Introduction
Malignant mesothelioma (MM) is an aggressive tumor
with a poor prognosis that arises most commonly in the pleura or
peritoneum (1,2). MM was once a rare disease, but its
incidence has increased worldwide, probably as a result of
widespread exposure to asbestos (3). However, a standard curative modality,
such as radiotherapy, conventional chemotherapy or molecular
targeting therapy, has not yet been established for advanced MM. In
addition, much less information regarding the molecular alterations
involved in MM is available, compared with other solid neoplasms
(4).
microRNAs (miRNAs) are a conserved class of
non-coding 20–22 nt small RNAs that regulate protein expression by
binding to mRNA, leading to mRNA degradation or the inhibition of
translation (5–7). Among the miRNAs, the microRNA-34
(miR-34) family members are direct transcriptional targets of p53,
constituting part of the p53 tumor-suppressive network and inducing
cell cycle arrest, apoptosis and senescence, which are the major
consequences of p53 activation (8,9). We
previously reported that miR-34b/c was frequently downregulated by
aberrant methylation in MM, resulting in the loss of
tumor-suppressive p53 function and the acquisition of a malignant
phenotype (10). One of the unique
molecular features of MM is that mutations and deletions of the
TP53 gene are rare (11), even
though MM generally exhibits cell cycle alterations and
anti-apoptosis, suggesting functional p53 deficiency (12).
In the present study, unlike our previous report, we
downregulated miR-34s in human mesothelial cells to investigate the
cellular biological effects of miR-34 inhibition in human
mesothelial cells and to elucidate the cancer mechanisms involved
in MM.
Materials and methods
Cell lines and cell culture
A human mesothelial cell line (LP-9, peritoneal
mesothelial cells) and three types of human primary-cultured
mesothelial cells (HPMCs) were used in this study. The LP-9 cell
line was purchased from the Coriell Cell Repository (Camden, NJ),
and the three HPMCs were established from pleural effusions
obtained from cancer-free patients treated at the Okayama
University Hospital (Okayama, Japan), as described in our previous
report (13). Approval from the
Institutional Review Board and informed consent from all the
patients were obtained.
LP-9 and HPMCs were cultured using Ham’s F12
medium/Medium 199 (1:1 mixture) with 15% fetal bovine serum, 2 mM
L-glutamine, 1.7 nM epidermal growth factor and 1100 nM
hydrocortisone. All the cells were incubated at 37°C in a
humidified atmosphere with 5% CO2.
Transfection of inhibitors of
anti-miRNA-34s
LP-9 and the three HPMCs were transfected with a
scramble control oligonucleotide or Anti-miR™ miR-34a, -34b and
-34c inhibitors (Ambion, Austin, TX) after being seeded in 6-well
plates. Each miRNA inhibitor (150 pmol) in 200 μl of serum-free
antibiotic-free medium was mixed with 5 μl of Lipofectamine 2000
transfection reagent (Invitrogen, Carlsbad, CA) dissolved in 200 μl
of the same medium and allowed to stand at room temperature for 20
min. The resulting 400 μl transfection solutions were then added to
each well containing 1.6 ml of medium supplemented with 15% FBS.
Cells were grown and harvested 48 h after the transfection for
additional analyses.
Expression of miR-34s as determined using
quantitative RT-PCR
The miRNA was isolated from LP-9 and mesothelial
cells using the TaqMan MicroRNA Cells-to-CT™ kit (Ambion) and
treated with DNase I (Ambion) to remove genomic DNA. A reverse
transcriptional (RT) reaction was performed to extract 0.5 μg of
miRNA using the TaqMan MicroRNA Reverse Transcriptional Kit system
(Applied Biosystems) and TaqMan single RT primers for each miRNA
(Applied Biosystems). Quantitative RT-PCR for miR-34a, -34b and
-34c was performed using TaqMan MicroRNA Assay technology
(Perkin-Elmer Corp., Foster City, CA) with the StepOnePlus™
Real-Time PCR system (Applied Biosystems). miR-374 expression was
used to normalize the expression of the miR-34s as an endogenous
control for the cell lines, following the manufacturer’s
recommendation (www.appliedbiosystems.com).
MTS assay
Cells were plated in 96-well plates at a density of
2.0×103 cells/well. Cell viability was evaluated at 0
and 3 days using an MTS assay with CellTiter 96® AQueous
One Solution reagent (Promega, Madison, WI).
Colony formation assay
The in vitro cell proliferation was assessed
by liquid colony formation assay. Viable cells (100) were plated
onto 6-well plates in triplicate. Cells were cultured and counted
14 days later after staining with 0.1% crystal violet in 20%
ethanol for 5 min at room temperature. The number of visible
colonies (>50 cells) was counted.
Immunohistochemistry for Ki-67
miR-34 inhibitor-transfected or scramble
control-transfected LP-9 cells were grown and treated in Lab-Tek
chamber slides (Nunc, Naperville, IL). Medium was aspirated and
cells were fixed in 4% paraformaldehyde for 10 min at room
temperature. Paraformaldehyde was aspirated, and the cells were
treated with a 0.2% Tween 20 in PBS for 15 min. Cells were then
washed in PBS, and Ki-67 (Novocastra, Newcastle, UK) was added at a
dilution of 1:2,000 in 1% bovine serum albumin and incubation was
carried out overnight at 4°C. Cells were washed in PBS before
incubating in the dark with an FITC-labeled secondary antibody
(Jackson ImmunoResearch, West Grove, PA) at a dilution of 1:100 in
1% bovine serum albumin for 1 h. The secondary antibody solution
was aspirated, and the cells were washed in PBS. Cells were
incubated in the dark with 4′,6-diamidino-2-phenylindole (1 μg/ml)
in PBS for 30 min and washed, and coverslips were mounted with an
anti-fade solution (Dako Corp., Carpinteria, CA). The Ki-67
staining was evaluated using labeling index. At least 1,000 cells
were counted under a microscope at a magnification of ×100.
Soft-agar colony formation assay
To investigate the anchorage-independent growth
potential of miR-34 inhibition, we performed soft agar colony
formation assay. Cells (7,500) in growth medium containing 0.4%
agarose were placed on a 60-mm dish with a 0.5% agarose base. After
3 weeks of incubation, the colonies were stained with 0.005%
crystal violet at room temperature for 1 h and were counted for
each dish. A549 cells (human lung adenocarcinoma cell line) were
used as a positive control.
Cell migration and invasion assays
Cell migration and invasion were assayed using a
Boyden chamber assay with filter inserts (pore size, 8 μm) in
6-well dishes (BD Biosciences Discovery Labware, Bedford, MA).
Tumor cells in 2 ml of serum-free medium (300 μl containing
0.75×105 cells for the Transwell migration assay and
1.5×105 cells for the Matrigel invasion assay) were
added to the top chamber. The bottom chamber was prepared with 15%
FBS as a chemoattractant. After a 24- and 48-h incubation for the
migration and invasion assays, respectively, the non-invasive cells
were removed by scrubbing with a cotton swab. The cells that
migrated through the membrane and adhered to the lower surface of
the membrane were fixed and stained using Diff-Quik stain (Sysmex,
Kobe, Japan). To quantify the migration and invasion, the cells
were counted under a microscope in 5 predetermined fields at a
magnification of ×100 and were represented as the average of three
independent experiments.
Flow cytometric analysis
Cells were harvested and resuspended in PBS
containing 0.2% Triton X-100 and 1 mg/ml RNase for 5 min at room
temperature and then stained with propidium iodide (PI) at 50 mg/ml
to determine subdiploid DNA content using a FACScan. Doublets, cell
debris and fixation artifacts were gated out, and cell cycle
analysis was carried out using CellQuest version 3.3 software.
Western blot analysis
Cells were grown to 80% confluence and harvested in
lysis buffer [20 mmol/l Tris-HCl (pH 7.5), 150 mmol/l NaCl, 1
mmol/l Na2EDTA, 1 mmol/l EGTA, 1% Triton, 2.5 mmol/l
sodium pyrophosphate, 1 mmol/l β-glycerophosphate, 1 mmol/l
Na3VO4, 1 μg/ml leupeptin] (Cell Signaling
Technology, Beverly, MA) supplemented with Complete Mini (Roche,
Basel, Switzerland) to extract the proteins. A total of 20 μg of
protein was separated using SDS-PAGE and was then transferred to
PVDF membranes. The proteins on the membranes were incubated
overnight at 4°C with the primary antibodies. The primary
antibodies used for western blotting were as follows: anti-MET
(25H2; Cell Signaling), anti-phospho-MET (3D7, Tyr1234/1235; Cell
Signaling) and anti-bcl-2 (human specific; Cell Signaling). The
following secondary antibodies were used: goat anti-rabbit or
anti-mouse IgG-conjugated horseradish peroxidase (Santa Cruz
Biotechnology, Santa Cruz, CA). To detect the specific signals, the
membranes were examined using ECL Plus Western Blotting Detection
reagents (Amersham Biosciences UK Ltd., Buckinghamshire, UK).
Statistical analysis
The statistical analysis was performed using SPSS
for Windows version 17.0 (SPSS Inc., Chicago, IL, USA). All of the
in vitro experiments were performed at least three times.
Data are represented as the means ± standard deviation. The
significance of the differences between the two groups was
determined using the Chi-square test and the Mann-Whitney U-test,
as appropriate. A 5% significance level (P<0.05) was considered
to indicate a statistically significant result.
Results
miR-34 inhibition by transfection with
miR-34 inhibitors
We transfected the LP-9 cells and HPMCs with a
scramble control and miR-34 inhibitors and confirmed that the
expression of the miR-34s was suppressed in all of the cells,
compared with the scramble control, using a real-time PCR method:
80–89% inhibition for the miR-34a inhibitor, 45–73% for miR-34b and
68–70% for miR-34c.
Impact of miR-34 inhibitors on cell
viability and proliferation
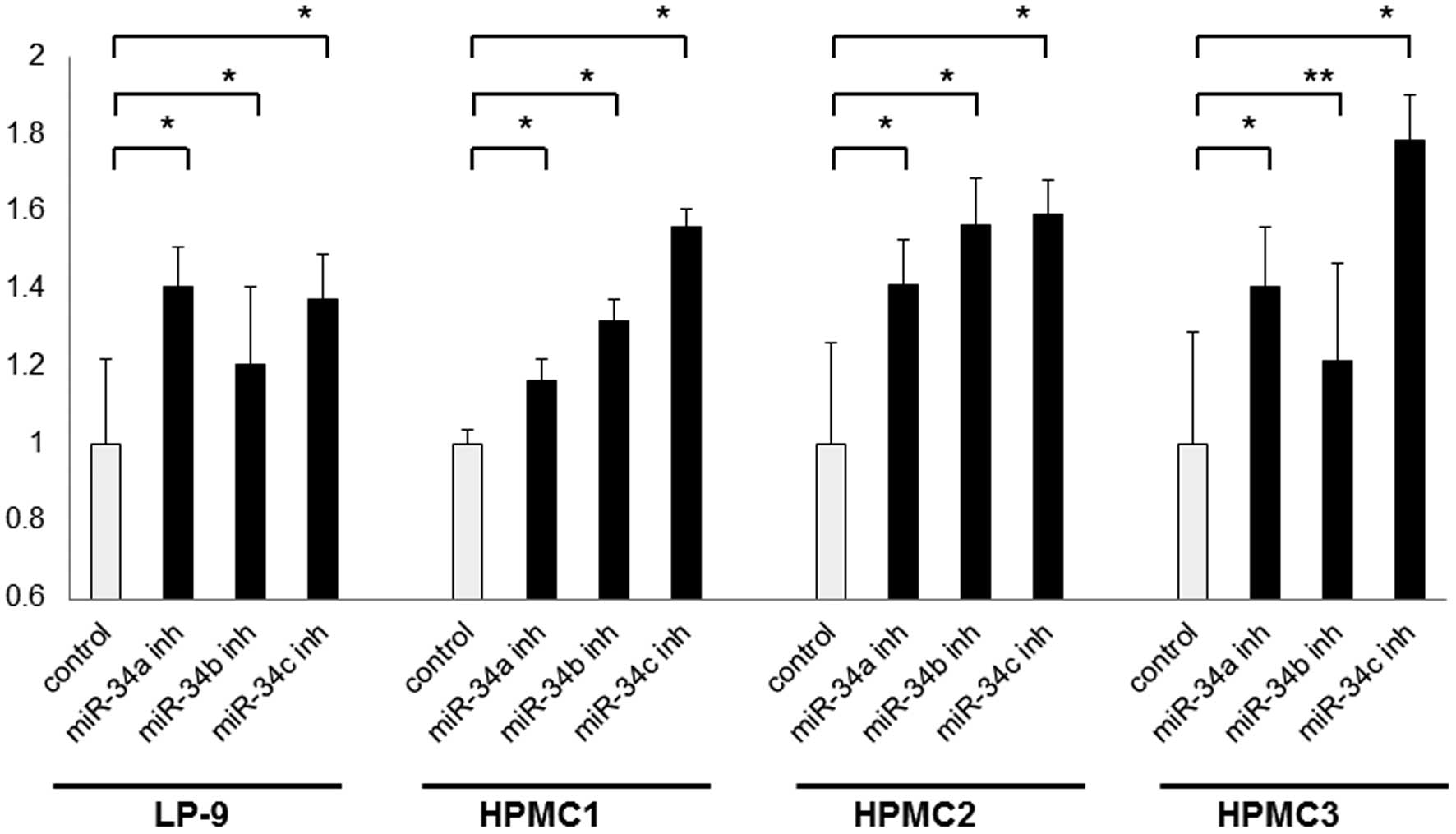
To screen for the cell viability effect of miR-34
inhibition, we performed MTS assays in LP-9 cells and the three
mesothelial cell lines using transient transfection. miR-34a, -34b
and -34c inhibitors significantly increased the cell viability of
all the examined cells, compared with the scramble control (1.2- to
1.4-fold for miR-34a, 1.2- to 1.6-fold for miR-34b, and 1.4- to
1.8-fold for miR-34c) (Fig. 1). In
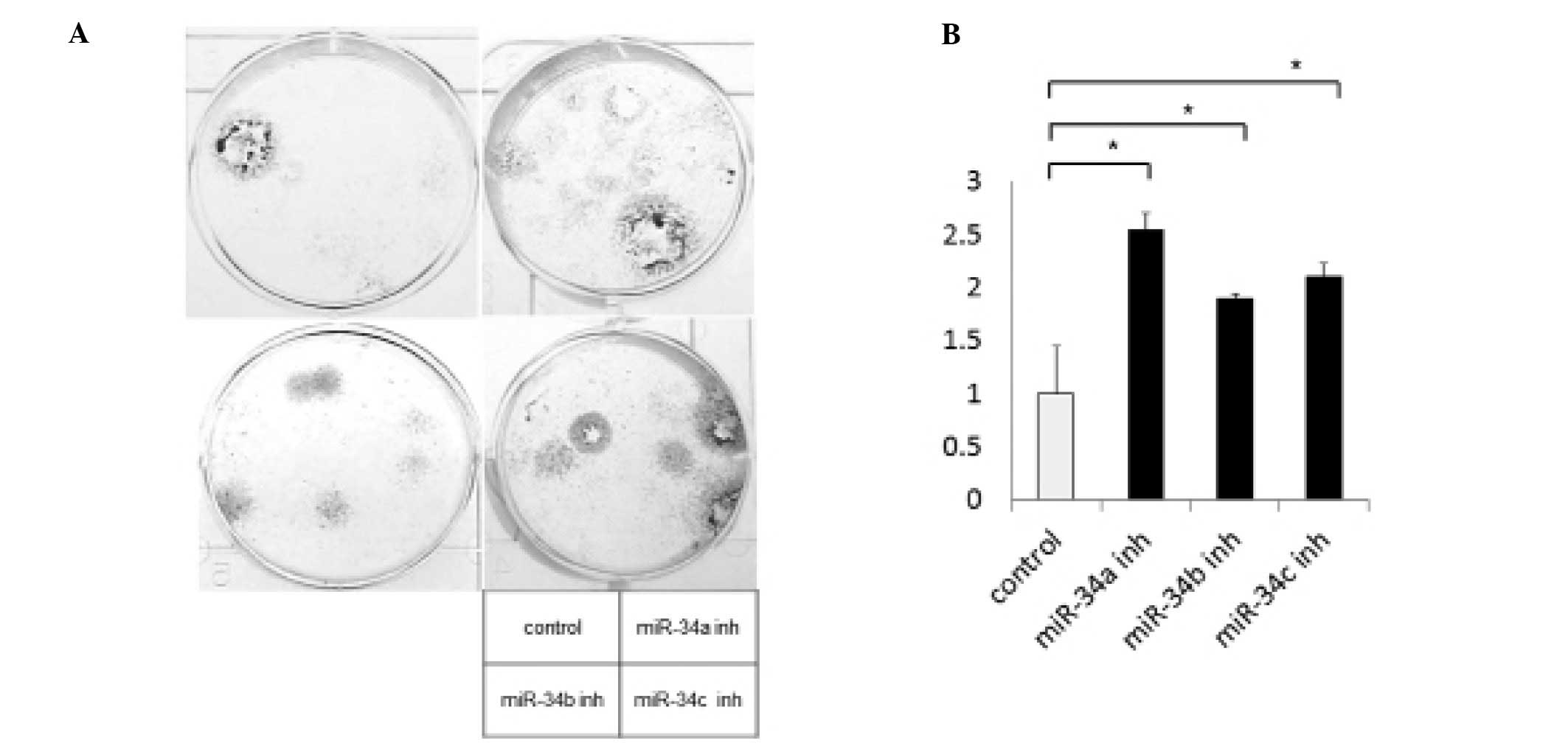
addition, to screen for the cell proliferation potential of miR-34
inhibition, we performed a colony formation assay and investigated
the expression of Ki-67 in the LP-9 cells. The number of visible
colonies was significantly increased in the cells transfected with
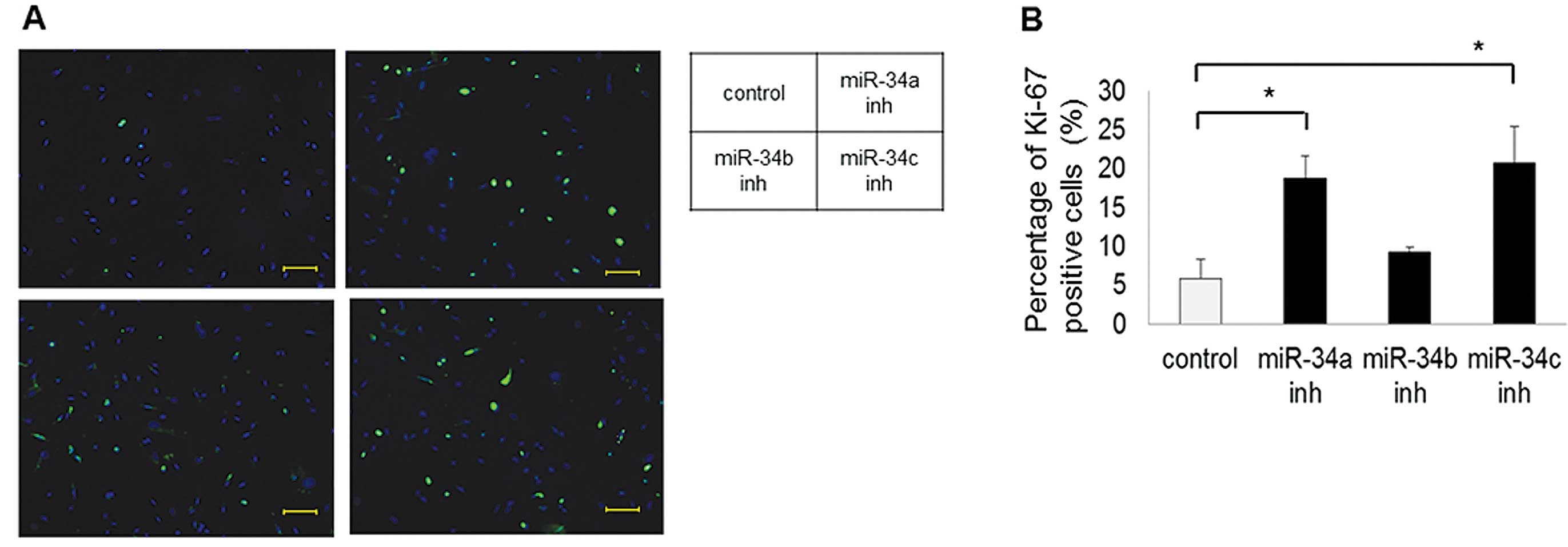
the miR-34 inhibitors, compared with the scramble control (Fig. 2). The number of Ki-67-stained cells
was significantly increased in cells transfected with the miR-34a
(P<0.01) and -34c (P<0.01) inhibitors, compared with the
scramble control. However, cells transfected with the miR-34b
inhibitor tended to have increased numbers of Ki-67-stained cells
(P=0.09) (Fig. 3). Regarding the
anchorage-independent growth potential of miR-34 inhibition, LP-9
cells transfected with both the scramble control and miR-34
inhibitors did not grow in soft agar.
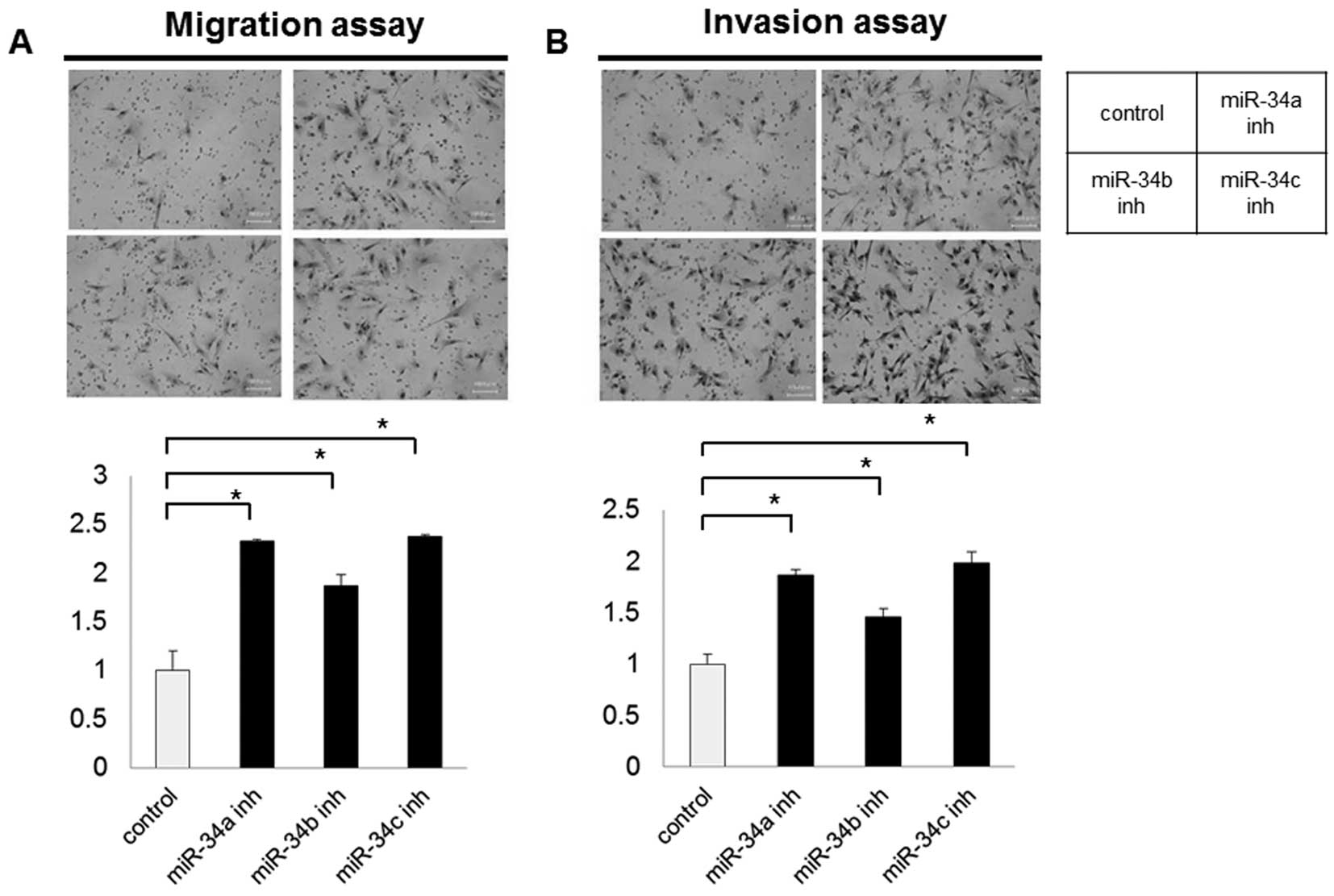
Impact of miR-34 inhibitors on migration
and invasion
Cell migration and invasion potential were examined
using a Boyden chamber. Microscopy images of the Boyden chamber
assay are shown in Fig. 4. Both
migration and invasion were significantly increased in the LP-9
cells transfected with all miR-34 inhibitors (P<0.01), compared
with the scramble control. Parental HPMCs did not exhibit migration
or invasion in this study, and such features were not acquired
after transfection with miR-34 inhibitors.
Cell cycle analysis of LP-9 cells
transfected with miR-34 inhibitors
Cell cycle analysis was conducted for LP-9 cells
transfected with the scramble control or miR-34 inhibitors. The
LP-9 cells transfected with the miR-34 inhibitors showed a slight
decrease in the G0–G1 cell fraction, indicating that miR-34
inhibitors reduced G1 arrest (2.0–4.6% decrease for miR inhibitors)
(data not shown).
Protein expression of LP-9 cells
transfected with miR-34 inhibitors
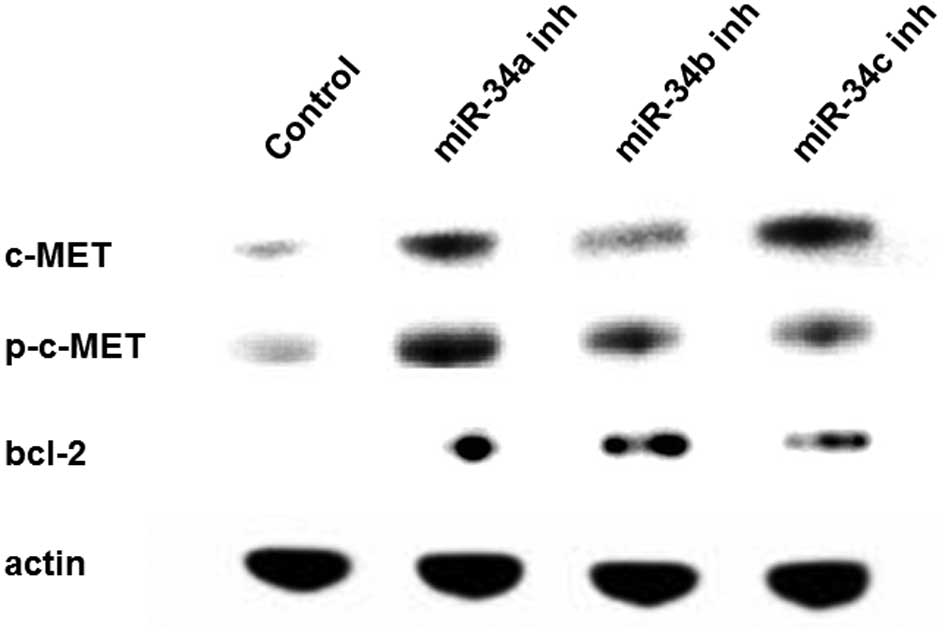
To examine the effect of miR-34 inhibition, we
focused on c-MET (both total and phosphorylated types) and Bcl-2,
which are putative targets of miR-34s. Western blotting was
performed using LP-9 cells transfected with the scramble control or
miR-34 inhibitors. The total and phosphorylated c-MET and Bcl-2
expression levels were upregulated in the LP-9 cells transfected
with the miR-34 inhibitors (Fig.
5).
Discussion
In the present study, we found that the
downregulation of miR-34s in human mesothelial cells prompted
increased cell viability, proliferation, resistance to apoptosis,
and invasive potential but failed to increase anchorage-independent
growth potential. We previously reported that the epigenetic
silencing of miR-34b/c by methylation was extremely common (100% of
cell lines) and played an important role in the tumorigenesis of
MM. In that study, miR-34a was also found to be downregulated by
methylation (30% of cell lines), suggesting a tumorigenic role in
MM (10). Of note, Ji et
al(14) reported that the
restoration of miR-34s significantly inhibited clonogenic growth,
while the inhibition of endogenous miR-34s by miR-34 inhibitors
promoted growth in human pancreatic cancer cell lines. These
results suggest that the inhibition of miR-34s is an important
factor contributing to MM carcinogenesis.
Regarding the study of the oncogenic transformation
of normal cells, the introduction of oncogenes, such as
K-RAS or HRAS and c-MYC, caused the induction
of a malignant phenotype in human bronchial epithelial cells
(15–17). Sato et al(18) also reported that additional genetic
changes, such as p53 knockdown, K-RASV12,
and mutant epidermal growth factor receptor (EGFR), either alone or
in combination, caused the progression of human bronchial
epithelial cells at least partly toward malignancy, including the
development of characteristics such as a higher saturation density,
anchorage-independent growth, and an invasive phenotype, but failed
to induce tumor formation. In our study, miR-34 inhibition induced
increased cell viability in all the human mesothelial cell lines,
and increased cell proliferation and invasive potential in LP-9
cells. However, miR-34 inhibition failed to promote
anchorage-independent growth potential, suggesting that the
inhibition of miR-34s was not only sufficient to cause crude
mesothelial cells to undergo apparent malignant transformation but
that other molecular alterations are required for the carcinogenic
process in human mesothelial cells. Analysis of the
immunofluorescent staining of Ki-67 demonstrated that cells
transfected with miR-34a and -34c inhibitors exhibited
significantly increased numbers of Ki-67-positive stained cells,
compared with the scramble control, although the cells transfected
with the miR-34b inhibitor did not. The absence of significant
differences in the miR-34b inhibitor suggest that the inhibition
efficiency of the miR-34b inhibitor was lower than that of the
miR-34a and 34c inhibitors in this study.
Several genes have been identified as targets of the
miR-34s (9). In this study,
c-MET and Bcl-2 genes were examined as these gene
products are considered to be important molecules in MM and were
upregulated after miR-34 inhibition. c-MET was found to be
activated in MM through overexpression or mutation, and its ligand,
hepatocyte growth factor, was found to be overexpressed in MMs
(19). Indeed, the suppression of
c-MET using MET inhibitors revealed a potent inhibition of
proliferation, invasion and migration in several MM cell lines
(20). Bcl-2 is an anti-apoptotic
protein located downstream of p53. The overexpression of Bcl-2 in
MM has been reported in immunohistochemical analysis (21,22)
and is considered to be responsible for the anti-apoptotic feature
of MM. Considering the results of western blot analysis together
with colony formation assay and cell cycle analysis, our findings
suggest that the inhibition of miR-34s increases the grade of
malignancy in mesothelial cells through c-MET and Bcl-2.
In conclusion, the present study, together with the
findings of our previous report, strongly suggest that miR-34s play
an important role in the early carcinogenic progression of human
mesothelial cells to malignant mesothelioma.
Acknowledgements
We thank the Central Research Laboratory of the
Okayama University Medical School for the technical support for the
immunohistochemical staining. This study was supported by
Grant-in-Aids for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology of Japan
22591566 (S.T.).
Abbreviations:
|
MM
|
malignant mesothelioma
|
|
miR
|
microRNA
|
|
HPMCs
|
human primary-cultured mesothelial
cells
|
References
|
1
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jakobsen JN and Sorensen JB: Review on
clinical trials of targeted treatments in malignant mesothelioma.
Cancer Chemother Pharmacol. 68:1–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hansen J, de Klerk NH, Musk AW and Hobbs
MS: Environmental exposure to crocidolite and mesothelioma:
exposure-response relationships. Am J Respir Crit Care Med.
157:69–75. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toyooka S, Kishimoto T and Date H:
Advances in the molecular biology of malignant mesothelioma. Acta
Med Okayama. 62:1–7. 2008.PubMed/NCBI
|
|
5
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hatfield S and Ruohola-Baker H: microRNA
and stem cell function. Cell Tissue Res. 331:57–66. 2008.
View Article : Google Scholar
|
|
7
|
Zhang W, Dahlberg JE and Tam W: MicroRNAs
in tumorigenesis: a primer. Am J Pathol. 171:728–738. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He L, He X, Lim LP, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hermeking H: p53 enters the microRNA
world. Cancer Cell. 12:414–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kubo T, Toyooka S, Tsukuda K, et al:
Epigenetic silencing of microRNA-34b/c plays an important role in
the pathogenesis of malignant pleural mesothelioma. Clin Cancer
Res. 17:4965–4974. 2011. View Article : Google Scholar
|
|
11
|
Carbone M, Rizzo P, Grimley PM, et al:
Simian virus-40 large-T antigen binds p53 in human mesotheliomas.
Nat Med. 3:908–912. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimamura A and Fisher DE: p53 in life and
death. Clin Cancer Res. 2:435–440. 1996.PubMed/NCBI
|
|
13
|
Toyooka S, Pass HI, Shivapurkar N, et al:
Aberrant methylation and simian virus 40 tag sequences in malignant
mesothelioma. Cancer Res. 61:5727–5730. 2001.PubMed/NCBI
|
|
14
|
Ji Q, Hao X, Zhang M, et al: MicroRNA
miR-34 inhibits human pancreatic cancer tumor-initiating cells.
PLoS One. 4:e68162009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reddel RR, Ke Y, Kaighn ME, et al: Human
bronchial epithelial cells neoplastically transformed by v-Ki-ras:
altered response to inducers of terminal squamous differentiation.
Oncogene Res. 3:401–408. 1988.
|
|
16
|
Ura H, Bonfil RD, Reich R, et al:
Expression of type IV collagenase and procollagen genes and its
correlation with the tumorigenic, invasive, and metastatic
abilities of oncogene-transformed human bronchial epithelial cells.
Cancer Res. 49:4615–4621. 1989.
|
|
17
|
Yoakum GH, Lechner JF, Gabrielson EW, et
al: Transformation of human bronchial epithelial cells transfected
by Harvey ras oncogene. Science. 227:1174–1179. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato M, Vaughan MB, Girard L, et al:
Multiple oncogenic changes (K-RAS(V12), p53 knockdown, mutant
EGFRs, p16 bypass, telomerase) are not sufficient to confer a full
malignant phenotype on human bronchial epithelial cells. Cancer
Res. 66:2116–2128. 2006. View Article : Google Scholar
|
|
19
|
Harvey P, Warn A, Newman P, Perry LJ, Ball
RY and Warn RM: Immunoreactivity for hepatocyte growth
factor/scatter factor and its receptor, met, in human lung
carcinomas and malignant mesotheliomas. J Pathol. 180:389–394.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jagadeeswaran R, Ma PC, Seiwert TY, et al:
Functional analysis of c-Met/hepatocyte growth factor pathway in
malignant pleural mesothelioma. Cancer Res. 66:352–361. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soini Y, Kinnula V, Kaarteenaho-Wiik R,
Kurttila E, Linnainmaa K and Paakko P: Apoptosis and expression of
apoptosis regulating proteins bcl-2, mcl-1, bcl-X, and bax in
malignant mesothelioma. Clin Cancer Res. 5:3508–3515.
1999.PubMed/NCBI
|
|
22
|
O’Kane SL, Pound RJ, Campbell A, Chaudhuri
N, Lind MJ and Cawkwell L: Expression of Bcl-2 family members in
malignant pleural mesothelioma. Acta Oncol. 45:449–453.
2006.PubMed/NCBI
|