Introduction
Tumors derived from epithelial tissues as lung,
prostate, colon or breast are the most prevalent in industrialized
countries. Colorectal cancer is one of the most common causes of
cancer-related mortality worldwide. For colorectal cancer, surgical
resection of the tumor is commonly the first therapeutic action
with curative intent. However, ~50% of patients develop metastatic
disease which is incurable with current treatments. Although the
median overall survival has increased as a result of the
improvement in systemic therapies such as new chemotherapy agents
and monoclonal antibodies, the median overall survival has reached
a plateau at 24 months. Therefore, one of the main challenges in
colorectal cancer is to develop new strategies to inhibit disease
progression (1,2).
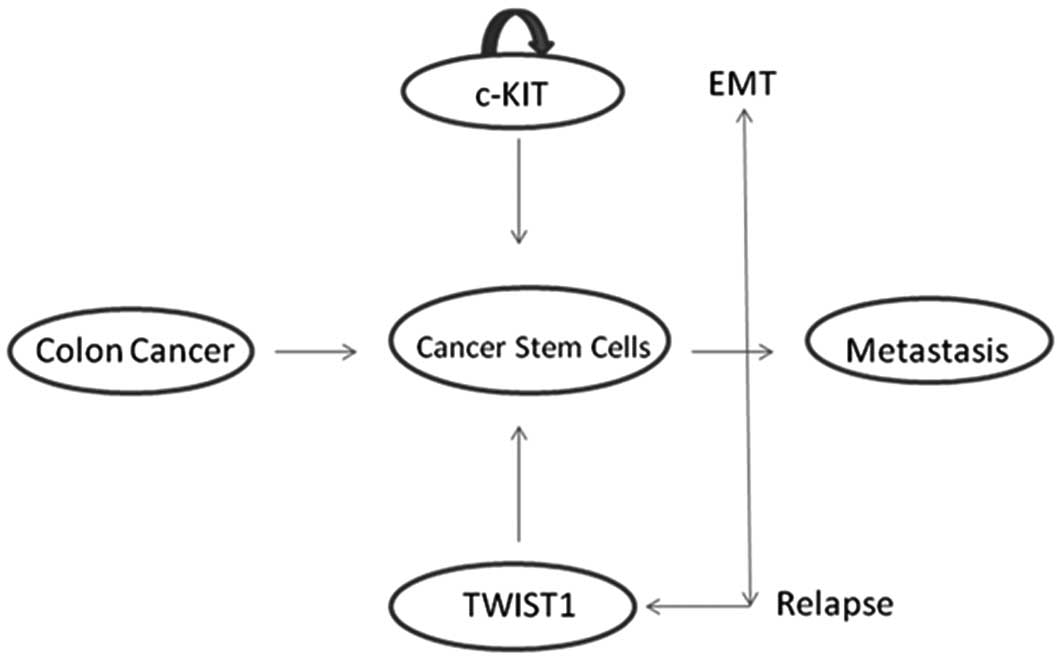
The stem cell hypothesis postulates that cancers, as
they occur in normal tissues, consist of at least two types of cell
populations: cancer stem cells (CSCs) and adult stem cells. CSCs
exhibit all of the characteristics of stem cells: the capability of
self-renewal, unlimited proliferation potential, multi-line
differentiation and the capability to form new cancer stem cells
and adult cancer stem cells through asymmetric division. It has
been hypothesized that CSCs play a role in tumor chemoresistance.
They have also been termed cancer initiating cells as they retain
the ability to form colonies, and are also responsible for tumor
recurrence and refractoriness (3–9). It
has been suggested that colorectal cancer is caused by an
alteration in the normal homeostasis of stem cells (Fig. 1).
The standard procedure for isolating circulating
stem cells involves cell sorting of the cell subpopulation based on
the presence of cell surface markers. Many of these surface markers
have been reported to be present in CSCs. The expression of these
markers change depending on the organ that is consider. One of the
putative markers highly specific in colon cancer is CD133 (also
known as prominin-1). The CD133 gene is located on chromosome
4p15.32 and encodes a cell surface glycoprotein consisting of 5
transmembrane domains and 2 large glycosylated extracellular loops.
Yet, the function of CD133 has not been established to date. An
association between levels of CD133 and the prognosis of colorectal
cancer patients have been established. Several authors have
reported that high expression of CD133 is associated with poor
prognosis and distant metastasis, while another study indicates it
is an independent factor. Initially identified in hematopoietic
stem cells, CD133 is a shared cancer stem cell marker in multiple
solid tumors including brain, breast, lung, liver, prostate,
pancreas, medulloblastoma and melanoma (10–24).
c-Kit, also called KIT, CD117 or c-Kit receptor, is
a cytokine receptor expressed on the surface of several types of
stem cells. c-Kit was first identified as the cellular homolog of
the feline sarcoma viral oncogene V-kit. Altered forms of this
receptor may be associated with certain types of cancer. This
protein is a type 3 transmembrane receptor for mast cell growth
factor, also known as stem cell factor (MGF). The kit gene encodes
the human homolog of the proto-oncogene c-Kit. Mutations in this
gene are associated with gastrointestinal stromal tumors,
testicular seminoma, mast cell disease, melanoma, acute myeloid
leukemia, and benign disease such as piebaldism. Multiple
transcript variants encoding different isoforms have been found for
this gene (25).
OCT4 is a commonly used synonym for POU5F1 (POU
class 5 homeobox 1). It is a homeodomain transcription factor of
the POU family. This protein is critically involved in the
self-renewal of undifferentiated embryonic stem cells; thus, it is
commonly used as a marker of undifferentiated cells. OCT4
expression must be strictly regulated; excess or default causes
cell differentiation. The OCT4 transcription factor is initially
active as a maternal factor in the oocyte but remains active in
embryos throughout the preimplantation period. OCT4 expression is
associated with the undifferentiated phenotype and tumors. OCT4 can
form a heterodimer with SOX2; thus, these two proteins bind DNA
together (26,27).
OCT4 and SOX2 transcription factors are essential
for normal pluripotent cell development and maintenance. They are
also known as reprogramming genes, inducing an embryonic stem
cell-like state. SOX2 plays a critical role in cell fate
determination, differentiation and proliferation; is involved in
cancer events such as invasion and metastasis, and plays a role in
conferring a low differentiation phenotype in tumors. TWIST1, a
master regulator of embryonic morphogenesis, plays an essential
role in metastasis. Ectopic expression of TWIST1 results in loss of
E-cadherin-mediated cell-cell adhesion, activation of
mesenchymal markers, and induction of cell motility, suggesting
TWIST contributes to metastasis by promoting an
epithelial-mesenchymal transition (EMT) (28–30).
For epithelial tumors, EMT is consider to be a
crucial event in the metastatic process, which involves the
disruption of epithelial cell homeostasis and the acquisition of a
migratory mesenchymal phenotype allowing cells to travel to the
site of metastasis formation without being affected by conventional
treatment. Assuming metastasis requires dissemination of tumor stem
cells showing EMT, it seems likely that this type of cell is
detectable among circulating tumor cells found in cancer patients
(31).
The objective of our study was to determine the
expression of markers of CSCs in circulating tumor cells (CTCs) in
the peripheral blood of colon cancer patients, and assess the
pathway expression at various time points during treatment in
respect to the response of the disease to chemotherapy.
Materials and methods
We enrolled 29 metastatic colorectal cancer
patients. Eligibility patient criteria were as follows: age ≥18
years of age, clinical stage IV based on the International TNM
classification (40), performance
status of 0–2, and the presence of no other malignancies. All of
the patients were treated with fluoropyrimides. A second group of
16 healthy volunteers, matched for age, were used as the control
group. All patients and volunteers signed informed consent forms.
This study was approved by the Ethics Committee for Clinical
Research of Galicia, Spain (CEIC Galicia: 2010/238).
Samples of EDTA blood (10 ml) were collected for
detection of circulating CSC markers. Blood samples were evaluated
for expression of c-Kit, CD133, SOX2, OCT4 and TWIST1 before and
during chemotherapy.
In the patient group, samples were collected at 3
different time points during therapy: prior to chemotherapy; before
the second cycle of treatment, and finally before the fifth cycle
of treatment. The patient response was evaluated following the
Response Evaluation Criteria In Solid Tumors (RECIST 1.1) (39) and was confirmed by computed
tomography (CT) according to 4 categories: complete response (CR),
partial response (PR), stable disease (ED) and progressive disease
(PD).
Samples were centrifuged at 2,000 × g, for 10 min;
the serum phase was separated and frozen at −80°C. Buffy coat was
collected and processed with lysis (ammonium chloride, Tris,
ddH2O) and washed with PBS. The dry pellet was
maintained at −80°C until RNA isolation. RNA was purified using the
QIAamp RNA Blood Mini kit (Qiagen Inc., USA), according to the
manufacturer’s instructions. Complementary DNA (cDNA) was
synthesized with random hexamer primers (Deoxynucleoside
Triphosphate Set; Roche, Germany) with 10 mM MgCl2, MuLV
reverse transcriptase, PCR buffer, RNAse inhibitor and random
hexamers (Applied Biosystems, USA). The resulting cDNA was stored
at −20°C until further use.
Quantitative RT-PCR analysis was carried out with
SYBR-Green Master Mix (Applied Biosystems), using Applied
Biosystems 7500 Real-Time PCR system according to the
manufacturer’s instructions. Primers for c-Kit and GAPDH were
designed with Vector NTI Advance™ 11 (Invitrogen), and primers for
CD133, SOX2, OCT4 and TWIST1 were designed with Primer3 software
(version 0.4.0; Biology Workbench) (Table I). To avoid the influence of genomic
contamination, forward and reverse primers for each gene were
located in different exons. PCR was performed using a final volume
of 20 μl with SYBR-Green PCR Master Mix, using 1 μl cDNA. Cycling
conditions consisted of 95°C for 10 min, followed by 40 cycles at
95°C for 15 sec and 60°C for 1 min each. A melting curve was then
obtained.
 | Table ISequences of the specific primers
designed to perform RT-PCR for the stem cell markers. |
Table I
Sequences of the specific primers
designed to perform RT-PCR for the stem cell markers.
| | Primer sequences |
|---|
| c-Kit | Sense |
5′-GATGGCACCTGAAAGCATTT-3′ |
| Antisense |
5′-TCCGGAAGCCTTCCTTGATC-3′ |
| GAPDH | Sense |
5′-AGGTCATCCATGACAACTTTG-3′ |
| Antisense |
5′-TTCAGCTCAGGGATGACCTT-3′ |
| CD133 | Sense |
5′-TTTCCAAATCCAGGAGCAAC-3′ |
| Antisense |
5′-ACAGGAAGGGAGGGAGTCAT-3′ |
| SOX2 | Sense |
5′-ACCAGCGCATGGACAGTTAC-3′ |
| Antisense |
5′-TCATGTAGGTCTGCGAGCTG-3′ |
| OCT4 | Sense |
5′-AGTGAGAGGCAACCTGGAGA-3′ |
| Antisense |
5′-ACACTCGGACCACATCCTTC-3′ |
| TWIST1 | Sense |
5′-ACTGGCCTGCAAAACCATAG-3′ |
| Antisense |
5′-TGCATTTTACCATGGGTCCT-3′ |
Relative gene expression levels were determined
using the quantitative curve method. Quantitative normalization of
cDNA in each sample was performed using GAPDH gene expression as an
internal control. Target gene messenger RNA (mRNA) levels were
expressed as ratios to GAPDH mRNA levels. RT-PCR assays were
carried out in duplicate for each sample, and the mean value was
used for calculation of the mRNA expression levels.
Data are presented as median, range and mean ±
standard deviation. Significance between two groups was determined
by an unpaired two-tailed Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Spearman’s correlation coefficient non-parametric measure of
statistical dependence between two variables was used; a perfect
Spearman correlation of +1 or −1 occurs when each of the variables
is a perfect monotone function of the other. All statistical
analyses were carried out using GraphPad Prism 5.03 software.
Results
Patient characteristics
We evaluated the expression of CSC markers in the
peripheral blood of 29 colon cancer patients. The patients had
stage IV disease and consisted of 16 males and 13 females with a
median age of 63 years (range 42–85). Three patients dropped out of
the study due to disease progression.
Distribution of the localization of the tumors is as
follows: 6 were situated in the ascending colon, 2 were in the
transverse colon, 6 were in the descending colon, 5 were in the
sigmoid colon, 8 were in the rectum, and the sites was unknown for
2 patients diagnosed at other hospitals. All of the cases were
adenocarcinomas: 9 well differentiated, 19 moderately
differentiated and 1 poorly differentiated.
The control group included 16 volunteers with a
median age of 60 years (range 42–83).
We analyzed the CEA values at three different time
points during the sample collection, but no statistical differences
were noted, although a reduction in the levels of CEA was noted
when patients demonstrated a response to chemotherapy.
Expression of the various markers
DNA obtained from cells isolated from the peripheral
blood samples was analyzed by RT-PCR. The signal expression of stem
cell markers, c-Kit, CD133, SOX2, OCT4 and TWIST1, in the patients
were compared with the healthy control group.
The expression of the markers was also assessed in
colon tumor tissue samples, following the same procedure to obtain
DNA for RT-PCR analysis.
When we compared the patients not yet treated with
chemotherapy with the controls, significant differences in the
expression of CD133, SOX2, OCT4 and TWIST1 (p=0.0002; p=0.0182;
p=0.0217; p=0.0031, respectively) (Fig.
2) were noted. Expression of c-Kit was not significantly
different.
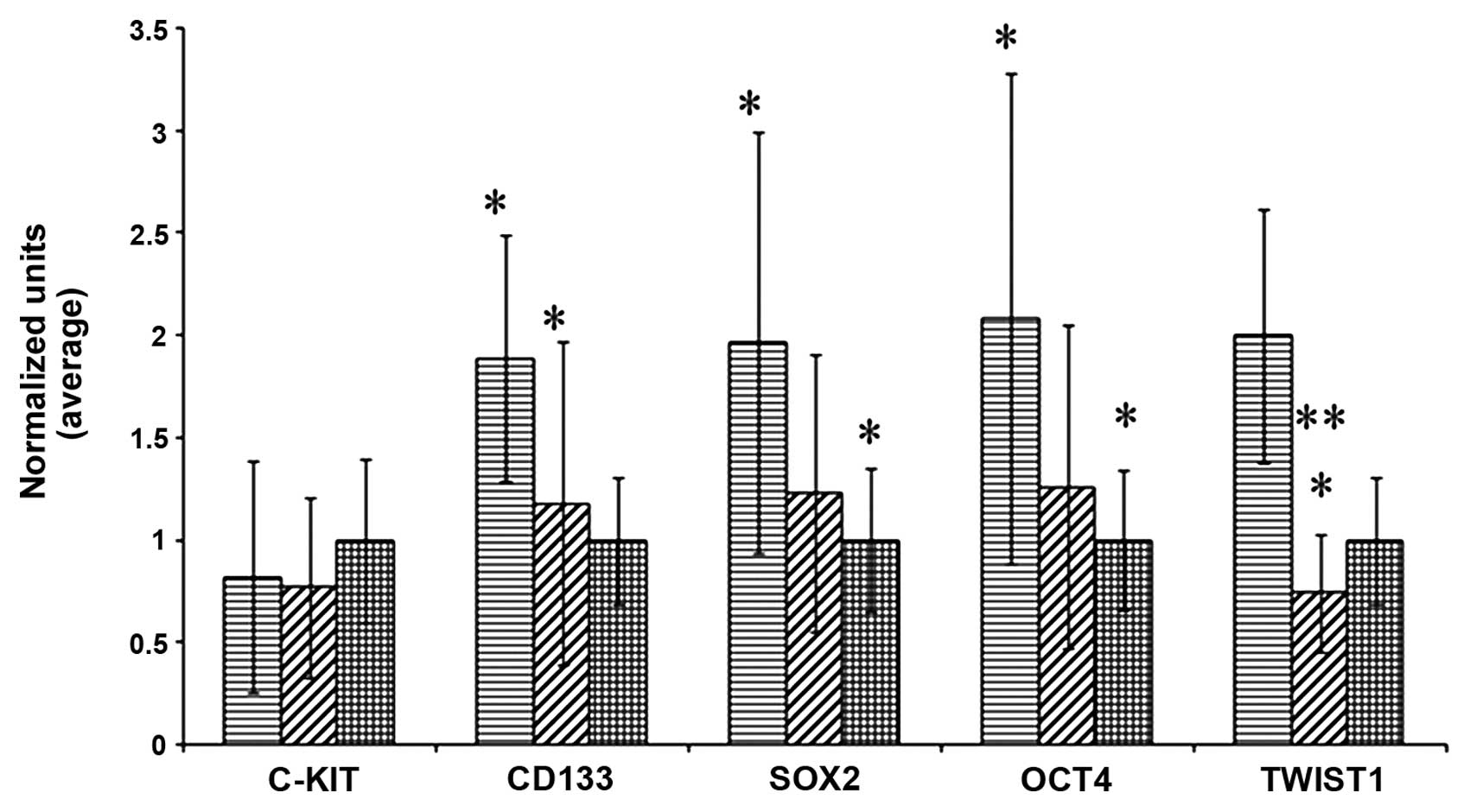 | Figure 2Expression of c-Kit, CD133, SOX2, OCT4
and TWIST1 in the DNA of cells isolated from the peripheral blood
of patients prior to chemotherapy (columns with horizontal lines,
left) and following the first dose of chemotherapy (columns with
oblique lines, middle), compared with healthy donors (columns with
squares, right). Prior to chemotherapy, apart from c-Kit, all of
the markers demonstrated differences between the patients and
controls; levels were higher than in the healthy donors, but they
were reduced after treatment. There were no significant differences
between the controls and patients who received one dose of
chemotherapy. When comparing patients before and after
chemotherapy, the levels of markers were significantly reduced, in
particular TWIST1. Data were normalized to establish the
comparison. *Indicates differences with statistical
significance between patients and controls. ***Indicates
differences with statistical significance between patients and
controls at P<0.001. |
When we analyzed the same marker levels in the
peripheral blood in the patients following administration of the
first cycle of chemotherapy and prior to the administration of the
second, the results obtained were similar. There were significant
differences in the expression levels of the same markers: CD133
(p=0.008), SOX2 (p=0.036), OCT4 (p=0.04) and TWIST1 (p=0.0002). No
difference was found in c-Kit expression (Fig. 2).
We next divided the patients according to their
response to treatment. There were two groups: one group included
patients with no change, and another group exhibited PR or CR to
treatment. We found differences in the expression pattern of the
markers in the patients ‘in progression’ compared to those ‘with
response’. In the patient with tumor progression, we found
differences in the levels of all markers, including c-Kit. The
differences were significant: for c-Kit (p=0.0046), for CD133
(p=0.0001), for SOX2 (p=0.0042), for OCT4 (p=0.0094) and for TWIST1
(p<0.001). In the patient group with no progression, there were
no differences in expression levels when compared with the controls
(Fig. 3).
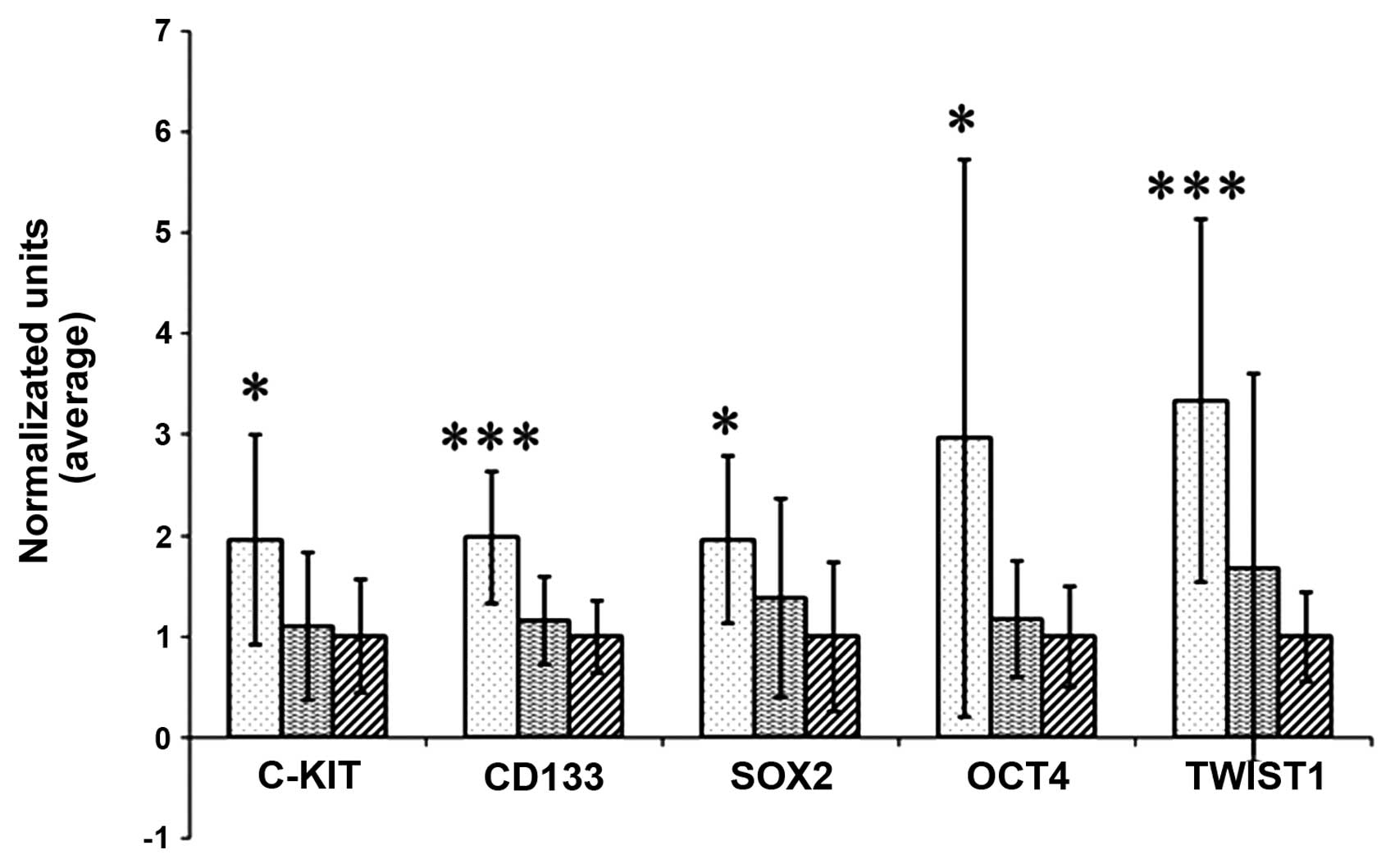 | Figure 3Expression of c-Kit, CD133, SOX2, OCT4
and TWIST1 in the DNA of cells isolated from the peripheral blood
of patients prior to chemotherapy (column with dots, left) and
after the fourth dose of chemotherapy (column with horizontal
lines, middle), compared with healthy controls (columns with
oblique lines, right). The levels of all markers were increased in
patients with disease progression when compared with the controls,
indicating no response to chemotherapy and tumor spread. Notable
were the levels of CD133 and TWIST1, a colon stem cell marker and
epithelial-mesenchymal transition marker, respectively. Conversely,
there were no differences between the levels of markers between
patients responsive to chemotherapy and the controls. Data were
normalized to establish the comparison. *Indicates
differences with statistical significance between patients and
controls. ***Indicates differences with statistical
significance between patients and controls at P<0.001. |
In addition to the comparison between patients and
controls, we compared the different groups of patients in regards
to response to therapy.
When comparing patients prior to chemotherapy with
patients who had tumor progression after 4 cycles of treatment,
differences only in the expression of TWIST1 (p<0.0001) were
noted. When we compared the patients prior to treatment with those
showing a response after 4 cycles of chemotherapy, differences in
the levels of CD133 (p=0.0002) and OCT4, (p=0.0073) were noted.
Comparing patients following administration of 4
cycles of treatment with those who were administered only one dose,
the patient group with tumor progression had high expression levels
of all the markers with significant P-values [c-Kit (p=0.0011),
CD133 (p=0.01), SOX2 (p=0.02), OCT4 (p=0.04)and TWIST1
(p<0.0001)]. In the case of the group who showed respond to
therapy, there were no significant differences.
Finally, we compared the patients after the fourth
cycle of chemotherapy with tumor progression and without
progression; expression of all markers demonstrated significant
differences excluding SOX2.
Correlation between markers
After evaluation of marker expression, we applied
the Spearman’s test to ascertain the correlation between them. In
the patients prior to chemotherapy, we found a negative correlation
between c-Kit and SOX2 (r=0.5, p=0.03), while positive correlations
were noted between CD133 and SOX2 (r=0.6, p=0.01), CD133 and OCT4
(r=0.6, p=0.01), CD133 and TWIST1 (r=0.9, p=0.002), and SOX2 and
OCT4 (r=0.8, p=0.0003).
In the patients who received one dose of
chemotherapy a correlation was noted between CD133 and SOX2, OCT4
and TWIST1, with the following values: CD133 and SOX2 (r=0.55,
p=0.04); CD133 and OCT4 (r=0.88, p<0.0001), SOX2 and OCT4
(r=0.65, p=0.01). Finally, there was no correlation between TWIST1
and CD133, TWIST1 and SOX2, TWIST1 and OCT4 and between c-Kit and
the rest of the markers.
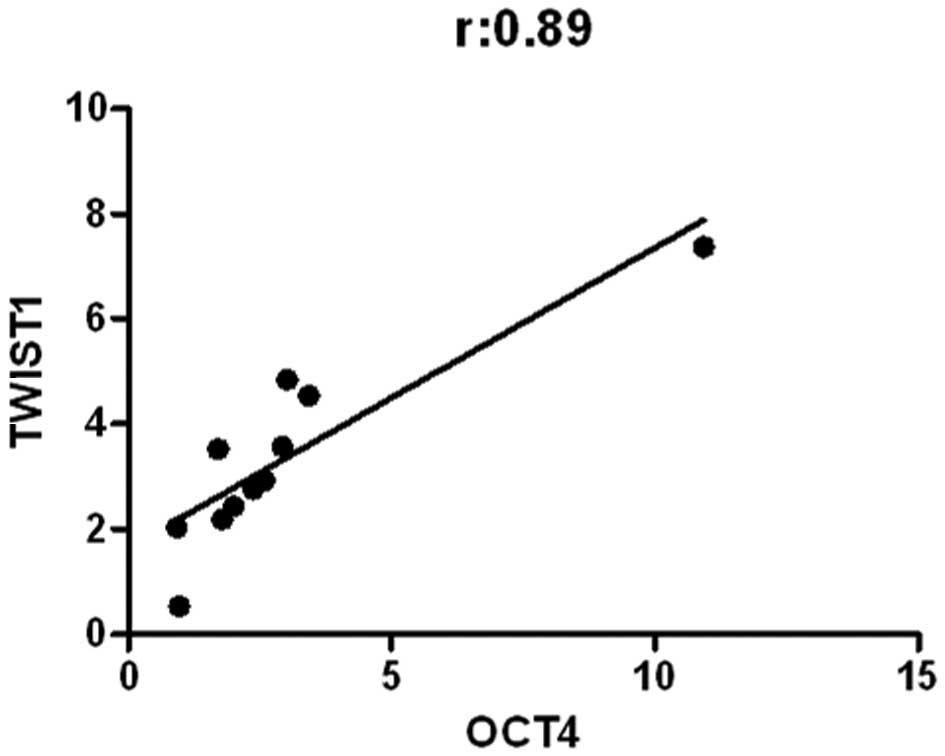
In the patients who received 4 cycles of
chemotherapy with tumor progression, a positive correlation was
noted only between OCT4 and TWIST1 (r=0.89, p=0.0002) (Fig. 4). In the same patient group but
without tumor progression, a significant correlation was found
between c-Kit and CD133 (r=0.55, p=0.001), c-Kit and OCT4 (r=0.47,
p=0.04), c-Kit and TWIST1 (r=0.49, p=0.03), CD133 and OCT4 (r=0.53,
p=0.02), CD133 and TWIST1 (r=0.61, p=0.006), OCT4 and TWIST1
(r=0.68, p=0.001).
Discussion
In the present study, we found significant
differences in the levels of various stem cell markers (CD133,
SOX2, OCT4 and TWIST1) correlated with colon cancer behavior. There
was a reduction in expression when the tumors were responsive to
the chemotherapy, and in contast, increased levels were noted when
there was no response to treatment. Thus, the expression of these
markers predicted the behavior of the tumor.
Research has focused on the study of tumor stem
cells, in particular, in regards to the expression of markers to
facilitate the identification of putative stem cells in tumors.
Most of them are developed in tissue from a surgical tumor
resection using a histological study. According to the cancer stem
cell theory, CSCs are initiators of metastasis. Thus, our objective
was to isolate them in peripheral blood. This procedure was used
previously, and it affords the advantages of simplicity and easy
accessibility to patient samples, without biopsy. RT-PCR has been
widely used for the detection of CSCs in the peripheral blood for a
variety of cancer types. RT-PCR is extremely sensitive, less
subjective and can be automated, but its specificity depends on the
cancer marker used (32–34).
In the present study, expression of c-Kit and CD133
was found in primary tumors, and CD133 expression was consistently
higher in all colon cancer cases as compared to the controls, thus
ruling out the possibility that CD133+ cells came from
bone marrow. This difference in expression could be used as a
biomarker, since the level in patients was almost 2-fold when
compared to that in the controls. The level was decreased in
patients responsive to treatment, whereas in patients exhibiting
disease progression an increase in levels was noted, due to an
increase in the cell numbers in the tumor. There are many putative
markers of cancer stem cells. Expression of these markers change
depending on the specific tumor. Yet, at present, there is no doubt
that CD133 is an effective stem cell marker for colon cancer. There
are discrepancies concerning the pattern and frequency of CD133
expression in colon cancer. Horst et al(13) and Kojima et al(14) reported that CD133 expression is
localized on glandular-luminal surface of colorectal cancer.
However, Chun-Yan et al(8)
showed that CD133 expression is located not only on the apical
membrane but also on the basal surface of tumor cells, both in the
invasive front. Nevertheless, we must consider the heterogeneous
pathways and that CD133 expression levels are variable depending on
the study.
Our data demonstrated that levels of SOX2 and OCT4
were highly correlated in patients and their trend was similar to
CD133. It is well known that SOX2 and OCT4 are associated with the
maintenance of stem cells, and play a critical role in the
determination, differentiation and proliferation of stem cells,
explicating the correlation between them and CD133. This positive
correlation means that there is an increase in the number of stem
cells and all the mechanisms implicated in proliferation and
maintenance are activated (28,29–35).
The levels of the three markers, CD133, SOX2 and
OCT4, were higher than in the controls in all cases, indicating
that this was not due to maintenance of the physiological stem
cells and is in relation with circulating cancer stem cells.
Furthermore, their levels were reduced in those patients who were
responsive to treatment, and increased in patients who were not
responsive. We also observed a statistically significant
correlation of the three markers at all sample collection points.
This was in accordance with Saigusa et al(36) who reported that expression of the 3
genes (CD133, SOX2 and OCT4) was identified in residual CSCs and
was associated with distal recurrences and poor disease-free
survival which is linked to cancer cells that survive radical
treatment, in support of the theory of metastasis due to stem
cells. Our data indicate that CD133, OCT4, SOX2 had a predictive
value for colon cancer behavior, and the expression may be useful
for predicting the evolution of the treatment process.
Similar results were found for TWIST1 which
regulates EMT in vertebrates. TWIST1 is highly expressed in several
tumor types and its upregulation is considered an unfavorable
prognostic factor in colorectal cancer. We demonstrated that the
levels of TWIST1 exhibited the same trend as that of the other
markers, but compared to all the markers, it indicated a favorable
response to treatment. This suggests that it is a good indicator of
disease progression and response to therapy. Furthermore, TWIST2
was highly correlated with the other markers. This indicated that
EMT occurs in stem cells in order to facilitate circulation in the
peripheral blood, which is a protective mechanism for transit to
distant organs. OCT4 and SOX2 are required for their maintenance,
and once the cells reached a distant site they again began to show
more specific markers. TWIST1 had predictive value for colon cancer
behavior, and its correlation with OCT4 and the other markers may
be useful for predicting the evolution of the treatment process
(37).
The gene c-Kit, or stem cell factor, was the only
marker exhibiting a different trend. In patients prior to treatment
and after one dose of chemotherapy, the levels of c-Kit were lower
in the patients when compared with that in the controls, while
after the fourth dose of treatment the levels increased. This
suggests that expression of c-Kit in colon tumors is rare; thus, it
is not a marker of choice in the colon. These findings are in
accordance with Friederichs et al(38). Yet, the increased expression after
several dose of chemotherapy can indicate a change in receptor
expression pathway of stem cells, or an alteration in the
tyrosine-kinase receptors.
In conclusion, expression of CD133, OCT4, SOX2 and
TWIST1 had a predictive value for colon cancer behavior. Evaluation
of the expression of these stem cell gene markers may be useful for
predicting the evolution of the treatment process, and the relative
easy access to samples facilitates this method. Moreover, the
correlation between CD133 and TWIST1 expression may be associated
with tumor recurrence and metastatic relapse. These findings open
new lines of research for the development of techniques for the
study of circulating cancer stem cells and have implications in the
research of the progression of colon cancer and have predictive
value in translational oncology.
References
|
1
|
Howlader N, Noone AM, Krapcho M, Neyman N,
Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z,
Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin
KA and Edwards BK: SEER Cancer Statistics Review, 1975–2008.
National Cancer Institute; Bethesda, MD: 2011
|
|
2
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar
|
|
3
|
Liao Y, Hu X, Huanq X and He C:
Quantitative analyses of CD133 expression facilitate researches on
tumor stem cells. Biol Pharm Bull. 33:738–742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boman BM, Wicha MS, Fields JZ, et al:
Symmetric division of cancer stem cells - a key mechanism in tumor
growth that should be targeted in future therapeutic approaches.
Clin Pharmacol Ther. 81:893–898. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boman BM, Fields JZ, Cavanaugh KL, Guetter
A and Runquist OA: How dysregulated colonic crypt dynamics cause
stem cell overpopulation and initiate colon cancer. Cancer Res.
68:3304–3313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morrison SJ and Kimble J: Asymmetric and
symmetric stem-cell divisions in development and cancer. Nature.
441:1068–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnston MD, Edwards CM, Bodmer WF, Maini
PK and Chapman SJ: Mathematical modeling of cell population
dynamics in the colonic crypt and in colorectal cancer. Proc Natl
Acad Sci USA. 104:4008–4013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li CY, Li BX, Liang Y, et al: Higher
percentage of CD133+ cells is associated with poor
prognosis in colon carcinoma patients with stage IIIB. J Transl
Med. 7:562009.PubMed/NCBI
|
|
9
|
Huff CA, Matsui WH, Smith DB and Jones RJ:
Strategies to eliminate cancer stem cells: clinical implications.
Eur J Cancer. 42:1293–1297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klonisch T, Wiechec E, Hombach-Klonisch S,
Ande SR, Wesselborg S, Schulze-Osthoff K and Los M: Cancer stem
cell markers in common cancers - therapeutic implications. Trends
Mol Med. 14:450–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shmelkov SV, Butler JM, Hooper AT, et al:
CD133 expression is not restricted to stem cells, and both
CD133+ and CD133− metastatic colon cancer
cells initiate tumors. J Clin Invest. 118:2111–2120.
2008.PubMed/NCBI
|
|
12
|
Saigusa S, Tanaka K, Toiyama Y, et al:
Clinical significance of CD133 and hypoxia inducible factor-1α gene
expression in rectal cancer after preoperative chemoradiotherapy.
Clin Oncol (R Coll Radiol). 23:323–332. 2011.PubMed/NCBI
|
|
13
|
Horst D, Kriegl L, Engel J, Kirchner T and
Junq A: Prognostic significance of the cancer stem cell markers
CD133, CD44, and CD166 in colorectal cancer. Cancer Invest.
27:844–850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kojima M, Ishii G, Atsumi N, Fujii S,
Saito N and Ochiai A: Immunohistochemical detection of CD133
expression in colorectal cancer: a clinicopathological study.
Cancer Sci. 99:1578–1583. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boman BM and Wicha MS: Cancer stem cells:
a step toward the cure. J Clin Oncol. 26:2795–2799. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin AH, Miraglia S, Zanjani ED, et al:
AC133, a novel marker for human hematopoietic stem and progenitor
cells. Blood. 90:5002–5012. 1997.PubMed/NCBI
|
|
19
|
Zeki SS, Graham TA and Wright NA: Stem
cells and their implications for colorectal cancer. Nat Rev
Gastroenterol Hepatol. 8:90–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lau CK, Yang ZF and Fan ST: Role of stem
cells in normal liver and cancer. Anticancer Agents Med Chem.
11:522–528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monzani E, Facchetti F, Galmozzi E, et al:
Melanoma contains CD133 and ABCG2 positive cells with enhanced
tumourigenic potential. Eur J Cancer. 43:935–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eramo A, Haas TL and De Maria R: Lung
cancer stem cells: tools and targets to fight lung cancer.
Oncogene. 29:4625–4635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma S, Chan KW, Hu L, et al: Identification
and characterization of tumorigenic liver cancer stem/progenitor
cells. Gastroenterology. 132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maitland NJ and Collins AT: Cancer stem
cells - A therapeutic target? Curr Opin Mol Ther. 12:662–673.
2010.PubMed/NCBI
|
|
25
|
Edling CE and Hallberg B: c-Kit - a
hematopoietic cell essential receptor tyrosine kinase. Int J
Biochem Cell Biol. 39:1995–1998. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niwa H, Miyazaki J and Smith AG:
Quantitative expression of Oct-3/4 defines differentiation,
dedifferentiation or self-renewal of ES cells. Nat Genet.
24:372–376. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Looijenga LH, Stoop H, de Leeuw HP, et al:
POU5F1 (OCT3/4) identifies cells with pluripotent potential in
human germ cell tumors. Cancer Res. 63:2244–2250. 2003.PubMed/NCBI
|
|
28
|
Tsukamoto T, Mizoshita T, Mihara M, et al:
Sox2 expression in human stomach adenocarcinomas with gastric and
gastric-and-intestinal-mixed phenotypes. Histopathology.
46:649–658. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia X, Li X, Xu Y, et al: SOX2 promotes
tumorigenesis and increases the anti-apoptotic property of human
prostate cancer cells. J Mol Cell Biol. 3:230–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Casas E, Kim J, Bendesky A, Ohno-Machado
L, Wolfe CJ and Yang J: Snail2 is an essential mediator of
Twist1-induced epithelial mesenchymal transition and metastasis.
Cancer Res. 71:245–254. 2011. View Article : Google Scholar
|
|
31
|
Aktas B, Tewes M, Fehm T, Hausch S, Kimmig
R and Kasimir-Bauer S: Stem cell and epithelial-mesenchymal
transition markers are frequently overexpressed in circulating
tumor cells of metastatic breast cancer patients. Breast Cancer
Res. 11:R462009. View
Article : Google Scholar
|
|
32
|
Xi L, Nicastri DG, El-Hefnawy T, Hughes
SJ, Luketich JD and Godfrey TE: Optimal markers for real-time
quantitative reverse transcription PCR detection of circulating
tumor cells from melanoma, breast, colon, esophageal, head and
neck, and lung cancers. Clin Chem. 53:1206–1215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iinuma H, Watanabe T, Mimori K, et al:
Clinical significance of circulating tumor cells, including cancer
stem-like cells, in peripheral blood for recurrence and prognosis
in patients with Dukes’ stage B and C colorectal cancer. J Clin
Oncol. 29:1547–1555. 2011.PubMed/NCBI
|
|
34
|
Sher YP, Shih JY, Yanq PC, et al:
Prognosis of non-small cell lung cancer patients by detecting
circulating cancer cells in the peripheral blood with multiple
marker genes. Clin Cancer Res. 11:173–179. 2005.PubMed/NCBI
|
|
35
|
Ong CW, Kim LG, Kong HH, et al: CD133
expression predicts for non-response to chemotherapy in colorectal
cancer. Mod Pathol. 23:450–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saigusa S, Tanaka K, Toiyama Y, et al:
Correlation of CD133, OCT4, and SOX2 in rectal cancer and their
association with distant recurrence after chemoradiotherapy. Ann
Surg Oncol. 16:3488–3498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li QQ, Xu JD, Wanq WJ, et al:
Twist1-mediated adriamycin-induced epithelial-mesenchymal
transition relates to multidrug resistance and invasive potential
in breast cancer cells. Clin Cancer Res. 15:2657–2665. 2009.
View Article : Google Scholar
|
|
38
|
Friederichs J, von Weyhern CW, Rosenberg
R, et al: Immunohistochemical detection of receptor tyrosine
kinases c-kit, EGF-R, and PDGF-R in colorectal adenocarcinomas.
Langenbecks Arch Surg. 395:373–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Costelloe CM, Chuang HH, Madewell JE and
Ueno NT: Cancer response criteria and bone metastases: RECIST 1.1,
MDA and PERCIST. J Cancer. 28:80–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; New York: 2009
|