Introduction
Toll-like receptors (TLRs) play a critical role in
innate immunity against microbial pathogens, as well as in the
subsequent induction of adaptive immune responses (1–3). These
receptors are the major pattern-recognition transducers in response
to microbial intruders such as bacteria, protozoa, fungi or viruses
(4). Stimulation of TLRs induces a
range of innate and adaptive immune responses through cytokines,
interferons, chemokines and cell surface molecules, in addition to
increasing the effector functions of TLR-expressing cells.
TLR2 and TLR4 play a key role in the recognition of
various bacteria. Viral glycoproteins are recognized by TLRs 2 and
4, virus-related double-stranded RNA (dsRNA) by TLR3 and
single-stranded RNA (ssRNA) by TLRs 7 and 8. TLR9 recognizes
specific oligodeoxynucleotide (ODN) sequences that consist of
unmethylated CpG-ODNs, which are frequently present in bacterial
and viral DNA (5).
TLRs are broadly distributed in various cells of the
immune system, including polymorphonuclear phagocytes, monocytes,
dendritic cells and natural killer cells, as well as in some
epithelial and endothelial cells (6,7). The
specific subcellular localization of TLRs remains unclear. TLRs 1,
2, 4, 5 and 6 are present in the plasma membrane. TLRs 3, 7, 8 and
9 are mainly present in endosomes and are likely to signal from
acidic endosomes (8).
Signaling pathways activated by specific TLRs are
largely dictated by the adaptor proteins that are recruited to the
intracellular domain of the TLR. MyD88 is involved in the majority
of TLR pathways, except for TLRs 3 and 8. TLR3 signaling depends
solely on its binding to the adaptor protein termed the
Toll/Interleukin-1 receptor domain-containing adaptor-inducing
IFN-β (TRIF) adaptor protein. Binding of TRIF to TLR3 leads to the
activation of NF-κB and IRF3 transcription factors, thereby
inducing the antiviral interferon response (9,10).
In humans, TLR9 is expressed in B-lymphocytes,
monocytes and plasmacytoid dendritic cells. TLR9 recognizes
specific oligodeoxynucleotide (ODN) sequences consisting of
unmethylated CpG-ODNs, which are frequently present in bacterial
and viral DNA (5).
TLRs have recently been found to be expressed in
various normal epithelial and cancer cells (11–18).
Of these cells, gastrointestinal epithelial cells in particular are
constantly exposed to external microbial pathogens or viruses.
Therefore, TLRs play a critical role not only in immune defense but
also in other biological functions.
In addition, we have previously reported that human
hepatocellular carcinoma (HCC) cells express TLR3 and TLR9 and that
these receptors function mainly in cell survival and in
anti-apoptotic pathways (15,16).
Previous reports indicated that colorectal cancer cells express
TLR2, TLR3, TLR4, TLR5 and TLR9. These findings indicated that TLRs
might play an important role also in colorectal cancer cells.
Recent reports have suggested that the neoplastic
process may sabotage TLR signaling pathways to favor cancer
progression. TLRs on tumor cells facilitate their evasion from
immune surveillance via suppression of T-cell proliferation and
natural killer cell activity. These studies suggest that TLR
signaling in tumor cells is associated with the progression of
cancer (12,14). However, the functions of TLRs in
colon carcinoma cells are not well understood. We therefore
investigated the expression of TLR3 and TLR9 in colon carcinoma
cell lines and in primary human normal and carcinogenic colon
tissues, and attempted to clarify the function of TLR3 and TLR9 in
colon carcinoma cells.
Materials and methods
Cells and HCC tissues
The colon adenocarcinoma cells Colo320 and SW480
were purchased from the American Type Culture Collection
(Rockville, MD, USA). All cells were cultured in DMEM at 37°C,
supplemented with 1% penicillin/streptomycin (Gibco BRL, Grand
Island, NY, USA) and 10% heat-inactivated fetal calf serum (Gibco
BRL).
A total of 42 colon carcinoma tissues (9 non-tumor
tissues, and 8 tissues with metastasis from colon carcinoma
tissues) were obtained from tissue array slides (SuperBioChips
Laboratories, Seoul, Korea). We obtained informed consent from all
patients prior to the subsequent use of their resected tissues.
Resected tissues were frozen immediately at −80°C or were fixed in
10% formalin.
Reagents
Polyinosinic-polycytidylic acid (Poly I:C), which
was used as a human TLR3 ligand, was obtained from Sigma (St.
Louis, MO, USA), and Lipofectamine LTX (Lipo) was obtained from
Invitrogen (Carlsbad, CA, USA). The type C CpG oligonucleotide (ODN
M362: 5′-tcg tcg tcg ttc gaa cga cgt tga t-3′), which was used as a
human TLR9 ligand, and the control, non-stimulatory oligonucleotide
ODN M362 (ODN M362 control: 5′-tgc tgc tgc ttg caa gca gct tga
t-3′), were purchased from InvivoGen (San Diego, CA, USA).
Adriamycin (ADM) was purchased from Wako (Osaka, Japan).
Immunohistochemical staining
Immunohistochemical staining of TLR3 and TLR9
expression in colon carcinoma and non-colon carcinoma tissues was
performed using the labeled streptavidin-biotin method.
Deparaffinized sections were heated for 5 min at 120°C in a
pressure cooker to reactivate the antigen. Sections were blocked
and incubated with anti-TLR3 or 9 antibodies (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), overnight at 4°C. The sections
were then incubated with a second biotinylated antibody, followed
by an avidin-biotin-peroxidase complex. The sections were then
developed in a substrate solution of 0.01%
3,3′-diaminobenzidene-hydrogen peroxidase and counterstained with
10% hematoxylin.
Detection of TLR 3 and 9 protein
expression by immunoblotting
The expression of TLRs 3 and 9, and that of the
loading control α-tubulin, in colon adenocarcinoma cell lines
(Colo320, SW480) was analyzed by immunoblotting. Briefly, after
incubation of the cells on 6-well plates (Nunc™ Brand Products,
Denmark) for 48 h, the cells were washed twice with
phosphate-buffered saline (PBS) and were then lysed by the addition
of SDS sample buffer (50 mmol/l Tris-HCl, pH 6.8, 2.5% SDS, 5%
glycerol, 5% 2-mercaptoethanol and 0.01% bromophenol blue). Equal
amounts of extracted proteins were separated by SDS-PAGE and were
then transferred to PVDF membranes (Millipore, Billerica, MA, USA).
Blots were blocked by incubation in Tris-HCl (pH 7.5) containing 5%
milk and 0.1% Tween-20 for 30 min at room temperature, and were
probed overnight at 4°C with the primary antibodies. The following
primary antibodies were used: anti-TLR3 or TLR9 monoclonal antibody
(Imgenex, San Diego, CA, USA) and anti-α-tubulin monoclonal
antibody (Oncogene Research Products, San Diego, CA, USA). The
antibodies were diluted in 5% milk or in BSA in Tris-HCl (pH 7.5)
containing 0.1% Tween-20. The immunoblots were then probed with
horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG)
(diluted 1:1000 in 1% milk or in BSA in Tris-HCl, pH 7.5) (Amersham
Biosciences, Buckinghamshire, UK) After the final wash, signals
were detected using an ECL kit (Amersham Pharmacia Biotech).
Detection of cell viability and apoptosis
assay
The colon carcinoma cell lines were seeded at a
density of 1.0×104 cells/well in 96-well flat-bottom
microtiter plates (Corning Glass Works, Corning, NY, USA) and were
incubated at 37°C in 5% CO2. After incubation for 24 h,
reagents were added, and the plates were incubated for a further 48
h. To assess the viability of the colon carcinoma cells, a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay was performed using the Cell Titer 96-assay kit (Promega,
Madison, WI, USA) according to the manufacturer’s instructions. For
analysis of apoptosis, a total of 2×104 SW480 cells were
cultured in a chamber slide for 24 h followed by no addition, or by
the addition of 1 μg/ml of either Poly I:C or Lipo, or of 1 μg/ml
Poly I:C plus Lipo. After incubation for 24 h, cell nuclei were
stained with 4’6,-diamidino-2-phenylindole (DAPI; Sigma) and the
cells were analyzed under a fluorescent microscope.
NF-κB activity assays
The NF-κB activity assay was performed using the
Dual-Glo™ Luciferase Assay System (Promega) and the pGL4.32
(luc2P/NF-κB-RE/Hygro) Vector (Promega) according to the
manufacturer’s instructions.
Results
TLR3 is expressed in colon carcinoma cell
lines and colon tissues
As shown in Fig. 1,
TLR3 expression was detected by western blotting in both of the
colon carcinoma cell lines tested. TLR3 expression in primary human
colon tissue was then investigated by immunohistochemical staining
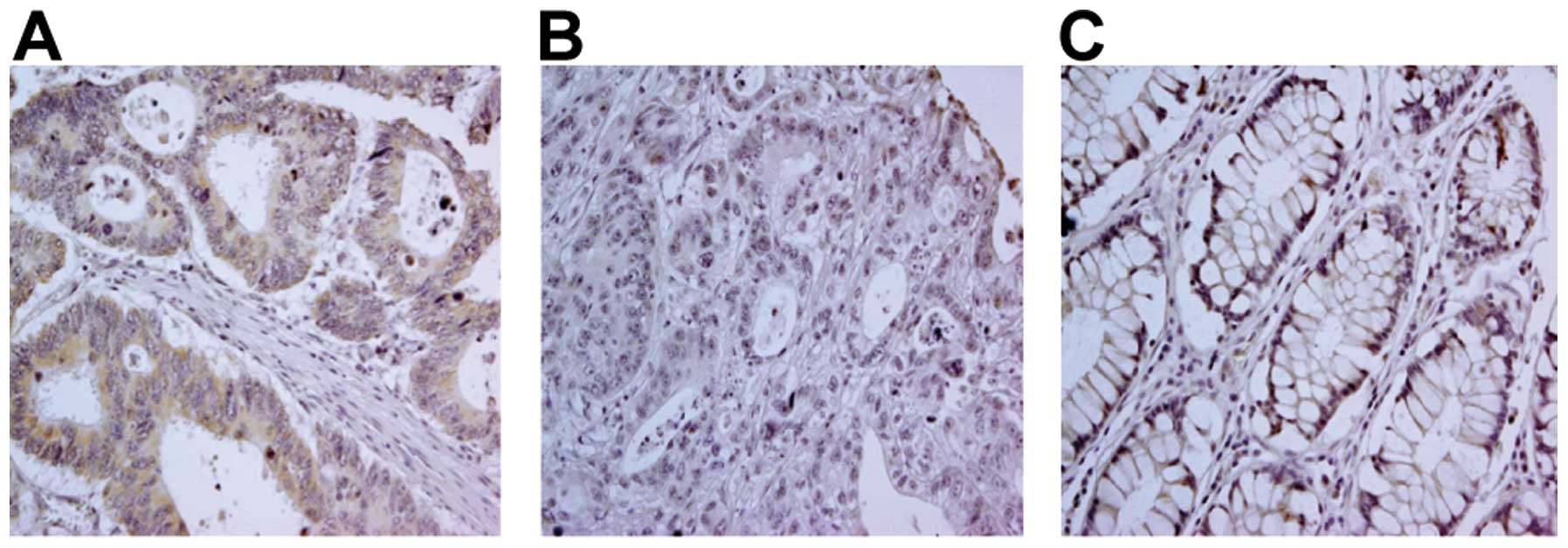
of both non-colon carcinoma and colon carcinoma lesions (Fig. 2, representative staining). While
there was a clear positive TLR3 signal in 91/100 colon carcinoma
cases (91%), no major differences in TLR3 expression were observed
between different histological tumor grades (Table I). TLR3 staining was detected not
only in the cytoplasm, but also in the cell membrane. Few
differences were noted in the TLR3 staining patterns between
non-tumor and tumor tissues.
 | Table IExpression of TLR3 in colon carcinoma
and non-tumor tissues. |
Table I
Expression of TLR3 in colon carcinoma
and non-tumor tissues.
| Staining |
|---|
|
|
|---|
| Histology | Absent or weak
(%) | Moderate (%) | Strong (%) |
|---|
| Non-tumor tissue | 1 (11.1) | 8 (88.9) | 0 (0) |
| Colon carcinoma |
| Poorly
differentiated | 1 (33.3) | 2 (66.7) | 0 (0) |
| Moderately
differentiated | 4 (6.4) | 36 (57.1) | 23 (36.5) |
| Well
differentiated | 3 (12.0) | 15 (60.0) | 7 (28.0) |
Transfected Poly I:C-induced apoptosis in
colon carcinoma cells
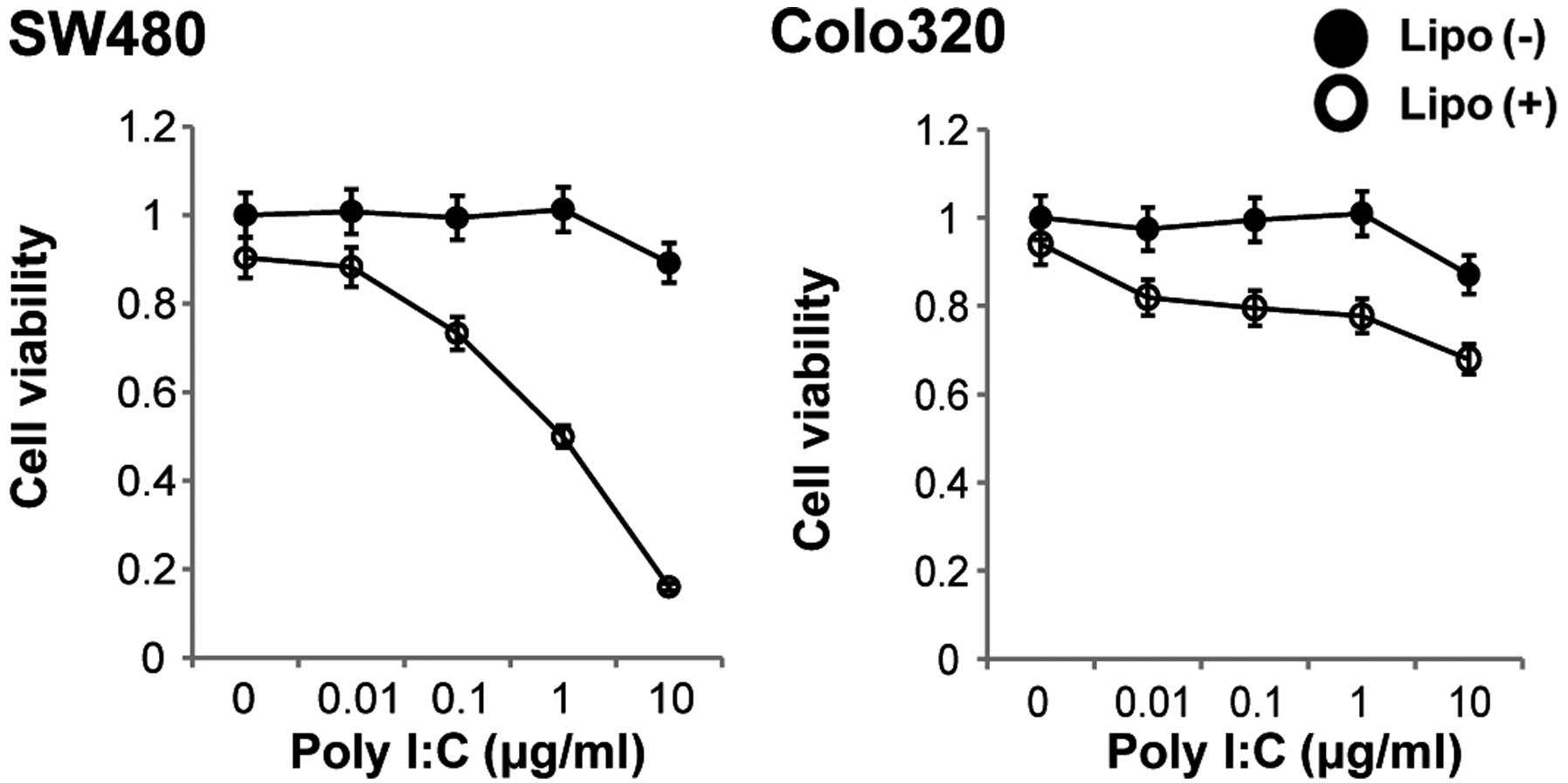
Cellular TLR3 is stimulated by Poly I:C. We
therefore next investigated the cytotoxicity of non-transfected or
of transfected Poly I:C for these colon carcinoma cells.
Stimulation of both cell lines with various concentrations of
non-transfected Poly I:C for 48 h resulted in no change in cell
viability, whereas stimulation of the cells with transfected Poly
I:C for 48 h resulted in a decrease in cell viability in a
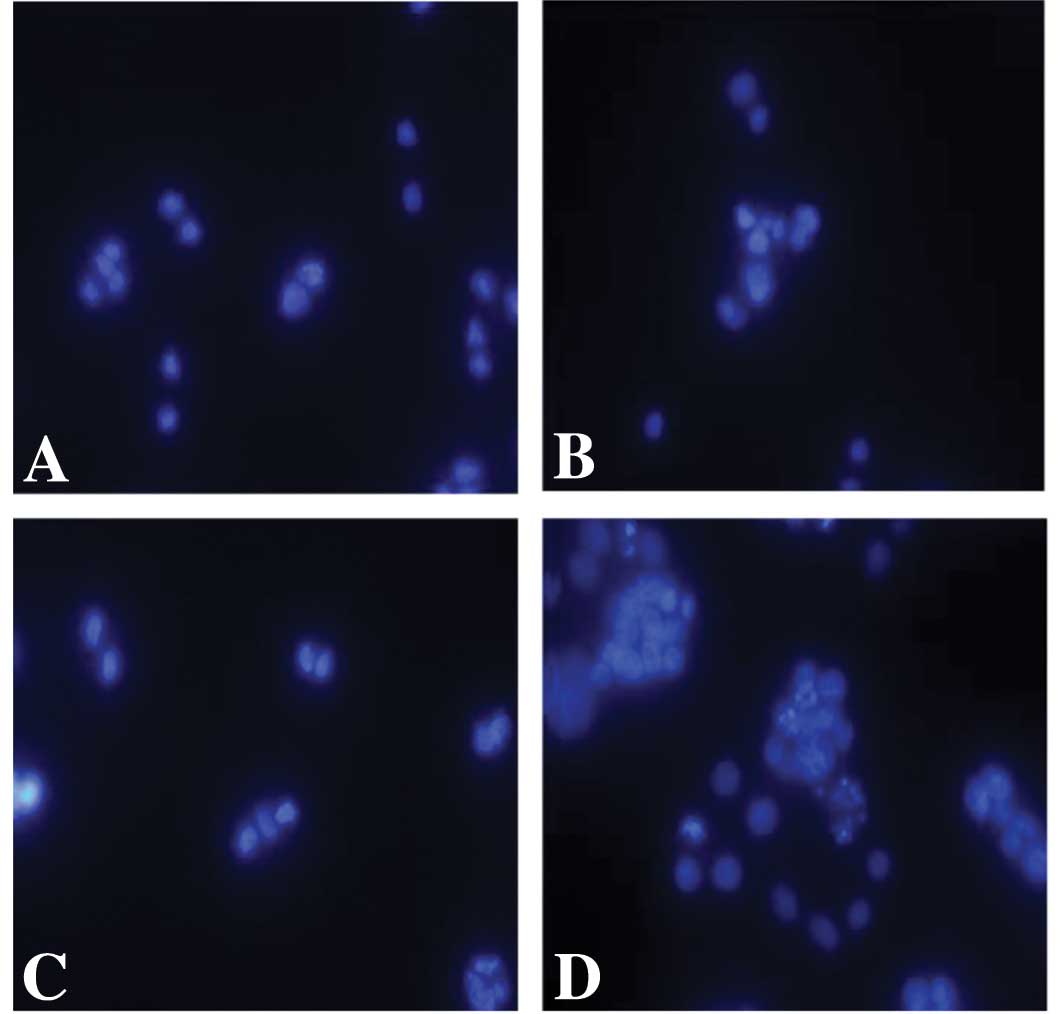
dose-dependent manner in the SW480 cell line (Fig. 3). We then determined whether
transfection of Poly I:C induced apoptosis in these SW480 cells by
staining the nuclei with DAPI. Whereas apoptosis was not induced in
the cells following treatment with non-transfected Poly I:C,
typical apoptotic features of the nuclei were observed in the colon
carcinoma cells that were treated with transfected Poly I:C
(Fig. 4).
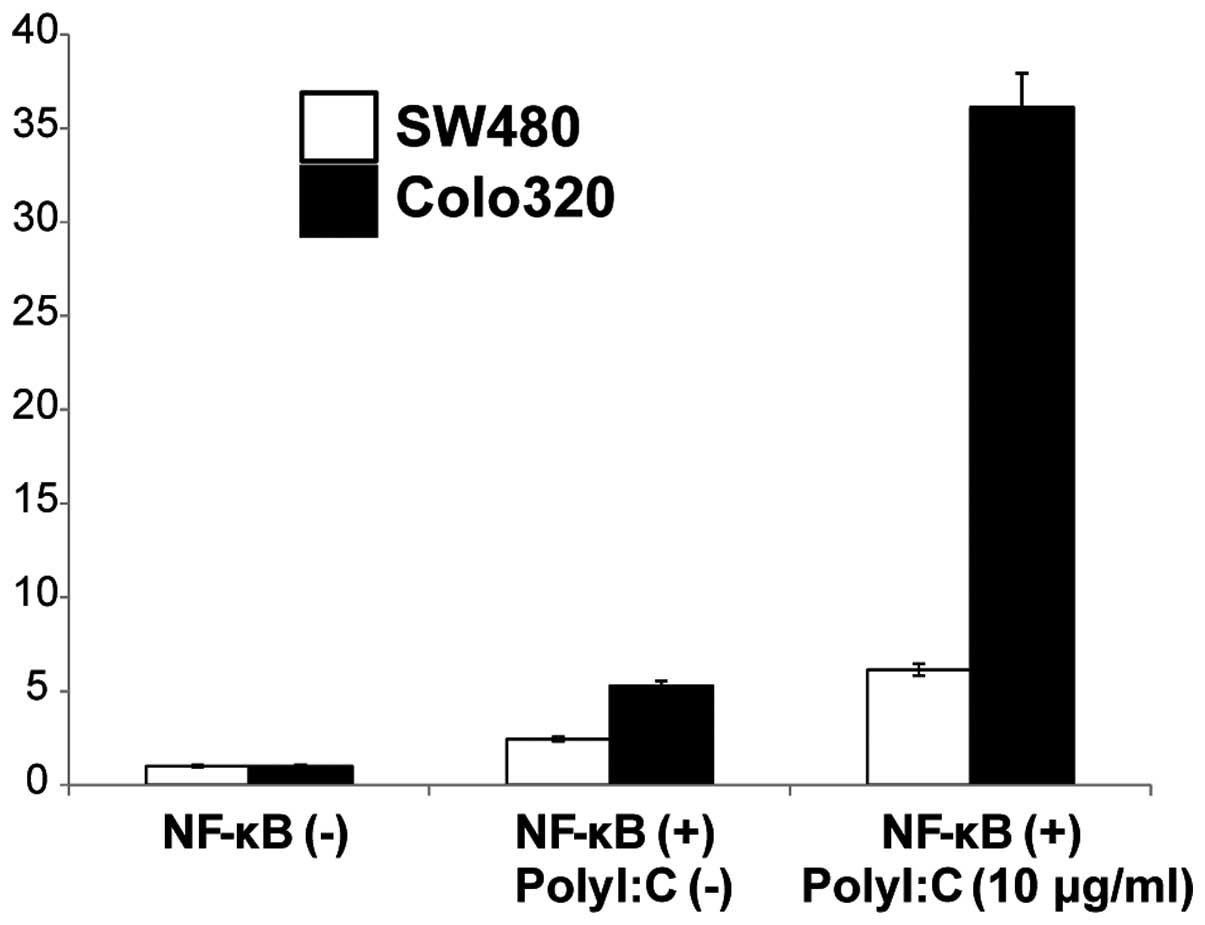
Poly I:C-induced NF-κB activation
Since TLR3 signaling is known to modulate NF-κB
signaling pathways we further examined if activation of TLR3 by
Poly I:C stimulation mediated induction of NF-κB in the two cell
lines. NF-κB activity following cell surface stimulation with Poly
I:C was assayed using a luciferase reporter assay and was compared
to that in the absence of stimulation. Stimulation of the cells
with Poly I:C resulted in increased NF-κB activity in both the
SW480 and the Colo320 cell lines compared to the non-stimulated
cells (Fig. 5). These results
indicated that cell surface stimulation with Poly I:C activates the
NF-κB signaling pathway.
TLR9 is expressed in colon carcinoma cell
lines and colon tissues
As shown in Fig. 6,
TLR9 protein expression was detected in both colon carcinoma cell
lines by western blotting. TLR9 expression in colon tissue was
further investigated by immunohistochemical staining of both
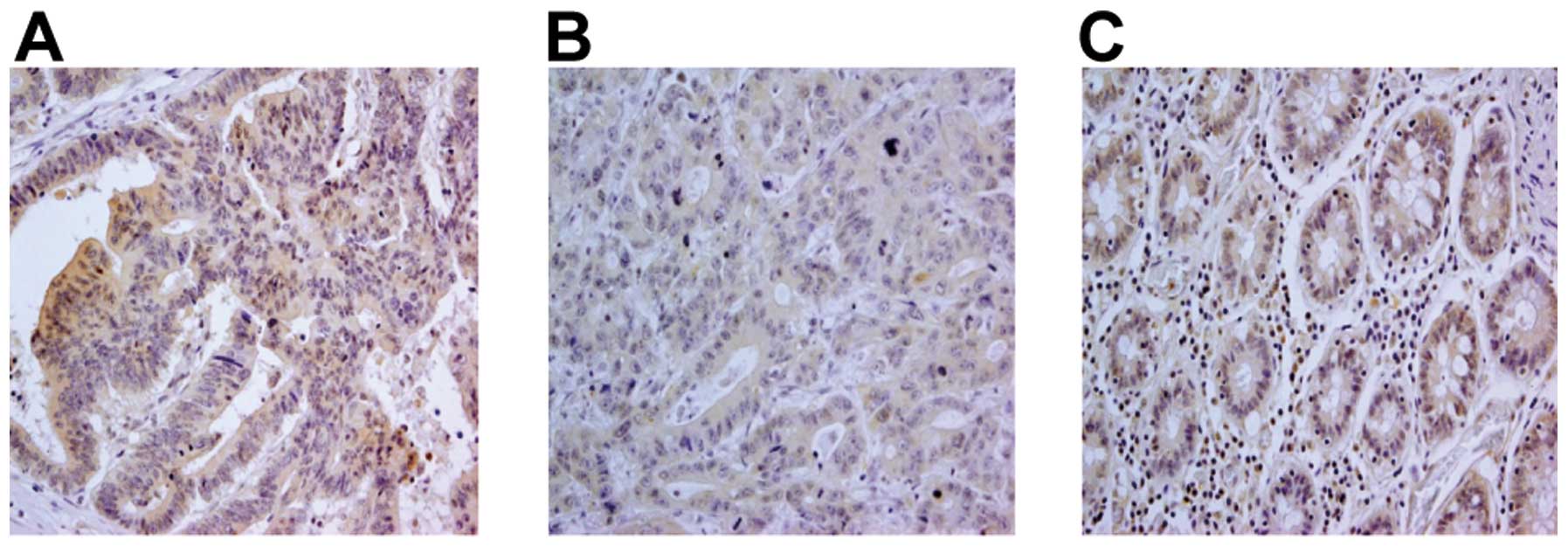
non-colon carcinoma tissue and colon carcinoma lesions (Fig. 7, representative staining). While
86/100 colon carcinoma cases (86%) displayed a clear positive TLR9
signal, no major differences in TLR9 signals were observed between
different histological grades of the tumors (Table II). TLR9 staining was detected not
only in the cytoplasm, but also in the cell membrane.
 | Table IIExpression of TLR9 in colon carcinoma
and non-tumor tissues. |
Table II
Expression of TLR9 in colon carcinoma
and non-tumor tissues.
| Staining |
|---|
|
|
|---|
| Histology | Absent or weak
(%) | Moderate (%) | Strong (%) |
|---|
| Non-tumor tissue | 1 (11.1) | 8 (88.9) | 0 (0) |
| Colon carcinoma |
| Poorly
differentiated | 3 (100) | 0 (0) | 0 (0) |
| Moderately
differentiated | 7 (10.8) | 35 (53.8) | 23 (35.4) |
| Well
differentiated | 3 (13.0) | 12 (52.2) | 8 (34.8) |
CpG-ODNs affect cell proliferation
In order to determine the biological significance of
the signaling that occurs via the cell surface TLR9 in colon
carcinoma cells, we investigated the cytotoxicity of treatment of
the two colon carcinoma cell lines with the TLR9 ligand CpG-ODNs.
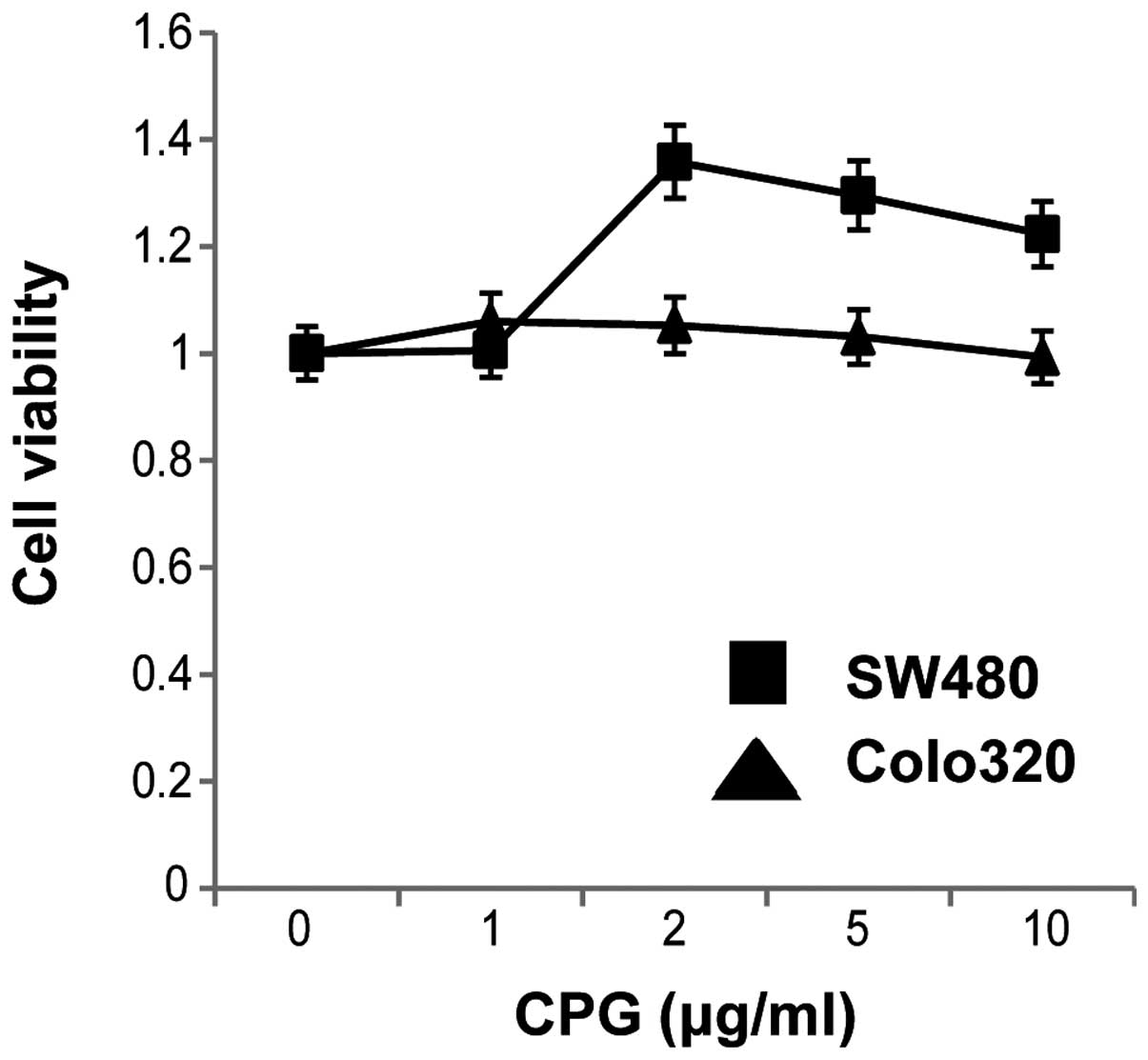
As shown in Fig. 8, stimulation of
the two cell lines with CpG-ODNs for 48 h increased the viability
of SW480 cells. This result suggested that cell surface stimulation
with CpG-ODNs might affect cell proliferation and survival in colon
carcinoma cells.
CpG-ODNs reduce the cytotoxicity of
ADM
We next examined possible interactions between the
effect of CpG-ODNs and that of the anticancer reagent, adriamycin
(ADM), on the viability of the colon carcinoma cell line Colo320.
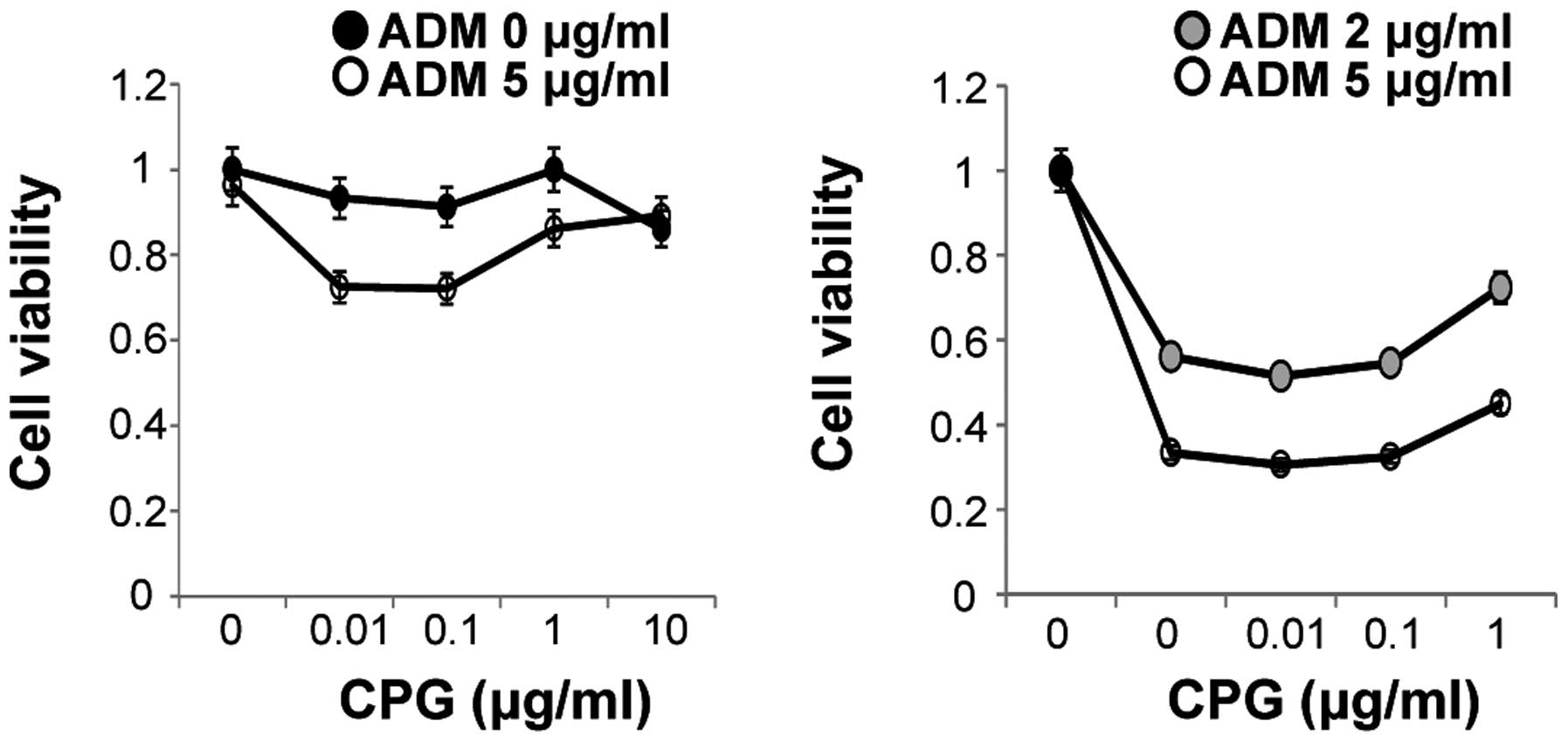
Cell surface stimulation of Colo320 cells with a combination of 1
μM CpG-ODNs and 2 μg/ml ADM for 48 h resulted in an increase in
cell viability of ~23% compared to ADM treatment alone.
Furthermore, cell surface stimulation with a combination of 1 μM
CpG-ODNs and 5 μg/ml ADM for 48 h resulted in an increase in cell
viability of ~12% compared to stimulation with ADM alone (Fig. 9). These results suggest that
CpG-ODNs might contribute to a reduction in the cytotoxicity of
ADM.
CpG-ODNs activate the NF-κB signaling
pathway
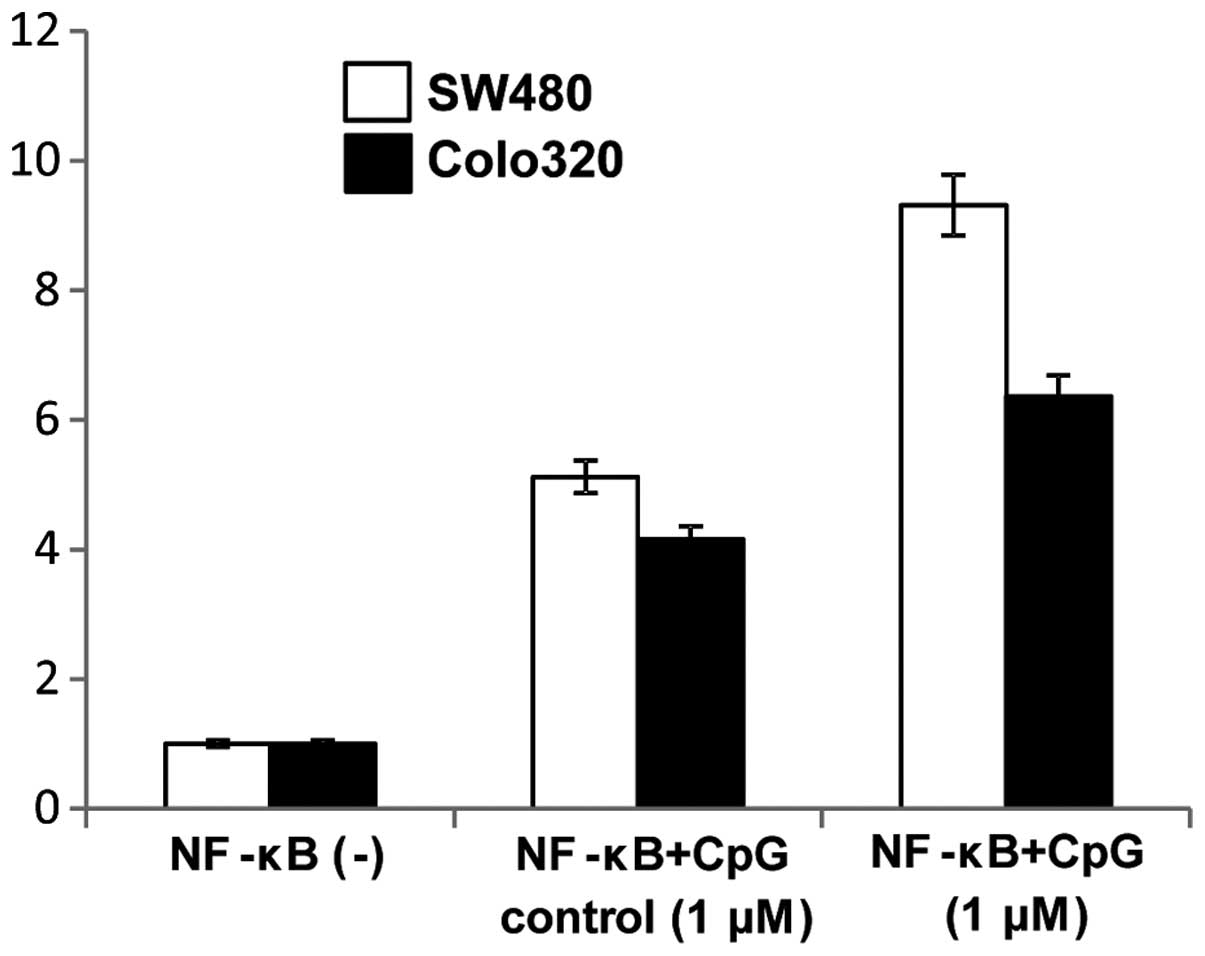
Since TLR9 signaling is known to modulate NF-κB
signaling pathways we further examined if CpG-ODN stimulation of
TLR9 mediated induction of NF-κB activity. Induction of NF-κB
activity following cell surface stimulation with 1 μM CpG-ODNs was
assayed using a luciferase reporter assay and was compared to the
NF-κB activity of non-simulated cells. NF-κB activity of both colon
carcinoma cell lines was increased by treatment with 1 μM CpG-ODNs
(Fig. 10).
Discussion
TLRs are essential for immune defense against
microbes and viruses. TLR agonists enhance tumor immunotherapy by
stimulating TLR signaling in immune cells thereby activating both
innate and adaptive immune responses. By contrast, recent studies
have indicated that some tumor cells express TLRs and that TLR
expression is associated with cancer risk, suggesting that TLRs may
also play important roles in tumor biology.
We previously found that human tumor cell lines and
tumor tissues, particularly human HCCs, express multiple TLRs
(13–16). In the present study,
immunohistochemical analysis revealed that 91.2% of colon carcinoma
tissues as well as two different colon carcinoma cell lines express
TLR3 in addition to its expression in non-tumor tissues such as
cirrhotic or normal tissues. We previously reported the high
prevalence of TLR3 in human HCC. In these previous reports, flow
cytometric analysis and immune fluorescence staining indicated that
TLR3 was clearly expressed both on the cell surface and in the
cytoplasm of HCC cells (15). TLRs
3, 7, 8 and 9, which recognize nucleic acid ligands, are known to
be expressed in the endosomes and in the endoplasmic reticulum. In
the present study, a similar staining pattern of TLRs, i.e., on the
cell surface and in the cytoplasm, was observed in colon carcinoma
tissues as was observed in the HCCs of the previous study.
The present study indicated that stimulation of
SW480 colon carcinoma cells with non-transfected Poly I:C resulted
in no change in cell viability, whereas stimulation of the cells
with transfected Poly I:C resulted in decreased cell viability in a
dose-dependent manner. These results indicate that activation of
intracellular TLR3 may predominate over that of cell surface TLR3
in this cell line.
However, the expression and function of TLR3 in
cancer cells are not well understood. Although the findings of the
present study indicate that cell surface stimulation of TLR3 did
not affect cell viability, cell surface stimulation with Poly I:C
did activate the NF-κB signaling pathway. These results suggest
that cell surface TLR3 may be functional. In cancer cells, upon
activation of NF-κB, the NF-κB dimers typically enter the nucleus
and induce the production of cytokines, growth factors and
antiapoptotic proteins. It appears that, in tumors, NF-κB can
convert inflammatory stimuli into tumor cell survival and growth
signals (13–16).
Stimulation of intracellular TLR3 by transfection of
the cells with Poly I:C showed that transfected Poly I:C caused
apoptotic cell death in colon carcinoma cells in a dose-dependent
manner. These findings indicate that TLR3 signals may be linked to
apoptotic signals. The combined results indicate that TLR3 signals
are different depending on the localization of TLR3 i.e., whether
it is on the cell surface or intracellular, and that these signals
play an important role in cell survival and cell death. Similar
observations have been reported for several other types of cancer
cells (13–16).
Regarding TLR9, we found that TLR9 expression,
similar to TLR3 expression, was prevalent in human colon carcinoma
cells. There was a clear positive TLR9 signal in 86 of 100 colon
carcinoma cases (86%). TLR9 staining was detected not only in the
cytoplasm, but also in the cell membrane. We previously reported
that there was high expression of TLR9 in human HCC. In that
previous study, western blot analysis of subcellular fractions, and
flow cytometric analysis of intact cells, clearly demonstrated the
expression of TLR9 on both the cell surface and in the cytoplasm of
human HCC cells (16). In the
present study, the staining pattern of TLR3 in colon carcinoma
cells was similar to that of TLR9 in the previous study of
HCCs.
We then investigated the function of TLR9 in colon
carcinoma cells. We demonstrated that stimulation with CpG-ODNs
increased the cell viability of SW480 cells. This result suggested
that cell surface stimulation with CpG-ODNs might affect cell
proliferation and survival in these colon carcinoma cells. This
result is consistent with the fact that the enhanced cell
proliferation and survival of HCC cells (16). We further found that activation of
TLR9 with CpG-ODNs in both colon carcinoma cell lines upregulated
NF-κB activity. In general, engagement of the TLR9 signaling
pathway leads to the activation of two major transcription factors
that have central roles in innate immunity, i.e., NF-κB and IRF-7.
TLR9 requires the adaptor molecule MyD88 for initiation of these
signals, and MyD88 can directly associate with and activate IRF-7,
leading to type I-IFN production (19,20).
NF-κB usually plays an important role in regulating immune and
inflammatory responses, apoptosis and oncogenes (21,22).
Thus the CpG-ODN-activation of NF-κB activity is consistent with
other studies of TLR9 signaling.
Finally, we found that cell surface stimulation with
CpG-ODNs reduced the cytotoxicity of ADM. We previously showed that
cell surface stimulation with CpG-ODNs might contribute to a
reduction in the cytotoxicity of ADM towards HepG2 cells via the
upregulation of apoptosis inhibitors (16). We therefore speculated that the
reduction in ADM cytotoxicity in colon carcinoma cells by CpG-ODNs
may be mediated by the same mechanism.
In conclusion, functional TLR3 and TLR9 are
expressed in colon carcinoma cells. TLR3 and TLR9 activate NF-κB
and the activation of these TLRs is closely related to cell death
and survival. Further evaluation of the possible roles and
regulation of TLR3 and TLR9 are critical for controlling cell
death, proliferation and immune escape of malignant cells.
References
|
1
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003. View Article : Google Scholar
|
|
2
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar
|
|
3
|
Beutler B, Jiang Z, Georgel P, Crozat K,
Croker B, Rutschmann S, et al: Genetic analysis of host resistance:
Toll-like receptor signaling and immunity at large. Annu Rev
Immunol. 24:353–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bauer S, Kirschning CJ, Häcker H, Redecke
V, Hausmann S, Akira S, Wagner H and Lipford GB: Human TLR9 confers
responsiveness to bacterial DNA via species-specific CpG motif
recognition. Proc Natl Acad Sci USA. 98:9237–9242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Becker MN, Diamond G, Verghese MW and
Randell SH: CD14-dependent lipopolysaccharide-induced
beta-defensin-2 expression in human tracheobronchial epithelium. J
Biol Chem. 275:29731–29736. 2000. View Article : Google Scholar
|
|
7
|
Faure E, Thomas L, Xu H, Medvedev A,
Equils O and Arditi M: Bacterial lipopolysaccharide and IFN-gamma
induce Toll-like receptor 2 and Toll-like receptor 4 expression in
human endothelial cells: role of NF-kappa B activation. J Immunol.
166:2018–2024. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barton GM, Kagan JC and Medzhitov R:
Intracellular localization of Toll-like receptor 9 prevents
recognition of self-DNA but facilitates access to viral DNA. Nat
Immunol. 7:49–56. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto M, Sato S, Hemmi H, Hoshino K,
Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K and
Akira S: Role of adaptor TRIF in the MyD88-independent toll-like
receptor signaling pathway. Science. 301:640–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alexopoulou L, Holt AC, Medzhitov R and
Flavell RA: Recognition of double-stranded RNA and activation of
NF-kappaB by Toll-like receptor 3. Nature. 413:732–738. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khvalevsky E, Rivkin L, Rachmilewitz J,
Galun E and Giladi H: TLR3 signaling in a hepatoma cell line is
skewed towards apoptosis. J Cell Biochem. 100:1301–1312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang B, Zhao J, Unkeless JC, Feng ZH and
Xiong H: TLR signaling by tumor and immune cells: a double-edged
sword. Oncogene. 27:218–224. 2008. View Article : Google Scholar
|
|
13
|
Yu L and Chen S: Toll-like receptors
expressed in tumor cells: targets for therapy. Cancer Immunol
Immunother. 57:1271–1278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato Y, Goto Y, Narita N and Hoon DS:
Cancer cells expressing Toll-like receptors and the tumor
microenvironment. Cancer Microenviron. 2:205–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoneda K, Sugimoto K, Shiraki K, Tanaka J,
Beppu T, Fuke H, Yamamoto N, Masuya M, Horie R, Uchida K and Takei
Y: Dual topology of functional Toll-like receptor 3 expression in
human hepatocellular carcinoma: Differential signaling mechanisms
of TLR3-induced NF-kappaB activation and apoptosis. Int J Oncol.
33:929–936. 2008.PubMed/NCBI
|
|
16
|
Tanaka J, Sugimoto K, Shiraki K, Tameda M,
Kusagawa S, Nojiri K, Beppu T, Yoneda K, Yamamoto N, Uchida K,
Kojima T and Takei Y: Functional cell surface expression of
toll-like receptor 9 promotes cell proliferation and survival in
human hepatocellular carcinomas. Int J Oncol. 37:805–814.
2010.PubMed/NCBI
|
|
17
|
Sun R, Zhang Y, Lv Q, Liu B, Jin M, Zhang
W, He Q, Deng M, Liu X, Li G, Li Y, Zhou G, Xie P, Xie X, Hu J and
Duan Z: Toll-like receptor 3 (TLR3) induces apoptosis via death
receptors and mitochondria by up-regulating the transactivating p63
isoform alpha (TAP63alpha). J Biol Chem. 286:15918–15928. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salaun B, Lebecque S, Matikainen S,
Rimoldi D and Romero P: Toll-like receptor 3 expressed by melanoma
cells as a target for therapy? Clin Cancer Res. 13:4565–4574. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Honda K, Yanai H, Mizutani T, Negishi H,
Shimada N, Suzuki N, Ohba Y, Takaoka A, Yeh WC and Taniguchi T:
Role of a transductional-transcriptional processor complex
involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc
Natl Acad Sci USA. 101:15416–15421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawai T, Sato S, Ishii KJ, Coban C, Hemmi
H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uematsu S, Takeuchi O
and Akira S: Interferon-alpha induction through Toll-like receptors
involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat
Immunol. 5:1061–1068. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: from innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghosh S and Karin M: Missing pieces in the
NF-κB puzzle. Cell. 109:S81–S96. 2002.
|