Introduction
The incidence rate of nasopharyngeal carcinoma (NPC)
in men is higher in rural than in urban China. NPC is the seventh
most common cancer in men in rural areas with an incidence rate of
6.08/100,000, followed by bladder cancer, brain cancer and lymphoma
(1). Despite significant advances
in the therapy and early diagnosis, the prognosis of patients with
stage III and IV NPC remains poor, usually due to a relatively high
incidence of locoregional recurrence or metastasis (2). Thus, there is an urgent need to
develop effective and safe therapeutics for this malignancy.
Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid (5F)
is an active compound in Pteris semipinnata L (PsL). In
vitro, 5F has been shown to kill various human cancer cells
including lung cancer cells, laryngeal cancer cells, thyroid
carcinoma cells, gastric cancer cells and colorectal cancer cells
via apoptotic pathways (3–7). Since 5F can induce apoptosis in cells
of various types of cancer, it may also be a potential
apoptosis-inducing drug in NPC therapy. In the present study, we
examined the potential antitumor action of 5F in a human NPC cell
line, CNE-2Z, and explored the possible synergism between 5F and
cisplatin.
Materials and methods
Cell culture
CNE-2Z is a poorly differentiated human NPC cell
line (8). CNE-2Z cells were
cultured in RPMI-1640 medium (Sigma-Aldrich) containing 10% fetal
bovine serum (FBS) (Sigma-Aldrich), 100 U/ml penicillin and 100
μg/ml streptomycin. Cells were cultured at 37°C in a humidified 5%
CO2 incubator.
Mutational analysis of p53
Genomic DNA was extracted from CNE-2Z using a
genomic DNA isolation kit (Sangon, Shanghai, China) following the
manufacturer’s instructions, and quantified using a NanoDrop
spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The
primers for PCR are listed in Table
I. A 300 ng aliquot of genomic DNA was added to a PCR master
mixture containing 1X PCR buffer (100 mM Tris-HCl, 500 mM KCl, pH
8.3), 200 μM each of deoxynucleoside triphosphate, 200 nM primer,
1.5 mM MgCl2, and 2.5 units of Taq polymerase. PCR was
performed under the following cycling conditions: 4 min at 95°C,
followed by 45 sec at 94°C, 45 sec at 68°C, 1 min at 72°C for 35
cycles and final extension for 8 min at 72°C. The PCR amplicons
were purified with Gel/PCR DNA Fragments Extraction kit (Sangon)
and sequenced with the BigDye Terminator kit (Applied Biosystems,
Foster City, CA, USA) and ABI Prism 3730 DNA Analyzer (Applied
Biosystems) according to the manufacturer’s instructions.
 | Table IPrimer sequences used to amplify p53
tumor suppressor gene. |
Table I
Primer sequences used to amplify p53
tumor suppressor gene.
| Exons | Primer sequences
(5′-3′) | Product size
(bp) |
|---|
| 2–4 | F:
GAAGTCTTGGGGATTTAGTGGT
R: CAAAAGCCAAGGAATACACG | 1192 |
| 5–6 | F:
GGTTGCAGGAGGTGCTTACG
R: GTTTCACCGTTAGCCAGGAT | 991 |
| 7 | F:
AGGCTGAGGAAGGAGAATGG
R: GGGTAGTAGTATGGAAGAAATCGG | 406 |
| 8–9 | F:
AGGGTGGTTGGGAGTAGATG
R: GCAGGCTAGGCTAAGCTATGAT | 791 |
| 10 | F:
GAGGCTGAGGCACAAGAATC
R: CCCTGGGTTTGGATGTTCTG | 601 |
| 11 | F:
GCAACAAGAGTGAAACTCCGT
R: TTACATCTCCCAAACATCCCT | 594 |
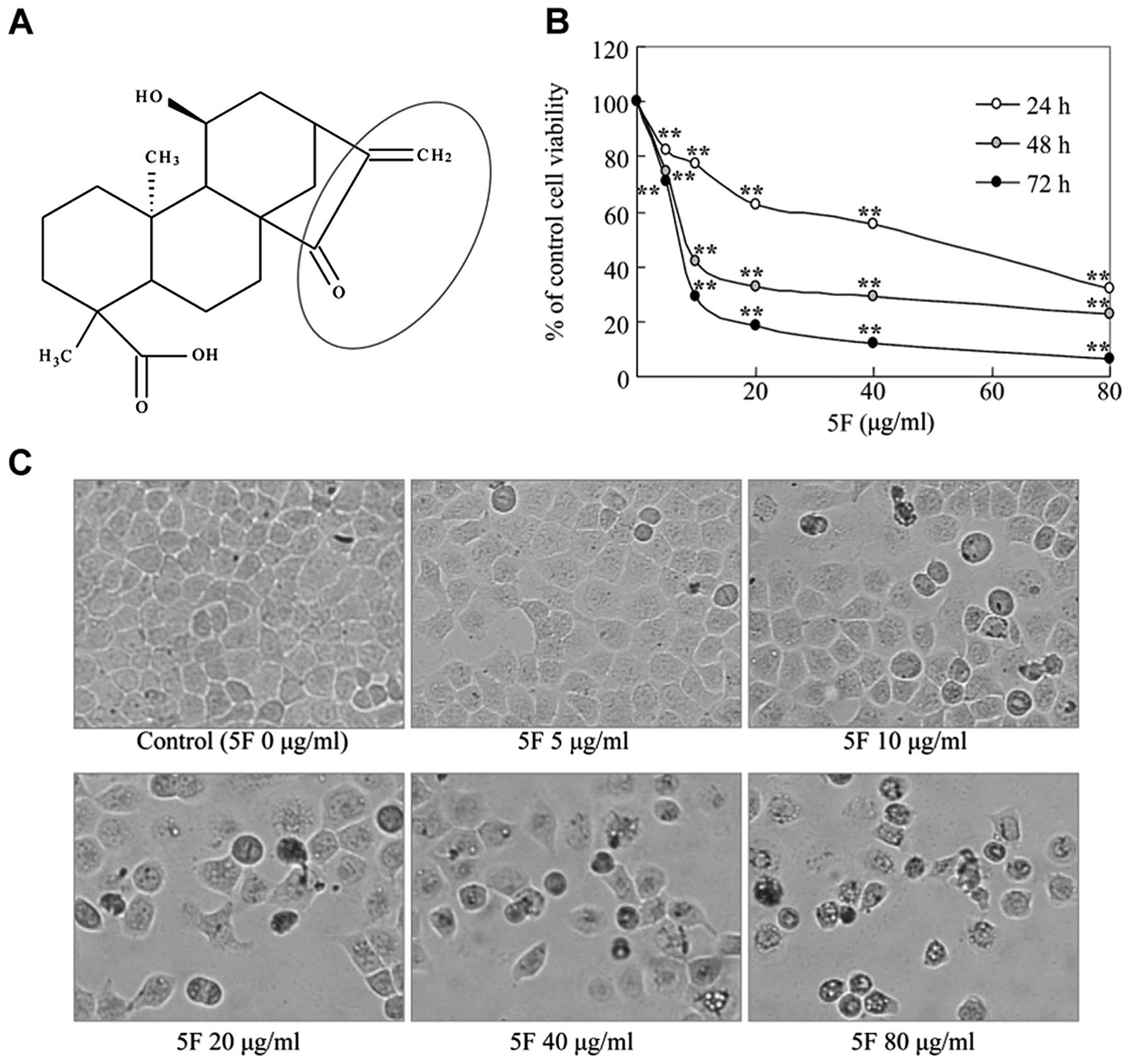
Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid (5F)
5F was isolated from PsL as previously described
(9) (Fig. 1A). 5F was dissolved in propylene
glycol (PG) and diluted with the culture medium immediately prior
to use (final PG concentration ≤1.2%). In all experiments, the
cells in RPMI-1640 medium plus PG only were used as the
control.
Cell viability assay
CNE-2Z cells (5×103) were seeded in each
well of a 96-well plate in 200 μl medium. At 24 h, the cells were
exposed to 5F (0–80 μg/ml), cisplatin (10 μg/ml), or a combination
of 5F (10 μg/ml) and cisplatin (10 μg/ml). Cell viability was
examined after an additional 24, 48 or 72 h using MTT assay. Drug
effect was expressed as percentage relative to the controls.
Morphology of the cells was examined 24 h after 5F exposure under
an inverted phase contrast microscope.
DAPI staining assay
Cells were fixed with 4% paraformaldehyde for 20
min, washed with PBS, and then incubated with DAPI (2 μg/ml)
(Beyotime Institute Biotechnology, Haimen, China) at room
temperature (RT) for 10 min. Following removal of free DAPI with
PBS, cells were observed under a fluorescence microscope.
Cell cycle distribution
CNE-2Z cells were seeded at a density of
1×105 cells/well in a 6-well plate. Following treatment,
cells were fixed overnight with 70% ethanol at −20°C and stained
with PI solution (100 μg/ml) (Sigma-Aldrich). Cell cycle
distribution analysis was performed using a flow cytometer.
Measurement of apoptosis
Following treatment, cells were harvested, washed
with PBS and resuspended in 195-μl binding buffer. The samples were
then incubated with 5 μl Annexin V-FITC for 15 min in the dark at
4°C and subjected to flow cytometry analysis immediately. Data
acquisition and analysis were performed on a Becton-Dickinson
FACSCalibur flow cytometer using CellQuest software. Caspase-3
activity was measured using Caspase-3 Colorimetric Assay kit
(Nanjing KeyGen Biotech. Co., Ltd., Nanjing, China) according to
the manufacturer’s instructions. Caspase-3 activity was expressed
as: ODtest compound/ODcontrol.
Reactive oxygen species (ROS)
generation
The intracellular ROS level was measured using a
fluorescent dye DCFH-DA (ROS Assay kit, Beyotime Institute
Biotechnology). Cells were exposed to 5F (0 and 10 μg/ml),
cisplatin (10 μg/ml), or a combination of 5F (10 μg/ml) and
cisplatin (10 μg/ml) for 3 h, and incubated in the dark with 10 μM
DCFH-DA in serum-free medium for 20 min at 37°C. ROS generation was
detected using a FACScan flow cytometer with the excitation and
emission settings at 488 and 530 nm, respectively.
Western blot analysis
Cells were lysed on ice in SDS Lysis Buffer
(Beyotime Institute Biotechnology) supplemented with protease
inhibitors and phosphatase inhibitor (Roche), and 1 mM PMSF
(Sigma). Cytoplasmic extract was obtained using a Cytoplasmic
Protein Extraction kit (Beyotime Institute Biotechnology). The
protein concentration was determined using a BCA Protein Assay kit
(Beyotime Institute Biotechnology). Immunoblots of 50 μg total
protein were probed with the following antibodies: cytochrome
c, IκB, actin (Santa Cruz Biotechnology, Santa Cruz, CA,
USA), Bcl-2, Bax (Zhongshan Golden Bridge Biotechnology, Wuhan,
China), p21 (Boster Bio-Engineering Ltd., Wuhan, China), NF-κB-p65
(Phospho-Ser536) (SAB Signalway Antibody, Pearland, TX, USA).
Protein bands were visualized using chemiluminescent reagents.
Statistical analysis
Data are presented as the means ± SD. The
differences between the groups were examined with one-way analysis
of variance (ANOVA) using the SPSS 13 software (SPSS Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
5F inhibits the proliferation of CNE-2Z
NPC cells
In vitro, 5F has been shown to inhibit cell
viability of various human cancer cells (3–7). To
test if 5F prevents the proliferation and growth of NPC cells, we
treated CNE-2Z NPC cells with different concentrations of 5F for
24, 48 or 72 h and examined the cell proliferation using a standard
MTT method. 5F treatment led to a time- and dose-dependent decrease
of the cell viability of CNE-2Z cells (Fig. 1B). Moreover, 5F-treated cells
exhibited a rounded and granulated morphology, and were detached
from culture wall after a 24-h exposure (Fig. 1C). Thus, 5F also inhibits the
proliferation and growth of CNE-2Z NPC cells.
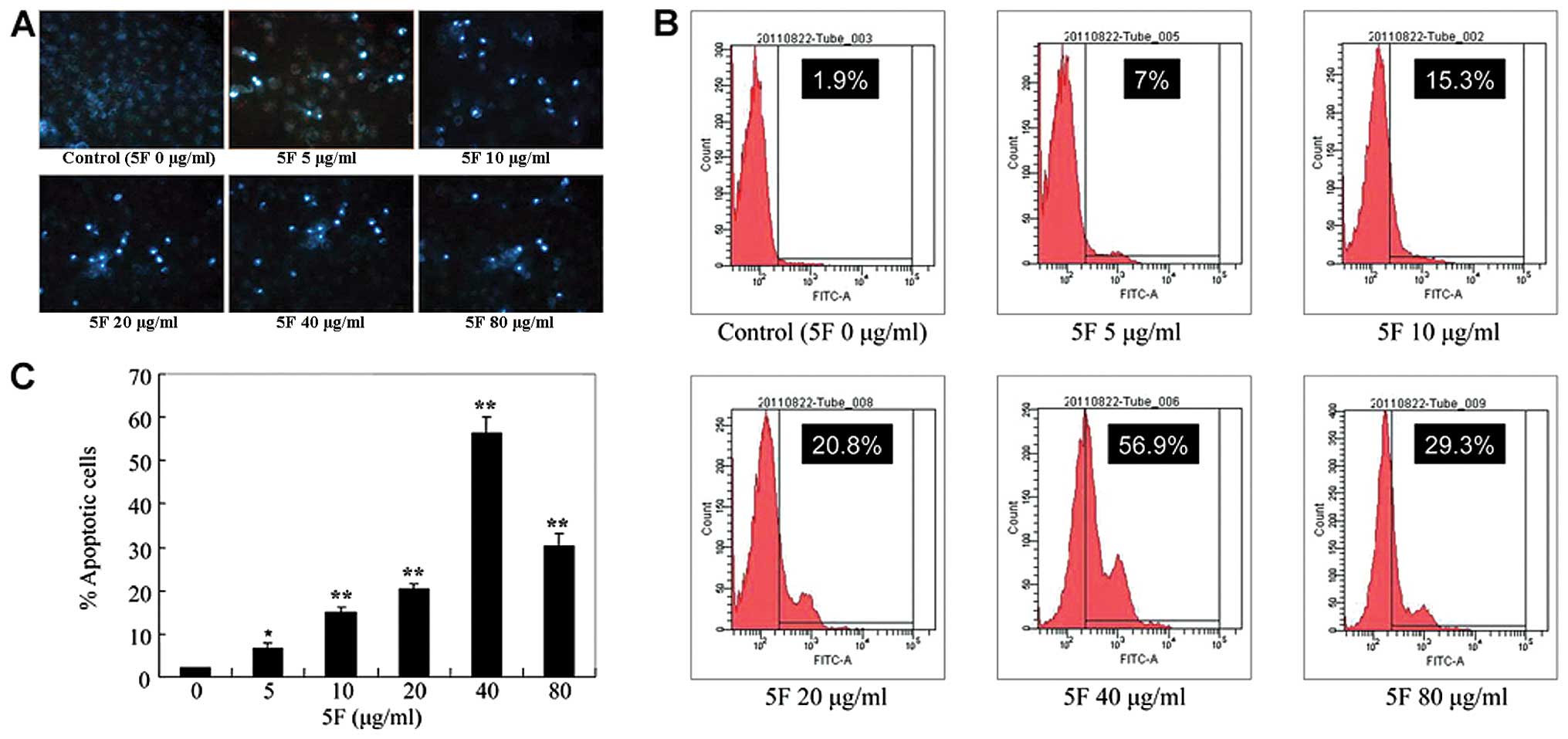
5F induces apoptosis of CNE-2Z cells
To test whether 5F inhibits the cell growth of
CNE-2Z cells by inducing apoptosis, we stained 5F-treated cells
with the fluorescent dye DAPI and observed nucleus changes under
the fluorescence microscope. Compared with untreated cells,
5F-treated cells demonstrated bright nuclear condensation, nuclear
pyknosis and apoptotic bodies, indicating that 5F induces apoptosis
in CNE-2Z cells (Fig. 2A). To
extend our observation, we further stained 5F-treated cells with
Annexin V-FITC, an early indicator of apoptosis. As shown in
Fig. 2B and C, 5F treatment of CNE-2Z cells resulted in a
5.1, 13.4, 18.9, 55 and 27.4% increase of Annexin V-positive cells
at 5, 10, 20, 40 and 80 μg/ml 5F, respectively. These results
clearly show that 5F induces significant apoptosis in CNE-2Z
cells.
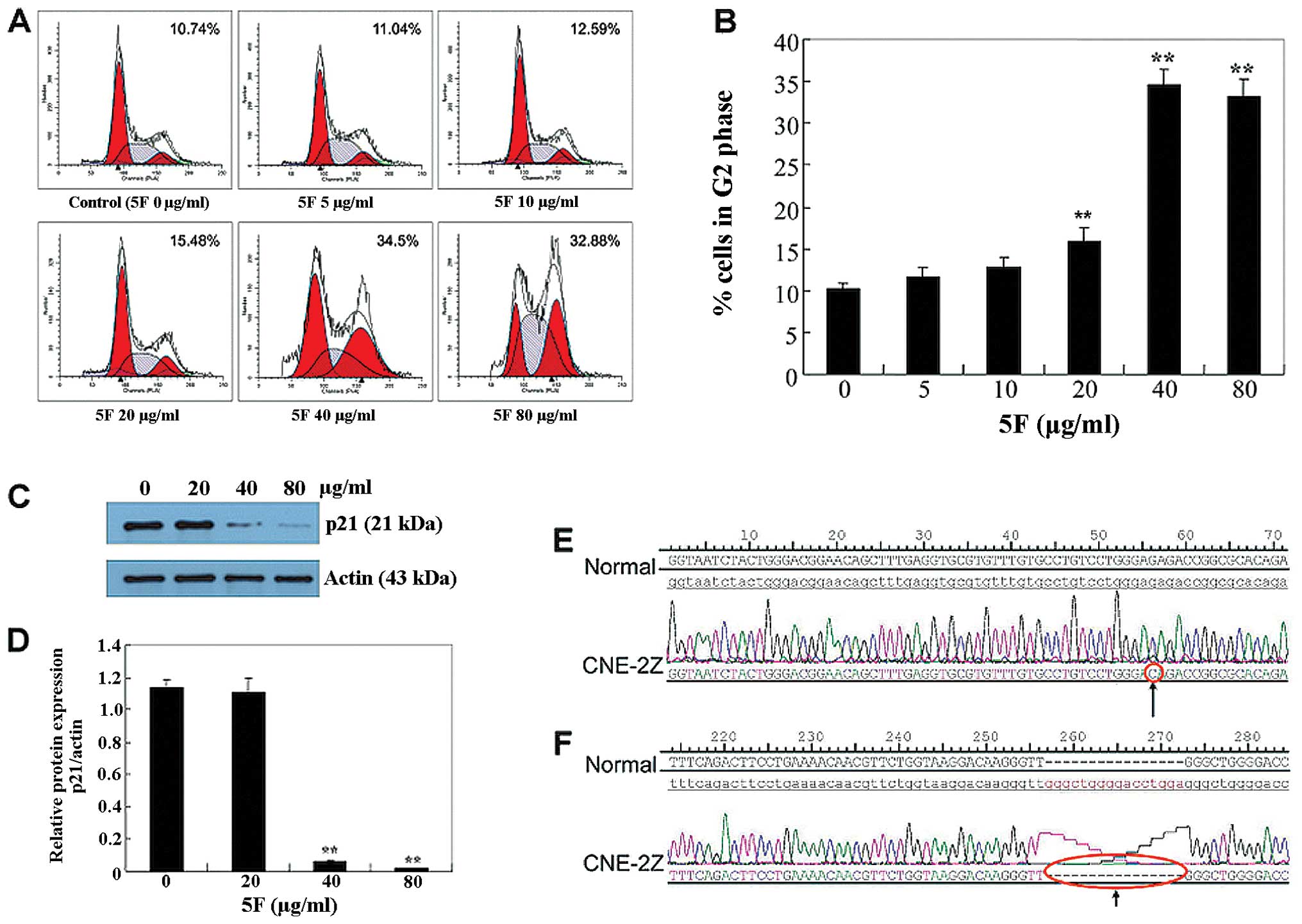
5F induces G2 cell cycle arrest in CNE-2Z
cells independently of p53-p21 axis
It has been well documented that 5F induces G2 phase
of cell cycle arrest in several cell lines. To determine whether
the growth-inhibitory effect of 5F in CNE-2Z cells is associated
with the induction of cell cycle arrest, we analyzed the
distribution of cells in the different phases of the cell cycle
using flow cytometry. Following treatment with 5, 10, 20, 40 and 80
μg/ml 5F for 24 h, the percentage of cells in the G2/M phase was
11.04, 12.59, 15.48, 34.5 and 32.88%, respectively (Fig. 3A and B), indicating that 5F induces
cell cycle arrest in the G2 phase in CNE-2Z cells.
To investigate the mechanism by which 5F causes the
G2-phase cell cycle arrest, we examined the effects of 5F on the
expression of p21, which regulates the G2-phase checkpoint
(10–12). Notably, 5F significantly reduced p21
protein levels at 40 and 80 μg/ml (Fig.
3C and D). p21 is the critical downstream transcriptional
target of p53, the reduction but not the increase of p21 by 5F
suggests that there may be a p53 mutation in CNE-2Z cells.
Therefore, we sequenced the gene of p53 of CNE-2Z cells and found
two types of changes. The first was a G-to-C change, at position 56
in exon 8 (Fig. 3E), and the other
was a deletion of GGGCTGGGGACCTGGA in the position 16–31 in intron
3 (Fig. 3F). Thereafter, the
failure to induce p21 by 5F is likely due to the loss-of-function
of p53.
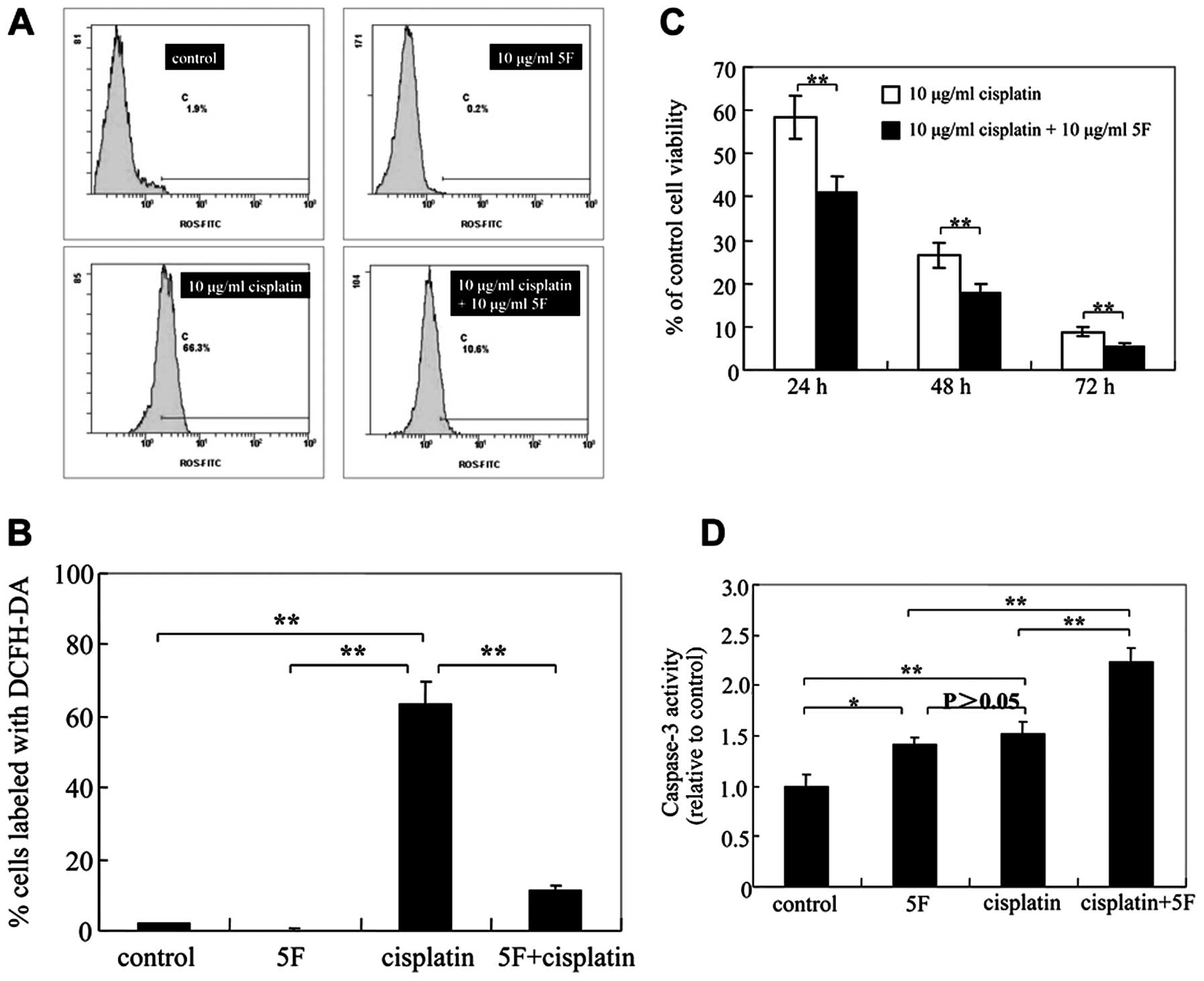
5F reduces intracellular ROS levels
ROS induces endogenous DNA damages and plays an
important role in apoptosis in a variety of cells. To test the
possibility that 5F induces cell apoptosis and G2 phase arrest by
inducing ROS generation in CNE-2Z cells, we measured intracellular
ROS levels. As shown in Fig. 4A and
B, 5F significantly decreased
ROS generation in CNE-2Z cells for 3 h. Thus, 5F reduces
intracellular ROS levels and the induction of the G2 phase might
not be due to the ROS-induced DNA damages.
5F sensitizes CNE-2Z cells to cisplatin
independently of its reduction of ROS
It has been reported that cisplatin cytotoxicity is
mediated by increased generation of ROS. Several reports have
demonstrated that cisplatin-induced cytotoxicity could be
ameliorated by using antioxidants or oxygen radical scavengers
(13–15). The reduction of ROS by 5F predicts
that 5F may attenuate cisplatin cytotoxicity. We thus determined
whether 5F antagonizes cisplatin-stimulated intracellular ROS
generation. We found that cisplatin increased ROS production
(Fig. 4A and B) and 5F reduced the ROS production in
cisplatin-treated CNE-2Z cells, whereas 5F significantly sensitized
CNE-2Z cells to cisplatin-induced cytotoxicity after 24, 48 and 72
h of treatment (Fig. 4C). Moreover,
a synergistic interaction in increasing caspase-3 activity was also
observed when 5F (10 μg/ml) was added to 10 μg/ml of cisplatin in
CNE-2Z NPC cells (Fig. 4D). Thus,
5F sensitizes CNE-2Z cells to cisplatin via the apoptosis
pathway.
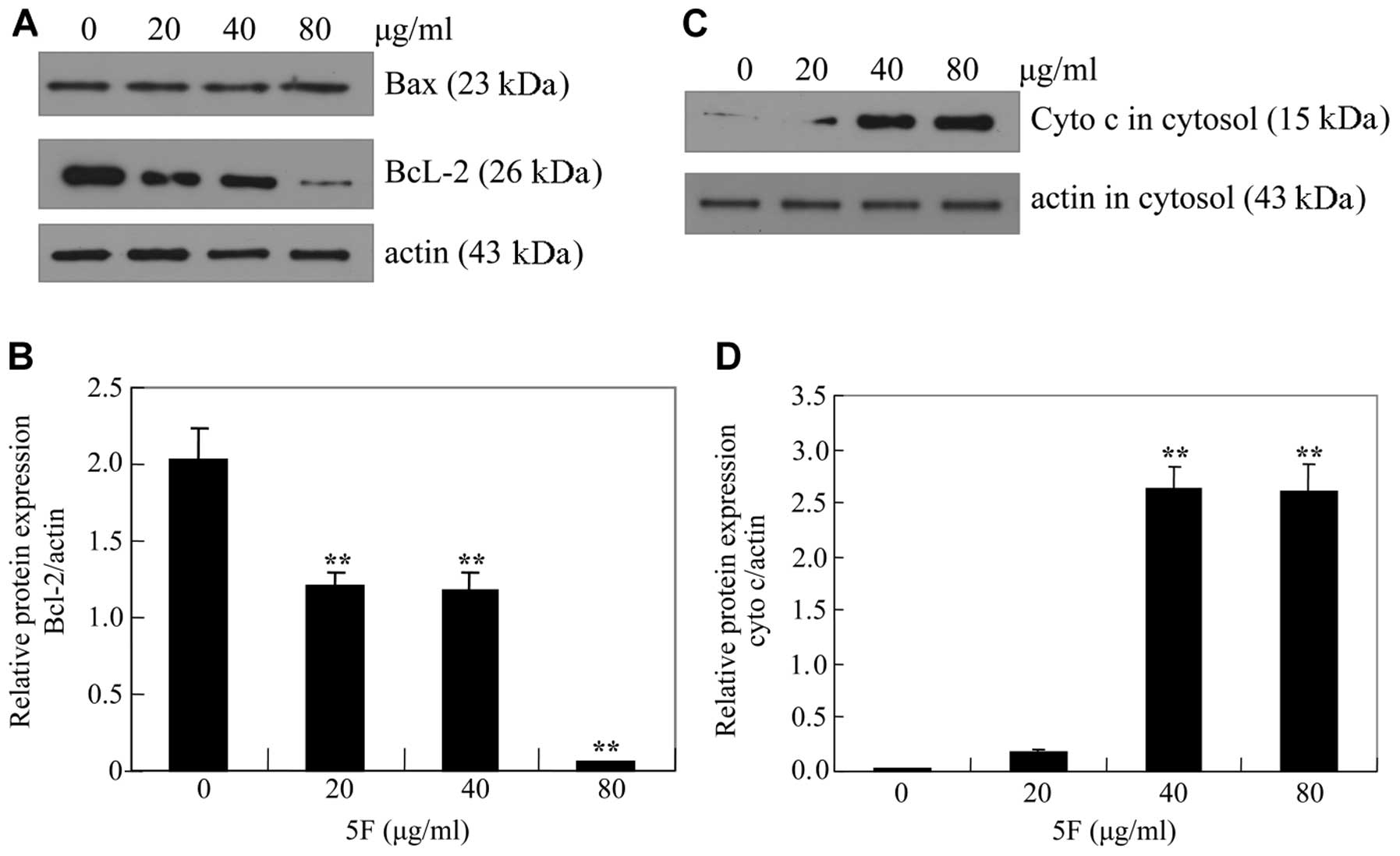
5F downregulates Bcl-2 and upregulates
IκBα
To elucidate the mechanism by which 5F induces
apoptosis in CNE-2Z cells, we measured the protein levels of Bcl-2
and Bax. Treatment of CNE-2Z cells with 5F resulted in a
dose-dependent decrease of anti-apoptotic Bcl-2 protein. By
contrast, 5F did not significantly influence the expression of the
pro-apoptotic Bax protein (Fig. 5A and
B). Accordingly, 5F induced a dose-dependent release of
cytochrome c from mitochondrion (Fig. 5C and D). These data indicated that
5F induces the activation of mitochondrial-mediated internal
apoptosis pathway by downregulating Bcl-2.
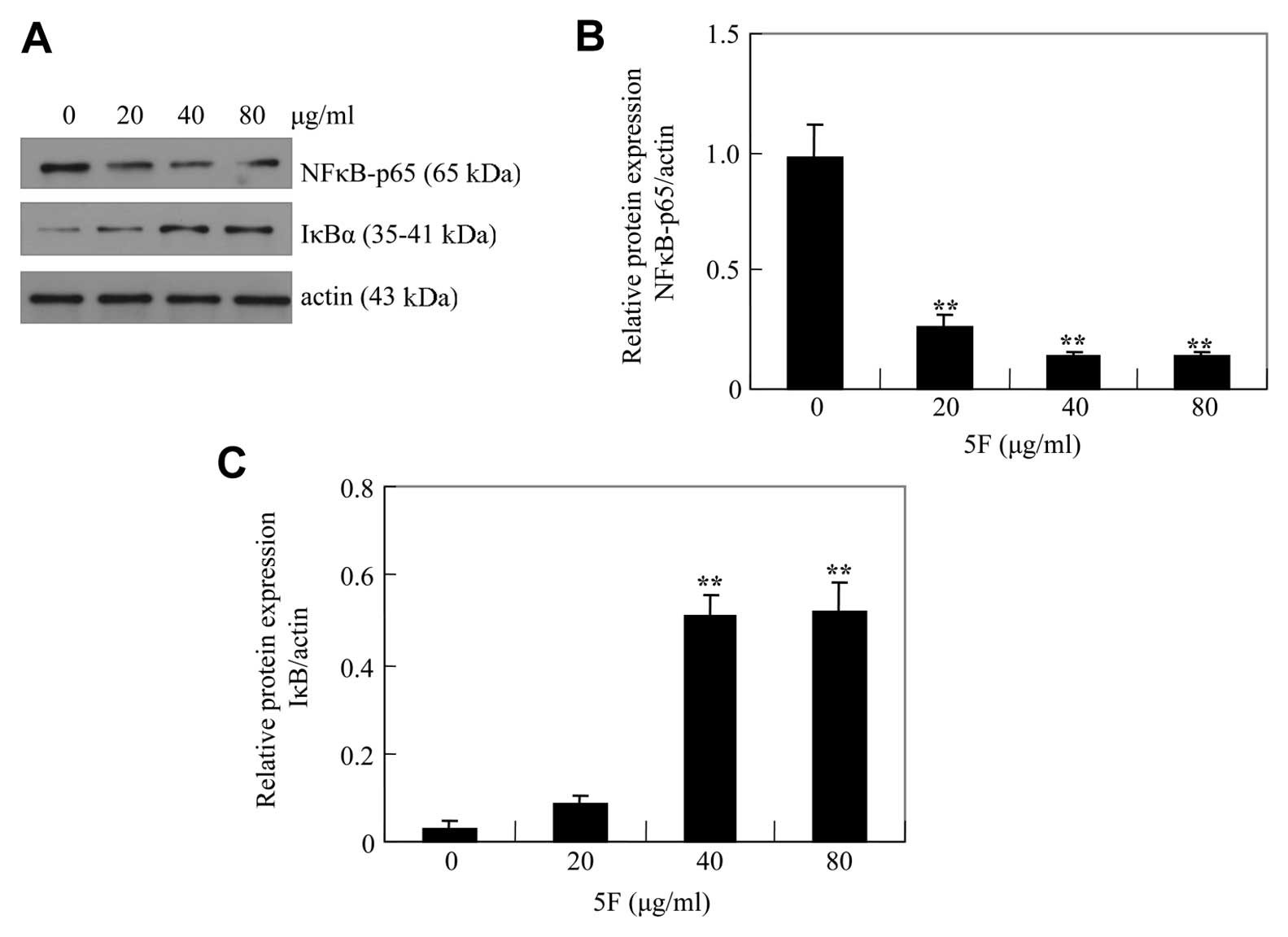
Bcl-2 is regulated by nuclear factor κB (NF-κB). To
test if 5F induces apoptosis by downregulating Bcl-2 through NF-κB,
we examined p65, a subunit of NF-κB, and IκBα, an inhibitor of
NF-κB (16). We found that the
level of p65 was reduced by 5F whereas the level of IκBα was
upregulated by 5F (Fig. 6). These
results suggest that 5F might induce apoptosis of CNE-2Z cells by
downregulating Bcl-2 by decreasing NFκB signaling.
Discussion
In the present study, we demonstrated that 5F
significantly inhibited the proliferation of CNE-2Z cells in a
dose- and time-dependent manner, and this inhibitory effect was p53
independent. Moreover, 5F induced mitochondrial-mediated apoptosis
in CNE-2Z cells, accompanied by a decrease of Bcl-2 and NF-κB.
Finally, we found that 5F sensitized CNE-2Z cells to cisplatin
independently of its reduction of ROS. Our data suggest that 5F may
be a potential anti-NPC agent.
Deregulation of cell cycle progression and evasion
of apoptosis are hallmarks of cancer cells (17). Accordingly, inhibition of cell cycle
progression may be particularly useful in the treatment of cancer.
5F has been demonstrated to arrest cells at the G2 phase in FRO
cells (an anaplastic thyroid carcinoma cell line) and A549 cells (a
non-small cell lung cancer cell line) (5,3).
Consistent with these reports, the present study showed that 5F
could induce G2-phase arrest in CNE-2Z cells. The G2 phase of the
cell cycle is controlled by cyclin-dependent kinase 1 (CDK1) and
can be inhibited by upregulation of the cyclin-dependent kinase
(CDK) inhibitor p21 (18). Several
studies have shown that anticancer drugs can increase the number of
cells in G2/M cell cycle phases, accompanied by upregulation of p21
(19–23). Therefore, we measured the expression
of p21 in CNE-2Z cells to further investigate the reason for the
G2/M-phase arrest mediated by 5F. Markedly, our results showed that
5F reduced the expression of p21 in CNE-2Z cells. In fact, the
functions of p21 are very complex. Despite the critical role of p21
in arresting cell cycle progression and promoting differentiation
and senescence, it was shown that p21 could also promote cellular
proliferation and tumorigenesis under certain conditions.
Consequently, depending on the cellular context and circumstances,
p21 is often deregulated in human cancer, suggesting that it can
act either as a tumor suppressor or as an oncogene (24). The tumor suppressor p53 is a nuclear
transcription factor that induces the expression of its numerous
downstream targets including p21, leading to cell cycle arrest,
senescence and apoptosis (25). In
the present study, we found p53 is a mutant in CNE-2Z cells. The
reduction of p21 by 5F in CNE-2Z cells may be partly due to the p53
mutation.
Previous reports have shown that several antitumor
agents (such as cisplatin) arrest cell cycle at the G2/M phase,
accompanied by apoptosis (26).
Induction of tumor cell death by apoptosis is a major mechanism
employed by antitumor agents. In the present study, we demonstrated
apoptosis-inducing effects of 5F in CNE-2Z cells using DAPI
staining and Annexin V-FITC staining assay. The
mitochondrial-mediated apoptotic pathway contributes to apoptosis
of cancer cells induced by several antitumor agents (27–29).
Elevated ratio of Bax/Bcl2 is an important marker of apoptosis in
several cancer cells (23,30–33).
Based on the quantification of western blot signals by
densitometry, an approximately 23-fold increase of Bax/Bcl2 was
observed following treatment with 80 μg/ml of 5F for 24 h (Fig. 5A and B). In addition, the release of
cytochrome c from mitochondria into the cytosol was also
observed. These results suggest that 5F-induced apoptosis in CNE-2Z
cells is mediated, at least in part, by the mitochondrial pathway.
On the other hand, caspase-3 plays a pivotal role in the terminal
execution phase of apoptosis (34).
An increasing caspase-3 activity was observed when 10 μg/ml of 5F
was added to 10 μg/ml of cisplatin in CNE-2Z cells, which suggests
that the sensitization of CNE-2Z cells by 5F to cisplatin is
associated with enhanced apoptosis.
The transcription factor NF-κB is an important
mediator of cell cycle progression and cell survival associated
with carcinogenesis (35).
Upregulation of NF-κB is positively associated with poor outcome of
several types of cancer, including NPC (36,37).
In resting cells, NF-κB exists in the cytoplasm and is inhibited by
complexing with IκB. Phosphorylation of IκB by IκB kinase (IKK)
causes ubiquitination and degradation of IκB. Subsequently, NF-κB
is released and translocates to the nucleus, where NF-κB regulates
the transcription of a number of genes which are involved in
tumorigenesis and cell growth (38,35).
These findings indicate that NF-κB is a potential therapeutic
target in cancer. In the present study, we investigated the effects
of 5F on the pattern of NF-κB activation. Our results showed that
treatment of CNE-2Z cells with 5F significantly inhibited the
expression of p-NF-κB/p65 protein and degradation of IκBα protein.
This study suggested that the effects of 5F on NF-κB/p65 might be
through inhibition of the phosphorylation and subsequent
proteolysis of IκBα.
Several antitumor agents such as cisplatin,
adriamycin (ADR), bleomycin and tumor necrosis factor (TNF) have
been reported to exert cytotoxic effects through ROS induction
(39–42), therefore, we determined the role of
ROS formation in 5F-induced cell death in CNE-2Z cells. Notably, 5F
induced apoptosis and, at the same time, reduced the ROS generation
in CNE-2Z cells. ROS scavenging of 5F might be related to
conjugated double bonds. Cisplatin is one of the most effective
chemotherapeutic agents, but has prominent side-effects such as
nephrotoxicity. These side-effects are believed to be related to
its ability to induce ROS production (43,44).
Animal studies have indicated that antioxidants or oxygen radical
scavengers could either ameliorate or protect against cisplatin
toxicity (44,45). Results from the current study
demonstrated that 5F possesses antioxidant properties in addition
to anticancer properties. Therefore, 5F combined with cisplatin
might improve cisplatin anticancer effects by reducing the
cisplatin-induced ROS.
In conclusion, 5F inhibited CNE-2Z cell
proliferation through cell cycle arrest at the G2/M phase and
apoptosis induction related to the mitochondrial-mediated apoptotic
pathway and NF-κB inhibition. Our results also indicate that 5F
sensitizes CNE-2Z cells to cisplatin-induced cytotoxicity and
reduces ROS production induced by cisplatin. Our previous studies
in mice showed that 5F could be effective against liver and lung
cancer with minimal side-effects (46,47).
These results suggest 5F is a promising anti-NPC agent.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 3987099), the Science and
Technology Innovation Fund of Guangdong Medical College
(STIF201104) and the Research Committee, Guangdong Medical College
(no. XB0601).
References
|
1
|
Chen WQ, Zeng HM, Zheng RS, Zhang SW and
He J: Cancer incidence and mortality in China, 2007. Chin J Cancer
Res. 24:1–8. 2012. View Article : Google Scholar
|
|
2
|
Chang JT, Ko JY and Hong RL: Recent
advances in the treatment of nasopharyngeal carcinoma. J Formos Med
Assoc. 103:496–510. 2004.
|
|
3
|
Li L, Chen GG, Lu YN, Liu Y, Wu KF, Gong
XL, Gou ZP, Li MY and Liang NC:
Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid inhibits the growth
of human lung cancer A549 cells by arresting cell cycle and
triggering apoptosis. Chin J Cancer Res. 24:109–115. 2012.
|
|
4
|
Vlantis AC, Lo CS, Chen GG, Ci Liang N,
Lui VW, Wu K, Deng YF, Gong X, Lu Y, Tong MC and van Hasselt CA:
Induction of laryngeal cancer cell death by
Ent-11-hydroxy-15-oxo-kaur-16-en-19-oic acid. Head Neck.
32:1506–1518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu ZM, Chen GG, Vlantis AC, Liang NC,
Deng YF and van Hasselt CA: Cell death induced by
ent-11alpha-hydroxy-15-oxo-kaur-16-en-19-oic-acid in anaplastic
thyroid carcinoma cells is via a mitochondrial-mediated pathway.
Apoptosis. 10:1345–1356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Z, Ng EK, Liang NC, Deng YF, Leung BC
and Chen GG: Cell death induced by Pteris semipinnataL. is
associated with p53 and oxidant stress in gastric cancer cells.
FEBS Lett. 579:1477–1487. 2005.PubMed/NCBI
|
|
7
|
Chen GG, Liang NC, Lee JF, Chan UP, Wang
SH, Leung BC and Leung KL: Over-expression of Bcl-2 against
Pteris semipinnataL-induced apoptosis of human colon cancer
cells via a NF-kappa B-related pathway. Apoptosis. 9:619–627.
2004.PubMed/NCBI
|
|
8
|
Gu SY, Zhao WP, Zeng Y, Tang WP, Zhao ML,
Deng HH and Li K: New human nasopharyngeal epithelial cell line
established from a patient with poorly differentiated
nasopharyngeal carcinoma. Chin J Cancer. 2:70–72. 1983.
|
|
9
|
Deng YF and Liang NC: Study on extraction
and separation of diterpenoids from Pteris semipinnata. Chin
Pharm J. 39:742–744. 2004.
|
|
10
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nigg EA: Cyclin-dependent protein kinases:
key regulators of the eukaryotic cell cycle. Bioessays. 17:471–480.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gautier J, Solomon MJ, Booher RN, Bazan JF
and Kirschner MW: cdc25 is a specific tyrosine phosphatase that
directly activates p34cdc2. Cell. 67:197–211. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang M, Wei Q, Pabla N, Dong G, Wang CY,
Yang T, Smith SB and Dong Z: Effects of hydroxyl radical scavenging
on cisplatin-induced p53 activation, tubular cell apoptosis and
nephrotoxicity. Biochem Pharmacol. 73:1499–1510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YH, Kim YW, Oh YJ, Back NI, Chung SA,
Chung HG, Jeong TS, Choi MS and Lee KT: Protective effect of the
ethanol extract of the roots of Brassica rapaon
cisplatin-induced nephrotoxicity in LLC-PK1cells and
rats. Biol Pharm Bull. 29:2436–2441. 2006.PubMed/NCBI
|
|
15
|
Satoh M, Kashihara N, Fujimoto S, Horike
H, Tokura T, Namikoshi T, Sasaki T and Makino H: A novel free
radical scavenger, edarabone, protects against cisplatin-induced
acute renal damage in vitro and in vivo. J Pharmacol Exp Ther.
305:1183–1190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van Waes C: Nuclear factor-kappaB in
development, prevention, and therapy of cancer. Clin Cancer Res.
13:1076–1082. 2007.PubMed/NCBI
|
|
17
|
Senderowicz AM: Targeting cell cycle and
apoptosis for the treatment of human malignancies. Curr Opin Cell
Biol. 16:670–678. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skladanowski A, Côme MG, Sabisz M,
Escargueil AE and Larsen AK: Down-regulation of DNA topoisomerase
IIalpha leads to prolonged cell cycle transit in G2 and early M
phases and increased survival to microtubule-interacting agents.
Mol Pharmacol. 68:625–634. 2005.PubMed/NCBI
|
|
19
|
Cho JH, Lee JG, Yang YI, Kim JH, Ahn JH,
Baek NI, Lee KT and Choi JH: Eupatilin, a dietary flavonoid,
induces G2/M cell cycle arrest in human endometrial cancer cells.
Food Chem Toxicol. 49:1737–1744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castro J, Ribó M, Navarro S, Nogués MV,
Vilanova M and Benito A: A human ribonuclease induces apoptosis
associated with p21WAF1/CIP1induction and JNK
inactivation. BMC Cancer. 11:92011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalimutho M, Minutolo A, Grelli S, Formosa
A, Sancesario G, Valentini A, Federici G and Bernardini S:
Satraplatin (JM-216) mediates G2/M cell cycle arrest and
potentiates apoptosis via multiple death pathways in colorectal
cancer cells thus overcoming platinum chemo-resistance. Cancer
Chemother Pharmacol. 67:1299–1312. 2011. View Article : Google Scholar
|
|
22
|
Huang WW, Ko SW, Tsai HY, Chung JG, Chiang
JH, Chen KT, Chen YC, Chen HY, Chen YF and Yang JS: Cantharidin
induces G2/M phase arrest and apoptosis in human colorectal cancer
colo 205 cells through inhibition of CDK1 activity and
caspase-dependent signaling pathways. Int J Oncol. 38:1067–1073.
2011.
|
|
23
|
Dia VP and Mejia EG: Lunasin promotes
apoptosis in human colon cancer cells by mitochondrial pathway
activation and induction of nuclear clusterin expression. Cancer
Lett. 295:44–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abbas T and Dutta A: p21 in cancer:
intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vousden KH and Prives C: Blinded by the
light: the growing complexity of p53. Cell. 137:413–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chu G: Cellular responses to cisplatin:
The roles of DNA-binding proteins and DNA repair. J Biol Chem.
269:787–790. 1994.PubMed/NCBI
|
|
27
|
Malugin A, Kopecková P and Kopecek J: HPMA
copolymer-bound doxorubicin induces apoptosis in ovarian carcinoma
cells by the disruption of mitochondrial function. Mol Pharm.
3:351–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yim EK, Lee KH, Bae JS, Namkoong SE, Um SJ
and Park JS: Proteomic analysis of antiproliferative effects by
treatment of 5-fluorouracil in cervical cancer cells. DNA Cell
Biol. 23:769–776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boulikas T and Vougiouka M: Cisplatin and
platinum drugs at the molecular level (Review). Oncol Rep.
10:1663–1682. 2003.PubMed/NCBI
|
|
30
|
Lin JP, Yang JS, Lee JH, Hsieh WT and
Chung JG: Berberine induces cell cycle arrest and apoptosis in
human gastric carcinoma SNU-5 cell line. World J Gastroenterol.
12:21–28. 2006.PubMed/NCBI
|
|
31
|
Hwang JM, Kuo HC, Tseng TH, Liu JY and Chu
CY: Berberine induces apoptosis through a mitochondria/caspases
pathway in human hepatoma cells. Arch Toxicol. 80:62–73. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mantena SK, Sharma SD and Katiyar SK:
Berberine, a natural product, induces G1-phase cell cycle arrest
and caspase-3-dependent apoptosis in human prostate carcinoma
cells. Mol Cancer Ther. 5:296–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuo CL, Chou CC and Yung BY: Berberine
complexes with DNA in the berberine-induced apoptosis in human
leukemic HL-60 cells. Cancer Lett. 93:193–200. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vaux DL and Korsmeyer SJ: Cell death in
development. Cell. 96:245–254. 1999. View Article : Google Scholar
|
|
35
|
Aggarwal BB: Nuclear factor-kappaB: the
enemy within. Cancer Cell. 6:203–208. 2004.PubMed/NCBI
|
|
36
|
Sun W, Guo MM, Han P, Lin JZ, Liang FY,
Tan GM, Li HB, Zeng M and Huang XM: Id-1 and the p65 subunit of
NF-κB promote migration of nasopharyngeal carcinoma cells and are
correlated with poor prognosis. Carcinogenesis. 33:810–817.
2012.
|
|
37
|
Zhang Y, Lang JY, Liu L, Wang J, Feng G,
Jiang Y, Deng YL, Wang XJ, Yang YH, Dai TZ, Xie G, Pu J and Du XB:
Association of nuclear factor κB expression with a poor outcome in
nasopharyngeal carcinoma. Med Oncol. 28:1338–1342. 2011.
|
|
38
|
Gupta S, Hastak K, Afaq F, Ahmad N and
Mukhtar H: Essential role of caspases in
epigallocatechin-3-gallate-mediated inhibition of nuclear factor
kappa B and induction of apoptosis. Oncogene. 23:2507–2522. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miyajima A, Nakashima J, Yoshioka K,
Tachibana M, Tazaki H and Murai M: Role of reactive oxygen species
in cis-dichlorodiammineplatinum-induced cytotoxicity on bladder
cancer cells. Br J Cancer. 76:206–210. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meijer C, Mulder NH, Timmer-Bosscha H,
Zijlstra JG and de Vries EG: Role of free radicals in an
adriamycin-resistant human small cell lung cancer cell line. Cancer
Res. 47:4613–4617. 1987.PubMed/NCBI
|
|
41
|
Sausville EA, Stein RW, Peisach J and
Horwitz SB: Properties and products of the degradation of DNA by
bleomycin and iron (II). Biochemistry. 17:2746–2754. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yamauchi N, Kuriyama H, Watanabe N, Neda
H, Maeda M and Niitsu Y: Intracellular hydroxyl radical production
induced by recombinant human tumor necrosis factor and its
implication in the killing of tumor cells in vitro. Cancer Res.
49:1671–1675. 1989.
|
|
43
|
Chirino YI and Pedraza-Chaverri J: Role of
oxidative and nitrosative stress in cisplatin-induced
nephrotoxicity. Exp Toxicol Pathol. 61:223–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rybak LP, Whitworth CA, Mukherjea D and
Ramkumar V: Mechanisms of cisplatin-induced ototoxicity and
prevention. Hear Res. 226:157–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
van den Berg JH, Beijnen JH, Balm AJ and
Schellens JH: Future opportunities in preventing cisplatin induced
ototoxicity. Cancer Treat Rev. 32:390–397. 2006.PubMed/NCBI
|
|
46
|
Chen GG, Leung J, Liang NC, Li L, Wu K,
Chan UP, Leung BC, Li M, Du J, Deng YF, Gong X, Lv Y, Chak EC and
Lai PB: Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid inhibits
hepatocellular carcinoma in vitro and in vivo via stabilizing IκBα.
Invest New Drugs. 30:2210–2218. 2012.
|
|
47
|
Li MY, Leung J, Kong AW, Liang NC, Wu K,
Hsin MK, Deng YF, Gong X, Lv Y, Mok TS, Underwood MJ and Chen GG:
Anticancer efficacy of 5F in NNK-induced lung cancer development of
A/J mice and human lung cancer cells. J Mol Med. 88:1265–1276.
2010. View Article : Google Scholar : PubMed/NCBI
|