Introduction
Neuroblastoma, the most common solid extracranial
tumor in children, accounts for 7% of pediatric cancers (1). Approximately 650 new cases of
neuroblastoma are diagnosed each year in the United States. The
cancer is usually diagnosed at 1 to 2 years of age and 90% of cases
are diagnosed by 5 years of age (1). This embryonal cancer typically arises
from the adrenal medulla or paraspinal sympathetic ganglia of the
abdomen, chest or neck and often metastasizes to the liver,
regional lymph nodes, bone marrow and bone (2). Neuroblastoma tumors that are benign,
localized and well differentiated are successfully treated by
surgical resection. Still, a majority of neuroblastoma patients
develop an aggressive disease that is refractory to intensive
therapies. Current treatment for high risk neuroblastoma has
reached an extreme toxic and virtually intolerable level that
includes intensive chemotherapy, radiotherapy, autologous bone
marrow transplantation and retinoid and immunomodulation among
others (3). Despite aggressive
conventional treatments, the majority of children older than one
year of age with advanced stage neuroblastoma die from progressive
disease, and only 40% of children over 4 years of age survive for 5
years, emphasizing an urgent need for the development of innovative
effective treatment strategies (4).
Advanced stages of neuroblastoma show increased
expression of the matrix metalloproteinase (MMP-2), and a higher
MMP-2 to TIMP-2 ratio has been shown to correlate with poorer
prognosis for neuroblastoma patients (5). Sugiura et al(6) reported higher levels of MMP-2 and
MMP-9 in patients with stage IV (metastatic) disease when compared
with those in stages I and II (non-invasive and non-metastatic).
MMP-2 was present in both tumor and stromal cells; however, MMP-9
was present in stromal, vascular and perivascular cells surrounding
nests of tumor cells.
We have developed strategies to inhibit cancer
development and its spread using naturally occurring nutrients such
as lysine, proline, ascorbic acid and green tea extract [nutrient
mixture (NM)]. This nutrient mixture has exhibited synergistic
anticancer activity in vivo and in vitro in a number
of cancer cell lines through inhibition of cancer cell growth, MMP
secretion, invasion, metastasis and angiogenesis (7–9). Our
main objective in this study was to evaluate the effectiveness of
NM on neuroblastoma cells in vivo using the nude mouse
xenograft model and in vitro, evaluating the effect of NM on
cell viability, MMP-2 and -9 secretion, TIMP-2 secretion, Matrigel
invasion and cellular apoptosis and morphology.
Materials and methods
In vivo
Animals
Male athymic mice (NCr-nu/nu), ~5 weeks of age on
arrival, were purchased from Simonsen Laboratories, Gilroy, CA, USA
and maintained in microisolator cages under pathogen-free
conditions on a 12-h light/12-h dark schedule for one week. All
procedures were performed according to humane and customary care
and use of experimental animals and followed a protocol approved by
the internal institutional animal safety review committee.
Experimental design
After housing for a week, the mice (n=16) were
inoculated subcutaneously with 3×106 neuroblastoma
SK-N-MC cells in 0.2 ml PBS and 0.1 ml Matrigel (BD Bioscience,
Bedford, MA, USA). After injection, the mice were randomly divided
into two groups of 8 mice each; group A mice were fed regular
Purina mouse chow and group B the regular diet supplemented with
0.5% NM (w/w). The regular diet was Laboratory Rodent Diet 5001
from Purina Mills, Inc. LLC/TestDiet® (Gray Summit, MO,
USA). The 0.5% NM diet was milled and pressed by Purina Mills and
generated by Vita-Tech (Tustin, CA, USA). During the study, the
mice consumed, on the average, 4 g of their respective diets/day.
Thus, the supplemented mice received ~20 mg of NM/day. After four
weeks, the mice were sacrificed and their tumors were excised,
weighed and processed for histology. The mean weight of mice at
initiation of the study and termination of the study did not differ
significantly between the groups.
Histology
Tissue samples were fixed in 10% buffered formalin.
All tissues were embedded in paraffin and cut at 4–5 μm. Sections
were deparaffinized through xylene and graduated alcohol series to
water and stained with hematoxylin and eosin (H&E) for
evaluation using a standard light microscope.
In vitro studies
Cell culture
Human neuronal epithelioma SK-N-MC cells (ATCC) were
grown in MEM, supplemented with 10% fetal bovine serum, penicillin
(100 U/ml) and streptomycin (100 mg/ml) in 24-well tissue culture
plates (Costar, Cambridge, MA, USA). Cells were incubated with 1 ml
of media at 37°C in a tissue culture incubator equilibrated with
95% air and 5% CO2. At near confluence, the cells were
treated with the nutrient mixture, dissolved in media and tested at
0, 10, 50, 100, 500 and 1,000 μg/ml in triplicate at each dose.
Phorbol 12-myristate 13-acetate (PMA) (100 ng/ml) was added to the
cells to induce MMP-9 secretion. The plates were then returned to
the incubator.
MTT assay
Cell viability was evaluated by MTT assay, a
colorimetric assay based on the ability of viable cells to reduce a
soluble yellow tetrazolium salt [3-(4,5-dimethylthiazol-2-yl)
2,5-diphenyl tetrazolium bromide] (MTT) to a blue formazan crystal
by mitochondrial succinate dehydrogenase activity of viable cells.
This test is a good index of mitochondrial activity and thus of
cell viability. After a 24-h incubation, the cells were washed with
phosphate-buffered saline (PBS) and 500 μl of MTT (#M-2128; Sigma)
0.5 mg/ml in media was added to each well. After MTT addition (0.5
mg/ml), the plates were covered and returned to the 37°C incubator
for 2 h, the optimal time for formazan product formation. Following
incubation, the supernatant was carefully removed from the wells,
the formazan product was dissolved in 1 ml DMSO, and absorbance was
measured at 570 nm in the BioSpec 1601 Shimadzu spectrometer. The
OD570 of the DMSO solution in each well was considered
to be proportional to the number of cells. The OD570 of
the control (treatment without supplement) was considered 100%.
Gelatinase zymography
Gelatinase zymography was performed in 10% Novex
Pre-Cast SDS polyacrylamide gel (Invitrogen) in the presence of
0.1% gelatin under non-reducing conditions. Culture media (20 μl)
were mixed with sample buffer and loaded for SDS-PAGE with Tris
glycine SDS buffer as suggested by the manufacturer (Novex).
Samples were not boiled before electrophoresis. Following
electrophoresis the gels were washed twice in 2.5% Triton X-100 for
30 min at room temperature to remove SDS. The gels were then
incubated at 37°C overnight in substrate buffer containing 50 mM
Tris-HCl and 10 mM CaCl2 at pH 8.0 and stained with 0.5%
Coomassie Blue R-250 in 50% methanol and 10% glacial acetic acid
for 30 min and destained. Upon renaturation of the enzyme, the
gelatinases digest the gelatin in the gel and provide clear bands
against an intensely stained background. Protein standards were run
concurrently, and approximate molecular weights were determined by
plotting the relative mobilities of known proteins.
Reverse zymography
TIMPs were analyzed by reverse zymography on 15% SDS
gels containing serum-free conditioned medium from cells. After
electrophoresis the gels were washed twice with 2.5% Triton X-100
for 30 min at room temperature to remove SDS. The gels were then
incubated at 37°C overnight in 50 mM Tris-HCl and 10 mM
CaCl2 at pH 7.6 and stained with 0.5% Coomassie Blue
R-25, destained and scanned.
Scanning of gelatinase and reverse
zymograms
Gelatinase and reverse zymograms were scanned using
CanoScan 9950F Canon scanner at 300 dpi. The intensity of the bands
was evaluated using the pixel-based densitometer program
Un-Scan-It, version 5.1, 32-bit, by Silk Scientific, Inc. (Orem,
UT, USA), at a resolution of 1 scanner unit (1/100 of an inch for
an image that was scanned at 100 dpi). The pixel densitometer
calculates the optical density of each pixel (values 0 to 255)
using the darkly stained background of the gel as a pixel value of
0. A logarithmic optical density scale was used since the optical
density of films and gels is logarithmically proportional to the
concentration. The pixel densitometer sums the optical density of
each pixel to give a band’s density. In all graphs, band densities
were reported as percentages of the sums of all pixels in a given
lane (treatment) of a gel.
Matrigel invasion
Invasion studies were conducted using Matrigel
(Becton-Dickinson) inserts in 24-well plates. Suspended in medium,
SK-N-MC cells were supplemented with nutrients, as specified in the
design of the experiment and seeded on the insert in the well.
Thus, both the medium on the insert and in the well contained the
same supplements. The plates with the inserts were then incubated
in a culture incubator equilibrated with 95% air and 5%
CO2 for 24 h. After incubation, the media from the wells
were withdrawn. The cells on the upper surface of the inserts were
gently scrubbed away with cotton swabs. The cells that had
penetrated the Matrigel membrane and migrated onto the lower
surface of the Matrigel were stained with H&E and visually
counted under a microscope.
Morphology and apoptosis
Morphology of cells cultured for 24 h in test
concentrations of NM were evaluated by H&E staining and
observed and photographed by microscopy. At near confluence,
SK-N-MC cells were challenged with NM dissolved in media at 0, 50,
100, 250, 500 and 1,000 μg/ml and incubated for 24 h. The cell
culture was washed with PBS and treated with the caspase reagent as
specified in the manufacturer’s protocol (Molecular Probes
Image-IT™ Live Green Poly Caspases Detection Kit 135104;
Invitrogen). The cells were photographed under a fluorescence
microscope and counted. Green-colored cells represented viable
cells, while yellow-orange colored cells were early apoptotic and
red, late apoptotic
Statistical analysis
Data are expressed as means ± SD, as indicated in
the results, for the groups. Data were analyzed by independent
sample t-test. Pearson’s correlation coefficients were determined
for toxicity and invasion correlations to NM concentration using
MedCalc Software (Markakerke, Belgium).
Results
In vivo
Tumor growth
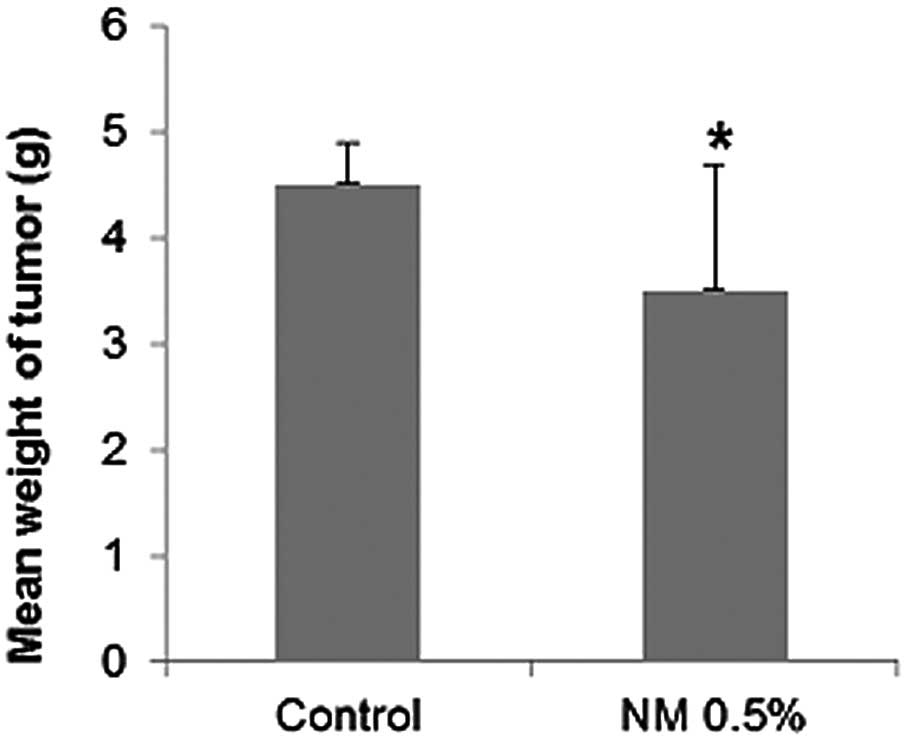
NM supplementation significantly inhibited
neuroblastoma SK-N-MC xenograft tumor growth. The mean weight of
tumors in the nude mice fed the 0.5% NM supplement was inhibited by
22% (P=0.04) in comparison to that of the control group of mice
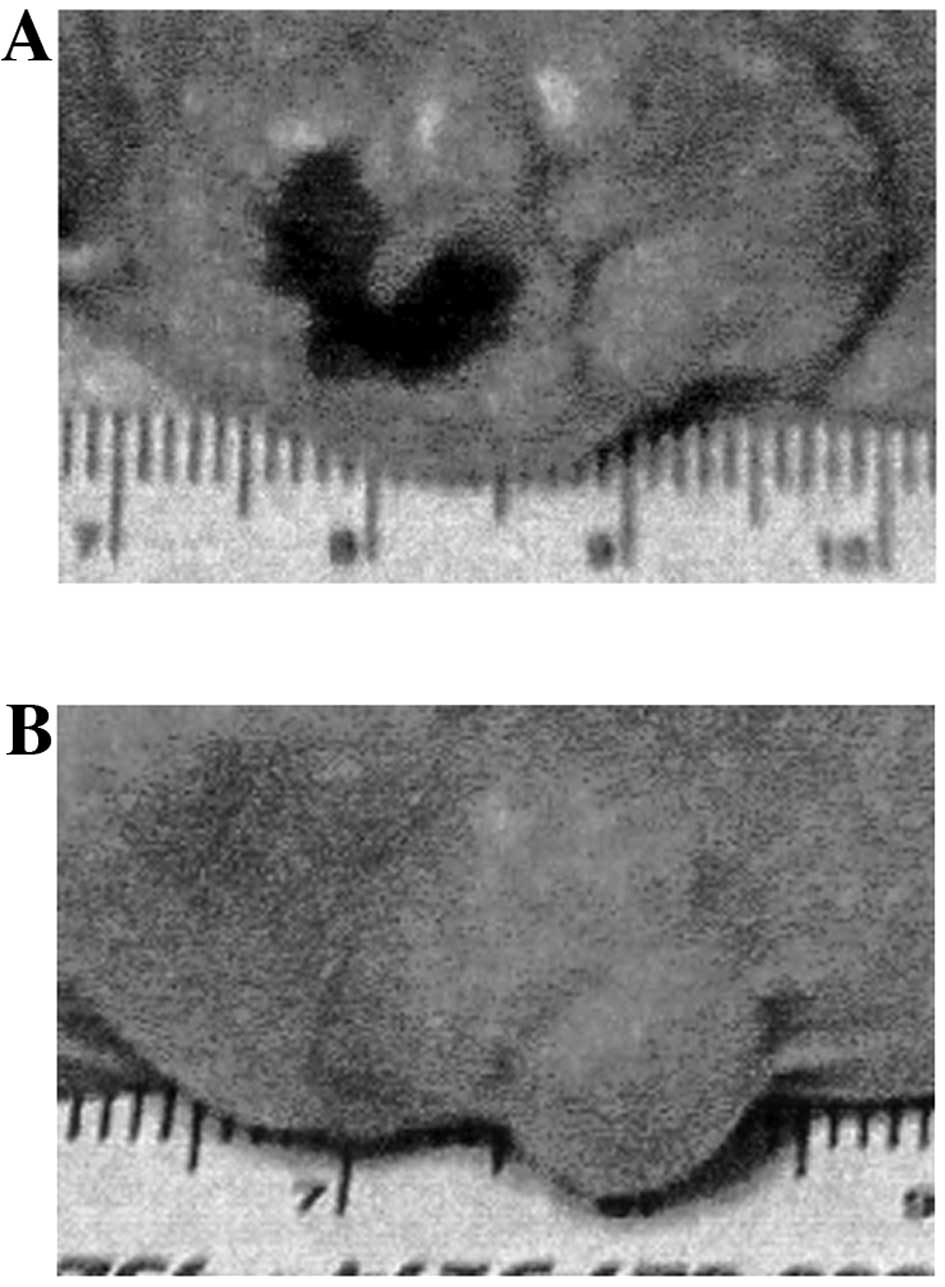
(Figs. 1 and 2).
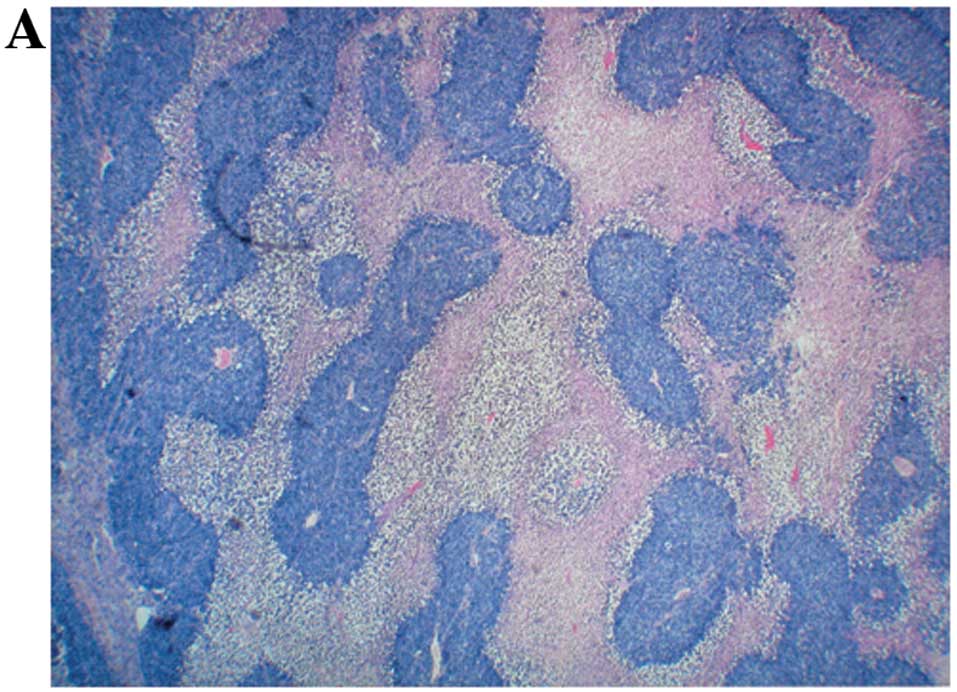
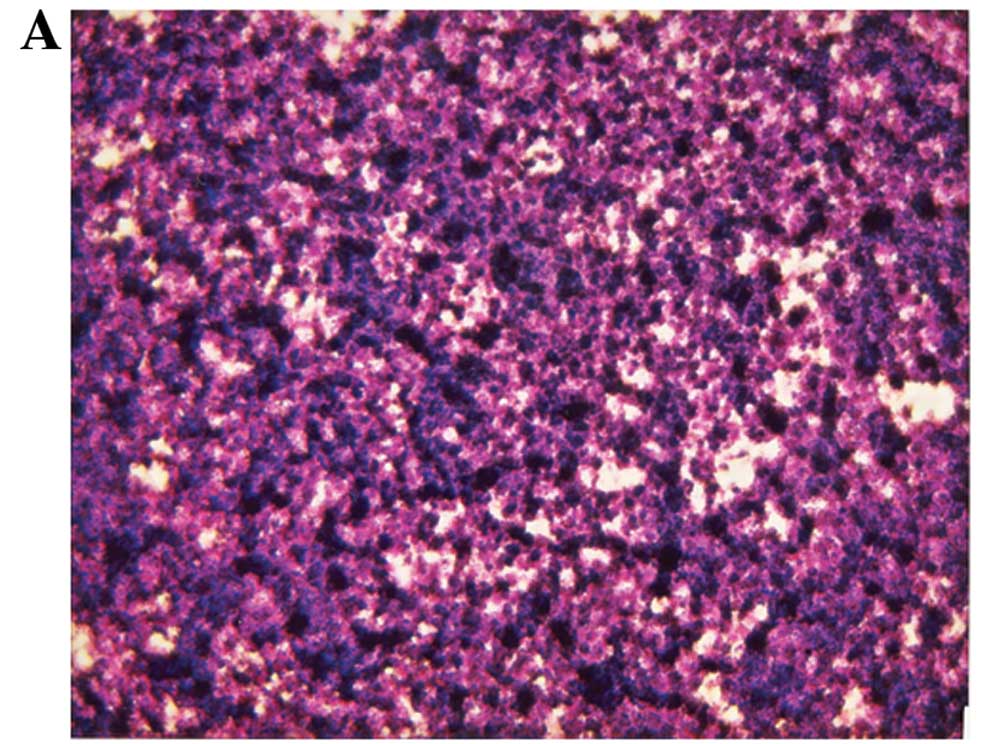
Histopathology
Histologically the tumors from both groups were
composed of necrotic, expansile, subcutaneous neoplastic masses
consistent with neuroblastoma (Fig.
3).
In vitro
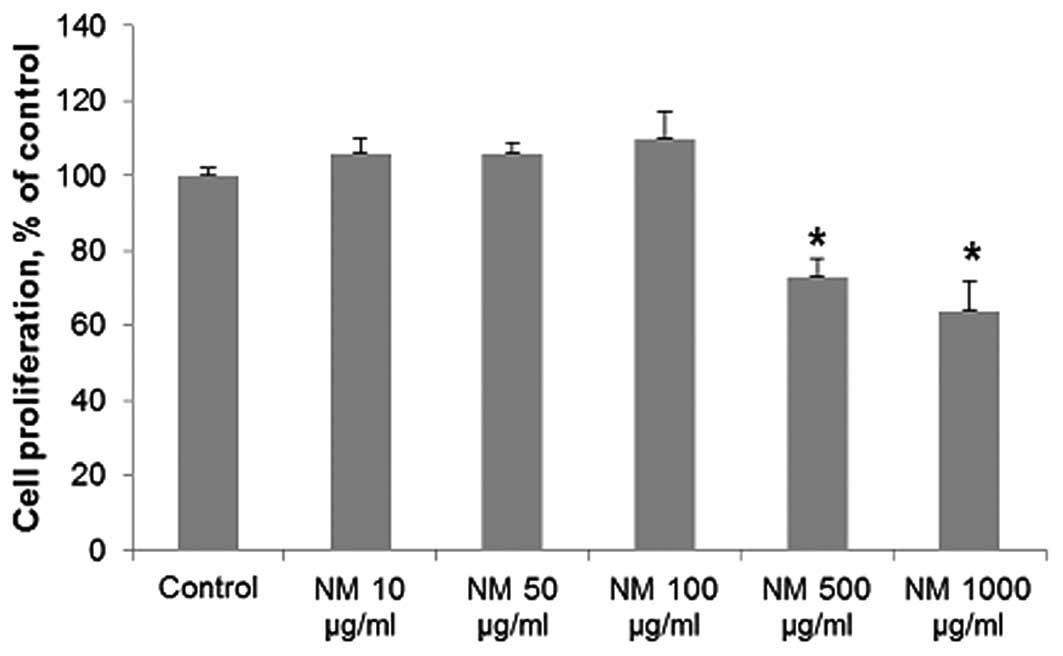
Cytotoxicity
NM exhibited no toxicity to human neuroblastoma
SK-N-MC cells at low concentrations of NM, but cytotoxicity of 27%
(P=0.001) was evident at 500 μg/ml NM and 36% (P=0.002) at 1,000
μg/ml NM (Fig. 4).
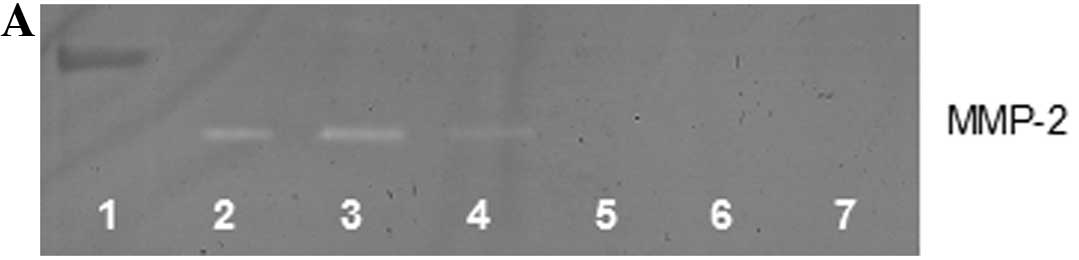
Gelatinase zymography
Zymography showed a faint band corresponding to
MMP-2 secretion, and PMA (100 ng/ml)-induced MMP-9 secretion. NM
inhibited the secretion of both MMP-2 and -9 with total blockage at
a concentration of 100 μg/ml (Fig.
5). MMP-2 secretion by normal SK-N-MC cells was inhibited by
50% by 50 μg/ml NM, and virtually blocked by NM 100–1,000 μg/ml
(linear trend R2=0.756). Secretion of MMP-2 by
PMA-treated cells was inhibited by 73% at 50 μg/ml NM and virtually
blocked at 100–1,000 μg/ml NM (linear trend R2=0.691).
MMP-9 secretion by PMA-treated cells was inhibited by 64% at 50
μg/ml NM and virtually blocked at 100–1,000 μg/ml NM (linear trend
R2=0.791).
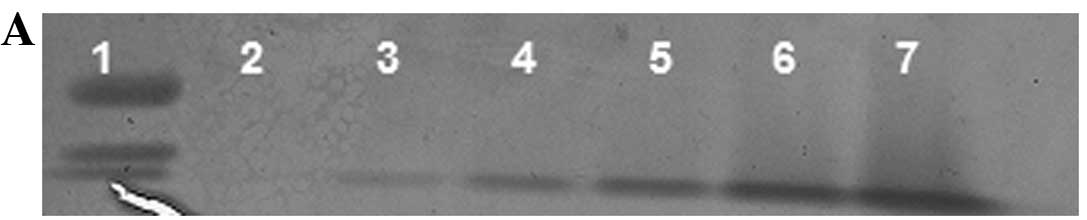
TIMP-2
Reverse zymography revealed upregulation of TIMP-2
activity following NM treatment of SK-N-MC cells in a
dose-dependent manner, with minimum activity expressed at 50 and
maximum activity at 1,000 μg/ml NM (linear trend
R2=0.877). Reverse zymogram and densitometry analysis
are shown in Fig. 6.
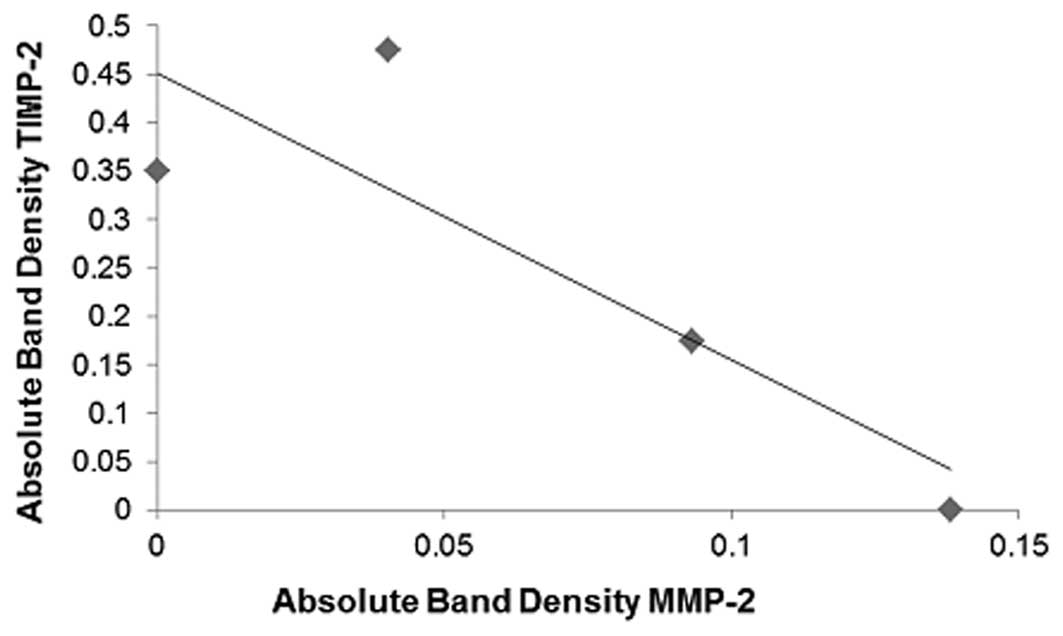
Correlation of MMP-2 and TIMP-2
A negative correlation (correlation coefficient
r=−0.8646) was found between MMP-2 and TIMP-2 expression in the
NM-treated SK-N-MC cells (Fig.
7).
Matrigel invasion
Notably, human neuroblastoma SK-N-MC cells were not
invasive through Matrigel.
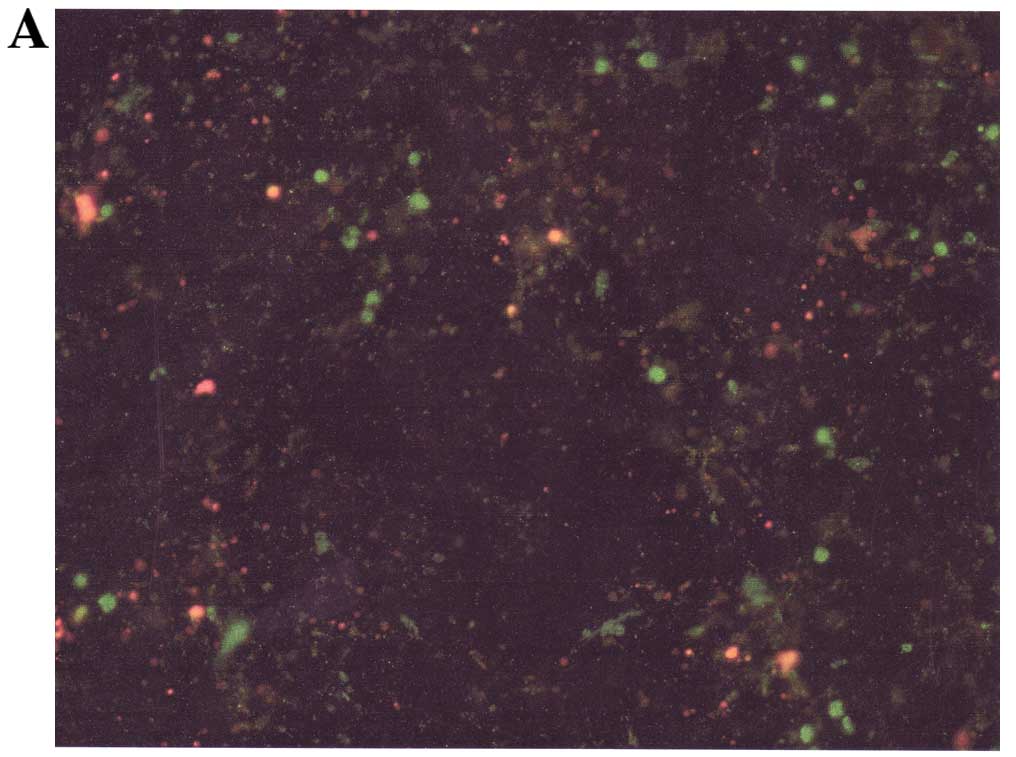
Cell morphology and apoptosis
Neuroblastoma cells exposed to various
concentrations of NM indicated no morphological changes at
concentrations <500 μg/ml as detected by H&E staining
(Fig. 8). Using the Live Green Poly
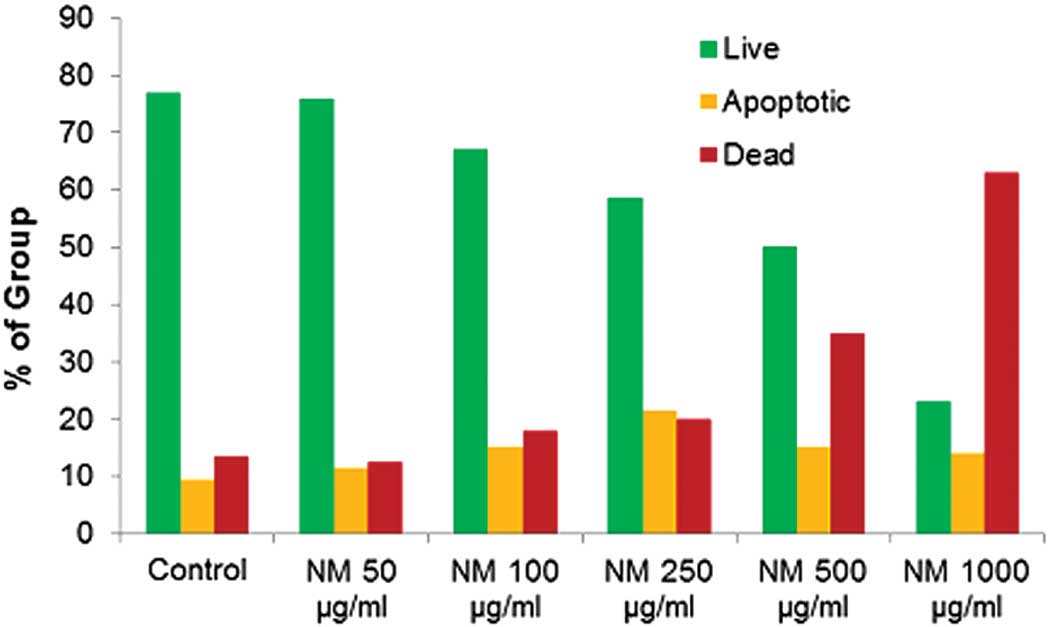
Caspases Detection kit, dose-dependent apoptosis of neuroblastoma
cells was evident following NM challenge (Fig. 9). At 100 μg/ml NM, 67% of cells were
viable, 15% of cells were early apoptotic and 18% of cells were
late apoptotic. At 500 μg/ml NM, 50% of cells were viable, 15% of
cells were early apoptotic, and 35% of cells were late apoptotic.
At 1,000 μg/ml NM, 23% of cells were viable, 14% of cells were
early apoptotic and 63% of cells were late apoptotic (Fig. 10).
Discussion
Dietary supplementation with 0.5% NM resulted in a
22% reduction in tumor growth in immune impaired (athymic) male
nude mice after subcutaneous administration of 3×106
human neuroblastoma SK-N-MC cells. Results from the cellular
proliferation and apoptosis studies support the in vivo
results, as NM showed dose-dependent toxicity in SK-N-MC cells and
induced apoptosis in a dose-dependent manner, with 36% inhibition
of cell growth and apoptotic induction of 77% in cells exposed to
1,000 μg/ml NM.
Malignant neuroblastoma is a highly vascularized
solid tumor that requires access to blood vessels for growth,
invasion and metastasis, and angiogenesis plays an important role
in determining tumor phenotype (10). High tumor vascularity is correlated
with widely disseminated disease and poor histology and outcome in
contrast to low tumor vascularity, which is associated with
favorable prognosis, such as localized disease and favorable
histology. Thus, researchers are focusing on targeting angiogenesis
for the treatment of neuroblastoma (10). Ribatti et al(11) reviewed the progress in pre-clinical
and clinical research of anti-angiogenic tumor therapy for
neuroblastoma. Angiogeneis is mediated by multiple regulating
factors, such as growth factors, adhesion molecules and matrix
degrading enzymes. In a previous study, NM significantly
(P<0.05) reduced bFGF-induced angiogenesis [utilizing a
chorioallantoic membrane (CAM) assay] in chick embryos, as well as
decreased human osteosarcoma U2OS cell expression of VEGF,
angiopoietin-2, bFGF, PDGF and TGFβ-1 (7).
Net matrix degradation and proteolysis depend on the
critical local balance between MMPs and TIMP-2. Ara et
al(5) reported that examination
of tumor tissues of 25 neuroblastoma patients for levels of MMPs
and TIMP-2 and correlation with stage of disease, revealed poor
prognosis with elevated MMP-2 expression and significantly higher
advanced stages of neuroblastoma with increased ratios of
MMP-2/TIMP-2. In the present study, NM demonstrated dose-dependent
inhibition of MMP-2 and -9 secretion by normal and PMA-treated
cells with total blockage of both MMPs at 100 μg/ml NM.
Furthermore, NM upregulated TIMP-2 activity in SK-N-MC cells in a
dose-dependent manner, with minimum activity expressed at 50 and
maximum activity at 1,000 μg/ml NM. A negative correlation
(correlation coefficient r=−0.8646) was found between MMP-2 and
TIMP-2 expression in the NM-treated SK-N-MC cells. The ratio of
MMP-2/TIMP-2 expression decreased significantly with increased NM
dose: 11.9 at 50 μg/ml NM, 1.9 at 100 μg/ml NM and 0 at 250–1,000
μg/ml NM.
NM was formulated by defining critical physiological
targets in cancer progression and metastasis, such as ECM integrity
and MMP activity. Adequate supplies of ascorbic acid and the amino
acids lysine and proline ensure proper synthesis and hydroxylation
of collagen fibers for optimal ECM structure. Manganese and copper
are also essential for collagen formation. Lysine, a natural
inhibitor of plasmin-induced proteolysis, plays an important role
in ECM stability (12,13). Green tea extract has been shown to
modulate cancer cell growth, metastasis, angiogenesis, and other
aspects of cancer progression (14–18).
N-acetyl cysteine has been shown to modulate MMP-9 and invasive
activities of tumor cells (19,20).
Selenium has been shown to inhibit MMP secretion, tumor invasion,
and migration of endothelial cells through ECM (21). Ascorbic acid demonstrates cytotoxic
and antimetastatic actions on neuroblastoma and other malignant
cell lines (22–27), and cancer patients have been found
to have low levels of ascorbic acid (28,29).
Low levels of arginine, a precursor of nitric oxide (NO), can limit
the production of NO, which has been shown to predominantly act as
an inducer of apoptosis (30).
In conclusion, current treatment methods for
neuroblastoma are generally ineffective and particularly toxic to
these patients. Thus, there is a need for the development of
effective therapeutic agents for these cancers with minimal
toxicity. Our studies demonstrated that NM significantly inhibited
the growth of xenograft tumors derived from the neuroblastoma cell
line SK-N-MC in vivo and significantly inhibited cell
proliferation and induced apoptosis in vitro. In addition,
invasive parameters, such as MMP-2 and -9 secretion, in the SK-N-MC
cell line were significantly inhibited by NM in vitro, while
TIMP-2 was enhanced. These findings suggest the potential of NM for
the treatment of neuroblastoma. Furthermore, in contrast to the
toxic side effects of chemotherapy, the nutrient mixture was shown
to be a safe therapeutic agent. In a previous in vivo study
addressing safety issues, we found that gavaging adult female ODS
rats (weighing 250–300 g) with the nutrient mixture (at 30, 90 or
150 mg/day for 7 days), had neither adverse effects on vital organs
(heart, liver and kidney) nor on associated functional serum
enzymes, indicating that this mixture is safe to use even at high
doses, which far exceed the normal equivalent dosage of the
nutrient (31).
Acknowledgements
The research study was funded by Dr. Rath Health
Foundation (Santa Clara, CA, USA), a non-profit organization.
Consulting pathologist Alexander de Paoli of IDEXX Reference
Laboratories provided the histopathology slides of the
neuroblastoma SK-N-MC tumors.
References
|
1
|
American Cancer Society. Neuroblastoma:
What are the key statistics about neuroblastoma? http://www.cancer.org/Cancer/Neuroblastoma/DetailedGuide/neuroblastoma-key-statistics.
Accessed Dec 6, 2012
|
|
2
|
Reynolds CP and Seeger RC: Neuroblastoma.
Cancer Treatment. Haskell CM: W.B. Saunders; Philadelphia, PA: pp.
860–871. 1994
|
|
3
|
Matthay KK, Villablanca JG, Seeger RC,
Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT,
Brodeur GM, Gerbing RB and Reynolds CP: Treatment of high-risk
neuroblastoma with intensive chemotherapy, radiotherapy, autologous
bone marrow transplantation and 13-cis-retinoic acid. Children’s
Cancer Group. N Engl J Med. 341:1165–1173. 1999.
|
|
4
|
Roy Choudhury S, Karmakar S, Banik NL and
Ray SK: Targeting angiogenesis for controlling neuroblastoma. J
Oncol. 2012:7820202012. View Article : Google Scholar
|
|
5
|
Ara T, Kusafuka T, Inoue M, Kuroda S,
Fukuzawa M and Okada A: Determination of imbalance between MMP-2
and TIMP-2 in human neuroblastoma by reverse-transcription
polymerase chain reaction and its correlation with tumor
progression. J Pediatr Surg. 35:432–437. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugiura Y, Shimada H, Seeger RC, Laug WE
and DeClerck YA: Matrix metalloproteinases-2 and-9 are expressed in
human neuroblastoma: contribution of stromal cells to their
production and correlation with metastasis. Cancer Res.
58:2209–2216. 1998.
|
|
7
|
Roomi MW, Roomi N, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Inhibitory effect of a mixture containing
ascorbic acid, lysine, proline and green tea extract on critical
parameters in angiogenesis. Oncol Rep. 14:807–815. 2005.PubMed/NCBI
|
|
8
|
Roomi MW, Roomi N, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Inhibition of pulmonary metastasis of
melanoma B16FO cells in C57BL/6 mice by a nutrient mixture
consisting of ascorbic acid, lysine, proline, arginine, and green
tea extract. Exp Lung Res. 32:517–530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ribatti D, Marimpietri D, Pastorino F,
Brignole C, Nico B, Vacca A and Ponzoni M: Angiogenesis in
neuroblastoma. Ann NY Acad Sci. 1028:133–142. 2004. View Article : Google Scholar
|
|
11
|
Ribatti D, Vacca A, Nico B, De Falco G,
Montaldo GP and Pnzoni M: Angiogenesis and anti-angiogenesis in
neuroblastoma. Eur J Cancer. 38:750–757. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
analogs. J Orthomolecular Med. 7:17–23. 1992.
|
|
13
|
Sun Z, Chen YH, Wang P, Zhang J, Gurewich
V, Zhang P and Liu JN: The blockage of high-affinity lysine binding
sites of plasminogen by EACA significantly inhibits
prourokinase-induced plasminogen activation. Biochem Biophys Acta.
1596:182–192. 2002.PubMed/NCBI
|
|
14
|
Valcic S, Timmermann BN, Alberts DS,
Wachter GA, Krutzsch M, Wymer J and Guillen JM: Inhibitory effect
of six green tea catechins and caffeine on the growth of four
selected human tumor cell lines. Anticancer Drugs. 7:461–468. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mukhtar H and Ahmad N: Tea polyphenols:
prevention of cancer and optimizing health. Am J Clin Nutr.
71(Suppl 6): S1698–S1704. 2000.PubMed/NCBI
|
|
16
|
Yang GY, Liao J, Kim K, Yurtow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taniguchi S, Fujiki H, Kobayashi H, Go H,
Miyado K, Sadano H and Shimokawa R: Effect of (−) epigallocatechin
gallate, the main constituent of green tea, on lung metastasis with
mouse B16 melanoma cell lines. Cancer Lett. 65:51–54. 1992.
|
|
18
|
Hara Y: Green Tea: Health Benefits and
Applications. Marcel Dekker, Inc; New York, Basel: 2001, View Article : Google Scholar
|
|
19
|
Kawakami S, Kageyama Y, Fujii Y, Kihara K
and Oshima H: Inhibitory effects of N-acetyl cysteine on invasion
and MMP-9 production of T24 human bladder cancer cells. Anticancer
Res. 21:213–219. 2001.PubMed/NCBI
|
|
20
|
Morini M, Cai T, Aluigi MG, Noonan DM,
Masiello L, De Floro S, D’Agostinin F, Albini A and Fassima G: The
role of the thiol N-acetyl cysteine in the prevention of tumor
invasion and angiogenesis. Int J Biol Markers. 14:268–271.
1999.PubMed/NCBI
|
|
21
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effects of selenite on invasion of HT1080 tumor cells. J Biol Chem.
276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carosio R, Zuccari G, Orienti I,
Mangraviti S and Montaldo PG: Sodium ascorbate induces apoptosis in
neuroblastoma cell lines by interfering with iron uptake. Mol
Cancer. 6:552007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maramag C, Menon M, Balaji KC, Reddy PG
and Laxmanan S: Effect of vitamin C on prostate cancer cells in
vitro: effect on cell number, viability and DNA synthesis.
Prostate. 32:188–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naidu KA, Karl RC, Naidu KA and Coppola D:
Antiproliferative and proapoptotic effect of ascorbyl stearate in
human pancreatic cancer cells: association with decreased
expression of insulin-like growth factor 1 receptor. Dig Dis Sci.
48:230–237. 2003. View Article : Google Scholar
|
|
25
|
Koh WS, Lee SJ, Lee H, Park C, Park MH,
Kim WS, Yoon SS, Park K, Hong SI, Chung MH and Park CH:
Differential effects and transport kinetics of ascorbate
derivatives in leukemic cell lines. Anticancer Res. 18:2487–2493.
1998.PubMed/NCBI
|
|
26
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurbacher CM, Wagner U, Kolster B,
Andreotti PE, Krebs D and Bruckner HW: Ascorbic acid (vitamin C)
improves the antineoplastic activity of doxorubicin, cisplatin and
paclitaxel in human breast carcinoma cells in vitro. Cancer Lett.
103:183–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anthony HM and Schorah CJ: Severe
hypovitaminosis C in lung-cancer patients: the utilization of
vitamin C in surgical repair and lymphocyte-related host
resistance. Br J Cancer. 46:354–367. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nunez C, Ortiz de Apodaca Y and Ruiz A:
Ascorbic acid in the plasma and blood cells of women with breast
cancer. The effect of consumption of food with an elevated content
of this vitamin. Nutr Hosp. 10:368–372. 1995.(In Spanish).
|
|
30
|
Cooke JP and Dzau VJ: Nitric oxide
synthase: role in the genesis of vascular disease. Annu Rev Med.
48:489–509. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roomi MW, Ivanov V, Netke SP, Niedzwiecki
A and Rath M: Serum markers of the liver, heart, and kidney and
lipid profile and histopathology in ODS rats treated with nutrient
synergy. J Am Coll Nutr. 22:4772003.
|