Introduction
Chronic lymphocytic leukemia (CLL) is the most
prevalent type of adult leukemia in western countries. The disease
is heterogeneous as regards prognosis and clinical outcome and
usually affects people over the age of 60. The median age at
diagnosis is 72 years (1). Although
significant progress has recently been made in the treatment of
CLL, the disease remains incurable (2,3).
The limited efficacy of CLL anti-leukemic therapy
may be associated with the particular nature of this type of
cancer, which is characterized by the accumulation of
apoptosis-defective leukemic cells in the blood, bone marrow and
lymph nodes of patients. Transformed cells circulating in the
peripheral blood of individuals who suffer from CLL are arrested in
the G0/G1 phase, whereas bone marrow, lymph nodes and lymph nodules
in peripheral lymphoid tissue may serve as reservoirs of cells
which divide and supply blood with the accumulating pool of cells
(4,5). Thus, effective therapeutic approaches
should be directed toward resting cells in peripheral blood as well
as toward the proliferating pool of cells in the germinal centers
of lymphocytes.
Statins are well-known drugs commonly used in the
treatment of hypercholesterolemia (6). They block the conversion of
3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) to L-mevalonic acid by the
competitive inhibition of HMG-CoA reductase, a key enzyme in the
cholesterol biosynthesis pathway. Statins simultaneously inhibit
the synthesis of all intermediates downstream of mevalonate, i.e.,
farnesyl pyrophosphate or geranyl pyrophosphate. Consistently, they
prevent protein prenylation leading to the modulation of their
cellular localization and function. The statin-mediated inhibition
of small G protein modification is thought to be responsible for
the cholesterol level-independent anti-proliferative activity of
these drugs in a variety of human cancer cell lines (7). Additionally, these drugs can induce
apoptosis in rapidly proliferating cells, as well as in cells which
do not proliferate; however, the mechanism behind this activity
remains unclear (8–10). Taking these facts into
consideration, statins may prove particularly useful in the
treatment of CLL. Moreover, many years of clinical experience with
statins have confirmed their safety and low toxicity against normal
cells (11).
In the present study, we evaluated ex vivo
the anti-leukemic potential of atorvastatin in peripheral blood
mononuclear cells (PBMCs) isolated from previously untreated CLL
patients. Additionally, the cytotoxicity of the tested drug was
also examined in mononuclear cells isolated from the blood of 4
healthy volunteers. Atorvastatin is a synthetic open-ring compound
that does not require β-lactone ring hydrolysis for its activity
(12). It has shown pro-apoptotic
potential in a large number of cancer cell lines (11,13–16).
However, the pro-apoptotic potential of atorvastatin in primary CLL
cells has yet not been established.
Materials and methods
Patients
Mononuclear cells were obtained from the peripheral
blood of 15 untreated CLL patients (3 females and 12 males) with
different stages of the disease (I-IV), classifed according to the
staging system described in the study by Rai et al(17). The median age of the patients at the
time of the study was 62 years (range, 51 to 80 years), and the
median leukocytosis, 182.67×109/l (range,
20×109/l to 600×109/l). All patients enrolled
in the study required anti-leukemic therapy.
CLL was diagnosed on the basis of standard clinical
and immunological criteria (17).
This study was approved by the Local Ethics Committee of the
Medical University of ŁódŸ (no. RNN/143/10/KE) and all patients
gave their written consent prior to enrollment.
Additionally, the peripheral cells from the blood of
4 healthy volunteers (1 male and 3 females, aged 23–65 years) with
normal leukocytosis were isolated to compare the activity of
atorvastatin in normal and primary tumor cells.
Isolation of mononuclear cells
PBMCs were isolated from peripheral blood samples
(collected into EDTA as the anticoagulant) obtained from CLL
patients or healthy donors by Histopaque-1077 density gradient
centrifugation (Sigma-Aldrich, St. Louis, MO, USA). The CLL or
control cell pellets were then resuspended in phosphate-buffered
saline (PBS) and divided as required for the planned
experiments.
Cell culture and drug treatment
The model cell samples were resuspended in RPMI-1640
medium with 10% fetal calf serum supplemented with 2 mM
L-glutamine, 100 μg/ml streptomycin, and 100 U/ml penicillin to a
final concentration of 2×106 cells/ml and incubated with
atorvastatin (LKT Laboratories, Inc.) at concentrations of 5, 10,
25, 50, 100 and 150 μM. The cells were incubated with this statin
(or without, controls) as well as with 0.15% DMSO (vehicle
controls) for 24 and 48 h at 37°C in an atmosphere of 5%
CO2.
Cell viability and apoptosis
determination
To evaluate the viability of leukemic (11 samples)
and normal mononuclear cells (4 samples), as well as the percentage
of apoptotic cells in the model cells exposed to atorvastatin, the
Vybrant Apoptosis Assay kit #4 from Invitrogen Molecular Probes
(Eugene, OR, USA) was used. The mononuclear cell population was
gated on the basis of forward scatter (FSC) and side scatter (SSC)
parameters. The percentage of viable cells was determined after 24
and 48 h of incubation with atorvastatin and quantified using the
LSR II Flow Cytometer (Becton-Dickinson, San Jose, CA, USA). The
number of viable cells was quantified in 4 experiments by a
colorimetric MTT assay based on MTT reduction, as previously
described (18) or propidium iodide
(PI) staining only (data not shown).
DNA content analysis
The DNA content in the PBMCs from the blood of 8 CLL
patients, as well as from healthy volunteers, was estimated after
48-h incubation with/without atorvastatin. Briefly,
1×106 cells were fixed with 70% ethanol and incubated at
−20°C for 2 h. Subsequently, the cells were incubated in the
presence of RNase A (at a final concentration in PBS of 0.5 mg/ml)
and PI (at a final concentration in PBS of 0.01 mg/ml) for 30 min,
at 37°C, in the dark. The fluorescence of PI was then measured by
flow cytometry (FACSCalibur; Becton-Dickinson) and the number of
sub-G1 cells was evaluated on the basis of FL-3 histograms using
CellQuest Pro software (Becton-Dickinson). Ten thousand events were
examined for each analysis.
DNA fragmentation analysis
The DNA fragmentation was assessed by agarose gel
electrophoresis performed according the procedure described in the
study by Bellosillo et al(19), with slight modifications. Briefly,
6×106 CLL cells (after washing with PBS) were lysed and
treated with proteinase K (0.2 mg/ml) in a buffer containing 5 mM
Tris-HCl, pH 8.0, 20 mM EDTA, 0.5% Triton X-100 for about 12 h at
37°C. DNA was extracted twice with buffered
phenol/chloroform/isoamyl alcohol (25:24:1), and precipitated with
0.1 volume of 3 M sodium acetate and 2 volumes of ethanol at −20°C,
overnight. The DNA precipitates were washed twice with 75% ethanol,
dissolved in triple-destilled water, and digested with RNase A (1
mg/ml) for 2 h at 37°C. Finally, the DNA samples were
electrophoresed by standard agarose gel (2.0%) electrophoresis.
Etidium bromide was used for DNA visualization under ultraviolet
light.
Protein separation and immunoblot
assay
Leukemic and normal PBMCs were lysed and prepared
for western blot analysis as previously described (18). Protein determination in the cell
lysates was performed according to the method described by Lowry
et al(20). Approximately 50
μg of protein was loaded per each lane and the proteins were
separated by SDS-PAGE into 8% and 12.5% slab gels, depending on the
molecular weights of the analyzed proteins. The proteins were then
transferred onto Immobilon-P membranes according to the method
described by Towbin et al(21) and stained reversibly with 0.05%
Ponceau S solution to confirm their equal loading. Subsequently,
the membranes were incubated in the presence of 5% non-fat dry milk
in TBST buffer [10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.05% Tween
20] for 1 h at room temperature with successive incubation with
appropriate antibodies, overnight. The following antibody dilutions
were used: anti-poly(ADP-ribose) polymerase-1 (PARP-1; 1:5,000),
anti-lamin B (1:2,000) and anti-caspase-9 (1:5,000) from Santa Cruz
Biotechnology (Santa Cruz, CA) and anti-actin (1:1,000) from Abcam
(Cambridge, UK). After being washed 3 times in TBST buffer, the
membranes were incubated with secondary antiserum conjugated with
alkaline phosphatase (Sigma-Aldrich) for 2 h and washed 3 times
again. The antigen-antibody complexes were visualized following
incubation of the membranes with a phosphatase substrate solution
containing 0.33 mg/ml of nitro blue tetrazolium and 0.17 mg/ml of
5-bromo-4-chloro-3-indolyl phosphate in 100 mM Tris-HCl (pH 9.5),
100 mM NaCl and 5 mM MgCl2.
Statistical analysis
All results are presented as the mean values ± SD.
The statistical data analysis was performed using the Wilcoxon
signed rank test. The results were considered statistically
significant at P≤0.05.
Results
Cell viability and apoptosis
induction
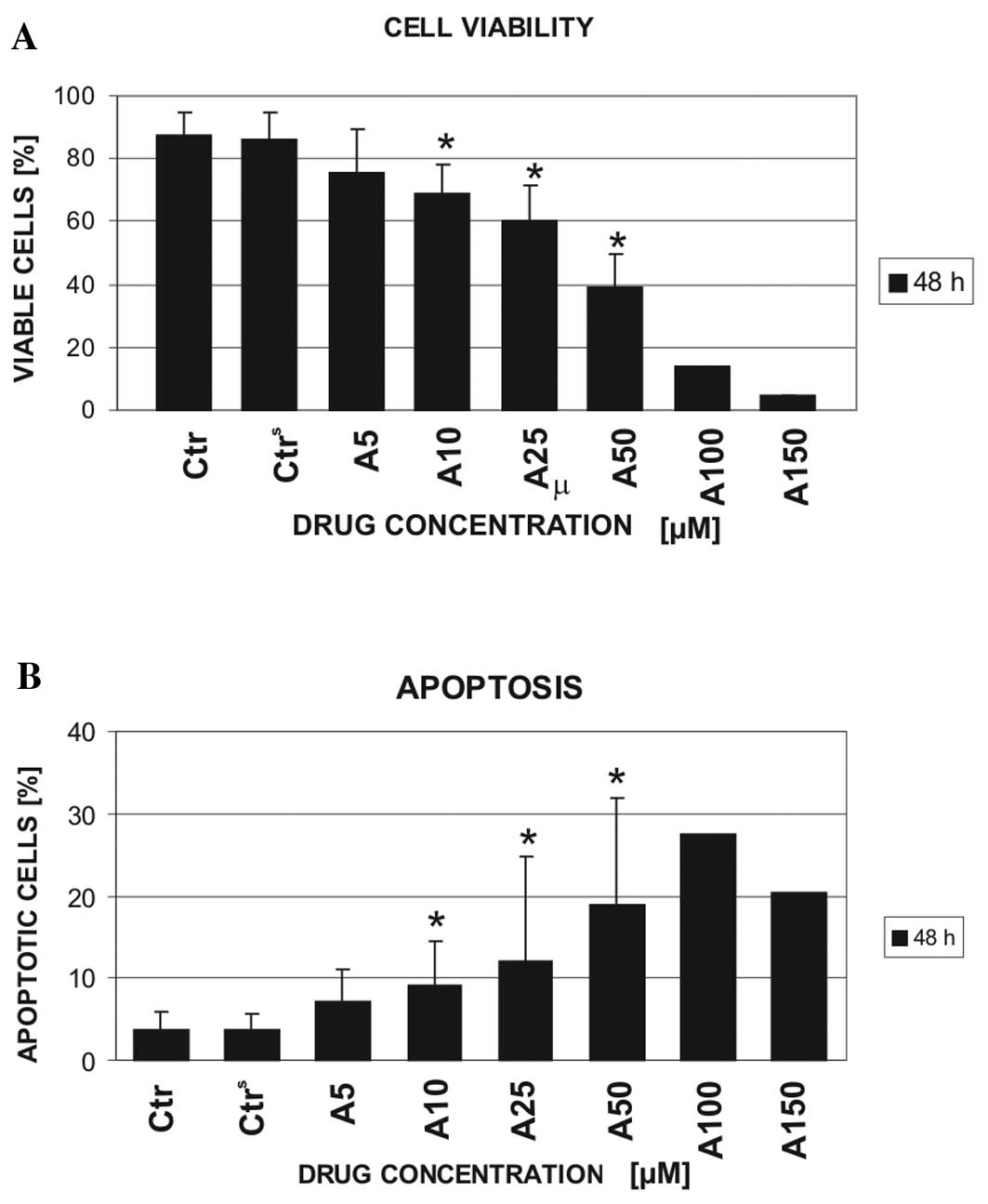
The Vybrant Apoptosis Assay kit #4 was used to
select the dose of atorvastatin that caused ~50% decrease in
leukemic cell viability in comparison to the cells incubated with
culture medium only (control). Examinations were performed on the
cell samples obtained from 11 CLL patients. The viability of CLL
cells was determined following a 24- (data not shown) and 48-h
exposure to atorvastatin at increasing concentrations (5, 10, 25
and 50 μM) (Fig. 1A). In 2 cases,
the exposure of the cells to 100 and 150 μM of atorvastatin was
also examined. Additionally, the effect of 0.15% DMSO (used as a
solvent for atorvastatin) on CLL cell viability was examined. The
obtained results indicated no significant effect of 0.15% DMSO on
model cell viability. By contrast, the leukemic cells were
sensitive to all tested drug concentrations. The effect was already
observed at 24 h of cell incubation with the statin (data not
shown). Approximately 50% (46.7±10.4) reduction in CLL cell
viability in comparison with the control cells was observed
following the 48-h exposure of the cells to 50 μM atorvastatin. A
further increase in the drug concentration (100 μM) decreased the
viability of the CLL cells by ~70% compared to the controls and the
vehicle controls (13.8 vs. 87.24 and 86.12%, respectively). The
decrease in CLL cell viability was primarily caused by the
induction of apoptosis (Fig. 1B).
However, the higher the concentration of atorvastatin used, the
greater the increase in the dead cell population. The Vybrant
Apoptosis Assay kit #4 does not distinguish between necrotic and
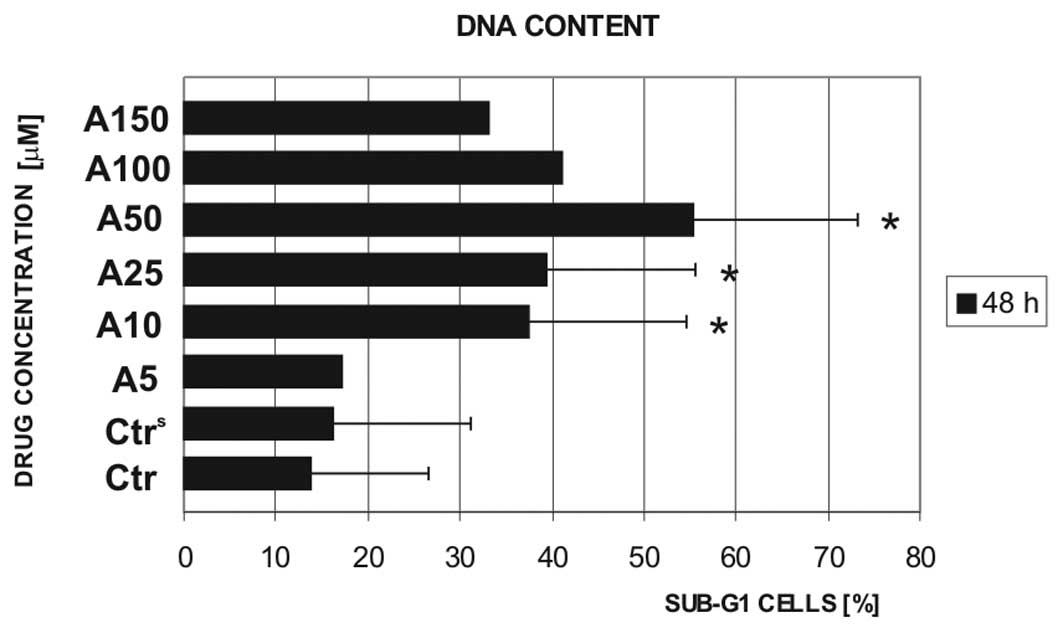
late apoptotic cells. Therefore, the DNA content was estimated to
better determine the apoptotic cell numbers in the population of
drug-exposed CLL cells. Since the majority of viable leukemic cells
do not proliferate, on DNA content histograms, they are viewed as a
diploid cell population following PI staining. By contrast,
apoptotic cells are characterized by the presence of
oligonucleosomal DNA fragments leaking from the cells after their
fixation (22). Hence, they are
revealed during cytometrical tests as the cells with a diminished
DNA content (sub-G1/hypodiploid cells) in comparison with viable
cells. The estimation of DNA content in the cells obtained from the
blood of 8 CLL patients confirmed the induction of apoptosis in the
cells treated with atorvastatin (Fig.
2). The exposure of CLL cells to 50 μM atorvastatin increased
the sub-G1 cell population from ~15% (control) to >55%. Of note,
the increase in the concentration of atorvastatin to ≥100 μM led to
a decrease in this population. These data, together with the
results obtained from the Vybrant Apoptosis Assay kit #4, suggest
that higher concentrations of atorvastatin induce cell death mainly
through necrosis.
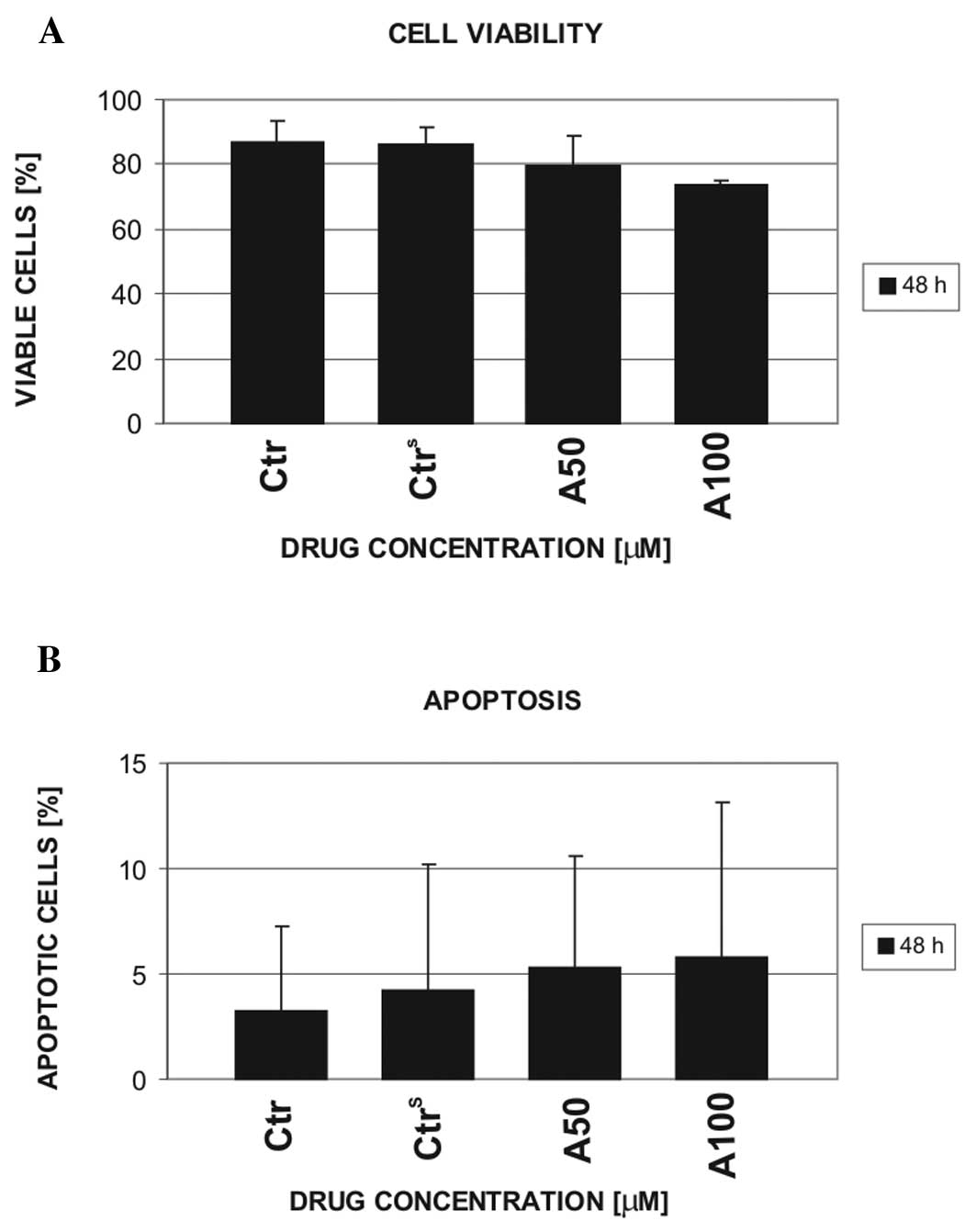
On the basis of CLL cell viability, the doses of 50
and 100 μM atorvastatin were selected for further experiments with
the use of normal mononuclear cells. In contrast to the leukemic
cells, normal cells were considerably resistant to 50 μM
atorvastatin, which caused ~50% decrease in leukemic cell viability
after 48 h of incubation (Fig. 3).
Furthermore, the viability and apoptosis of mononuclear cells from
the blood of 4 healthy volunteers was only slightly influenced by
100 μM atorvastatin.
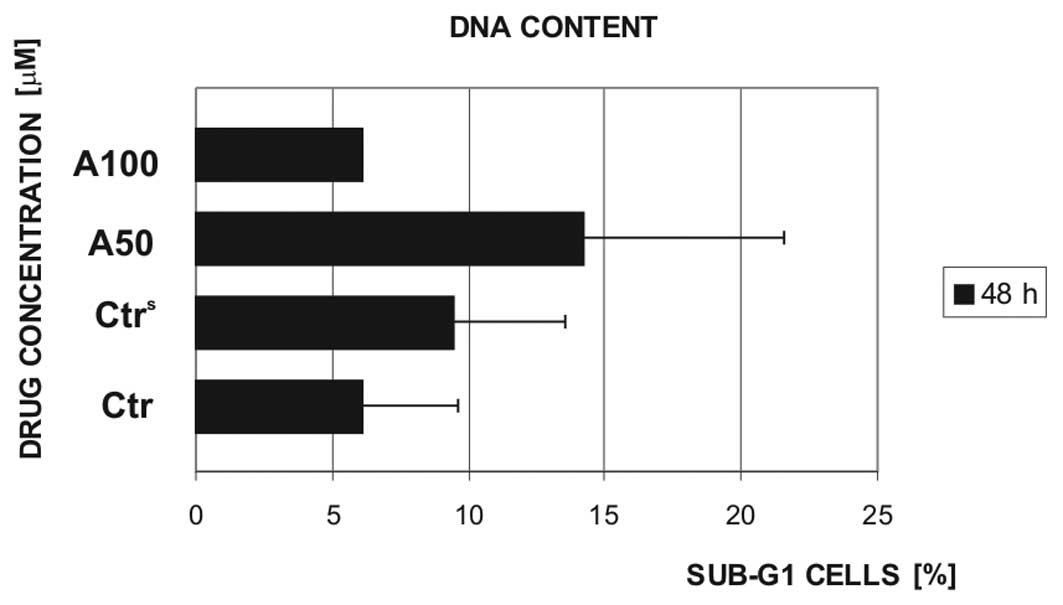
The cell viability results were partially confirmed
by sub-G1 cell population analyses. In normal mononuclear cells,
the DNA content was assessed following exposure to 50 and 100 μM
(in 2 cases) atorvastatin for 48 h (Fig. 4). The tested drug concentration (50
μM) caused an increase in the sub-G1 cell population of normal
PBMCs, on average, only by ~10% in comparison to the controls,
i.e., the cells which were not exposed to atorvastatin (controls,
Ctr) or exposed to 0.15% DMSO (vehicle control,
Ctrs).
DNA fragmentation
To confirm the pro-apoptotic activity of
atorvastatin in leukemic cells, analysis of DNA fragmentation was
performed. Fig. 5 illustrates the
representative results of DNA fragmentation analysis for PBMCs from
the blood of an exemplary CLL patient and treated ex vivo
with atorvastatin. As illustrated, the exposure of leukemic cells
to 25 and 50 μM atorvastatin resulted in DNA degradation to the
fragments visible as a DNA ladder, following electrophoretic
separation on agarose gels. DNA laddering is a known feature of
apoptosis. Notably, the dose of 50 and 100 μM atorvastatin did not
induce DNA fragmentation in normal PBMCs (data not shown).
Expression of apoptosis-related
proteins
Issues regarding the induction of apoptosis caused
by atorvastatin were addressed by comparative analysis of selected
nuclear proteins, as well as regulatory Bcl-2 family member
expression (Fig. 6). The western
blot analysis results of lamin B (67 kDa) and PARP1 (116 kDa),
known markers of apoptosis, revealed that atorvastatin effectively
induced apoptosis in CLL cells following treatment with the statin.
The representative results, shown in Fig. 6A, confirmed the cleavage of the
native forms of the nuclear proteins accompanied by an appearance
of their 44- and 85-kDa proteolytic products, respectively.
Moreover, immunoblot analysis revealed the proteolytic cleavage of
the cell cycle inhibitory protein precursor, p27Kip1, to
a 23-kDa product in CLL cells incubated with atorvastatin at all
tested drug concentrations. It is worth mentioning, however, that
the proteolytic level of these proteins varied noticeably between
samples from different CLL patients, depending on their individual
susceptibility to the used drug.
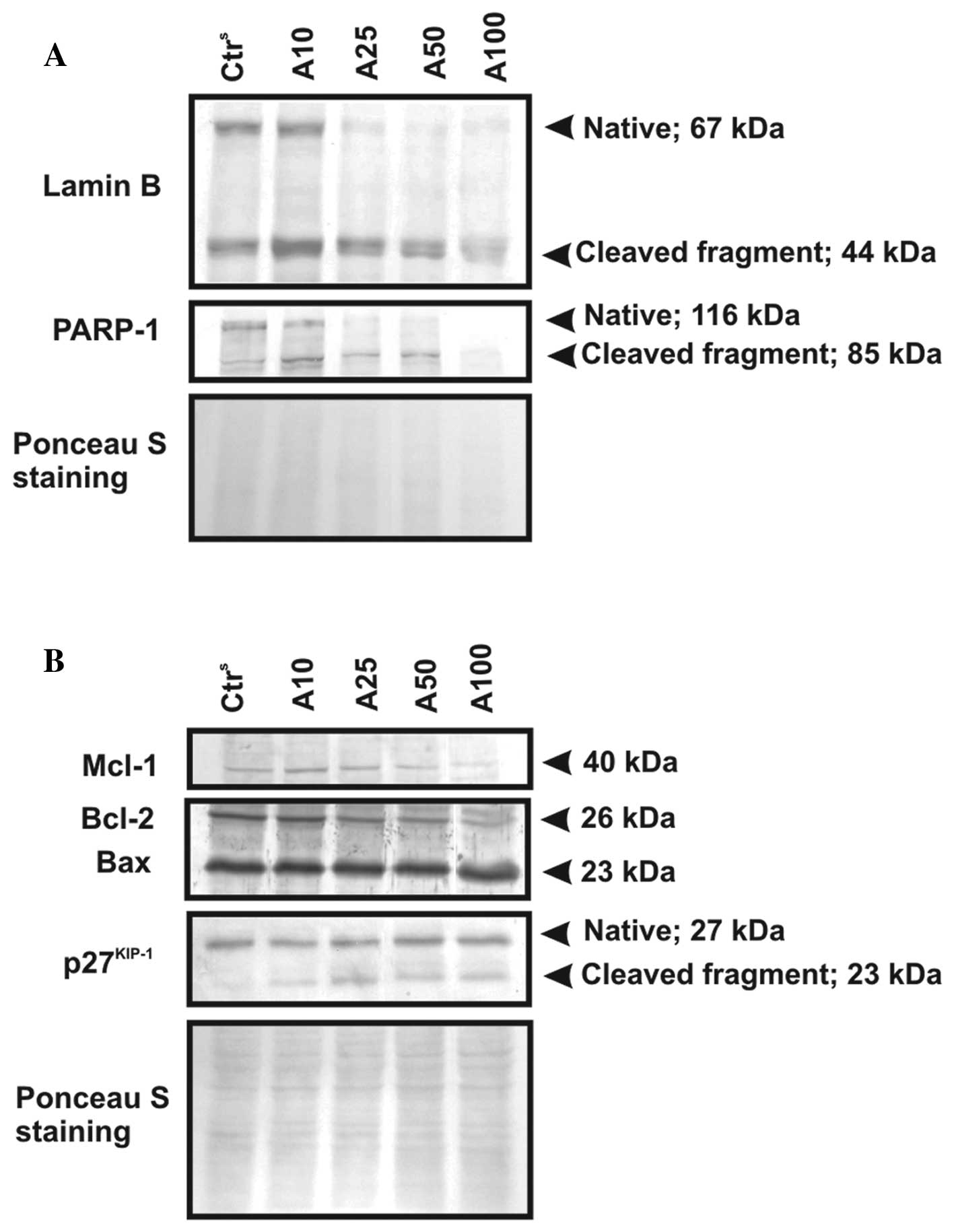 | Figure 6Ex vivo changes in the
expression level of selected apoptosis-related proteins in CLL cell
samples exposed to atorvastatin (10, 25, 50 and 100 μM) for 48 h.
Protein lysates (50 μg) from atorvastatin-treated CLL cells. After
electrophoretic separation and transfer, proteins immobilized on
Immobilon-P membranes were analyzed for the expression of (A) lamin
B and PARP-1, (separated on 8% polyacrylamide gels) and (B)
apoptosis-regulated proteins, i.e., p27Kip1, Mcl-1,
Bcl-2 and Bax, (separated on 12.5% polyacrylamide gels) by western
blot analysis. The obtained results from a selected CLL patient are
presented. Ctrs, CLL cells incubated in culture medium
with 0.15% DMSO. Ponceau staining was used as a loading
control. |
The expression levels of apoptosis-regulatory
proteins of the Bcl-2 family [myeloid cell leukemia-1 (Mcl-1),
B-cell leukemia/lymphoma 2 (Bcl-2) and Bcl-2-associated X protein
(Bax)] in the model cells exposed to the statin were evaluated. As
illustrated in Fig. 6B, a decreased
expression of Mcl-1 (40 kDa) following the exposure of leukemic
cells to atorvastatin was observed. The expression of the
full-length Mcl-1 protein diminished when the cells were treated
with 50 and 100 μM atorvastatin. The cellular level of another
anti-apoptotic protein, Bcl-2 (26 kDa), decreased, while the
pro-apoptotic protein, Bax (23 kDa), demonstrated a stable
expression following CLL cell incubation with atorvastatin at a
dose of up to 50 μM. The increase in the expression of the latter
protein was observed only in the lysates from the cells treated
with the highest studied drug concentration.
By contrast, even the highest tested concentration
of atorvastatin did not alter the expression of examined
apoptosis-related proteins in the mononuclear cells obtained from
healthy volunteers (data not shown).
Discussion
It has been well-established that statins exert
potent anticancer activity in a large number of cancer cell lines
(11,12,15,23).
Little is known, however, about the activity of these drugs in CLL
cells. Taking into consideration the pleiotropic mechanisms of
their action, as well as the nature of the disease, i.e., the
accumulation of quiescent cells in the blood accompanied by the
presence of a proliferating pool of cells in the bone marrow and
lymphatic organs, it seems that statins should be highly efficient
in the treatment of this hematological cancer. Recently, the
anti-leukemic potential of simvastatin against CLL cells used in
combination with the purine analogs, fludarabine and cladribine,
was demonstrated in the study by Podhorecka et al(24). The examined concentration of
simvastatin was 10 μM. The synergism of this drug with conventional
chemotherapeutics was revealed. Additionally, no significant effect
of simvastatin was observed on normal cells.
In this study, we evaluated the cytotoxic and
pro-apoptotic potential of another statin (atorvastatin) in primary
tumor cells obtained from the blood of CLL patients prior to
therapy. CLL cells are characterized by their strong resistance to
apoptosis (4,5). This lack of sensitivity to
death-inducing stimuli is mainly associated with the deregulation
of certain signaling pathways [mitogen-activated protein kinase
(MAPK) or protein kinase B (PKB) pathways] and the overexpression
of a number of pro-survival molecules [Bcl-2, Mcl-1, survivin and
inhibitor of apoptosis (IAP) proteins] in the transformed cells
(25–30). For this reason, it was of high
importance to examine the influence of increasing doses of
atorvastatin on normal and leukemic cells. Our results revealed an
increased cytotoxic activity of atorvastatin alone against PBMCs
from CLL patients with different clinical stages of the disease.
The susceptibility of CLL cells obtained from different patients
was diverse, although it was not dependent on the stage of
leukemia. Atorvastatin at the dose of 50 μM decreased the viability
of the tested leukemic cells, on average by ~50% in comparison to
the control cells. Importantly, the same dose of the drug did not
visibly affect the viability of normal cells. Moreover, a 2-fold
higher concentration of atorvastatin (100 μM), which caused
extensive necrosis in leukemic cells, caused only a slight decline
in the viable cell number in the population of PBMCs obtained from
the blood of healthy volunteers. By contrast, Salman et
al(31) reported a small, but
significant decrease in normal PBMC apoptosis following exposure to
atorvastatin at the concentration of 50 μM, which is confusing,
while taking into consideration the fact that the significant
influence of the drug has been limited only to early apoptotic
cells.
The results of our DNA fragmentation analysis and
DNA content in the drug-treated cells indicated the selective
pro-apoptotic potential of atorvastatin in leukemic, but not in
normal cells. The pro-apoptotic activity of this statin was
confirmed by western blot analysis of PARP-1 and lamin B
expression. It is widely accepted that the degradation of lamin B
and PARP-1 into 44- and 85-kDa fragments results from the
proteolytic activity of caspase-6, as well as caspase-3 and -7,
respectively (32,33). The proteolysis of lamin B and PARP-1
was observed in the leukemic cells following their incubation with
atorvastatin. In addition, we investigated the impact of this
statin on the expression level of the cyclin-dependent kinase
inhibitor, p27Kip1. Vrhovac et al(34) described a correlation between the
p27Kip1 expression level and CLL progression and drug
resistance. Moreover, the cleavage of p27Kip1, due to
caspase activity, may be a sensitive marker of apotosis in
conjunction with PARP-1 and lamin B proteolysis (35,36).
As expected, our results demonstrate that atorvastatin induces
changes in the expression level of p27Kip1 that are
accompanied by the appearance of a p23-kDa cleaved product of the
full-length protein. Thus, atorvastatin induces apoptosis in CLL
cells in a caspase-dependent manner.
To elucidate the mechanism behind the pro-apoptotic
action of atorvastatin, we evaluated the expression level of the
apoptosis-regulatory proteins, Mcl-1, Bcl-2 and Bax, in
drug-exposed CLL cells. Bcl-2 and Mcl-1 belong to the family of
anti-apoptotic proteins that regulate the function of pore-forming
proteins of the Bcl-2 family (Bax or Bak) (37). Bax molecules assemble the channels
in mitochondrial membranes in response to various apoptotic stimuli
and enable pro-apoptotic factors to be released from the
mitochondria to the cytosol (37,38).
Under physiological conditions, when the pro- and anti-apoptotic
members of Bcl-2 family retain a dynamic balance in the cells, Bax
can be bound by its counter partners, Bcl-2 and Mcl-1 (37). This interaction leads to the
inhibition of mitochondrial membrane permeability and inhibits
apoptosis. The expression of Mcl-1 and Bcl-2 proteins is known to
be elevated in CLL cells (26,28).
Of note, Mcl-1 has been reported to be capable of hampering
apoptosis in hematopoietic cells to a higher extent than Bcl-2.
Mcl-1 overexpression in leukemic cells is related to
chemoresistance and progression of the disease. We, as well as
others have previously confirmed the overexpression of
anti-apoptotic Bcl-2 family proteins in CLL cells in vivo,
which is considered to be one of the reasons of their resistance
towards apoptosis, as well as changes in the Bax/Bcl-2 ratio in
response to chemotherapy (27,28).
In this study, we revealed that the exposure of CLL
cells to atorvastatin ex vivo disturbs the dynamic balance
between pro- and anti-apoptotic proteins of Bcl-2 family via the
diminution of Bcl-2 and Mcl-1 expression level without altering Bax
expression.
Atorvastatin, similar to other statins, beyond its
cholesterol-reducing properties, has also demonstrated
pro-apoptotic properties against many types of cancer cells. The
anticancer potential of statins has encouraged their use in the
treatment of cancer. It has been suggested that these drugs may be
useful in combination with other therapeutic agents (24,39).
For this reason, further studies are required to establish the
potential benefits of statin alone/statin-combined therapy for
different types of cancer, including CLL.
In conclusion, atorvastatin induces the
mitochondrial pathway of apoptosis in leukemic PBMCs by affecting
the cellular concentration of anti-apoptotic proteins that are
deregulated in CLL cells, i.e., Mcl-1 and Bcl-2. Moreover, it
triggers alterations in drug-exposed leukemic cells, resulting in
the proteolysis of the cell cycle-related proteins,
p27Kip1, lamin B and PARP-1. Importantly, the
pro-apoptotic potential of atorvastatin is limitted specifically to
leukemic cells. Normal PBMCs from healthy volunteers do not exhibit
susceptibility to this statin, even at very high concentrations.
The obtained results suggest that atorvastatin may be use for the
treatment of CLL, possibly in conjunction with other
chemotherapeutic agents.
Acknowledgements
The present study was supported in part by a grant
from the University of Lodz (No. 545/479).
References
|
1
|
Eichhorst B, Dreyling M, Robak T,
Montserrat E and Hallek M; ESMO Guidelines Working Group. Chronic
lymphocytic leukemia: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 22(Suppl 6):
vi50–vi54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner H, Gondos A and Pulte D: Trends in
long-term survival of patients with chronic lymphocytic leukemia
from the 1980s to the early 21st century. Blood. 111:4916–4921.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kiliańska ZM and Rogalińska M: Potential
new agents for chronic lymphocytic leukemia treatment. Anticancer
Agents Med Chem. 10:666–682. 2010.
|
|
4
|
Burger JA, Tsukada N, Burger M, Zvaifler
NJ, Dell’Aquila M and Kipps TJ: Blood-derived nurse-like cells
protect chronic lymphocytic leukemia B cells from spontaneous
apoptosis through stromal cell-derived factor-1. Blood.
96:2655–2663. 2000.PubMed/NCBI
|
|
5
|
Decker T, Hipp S, Ringshausen I, Bogner C,
Oelsner M, Schneller F and Peschel C: Rapamycin-induced G1 arrest
in cycling B-CLL cells is associated with reduced expression of
cyclin D3, cyclin E, cyclin A, and survivin. Blood. 101:278–285.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Q and Liao JK: Pleiotropic effects of
statins. Basic research and clinical perspectives. Circ J.
74:818–826. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roy M, Kung HJ and Ghosh PM: Statins and
prostate cancer: role of cholesterol inhibition vs. prevention of
small GTP-binding proteins. Am J Cancer Res. 1:542–561.
2011.PubMed/NCBI
|
|
8
|
Chapman-Shimshoni D, Yuklea M, Radnay J,
Shapiro H and Lishner M: Simvastatin induces apoptosis of B-CLL
cells by activation of mitochondrial caspase 9. Exp Hematol.
31:779–783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pahan K: Lipid-lowering drugs. Cell Mol
Life Sci. 63:1165–1167. 2006. View Article : Google Scholar
|
|
10
|
Copajaa M, Venegasa D, Aránguiza P, et al:
Simvastatin induces apoptosis by a Rho-dependent mechanism in
cultured cardiac fibroblasts and myofibroblasts. Toxicol Appl
Pharmacol. 255:57–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wong WW, Dimitroulakos J, Minden MD and
Penn LZ: HMG-CoA reductase inhibitors and the malignant cell: the
statin family of drugs as triggers of tumor-specific apoptosis.
Leukemia. 16:508–519. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan KK, Oza AM and Siu LL: The statins as
anticancer agents. Clin Cancer Res. 9:10–19. 2003.
|
|
13
|
Agarwal B, Halmos B, Feoktistov AS, et al:
Mechanism of lovastatin-induced apoptosis in intestinal epithelial
cells. Carcinogenesis. 23:521–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van de Donk NW, Schotte D, Kamphuis MM,
van Marion AM, van Kessel B, Bloem AC and Lokhorst HM: Protein
geranylgeranylation is critical for the regulation of survival and
proliferation of lymphoma tumor cells. Clin Cancer Res.
9:5735–5748. 2003.PubMed/NCBI
|
|
15
|
Sleiffer S, van der Gaast A, Planting AS,
Stoter G and Verweij J: The potential of statins as part of
anti-cancer treatment. Eur J Cancer. 41:516–522. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhuang L, Kim J, Adam RM, Solomon KR and
Freeman MR: Cholesterol targeting alters lipid raft composition and
cell survival in prostate cancer cells and xenografts. J Clin
Invest. 115:959–968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rai KR, Sawitsky A, Cronkite EP, Chanana
AD, Levy RN and Pasternack BS: Clinical staging of chronic
lymphocytic leukemia. Blood. 46:219–234. 1975.PubMed/NCBI
|
|
18
|
Kobylinska A, Bednarek J, Blonski JZ,
Hanausek M, Walaszek Z, Robak T and Kilianska ZM: In vitro
sensitivity of B-cell chronic lymphocytic leukemia to cladribine
and its combinations with mafosfamide and/or mitoxantrone. Oncol
Rep. 16:1389–1395. 2006.
|
|
19
|
Bellosillo B, Villamor N, Colomer D, Pons
G, Montserrat E and Gil J: In vitro evaluation of fludarabine in
combination with cyclophosphamide and/or mitoxantrone in B-cell
chronic lymphocytic leukemia. Blood. 94:2836–2843. 1998.PubMed/NCBI
|
|
20
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
21
|
Towbin H, Staechlin T and Gordon J:
Electrophoretic transer of protein from polyacrylamide gels to
nitrocellulose sheets: procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kajstura M, Halicka HD, Pryjma J and
Darzynkiewicz Z: Discontinuous fragmentation of nuclear DNA during
apoptosis revealed by discrete ‘sub-G1’ peaks on DNA content
histograms. Cytometry A. 71:125–131. 2007.PubMed/NCBI
|
|
23
|
Kim J-S, Pirnia F, Choi YH, et al:
Lovastatin induces apoptosis in a primitive neuroectodermal tumor
cell line in association with RB down-regulation and loss of the G1
checkpoint. Oncogene. 19:6082–6090. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Podhorecka M, Halicka D, Klimek P, Kowal
M, Chocholska S and Dmoszyńska A: Simvastatin and purine analogs
have a synergic effect on apoptosis of chronic lymphocytic leukemia
cells. Ann Hematol. 89:1115–1124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nijhawan D, Fang M, Traer E, Zhong Q, Gao
W, Du F and Wang X: Elimination of Mcl-1 is required for the
initiation of apoptosis following ultraviolet irradiation. Genes
Dev. 17:1475–1486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnston JB, Paul JT, Neufeld NJ, et al:
Role of myeloid cell factor-1 (Mcl-1) in chronic lymphocytic
leukemia. Leuk Lymphoma. 45:2017–2027. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobylinska A, Blonski JZ, Hanausek M,
Walaszek Z, Robak T and Kilianska ZM: Determination of the in
vivo effects of cladribine alone and its combination with
cyclophosphamide or cyclophosphamide and mitoxantrone on Bax and
Bcl-2 protein expression in B-CLL cells. Oncol Rep. 11:699–705.
2004.
|
|
28
|
Saxena A, Viswanathan S, Moshynska O,
Tandon P, Sankaran K and Sheridan DP: Mcl-1 and Bcl-2/Bax ratio are
associated with treatment response but not with Rai stage in B-cell
chronic lymphocytic leukemia. Am J Hematol. 75:22–33. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muzio M, Apollonio B, Scielzo C, et al:
Constitutive activation of distinct BCR-signaling pathways in a
subset of CLL patients: a molecular signature of anergy. Blood.
112:188–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grzybowska-Izydorczyk O, Cebula B, Robak T
and Smolewski P: Expression and prognostic significance of the
inhibitor of apoptosis protein (IAP) family and its antagonists in
chronic lymphocytic leukaemia. Eur J Cancer. 46:800–810. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Salman H, Bergman M, Djaldetti M and
Bessler H: Hydrophobic but not hydrophilic statins enhance
phagocytosis and decrease apoptosis of human peripheral blood cells
in vitro. Biomed Pharmacother. 62:41–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McConkey DJ: Calcium-dependent,
interleukin 1-converting enzyme inhibitor-insensitive degradation
of lamin B1 and DNA fragmentation in isolated thymocyte nuclei. J
Biol Chem. 271:22398–22406. 1996.
|
|
33
|
Garnier P, Ying W and Swanson RA: Ischemic
preconditioning by caspase cleavage of poly(ADP-ribose)
polymerase-1. J Neurosci. 23:7967–7973. 2003.PubMed/NCBI
|
|
34
|
Vrhovac R, Delmer A, Tang R, Marie JP,
Zittoun R and Ajchenbaum-Cymbalista F: Prognostic significance of
the cell cycle inhibitor p27Kip1 in chronic B-cell
lymphocytic leukemia. Blood. 91:4694–4700. 1998.PubMed/NCBI
|
|
35
|
Eymin B, Sordet O, Droin N, et al:
Caspase-induced proteolysis of the cyclin-dependent kinase
inhibitor p27Kip1 mediates its anti-apoptotic activity.
Oncogene. 18:4839–4847. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zolnierczyk JD, Błoński JZ, Robak T,
Kiliańska ZM and Wesierska-Gadek J: Roscovitine triggers apoptosis
in B-cell chronic lymphocytic leukemia cells with similar
efficiency as combinations of conventional purine analogs with
cyclophosphamide. Ann NY Acad Sci. 1171:124–131. 2009. View Article : Google Scholar
|
|
37
|
Borner C: The Bcl-2 protein family:
sensors and checkpoints for life-or-death decisions. Mol Immunol.
39:615–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Skommer J, Wlodkowic D and Deptala A:
Larger than life: mitochondria and the Bcl-2 family. Leuk Res.
31:277–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Osmak M: Statin and cancer: current and
future prospects. Cancer Lett. 324:1–12. 2012. View Article : Google Scholar
|