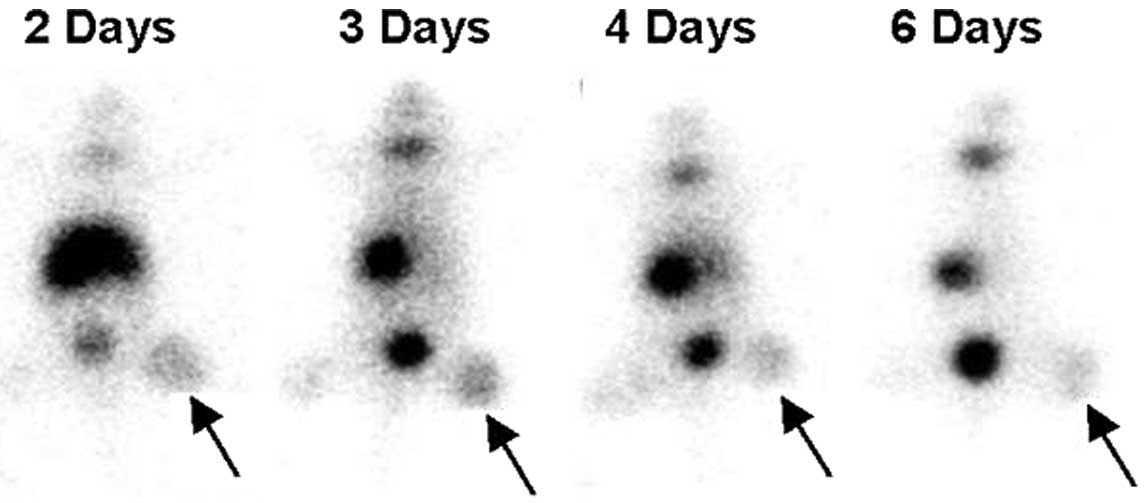
ARO cell xenografts in the right thighs of nude mice
were readily visualized 60 min after administering 131I
on days 2, 3, 4 and 6 following recombinant adenovirus injeciton
(Fig. 3). 131I
accumulation by xenografts was more prominent on the day 2 and 3
images compared to the day 4 and 6 images, which showed a gradual
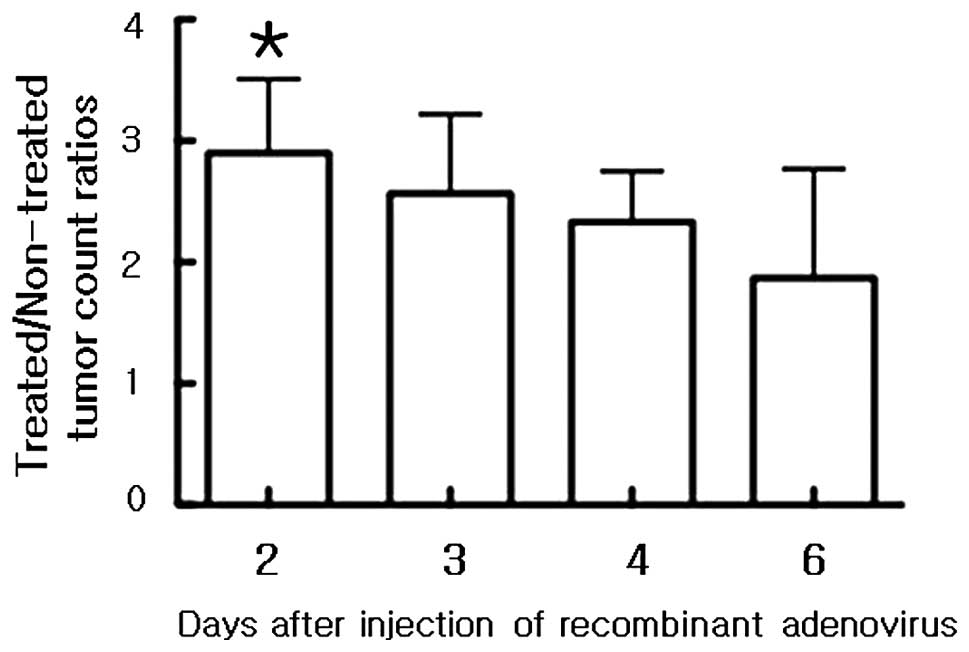
reduction. Mean T/NT count ratios of ARO cell xenografts of
131I images on days 2, 3, 4 and 6 were 2.85±0.61,
2.54±0.65, 2.31±0.42 and 2.18±0.90, respectively (Fig. 4).
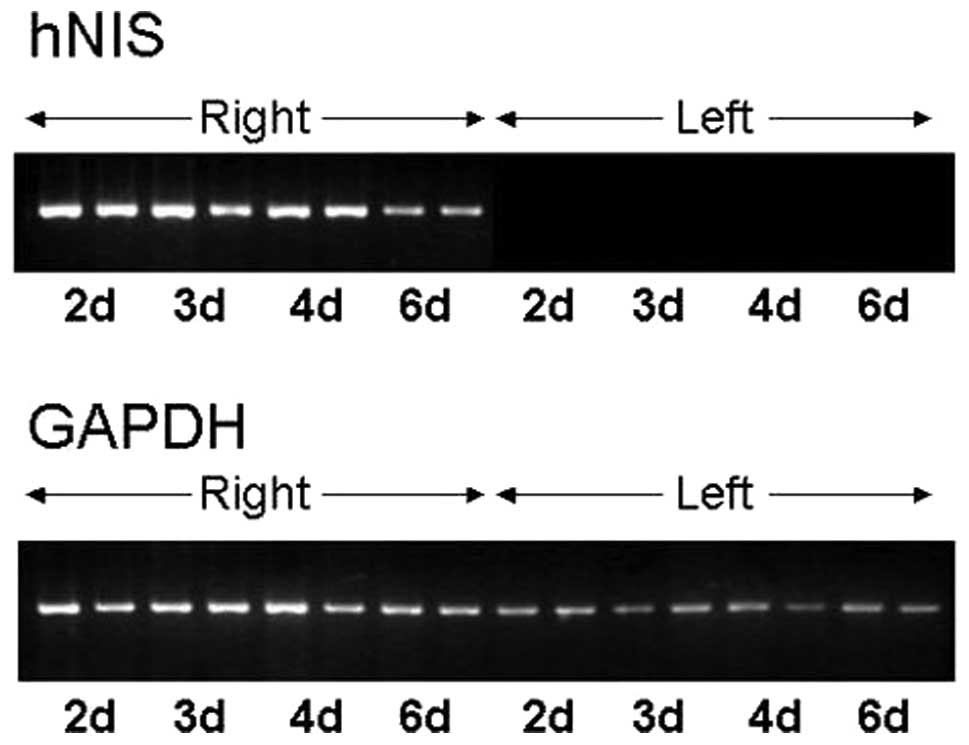
RT-PCR results of adenovirus-mediated
hNIS gene-transfected ARO cell xenografts
RT-PCR for hNIS mRNA, which was extracted from
adenovirus-transfected ARO cell xenografts excised from the right
thighs of nude mice, produced a 379-bp hNIS dsDNA RT-PCR product,
whereas RT-PCR for hNIS mRNA extracted from normal saline-injected
ARO cell xenografts into left thighs did not yield any product.
Amplified 379-bp hNIS dsDNA band intensities at 2, 3 and 4 days
post-recombinant adenovirus injection were higher compared to those
of amplified 468-bp GAPDH dsDNA bands in the same specimens.
Moreover, the band intensities of hNIS mRNA RT-PCR products were
the highest on day 2 and then gradually decreased, and on day 6
these intensities were lower compared to those of the GAPDH mRNA
RT-PCR products (Fig. 5).
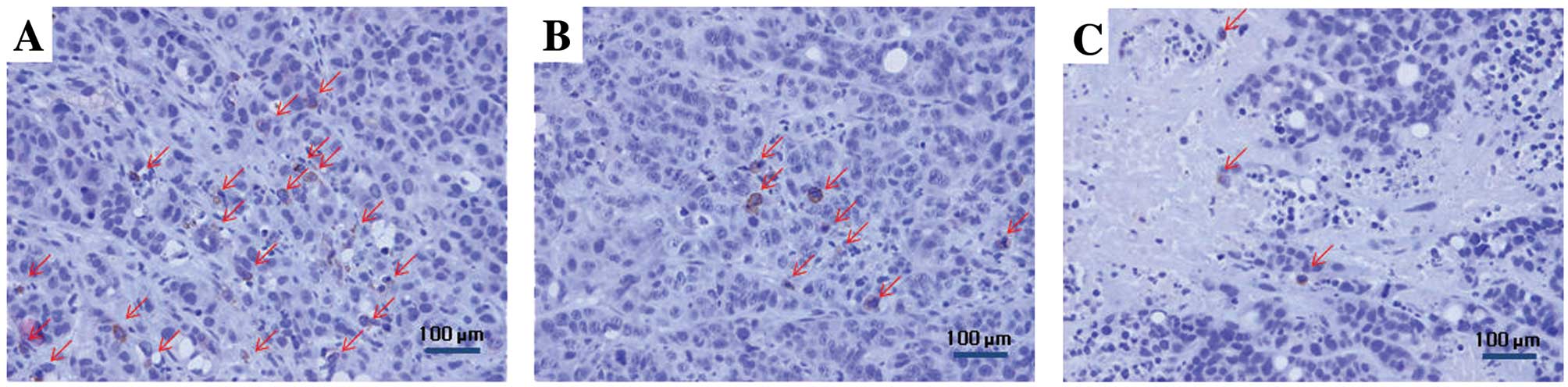
Immunohistochemical staining results of
adenovirus-mediated hNIS gene-transfected ARO cell xenografts
Immunohistochemical staining of excised ARO cell
xenografts was performed to determine the immunohistochemical
localization of hNIS in adenovirus-mediated hNIS gene-transfected
ARO cells. hNIS expression was the highest in ARO cell xenograft
tissue specimens excised 2 days post-recombinant adenovirus
injection, which then gradually decreased. Necrotic areas were most
abundant in ARO cell xenografts excised at 6 days post-recombinant
adenovirus injection. hNIS expression was predominantly found in
the cytoplasmic membranes of adenovirus-mediated hNIS
gene-transfected ARO cells (Fig.
6), whereas no hNIS expression was observed in tissue specimens
excised from ARO cell xenografts injected intratumorally with
normal saline.
Discussion
In the present study, we successfully transferred
the hNIS gene in vivo by intratumorally injecting
recombinant adenovirus encoding the hNIS gene (rAd-hNIS), and
serially measured transferred hNIS gene expression 2, 3, 4 and 6
days following recombinant virus injection using radioiodine
imaging, RT-PCR and immunohistochemistry. The results showed that 2
days post-intratumoral injection is an optimal time for radioiodine
therapy using the hNIS gene.
Thyroid cancer has been a target of gene therapy
using NIS gene and radioiodine. Shimura et al(15) and Smit et al(16) reported that transfection of the NIS
gene into thyroid cancer cells resulted in radioiodine
accumulation. However, those authors used cell lines stably
expressing the NIS gene. In the present study, we injected
recombinant adenovirus encoding hNIS in vivo intratumorally
to more accurately reflect perceived clinical applications.
In vivo NIS gene transfer has been
investigated in a variety of cancer cells using adenoviral vectors.
Boland et al(9) injected
131I i.p. 3 days following intratumoral injection of
recombinant adenovirus into a human cervical tumor cell xenograft.
Cho et al(10) injected
125I i.p. 43 h after recombinant adenovirus intratumoral
injection into a human glioma cell xenograft, and Spitzweg et
al(18) injected
123I i.p. 4 days after recombinant adenovirus
intratumoral injection into a human prostate cancer cell xenograft.
However, those studies did not mention the optimal timing of
radioiodine application since most were performed only at a single
time point.
When it comes to the use of adenoviral vector as a
vehicle, an initial high expression followed by a subsequent
reduction in hNIS expression delivered by adenovirus has been
reported in a non-tumor animal model (19). However, a thorough evaluation of
adenovirus-mediated hNIS expression in terms of the optimal timing
of radioiodine anti-tumor therapy has yet to be conducted.
Gene therapy using hNIS and 131I
administration have encountered several obstacles that need to be
overcome. One of these obstacles is the rapid washout of delivered
radioiodine. In their study, Spitzweg et al calculated that
the average biological half-life of 131I, which enters a
tumor-expressing hNIS, is only 5.6 h (18). However, the average radioiodine
half-life in metastatic thyroid cancer patients responding to
radioiodine therapy was reported to be as long as 5.5 days
(1). The application of
tissue-specific promoters, or cotransfection of the thyroperoxidase
gene, or the application of high-energy β-ray emitting
radioisotopes such as 188Re, may help resolve this
problem (13,20–22).
Increased knowledge of NIS and the development of gene therapy
techniques should enable the identification of a role for the NIS
gene in radionuclide gene therapy in the near future (4,5).
In conclusion, radioiodine uptake was successfully
increased in ARO tumors by adenovirus-mediated hNIS gene transfer
in vitro and in vivo. The optimal time for
radioiodine administration (day 2 post-recombinant adenovirus
injection) was determined by serial imaging and analysis. The
results obtained during this study suggest the possibility of
applying radioiodine therapy in iodine non-concentrating tumors by
hNIS gene transfer.
Acknowledgements
This study was supported in part by a grant of the
Korea Healthcare technology R&D Project, Ministry of Health and
Welfare (A111627), and by grants from the National Research
Foundation (NRF), Ministry of Education, Science and Technology
(MEST), (2012M2B2A9A02029612; Basic Science Research Program,
2012R1A1A2001060; and Global Core Research Center (GCRC) program,
2011-0030680), Republic of Korea.
References
|
1
|
Maxon HR, Thomas SR, Hertzberg VS,
Kereiakes JG, Chen IW, Sperling MI and Saenger EL: Relation between
effective radiation dose and outcome of radioiodine therapy for
thyroid cancer. N Engl J Med. 309:937–941. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeGroot LJ, Kaplan EL, McCormick M and
Straus FH: Natural history, treatment, and course of papillary
thyroid carcinoma. J Clin Endocrinol Metab. 71:414–424. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schlumberger MJ: Papillary and follicular
thyroid carcinoma. N Engl J Med. 338:297–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung JK: Sodium iodide symporter: its
role in nuclear medicine. J Nucl Med. 43:1188–1200. 2002.PubMed/NCBI
|
|
5
|
Chung JK, Youn HW, Kang JH, Lee HY and
Kang KW: Sodium iodide symporter and the radioiodine treatment of
thyroid carcinoma. Nucl Med Mol Imaging. 44:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dai G, Levy O and Carrasco N: Cloning and
characterization of the thyroid iodide transporter. Nature.
379:458–460. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smanik PA, Liu Q, Furminger TL, Ryu K,
Xing S, Mazzaferri EL and Jhiang SM: Cloning of the human sodium
iodide symporter. Biochem Biophys Res Commun. 226:339–345. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dohan O, De la Vieja A, Paroder V, et al:
The sodium/iodide Symporter (NIS): characterization, regulation,
and medical significance. Endocr Rev. 24:48–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boland A, Ricard M, Opolon P, et al:
Adenovirus-mediated transfer of the thyroid sodium/iodide symporter
gene into tumors for a targeted radiotherapy. Cancer Res.
60:3484–3492. 2000.PubMed/NCBI
|
|
10
|
Cho JY, Xing S, Liu X, et al: Expression
and activity of human Na+/I− symporter in
human glioma cells by adenovirus-mediated gene delivery. Gene Ther.
7:740–749. 2000.
|
|
11
|
Spitzweg C, O’Connor MK, Bergert ER,
Tindall DJ, Young CY and Morris JC: Treatment of prostate cancer by
radioiodine therapy after tissue-specific expression of the sodium
iodide symporter. Cancer Res. 60:6526–6530. 2000.PubMed/NCBI
|
|
12
|
Cho JY, Shen DH, Yang W, et al: In vivo
imaging and radioiodine therapy following sodium iodide symporter
gene transfer in animal model of intracerebral gliomas. Gene Ther.
9:1139–1145. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schipper ML, Weber A, Behe M, et al:
Radioiodide treatment after sodium iodide symporter gene transfer
is a highly effective therapy in neuroendocrine tumor cells. Cancer
Res. 63:1333–1338. 2003.PubMed/NCBI
|
|
14
|
Mandell RB, Mandell LZ and Link CJ Jr:
Radioisotope concentrator gene therapy using the sodium/iodide
symporter gene. Cancer Res. 59:661–668. 1999.PubMed/NCBI
|
|
15
|
Shimura H, Haraguchi K, Miyazaki A, Endo T
and Onaya T: Iodide uptake and experimental 131I therapy
in transplanted undifferentiated thyroid cancer cells expressing
the Na+/I− symporter gene. Endocrinology.
138:4493–4496. 1997.PubMed/NCBI
|
|
16
|
Smit JW, Shroder-van der Elst JP,
Karperien M, et al: Reestablishment of in vitro and in vivo iodide
uptake by transfection of the human sodium iodide symporter (hNIS)
in a hNIS defective human thyroid carcinoma cell line. Thyroid.
10:939–943. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spitzweg C, Dietz AB, O’Connor MK, Bergert
ER, Tindall DJ, Young CY and Morris JC: In vivo sodium iodide
symporter gene therapy of prostate cancer. Gene Ther. 8:1524–1531.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee WW, Moon DH, Park SY, Jin J, Kim SJ
and Lee H: Imaging of adenovirus-mediated expression of human
sodium iodide symporter gene by 99mTcO4 scintigraphy in mice. Nucl
Med Biol. 31:31–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang M, Batra RK, Kogai T, et al: Ectopic
expression of the thyroperoxidase gene augments radioiodide uptake
and retention mediated by the sodium iodide symporter in non-small
cell lung cancer. Cancer Fene Ther. 8:612–618. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Sharma S, Zhu LX, et al:
Nonradioactive iodide effectively induces apoptosis in genetically
modified lung cancer cells. Cancer Res. 63:5065–5072.
2003.PubMed/NCBI
|
|
22
|
Dadachova E, Bouzahzah B, Zuckier LS and
Pestell RG: Rhenium-188 as an alternative to Iodine-131 for
treatment of breast tumors expressing the sodium/iodide symporter
(NIS). Nucl Med Biol. 29:13–18. 2002. View Article : Google Scholar : PubMed/NCBI
|