Introduction
In spite of the marked spontaneous decline in the
incidence of stomach cancer in most Western countries, in Asia it
remains the second most common type of cancer, following lung
cancer, and accounts for 13% of all tumors (1–3). In
Italy, the 5-year relative survival rate is approximately 30%
(4). Although surgical techniques
have improved, only a small percentage of patients are diagnosed
with a localized disease amenable to surgery (5). The outcome of patients with clinically
advanced disease is usually poor although new chemotherapeutic
regimens are available. Bone metastases in gastric cancer are rare
and they usually appear late in the natural history of the disease;
however, micrometastatic seeding in the bone may be evident in the
early stage of the disease (5).
Nevertheless, when these metastatic lesions occur, the prognosis is
poor, as they can cause bone marrow suppression, anemia, cord
compression and intravascular coagulation and can quickly lead to
mortality (6). In early gastric
cancer, a poorly differentiated carcinoma and the presence of
signet-cells seem to be associated with bone metastases (7). The mechanism of bone metastases in
gastric cancer remains to be clarified, since there are currently
different hypotheses concerning the metastatic spread to the bone,
such as through portal vein, lymphatic channel (7) and vertebral vein system (8). In general, gastric cancer bone
metastases result from a diffuse metastatic spread in the bone
marrow, most frequently localized in the thoracic and lumbar
vertebrae (9), and they are
osteolytic or, less commonly, mixed osteolytic/osteoblastic.
In osteotropic tumors such as breast, prostate and
lung cancer, osteoclasts (OCs) are mainly responsible for bone
destruction in patients with metastatic bone disease. Several
molecules have been identified as the mediators of bone metastases
in solid tumors, for example the RANK/RANKL/ osteoprotegerin (OPG),
TNF-α and PTHrP system (10–12).
In addition to the known role of RANKL in the skeletal and immune
systems (13), recent studies also
reported a role of RANKL in angiogenesis. Certain authors support
an inhibitory role of RANKL (14,15),
whereas others showed a stimulating action on angiogenesis
(16). Contrary to RANKL, VEGF is
known as a potent mitogen angiogenic factor and is one of the most
important molecules involved in the vascularization of bone tissue
(17), thus its serum levels are
considered an index of angiogenesis. Moreover, increased VEGF serum
levels are associated with a poor clinical outcome in gastric
cancer (18).
In this study, we examined the mechanisms of bone
metastases by gastric cancer investigating the presence of
spontaneous osteoclastogenesis in vitro, the expression of
genes involved in bone metastasis by gastric tumor tissues and any
correlations among the serum levels of RANKL, VEGF and clinical
parameters in our cohort of patients.
Materials and methods
Patients
Between October 2008 and February 2011, we collected
blood samples and sera from 31 consecutive newly diagnosed gastric
cancer patients at the San Giovanni Battista Hospital in Turin. We
also collected samples from 45 healthy controls. The study design
was approved by the Ethics Committee of the hospital.
Clinicopathological data for all patients were collected in a
database.
Cell cultures
Peripheral blood (PB) samples were obtained from 16
gastric cancer patients and 19 healthy controls, processed as
previously described (19).
Briefly, peripheral blood mononuclear cells (PBMCs) were isolated
following centrifugation over a density gradient, according to the
Ficoll method. The number of PBMCs retrieved from the blood samples
varied among patients and controls, thus it was not possible to
perform all the experimental procedures on all the enrolled
patients and controls. PBMCs were plated in 24-well plates, using
α-minimal essential medium (α-MEM; Invitrogen, Carlsbad, CA, USA),
supplemented with 10% fetal bovine serum, benzylpenicillin (100
IU/ml) and streptomycin (100 mg/ml; Lonza, Basel, Switzerland) and
maintained at 37°C in a humidified atmosphere of 5% CO2.
To obtain fully differentiated human OCs, PBMCs were cultured in
the presence or absence of recombinant human M-CSF (25 ng/ml) and
RANKL (30 ng/ml), for 15 days. In 8 independent experiments, PBMCs
were cultured in the presence of increasing concentrations of
RANKFc (30–50 ng/ml) and anti-TNF-α (1.5-3-5 mg/ml; PeproTech,
London, UK).
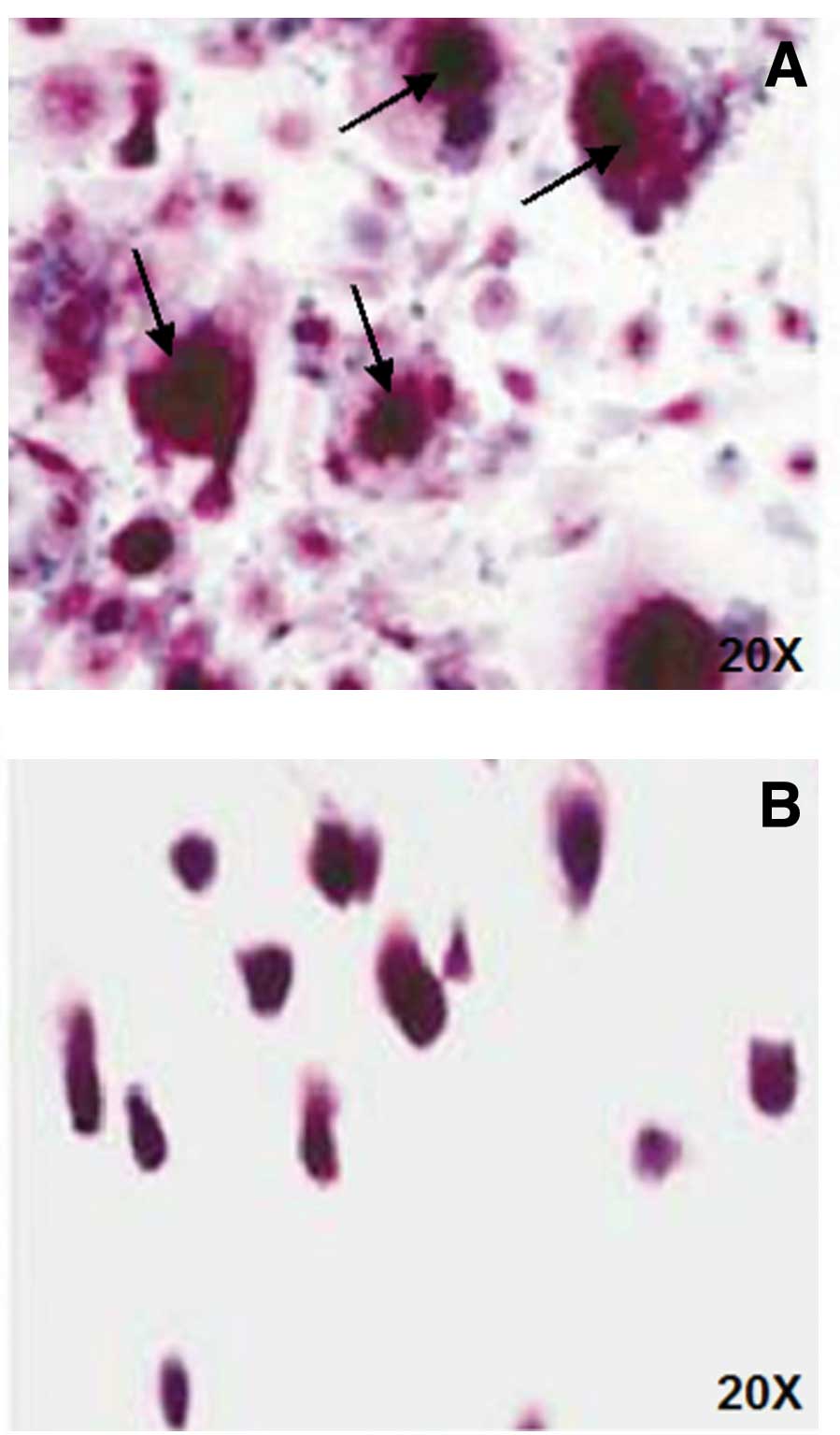
At the end of the culture period, cells were stained
for tartrate-resistant acid phosphatase (TRAP, kit supplied by
Sigma-Aldrich, St. Louis, MO, USA) and OCs were identified as TRAP
positive, multinucleated cells, containing three or more
nuclei.
Assay of bone resorption activity
To study OC resorbing activity, PBMCs from patients
and healthy controls were plated on a BioCoat osteologic bone cell
culture system provided by BD Biosciences (Bedford, MA, USA) and
cultured for 20 days. In order to visualize pits formed by OCs, the
cells were removed by washing each well with NaOCl and resorption
lacunae were identified by light microscopy. The quantification of
resorbing area was performed by a semi-automated image analyzing
system (20).
Real-time quantitative analysis of TGF-β,
OPG, DKK-1, SOST, RANKL and IL-7 gene expression
Total RNA was extracted by the TRIzol system
(Invitrogen) from surgically resected primary tumor and bone
biopsies from patients previously diagnosed with bone metastasis by
gastric cancer. The first-strand cDNA synthesis was performed as
previously described (21).
Quantitative analysis of transforming growth
factor-β (TGF-β), osteoprotegerin (OPG), dickkopf-1 (DKK-1),
sclerostin (SOST), RANKL and IL-7 were performed with real-time
quantitative PCR (RQ-PCR) using β-actin as the housekeeping
control. RT-PCR was carried out using the iCycler iQ™ system
(Bio-Rad, Hercules, CA, USA). TaqMan probes were designed using
Primer Express v2.0 software and synthesized by Applied Biosystems.
The sequences of the probes and primers were previously described
(22). All the probes were labeled
at the 5′-end with 6-carboxyfluorescein (FAM) and the 3′-end with
6-carboxytetramethylrhodamine (TAMRA). Reactions for gene
quantification were performed in a 25-μl final volume with 2 μl of
sample cDNA, 1X iQ Supermix (Bio-Rad), 0.3 μM of each primer and
0.4 μM of the probes. PCR primers were the same as those used for
gene cloning. The amplification conditions for quantization were:
95°C for 15 min, 50 cycles at 95°C for 15 sec, 58°C for 1 min.
ELISA
The serum levels of total RANKL (Biomedica
Medizinprodukte GmbH and Co. KG, Vienna, Austria), IL-7 (BenderMed
Systems, Vienna, Austria) and VEGF (R&D Systems, Abingdon, UK)
were determined by a commercially available ELISA kit according to
manufacturer’s instructions in all patients and healthy controls.
Samples were assayed in duplicate and data were expressed as mean
values.
Statistical analysis
The characteristics of the patients were described
using medians and range for the continuous variables and using
percentage frequencies for the categorical variables. We performed
the Mann-Whitney test to assess difference of number of culture
between patients and controls, difference of gene expression
between primary tumor and bone biopsies, and difference of RANKL
and VEGF between patients and healthy controls. Crude survival
probabilities were estimated with the Kaplan-Meier method. The
survival time was months since cancer diagnosis. A Cox proportional
hazard model was employed to estimate the crude and adjusted hazard
ratios (HRs) and 95% confidence intervals. Analyses were performed
using Stata 11.2.
Results
Patients
Among 31 patients newly diagnosed with gastric
cancer, 16 patients had a localized disease and were subjected to
surgery and 15 had a metastatic disease. The main clinical
characteristics of the patients are summarized in Table I.
 | Table IPatient baseline characteristics by
metastatic status. |
Table I
Patient baseline characteristics by
metastatic status.
| Non-metastatic | Metastatic | Total |
|---|
|
|
|
|
|---|
| No. | % | No. | % | No. | % |
|---|
| Gender |
| Female | 4 | 25 | 6 | 40 | 10 | 32.26 |
| Male | 12 | 75 | 9 | 60 | 21 | 67.74 |
| Age |
| <60 | 7 | 43.75 | 9 | 60 | 16 | 51.61 |
| ≥60 | 9 | 56.25 | 6 | 40 | 15 | 48.39 |
| Tumor site |
| Stomach | 16 | 100 | 14 | 93.33 | 30 | 96.77 |
| Cardias | 0 | 0 | 1 | 6.67 | 1 | 3.23 |
| Grading |
| 1 | 1 | 6.25 | 0 | 0 | 1 | 3.23 |
| 2 | 2 | 12.5 | 0 | 0 | 2 | 6.45 |
| 3 | 12 | 75.0 | 10 | 66.67 | 22 | 70.97 |
| Not evaluated | 1 | 6.25 | 5 | 33.33 | 6 | 19.35 |
| Histologic
type |
| Diffuse | 7 | 43.75 | 8 | 53.33 | 15 | 48.39 |
| Enteric | 6 | 37.5 | 1 | 6.67 | 7 | 22.58 |
| Mixed | 1 | 6.25 | 1 | 6.67 | 2 | 6.45 |
|
Adenocarcinoma | 2 | 12.5 | 5 | 33.33 | 7 | 22.58 |
| Vascular
invasion |
| Yes | 7 | 43.75 | 3 | 20 | 10 | 32.26 |
| No | 9 | 56.25 | 12 | 80 | 21 | 67.74 |
| Chemotherapy |
| In | 0 | 0 | 3 | 20 | 3 | 9.68 |
| Out | 16 | 100 | 12 | 80 | 28 | 90.32 |
| Lymph node
invasion |
| N0 | 7 | 43.75 | | | | |
| N1 | 3 | 18.75 | | | | |
| N2 | 3 | 18.75 | | | | |
| N3 | 3 | 18.75 | | | | |
| Total | 16 | 100 | 15 | 100 | 31 | 100 |
Evaluation of spontaneous
osteoclastogenesis in vitro and OC activity
OCs spontaneously differentiated in vitro
without the addition of M-CSF and RANKL (spontaneous
osteoclastogenesis) in half of the analyzed patients (8/16 cases),
whereas with PBMC derived from the other 8 patients, OCs only
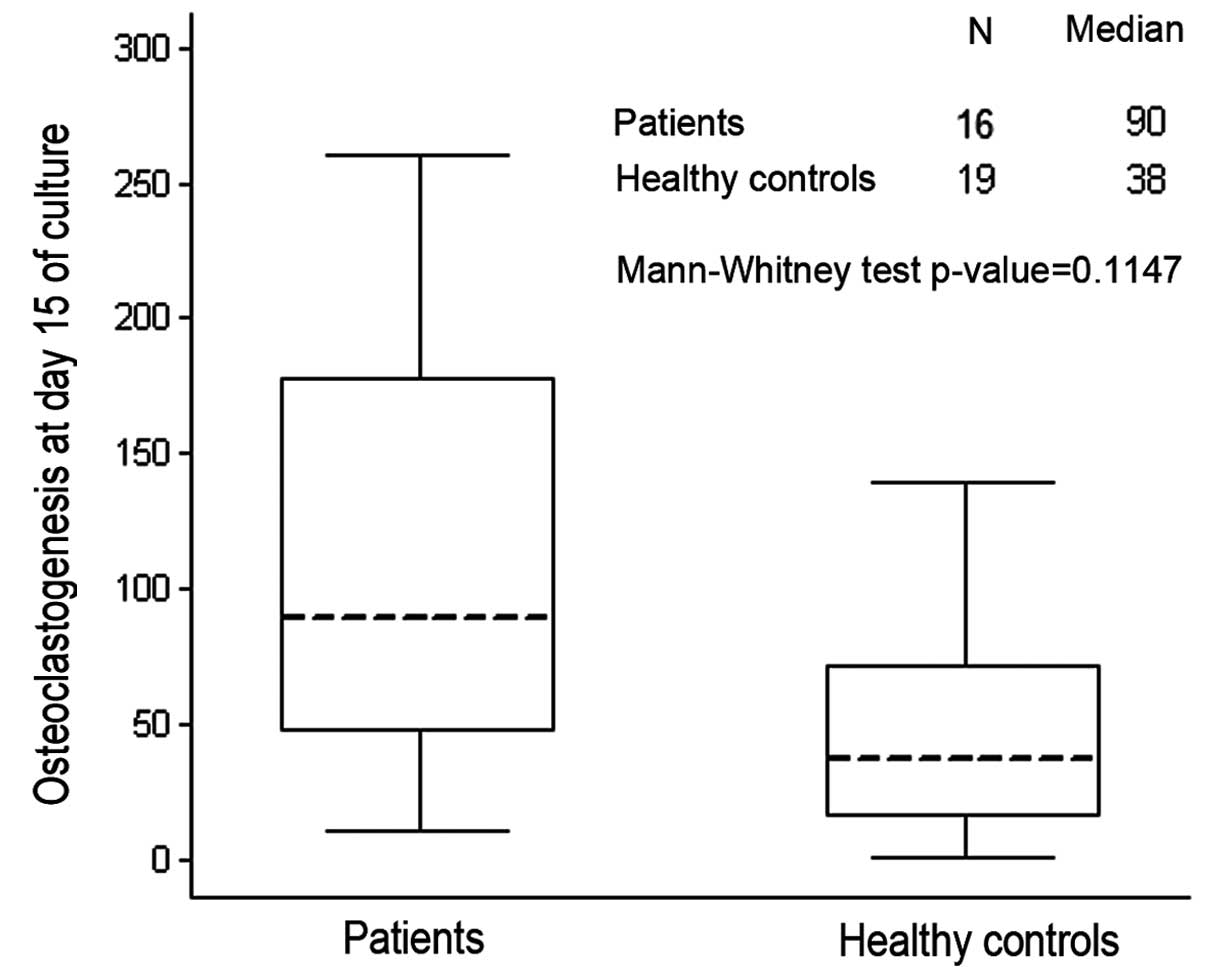
originated after the addition of exogenous factor (Figs. 1 and 2). Gastric patients showed an increase of
spontaneous osteoclastogenesis compared to healthy controls, but it
was not statistically significant (p=0.11) (Fig. 1).
The analysis of OC activity revealed that it did not
increase in patients compared to controls; indeed, we did not
observe significant variation in the percentage of resorption (data
not shown). Since osteoclastogenesis is commonly dependent on RANKL
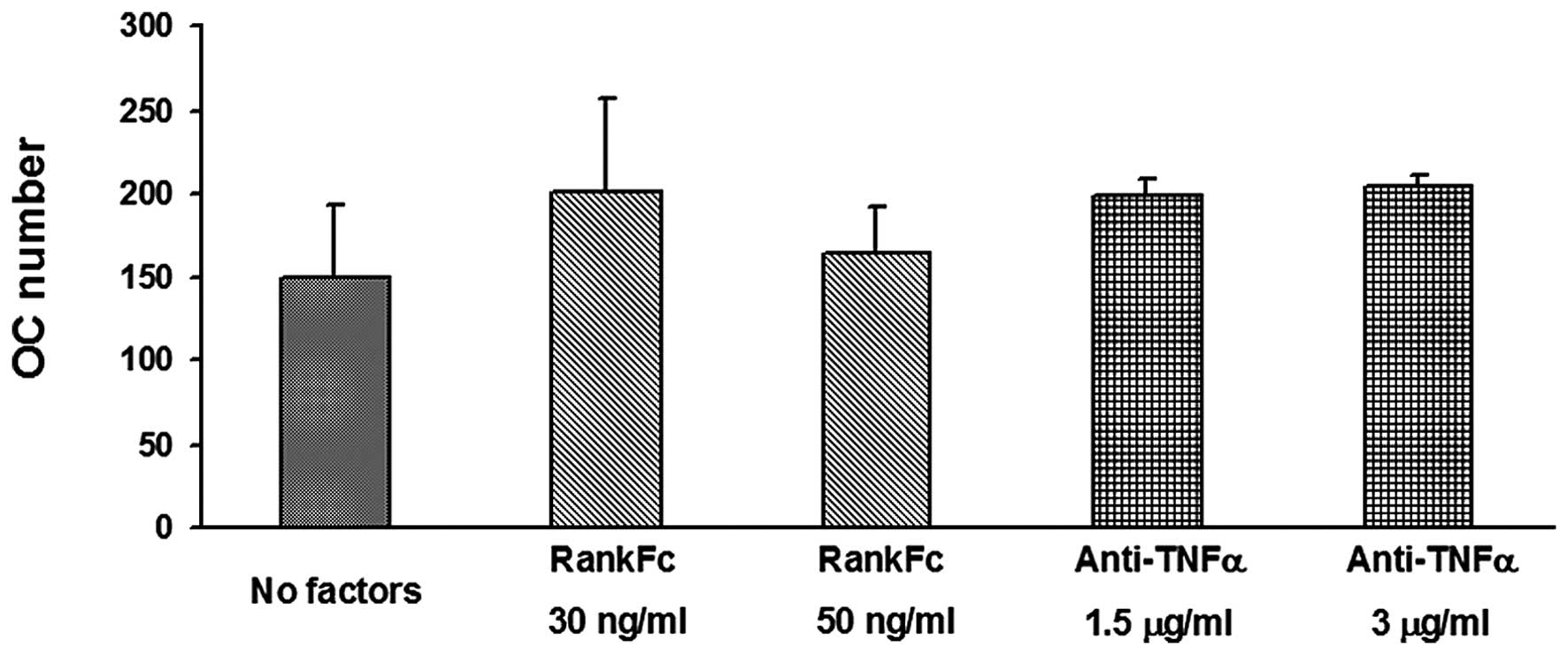
and TNF-α, we tested the effect of an anti-TNF-α antibody and
RANK-Fc in PBMC cultures, showing that osteoclastogenesis was not
inhibited, thus it was not dependent on these two factors (Fig. 3).
Expression analysis of genes involved in
osteoclastogenesis
In order to investigate whether gastric cancer cells
expressed genes which regulate osteoclastogenesis, we analyzed the
expression of TGF-β, RANKL, OPG, DKK-1, SOST and IL-7 in primary
and bone metastatic gastric cancer tissues. TGF-β, OPG and DKK-1
were higher in the primary tumors than in the bone metastatic
lesions. SOST was only expressed in the bone metastases, while IL-7
was only expressed in primary tumors (Table II). RANKL was not detectable in
either tissue.
 | Table IIExpression analysis of genes involved
in osteoclastogenesis. |
Table II
Expression analysis of genes involved
in osteoclastogenesis.
| Primary tumor | Bone
metastases | |
|---|
|
|
| |
|---|
| Median
Copy N. genes/β-actin | Range | Median
Copy N. genes/β-actin | Range | p-value |
|---|
| TGF-β/β-actin | 0.0154 | 0.0034–0.0236 | 0.0030 | 0.0015–0.0045 | 0.01 |
| OPG/β-actin | 0.0083 | 0.0027–0.3508 | 0.0010 | 0.0005–0.0019 | 0.46 |
| DKK-1/β-actin | 0.0004 | 0.0002–0.016 | 0.0001 | 0–0.0002 | 0.39 |
| SOST/β-actin | Not detected | | 0.0006 | 0.0003–0.003 | |
| IL-7/β-actin | 0.0954 | 0.0507–1.6417 | Not detected | | |
Serum level of IL-7, RANKL and VEGF
In previous studies on bone metastases by solid
tumors, we demonstrated a role of IL-7 in promoting
osteoclastogenesis (23). Thus, we
administered IL-7 serum levels in 31 gastric cancer patients.
Unlike other osteotropic tumors, such as lung cancer, in gastric
patients IL-7 levels were not significantly different between
patients with and without spontaneous osteoclastogenesis (the
median values were 10.1 and 12.8 pg/ml, respectively).
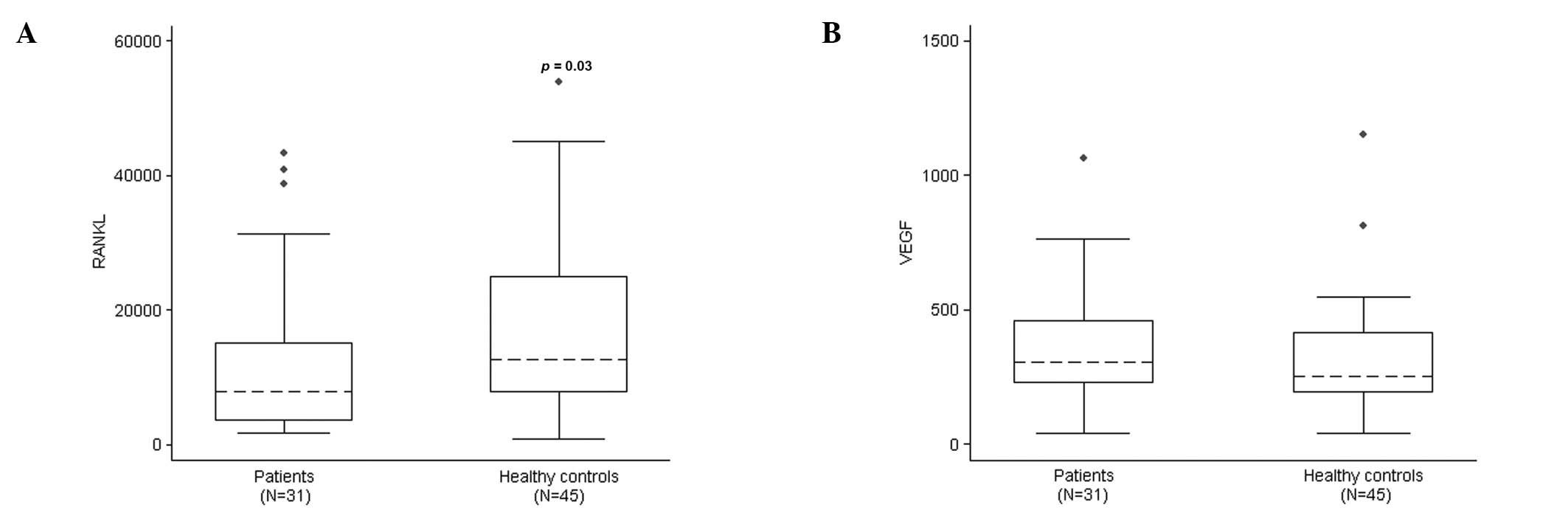
Serum RANKL levels were significantly higher in
healthy controls than in patients (median, 12635.40 and 7772.80
pg/ml; range, 818.7–53898.50 and 1643.00–43245.40 pg/ml,
respectively; p=0.03) (Fig. 4A). We
did not observe any correlation between serum RANKL and the
presence of spontaneous osteoclastogenesis, confirming the
RANKL-indipendence of the detected osteoclastogenesis in
vitro. The serum VEGF level was higher in patients than in
healthy controls, but the difference was not statistically
significant (median, 304 and 252 pg/ml; range, 38–1064 and 39–1151
pg/ml, respectively; p=0.14) (Fig.
4B).
Analysis of prognostic value of RANKL and
VEGF
In order to evaluate the prognostic value of RANKL
and VEGF, we calculated the overall survival (OS) of 31 patients
who were diagnosed with gastric cancer when the blood sample was
collected. As there is a lack of information regarding RANKL and
VEGF values, the median value of serum RANKL and VEGF was chosen as
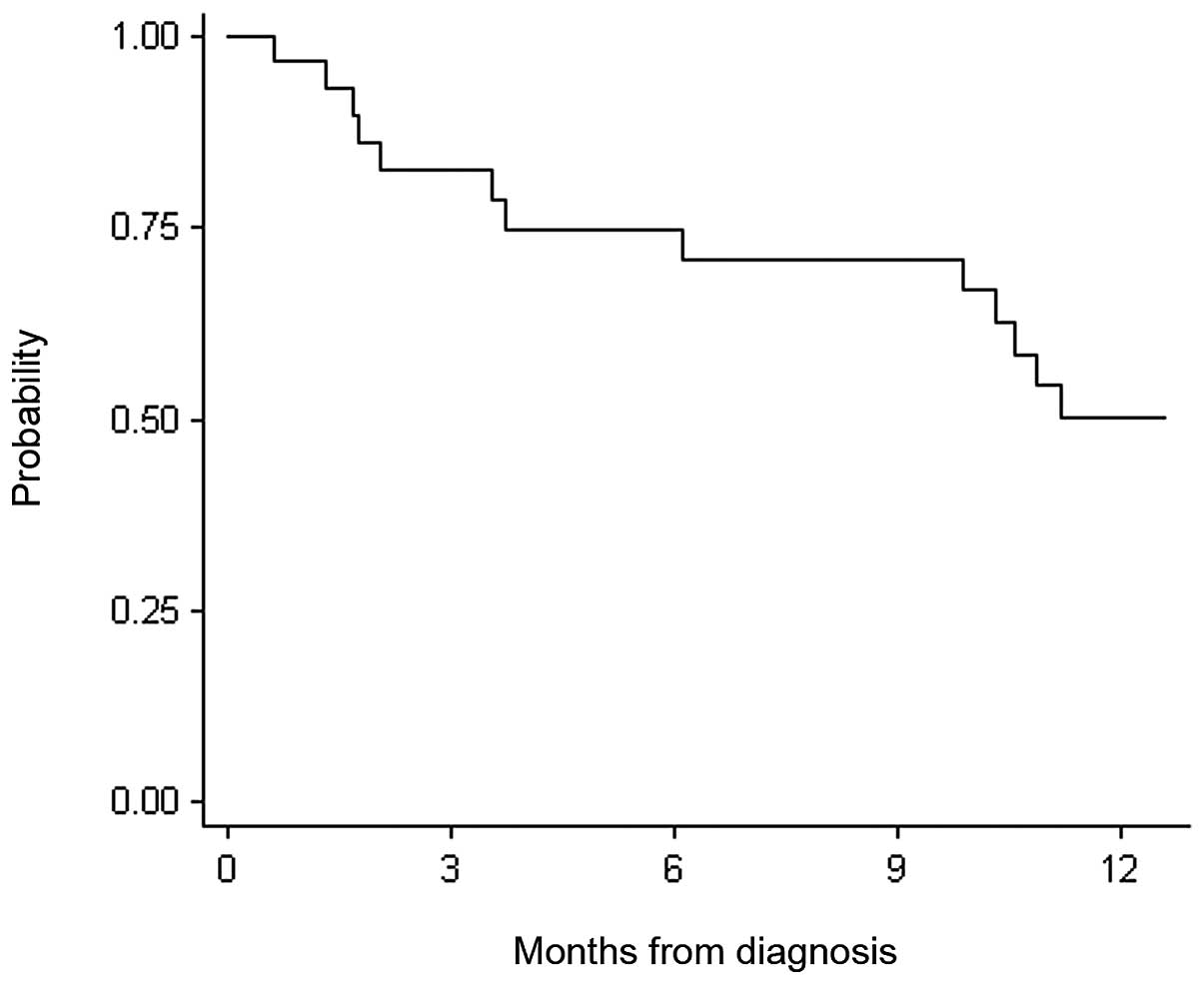
cut-off for the analyses. OS at 12 months was 0.50 (95% IC,
0.30–0.67) (Fig. 5). On
multivariable Cox analysis, RANKL and VEGF (adjusted for metastatic
status) were not predictive of OS (RANKL, HR, 1.10; 95% IC,
0.38–3.19; VEGF, HR, 1.19; 95% IC, 0.41–3.44). These results were
also confirmed when adjusted for age and using RANKL and VEGF as
continuous variables.
Discussion
One of the main characteristics of osteolytic bone
metastasis is the increase of OCs and their activity. We studied
the spontaneous osteoclastogenesis in in vitro cultures of
PBMCs derived from gastric cancer patients. Spontaneous
osteoclastogenesis was observed in half of the patients. Moreover,
unlike other osteotropic solid tumors such as breast and lung
cancer (19,24,25),
in gastric cancer the osteoclastogenesis was independent of RANKL
and TNF-α. We consider that other molecules could be involved in
the stimulation of osteoclastogenesis, such as IL-11, which has
been reported to support human osteoclastogenesis with IL-6 by a
RANKL-independent mechanism (26).
Moreover, IL-11 is key in gastric damage, mucosal repair and
gastric cancer progression (27,28).
Since the detected osteoclastogenesis did not
correlate with the presence of bone metastases, we suggested an
alternative hypothesis to explain it, considering a potential
relationship between an increased osteoclastogenesis and the
gastric acid levels, which are known to affect OC activity
(29). We observed that some
patients with increased osteoclastogenesis also showed chronic
gastritis and referred to taking proton-pump inhibitors (data not
shown). Although this requires further investigation, it might be
possible that hypochlorhydria induced by drugs is responsible for
the stimulation of OC resorption activity, leading to an increase
of osteoclastogenesis in vitro.
To elucidate the production of molecules stimulating
osteoclastogenesis by gastric cancer cells, we analyzed the mRNA
expression on primary tumors and bone metastases of gastric cancer.
TGF-β was higher in primary tumor than in bone metastatic samples.
TGF-β is released in active form by bone matrix, upon tumor-induced
osteoclastic bone resorption. It stimulates bone metastatic cells
to secrete factors that further drive osteolytic destruction of the
bone adjacent to the tumor (30).
Our result is in accordance with a previous study, reporting an
increased TGF-β expression in gastric cancer, which is closely
related to invasion and metastasis (31).
IL-7 was mainly expressed in the tumor tissue of
gastric cancer patients, but its mean serum level was not
significantly different between patients with or without
osteoclastogenesis. This result differs from our previously
published data on osteotropic tumors, where we demonstrated that
IL-7 was high in patients with spontaneous osteoclastogenesis, as
it stimulated OC differentiation through RANKL (23). In the present study, in gastric
cancer, we did not show the same mechanism since RANKL did not
promote osteoclastogenesis. Furthermore, we did not detect RANKL
expression in primary tumor or in bone metastatic samples, and
RANKL serum levels did not correlate with the presence of bone
metastases. In particular, serum RANKL values were lower in
patients than in controls, confirming our finding on
RANKL-independent osteoclastogenesis. OPG was expressed in primary
tumor and bone metastatic samples, according to a previous report
that OPG provides a survival advantage to cancer cells (32). OPG correlates with aggressiveness
and poor prognosis of gastric carcinoma (33). We did not observe any correlation
between OPG expression and osteoclastogenesis, likely due to the
RANKL-independence of osteoclastogenesis in our cohort of
patients.
DKK-1 and SOST are soluble inhibitors of canonical
WNT signaling (34), which plays an
important role in bone development by inhibiting OC differentiation
(35), stimulating
osteoblastogenesis and mineralizing activity of osteoblasts
(36). We detected DKK-1 expression
in primary tumor, according to previously published data reporting
its expression in some human specimens of tumors, and suggesting
that a cancer-mediated modulation of WNT activity affects the
metastatic phenotype (37–39). SOST was only detected in bone
metastatic samples, according to the specific expression of these
molecules by bone cells (34).
Since the collected data did not link RANKL to the
bone metastasis pathogenesis, we investigated the potential
involvement of RANKL in the tumor-induced angiogenesis. Previous
data reported an inhibitory role of RANKL in angiogenesis (14,15),
which could explain its lower serum levels in patients compared to
controls. To evaluate angiogenesis, we administered serum VEGF,
which is a key angiogenic factor mediating neo-vascularization and
could act as a surrogate marker of tumor angiogenesis (40). Unlike RANKL, the VEGF serum levels
were higher in gastric patients than in controls, suggesting a
block of the angiogenesis inhibition due to RANKL. We also
evaluated the prognostic value of serum RANKL and VEGF, as in
gastric cancer VEGF has been described as an independent prognostic
factor of survival (18,41). In our cohort of patients neither
RANKL nor VEGF showed significant differences in the OS.
To our knowledge, this is the first study to
investigate osteoclastogenesis and to evaluate the role of RANKL in
gastric cancer. Although this study has certain limitations with
regard to the single-centre design of this study and its small
sample size, we aim to open new perspectives in the investigation
of the molecular mechanisms involved in this process.
In conclusion, our results suggest that the common
parameters and molecules regulating bone metastases in osteotropic
solid tumors, such as breast, lung and prostate cancer, do not
appear to be involved in the pathogenesis of bone metastases from
gastric cancer. Indeed, neither spontaneous osteoclastogenesis
in vitro nor serum RANKL concentrations correlated with bone
metastases. RANKL could play a role in the angiogenesis of gastric
cancer, however further investigations to define its
pathophysiological role in gastric carcinoma are warranted.
References
|
1
|
Sasako M, Inoue M, Lin JT, Khor C, Yang HK
and Ohtsu A: Gastric Cancer Working Group Report. Jpn J Clin Oncol.
40(Suppl 1): i28–i37. 2010. View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.
|
|
4
|
AIRTUM Working Group. Italian cancer
figures, report 2011: survival of cancer patients in Italy.
Epidemiol Prev. 35(5–6 Suppl 3): 1–200. 2011.(In Italian).
|
|
5
|
Amadori D, Cascinu S, Conte PF and Ibrahim
T: Bone metastases. Osteo-Oncology Textbook. Cisalpina Grafiche and
Milanese S Giuliano: pp. 200–201. 2010
|
|
6
|
Kim HS, Yi SY, Jun HJ, et al: Clinical
outcome of gastric cancer patients with bone marrow metastases.
Oncology. 73:192–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi M, Okabayashi T, Sano T and
Araki K: Metastatic bone cancer as a recurrence of early gastric
cancer - characteristics and possible mechanisms. World J
Gastroenterol. 11:5587–5591. 2005.PubMed/NCBI
|
|
8
|
Batson OV: The function of the vertebral
veins and their role in the spread of metastases. 1940. Clin Orthop
Relat Res. (312): 4–9. 1995.PubMed/NCBI
|
|
9
|
Devkaran B, Jhobta R and Verma DK: Bony
metastasis of gastric adenocarcinoma. Saudi J Gastroenterol.
15:137–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hofbauer LC, Neubauer A and Heufelder AE:
Receptor activator of nuclear factor-kappaB ligand and
osteoprotegerin: potential implications for the pathogenesis and
treatment of malignant bone diseases. Cancer. 92:460–470. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kobayashi K, Takahashi N, Jimi E, et al:
Tumor necrosis factor alpha stimulates osteoclast differentiation
by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp
Med. 191:275–286. 2000. View Article : Google Scholar
|
|
13
|
Walsh MC and Choi Y: Biology of the TRANCE
axis. Cytokine Growth Factor Rev. 14:251–263. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McGonigle JS, Giachelli CM and Scatena M:
Osteoprotegerin and RANKL differentially regulate angiogenesis and
endothelial cell function. Angiogenesis. 12:35–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sfiridaki K, Pappa CA, Tsirakis G, et al:
Angiogenesis-related cytokines, RANKL, and osteoprotegerin in
multiple myeloma patients in relation to clinical features and
response to treatment. Mediators Inflamm. 2011:8675762011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Min JK, Cho YL, Choi JH, et al: Receptor
activator of nuclear factor (NF)-kappaB ligand (RANKL) increases
vascular permeability: impaired permeability and angiogenesis in
eNOS-deficient mice. Blood. 109:1495–1502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carlevaro MF, Cermelli S, Cancedda R and
Descalzi Cancedda F: Vascular endothelial growth factor (VEGF) in
cartilage neovascularization and chondrocyte differentiation:
auto-paracrine role during endochondral bone formation. J Cell Sci.
113:59–69. 2000.
|
|
18
|
Vidal O, Metges JP, Elizalde I, et al:
High preoperative serum vascular endothelial growth factor levels
predict poor clinical outcome after curative resection of gastric
cancer. Br J Surg. 96:1443–1451. 2009. View
Article : Google Scholar
|
|
19
|
Roato I, Grano M, Brunetti G, et al:
Mechanisms of spontaneous osteoclastogenesis in cancer with bone
involvement. FASEB J. 19:228–230. 2005.PubMed/NCBI
|
|
20
|
Brianza S, D’Amelio P, Cerrato M, Bignardi
C, Grimaldi A, Pescarmona G and Isaia G: A dedicated image analysis
software tool for the evaluation of the resorption activity of
cultured osteoclasts. JIST. 52:30508-1–30508-9. 2008. View Article : Google Scholar
|
|
21
|
Roato I, D’Amelio P, Gorassini E, et al:
Osteoclasts are active in bone forming metastases of prostate
cancer patients. PLoS One. 3:e36272008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
D’Amelio P, Roato I, D’Amico L, et al:
Bone and bone marrow pro-osteoclastogenic cytokines are
up-regulated in osteoporosis fragility fractures. Osteoporos Int.
22:2869–2877. 2011.PubMed/NCBI
|
|
23
|
Roato I, Brunetti G, Gorassini E, et al:
IL-7 up-regulates TNF-alpha-dependent osteoclastogenesis in
patients affected by solid tumor. PLoS One. 1:e1242006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roato I, Gorassini E, Buffoni L, et al:
Spontaneous osteoclastogenesis is a predictive factor for bone
metastases from non-small cell lung cancer. Lung Cancer.
61:109–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Colucci S, Brunetti G, Rizzi R, et al: T
cells support osteoclastogenesis in an in vitro model derived from
human multiple myeloma bone disease: the role of the OPG/TRAIL
interaction. Blood. 104:3722–3730. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kudo O, Sabokbar A, Pocock A, Itonaga I,
Fujikawa Y and Athanasou NA: Interleukin-6 and interleukin-11
support human osteoclast formation by a RANKL-independent
mechanism. Bone. 32:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Howlett M, Chalinor HV, Buzzelli JN, et
al: IL-11 is a parietal cell cytokine that induces atrophic
gastritis. Gut. 61:1398–1409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Necula LG, Chivu-Economescu M,
Stanciulescu EL, et al: IL-6 and IL-11 as markers for tumor
aggressiveness and prognosis in gastric adenocarcinoma patients
without mutations in Gp130 subunits. J Gastrointestin Liver Dis.
21:23–29. 2012.PubMed/NCBI
|
|
29
|
Schinke T, Schilling AF, Baranowsky A, et
al: Impaired gastric acidification negatively affects calcium
homeostasis and bone mass. Nat Med. 15:674–681. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Juarez P and Guise TA: TGF-β in cancer and
bone: implications for treatment of bone metastases. Bone.
48:23–29. 2011.
|
|
31
|
Maehara Y, Kakeji Y, Kabashima A, et al:
Role of transforming growth factor-beta 1 in invasion and
metastasis in gastric carcinoma. J Clin Oncol. 17:607–614.
1999.PubMed/NCBI
|
|
32
|
Holen I and Shipman CM: Role of
osteoprotegerin (OPG) in cancer. Clin Sci. 110:279–291. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ito R, Nakayama H, Yoshida K, et al:
Expression of osteoprotegerin correlates with aggressiveness and
poor prognosis of gastric carcinoma. Virchows Arch. 443:146–151.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Macsai CE, Foster BK and Xian CJ: Roles of
Wnt signalling in bone growth, remodelling, skeletal disorders and
fracture repair. J Cell Physiol. 215:578–587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Glass DA II, Bialek P, Ahn JD, et al:
Canonical Wnt signaling in differentiated osteoblasts controls
osteoclast differentiation. Dev Cell. 8:751–764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morvan F, Boulukos K, Clement-Lacroix P,
et al: Deletion of a single allele of the Dkk1 gene leads to an
increase in bone formation and bone mass. J Bone Miner Res.
21:934–945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tian E, Zhan F, Walker R, et al: The role
of the Wnt-signaling antagonist DKK1 in the development of
osteolytic lesions in multiple myeloma. N Engl J Med.
349:2483–2494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li ZG, Yang J, Vazquez ES, et al:
Low-density lipoprotein receptor-related protein 5 (LRP5) mediates
the prostate cancer-induced formation of new bone. Oncogene.
27:596–603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Voorzanger-Rousselot N, Goehrig D, Journe
F, et al: Increased Dickkopf-1 expression in breast cancer bone
metastases. Br J Cancer. 97:964–970. 2007.PubMed/NCBI
|
|
40
|
Poon RT, Fan ST and Wong J: Clinical
implications of circulating angiogenic factors in cancer patients.
J Clin Oncol. 19:1207–1225. 2001.PubMed/NCBI
|
|
41
|
Fondevila C, Metges JP, Fuster J, et al:
p53 and VEGF expression are independent predictors of tumour
recurrence and survival following curative resection of gastric
cancer. Br J Cancer. 90:206–215. 2004. View Article : Google Scholar : PubMed/NCBI
|