Introduction
Human nasopharyngeal carcinoma (NPC) is one of the
most common cancers in Southern China (1). The highest incidence is found among
Southern Chinese (~25–30 per 100,000 individuals per year), in
particular those of Cantonese origin (2). Although NPC is a relatively
radiosensitive disease, the therapeutic effect remains
unsatisfactory due to radioresistance (3). Aiming to identify the mechanisms of
radioresistance, our research group established a radioresistant
NPC cell line (CNE2R) derived from the CNE2 cell line. In recent
years, epigenetic changes in miRNAs have attracted increasing
attention as they regulate a wide variety of drug-resistant genes
(4). Emerging evidence suggests
that miRNAs are involved in cancer therapy resistance (5,6), and
several studies have focused on alterations in proteins and genes
which are regulated by miRNAs in order to explain the mechanisms of
resistance (7,8). We demonstrated that
irradiation-induced miR-205 determined the resistance of NPC by
targeting PTEN (9). However,
specific inhibitors targeting miRNAs remain largely
undetermined.
SZ-685C, an anthracycline analogue isolated from the
secondary metabolites of the mangrove endophytic fungus no. 1403
collected from the South China Sea (10), was found to exhibit an anticancer
effect on breast cancer in vivo and in vitro
involving the inhibition of Akt/FOXO (11). The upregulations of Akt and FOXO are
linked to drug resistance (12).
These studies suggest that SZ-685C is a potential anticancer drug
candidate for cancer therapy resistance. To date, no studies have
directly investigated whether NPC cells are sensitive to SZ-685C,
particularly the radioresistant nasopharyngeal carcinoma cell line
CNE2R. In the present study, we confirmed the anticancer activity
of SZ-685C in both CNE2 and CNE2R cells. Furthermore, we
demonstrated, for the first time, that protein changes in
anticancer pathways and also potent miRNA changes participated in
the anticancer effect of SZ-685C on the two NPC cell lines.
Materials and methods
Preparation of SZ-685C
SZ-685C was obtained from the Guangdong Province Key
Laboratory of Functional Molecules in Oceanic Microorganisms (Sun
Yatsen University). This product was dissolved in
dimethylsulphoxide (DMSO) at a concentration of 50 nM as a stock
solution and diluted according to experimental requirements when
used.
Cell culture
Human nasopharyngeal carcinoma cell line CNE2 was
obtained from the Cancer Center, Sun Yatsen University (Guangzhou,
China), and the stable radioresistant NPC cell line CNE2R was
established in our laboratory. The two cell lines were cultured in
RPMI-1640 medium containing 10% fetal bovine serum with
penicillin-streptomycin sulfate. All cell lines were incubated at
37°C in an atmosphere of 5% CO2.
Cell viability and colony formation
assays
MTT
(3,4,5-dimethylthiazol-2-yl)-2–5-diphenyltetrazolium bromide) assay
was used to determine the effect of SZ-685C on cell viability. The
cells were seeded in 200 μl media for 12 h and incubated with 0.1,
0.5, 1, 5, 10, 20, 30, 40 and 50 μM SZ-685C for 24, 48 and 72 h.
Cells were then incubated with MTT (Sigma-Aldrich, St. Louis, MO,
USA) reagent for 4 h, lysed with DMSO and quantitated using a plate
reader at 570 nm.
Clonogenic assays were performed by seeding cells
into 6-well plates, allowing adherence overnight, and the culture
medium was replaced with 3 ml of fresh media containing SZ-685C at
specified concentrations. After incubation for 14 days, the cells
were fixed with methanol, they were then stained with 0.2% Giemsa
(Sigma-Aldrich), and finally colonies consisting of >50 cells
were counted.
Apoptotic morphologic changes
CNE2 and CNE2R cells were plated into 6-well plates
at a density of 2×105 cells/well overnight, and then
SZ-685C was added at final concentrations of 0, 10, 20 and 30 μM.
After 48 h, cells were stained with Hoechst 33342 (Molecular
Probes, Life Technologies, USA) at 10 μg/ml for 15 min, fixed with
methanol and subsequently washed with PBS. A phase-contrast
fluorescence microscope was used to observe apoptotic morphologic
changes.
Determination of cell cycle and
apoptosis
Cells (2×105/well) in 6-well plates were
maintained with 20 and 30 μM of SZ-685C for 48 h. The cells were
harvested by centrifugation and fixed in cold 70% ethanol at 4°C
overnight (≥12 h). Fixed cells were washed with PBS and stained
with propidium iodide containing RNase A at 10 μg/ml. Cells were
collected by flow cytometry (FACSCalibur, Becton-Dickinson, San
Jose, CA, USA) and analyzed using ModFit Software (Verity Software
House Inc., Topsham, ME, USA).
For apoptosis experiments, cells were seeded and
cultured as above. Cell apoptosis was determined by flow cytometry.
Cells were stained with Annexin-V-FITC and propidium iodide (PI),
using an assay kit from Roche as described by the manufacturer
(Roche Diagnostics, Indianapolis, IN, USA).
Western blotting
Cells were seeded into 60-mm dishes overnight and
then treated with SZ-685C at different concentrations for 12, 24 or
48 h. Including the floating cells, all the cells were harvested
and lysed in 1X sampling buffer (Cell Signaling Technology,
Danvers, MA, USA) and protease inhibitor cocktail (Roche). The
concentrations of these lysates were determined by a bicinchoninic
acid protein assay kit (Pierce Biotech, Rockford, IL, USA).
Equivalent amounts of proteins were loaded on a 10, 12 or 15%
polyacrylamide SDS gel and were electrophoresed for 1.5–2 h.
Proteins were then transferred from the gel to polyvinylidene
fluoride (PVDF) membranes (EMD Millipore, Billerica, MA, USA).
Membranes were blocked with fat-free milk powder (Roche) (5%, w/v)
and Tween 20 (1%, w/v) in TBS-T for 1 h. Protein bands were
detected by exposing the membranes to primary antibodies overnight
at 4°C. After washing and secondary antibody incubation, the signal
from the target protein band was visualized on X-ray film using ECL
western blotting reagents (Biyuntian, Beijing, China).
The following primary antibodies were used: a
1:1,000 dilution of rabbit PTEN (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), total Akt (Cell Signaling Technology),
14-3-3ζ (Abcam Ltd., Hong Kong); a 1:1,000 dilution of mouse
caspase-3, Stat3, p27 (Cell Signaling Technology), Bcl-2/Bax (BD
Pharmingen, BD Biosciences, San Diego, CA, USA), caspase-4, Jab1
(Abcam); a 1:500 dilution of mouse monoclonal caspase-8, caspase-9
(Santa Cruz Biotechnology); a 1:10,000 dilution of mouse monoclonal
β-actin (Sigma) and an HRP-conjugated secondary antibody (Cell
Signaling Technology) followed by enhanced chemiluminescence
detection.
qRT-PCR
Total RNA was extracted with the TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). For miRNA quantification, the cDNA
was synthesized by SuperScript® III First-Strand
(Invitrogen) and Platinum® SYBR® Green qPCR
SuperMix-UDG (Invitrogen). The special miR-205 and miR-24 were
designed by RiboBio (Guangzhou, China). Expression levels were
normalized to that of U6 rRNA and then converted into relative
values calculated by the comparative CT method. The mRNA levels for
PTEN and Akt were measured by a method similar to miRNA
quantification. β-actin was used as an internal control for
normalization. qRT-PCR reactions were performed with the following
primers: PTEN forward, 5′-TGGATTCGACTTAGACTTGACCT-3′; reverse,
5′-TTTGGCGGTGTCATAATGTCTT-3′; Akt1 forward,
5′-TCTTTGCCGGTATCGTGT-3′; reverse, 5′-TGTC ATCTTGGTCAGGTGGT-3′. All
quantitative real-time PCR was performed using the iQ5 Bio-Rad
Real-Time PCR system (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
At least three independent experiments were
performed for statistical evaluation. Data are presented as means ±
SD. The statistical significance was evaluated using the Student’s
t-test. A P-value of <0.05 was considered to indicate a
statistically significant result.
Results
Cytotoxic effect of SZ-685C on CNE2 and
CNE2R cells
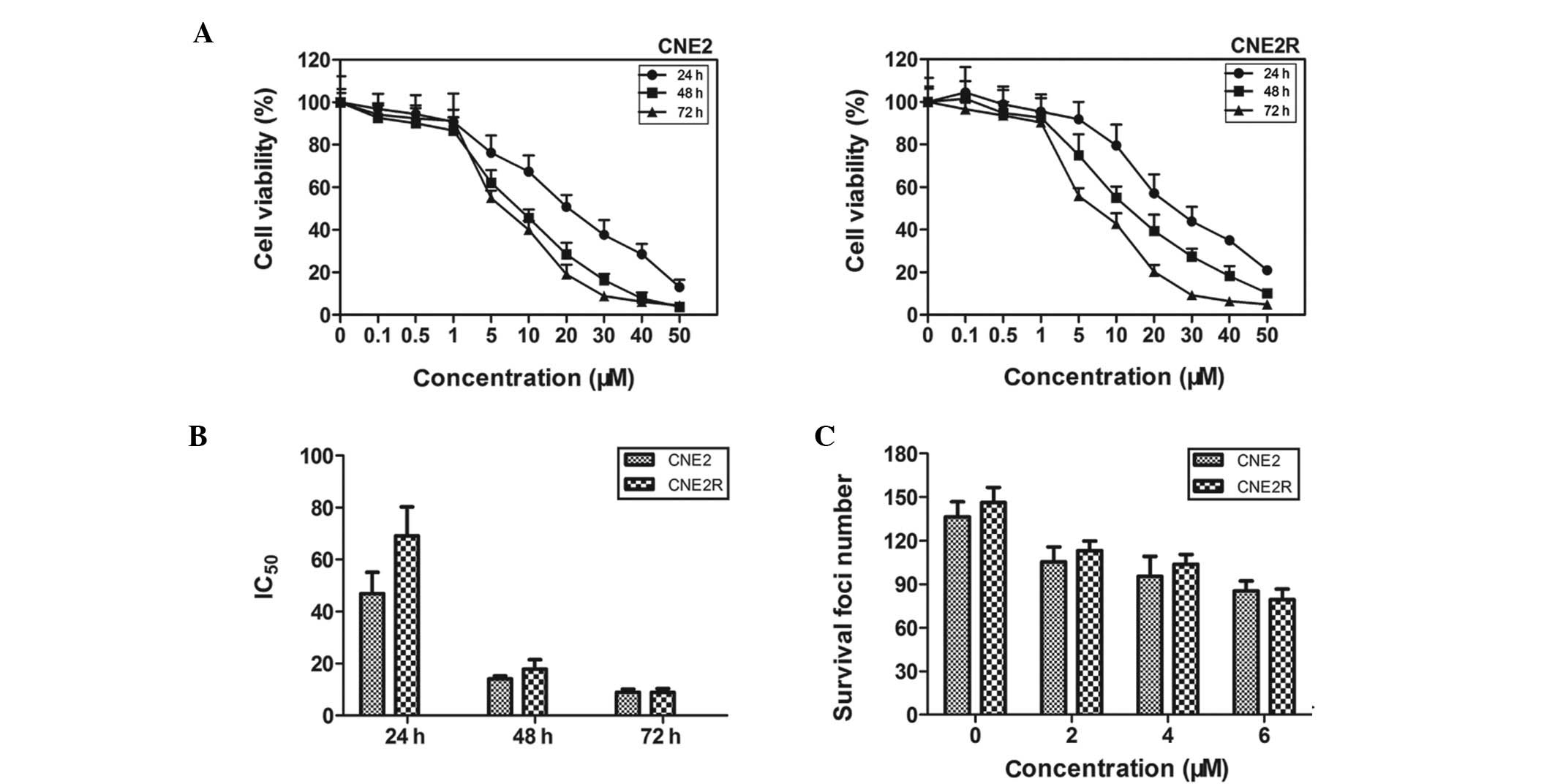
The viabilities of CNE2 and CNE2R cells decreased in
a dose- and time-dependent manner following treatment with SZ-685C
(Fig. 1A). As shown in Fig. 1B, after treatment with SZ-685C for
24, 48 and 72 h, the calculated IC50 value was 46.89,
14.13 and 8.97 μM for CNE2 cells and 69.11, 17.86 and 8.94 μM for
CNE2R cells, respectively. We further determined the clonogenic
abilities of the two cell lines treated with SZ-685C (Fig. 1C) and found that there were no
significant differences between CNE2 and CNE2R cells.
Effects of SZ-685C on apoptosis and the
cell cycle
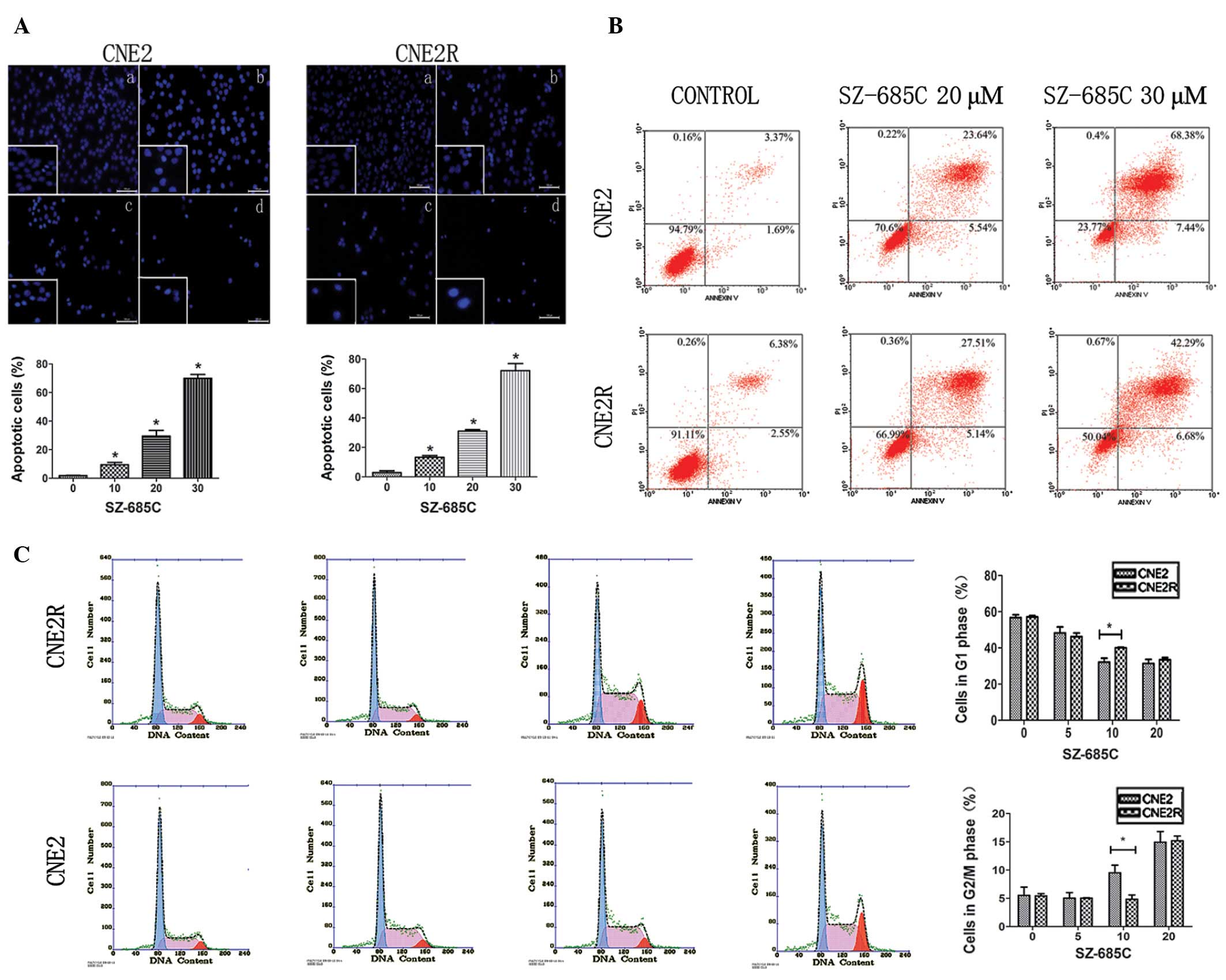
To confirm whether the decreased viability of CNE2
and CNE2R cells induced by SZ-685C was associated with apoptosis,
we imaged the morphologic changes of the two cell lines treated
with SZ-685C for 48 h. We observed nuclear fragmentation and bright
blue nuclei (Fig. 2A). In CNE2
cells, the percentage of apoptotic cells was ~70%, and the
percentage in CNE2R cells was 72%. The data indicated that the
pro-apoptotic effect of SZ-685C on the two cell lines was not
significantly different. We next analyzed the number of apoptotic
cells by flow cytometry. In the CNE2 cells, treatment with 20 and
30 μM SZ-685C for 48 h resulted in 29.18 and 75.83% of the cells
undergoing apoptosis, respectively. For the CNE2R cells, the
percentages of apoptotic cells were 32.65 and 48.97%, respectively
(Fig. 2B).
Cell cycle progression using flow cytometry was
determined. SZ-685C induced cell cycle arrest at the G2/M phase in
CNE2 cells, and the percentage of cells in the G2/M phase increased
from 5.5% at 0 μM SZ-685C to 14.93% at 20 μM SZ-685C at 24 h. At
the same time, in the CNE2R cells, similar results were found
showing that the proportion of cells in the G2/M phase increased
from 5.43 to 15.17% (Fig. 2C).
Activation of apoptotic pathways by
SZ-685C
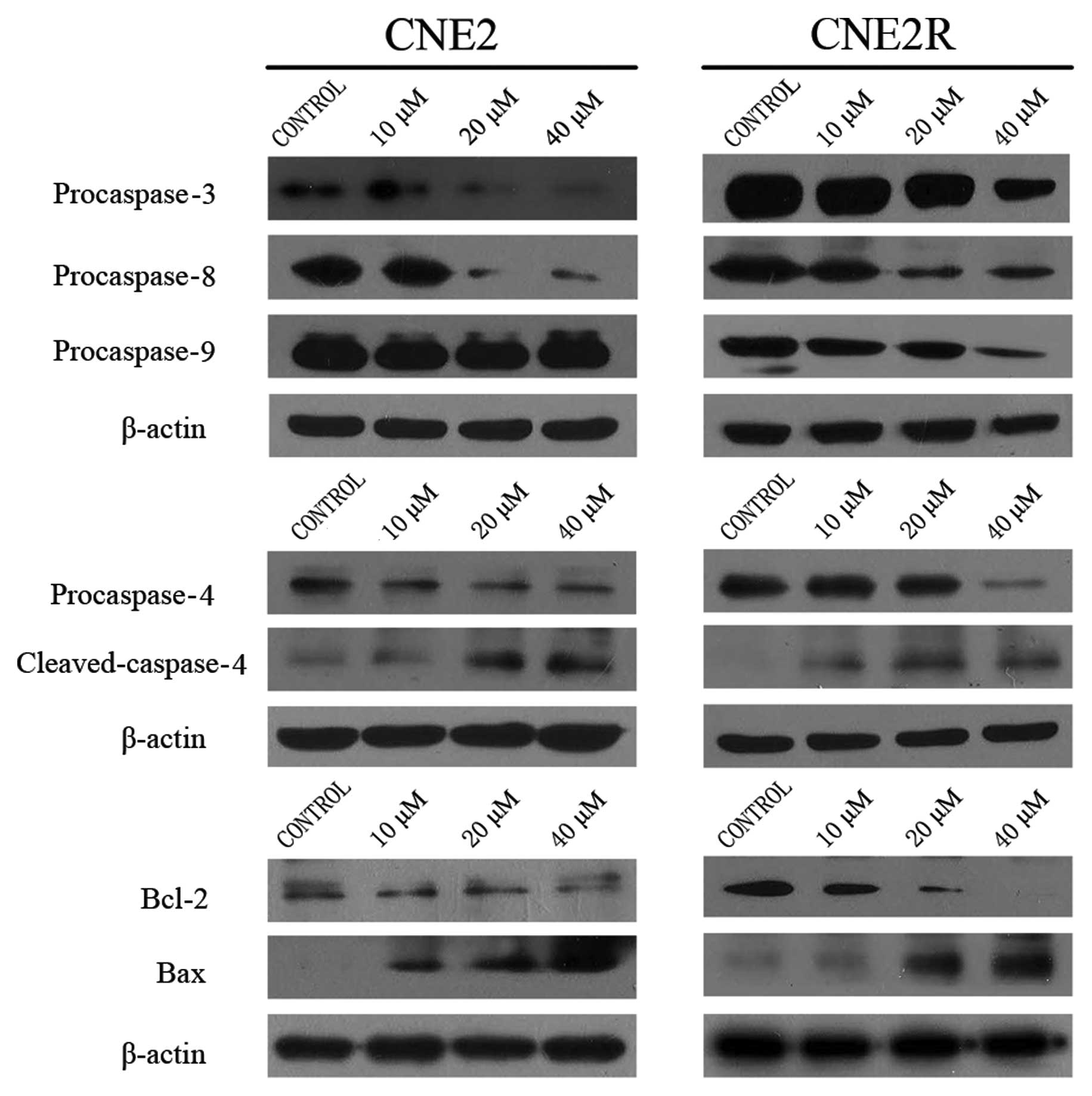
In order to elucidate the exact apoptotic pathways
involved, we assessed the levels of several key caspases. As shown
in Fig. 3, in CNE2 cells, the
levels of procaspase-3, -8 and -4 decreased in an obvious
dose-dependent manner, and the level of cleaved caspase-4 increased
in the same manner. We also detected changes in these proteins in
the CNE2R cells, and found similar data except procaspase-9 which
was decreased in the CNE2R cells. The data indicated that SZ-685C
induced apoptosis which involved the intrinsic and extrinsic
pathways in both cell lines.
We determined the levels of Bcl-2 and Bax in both
cell lines following SZ-685C treatment for 48 h. The level of Bcl-2
obviously decreased while the level of Bax was increased in the
CNE2 and CNE2R cell lines (Fig.
3).
SZ-685C induces apoptosis via the
miR-205-PTEN-AKT pathway
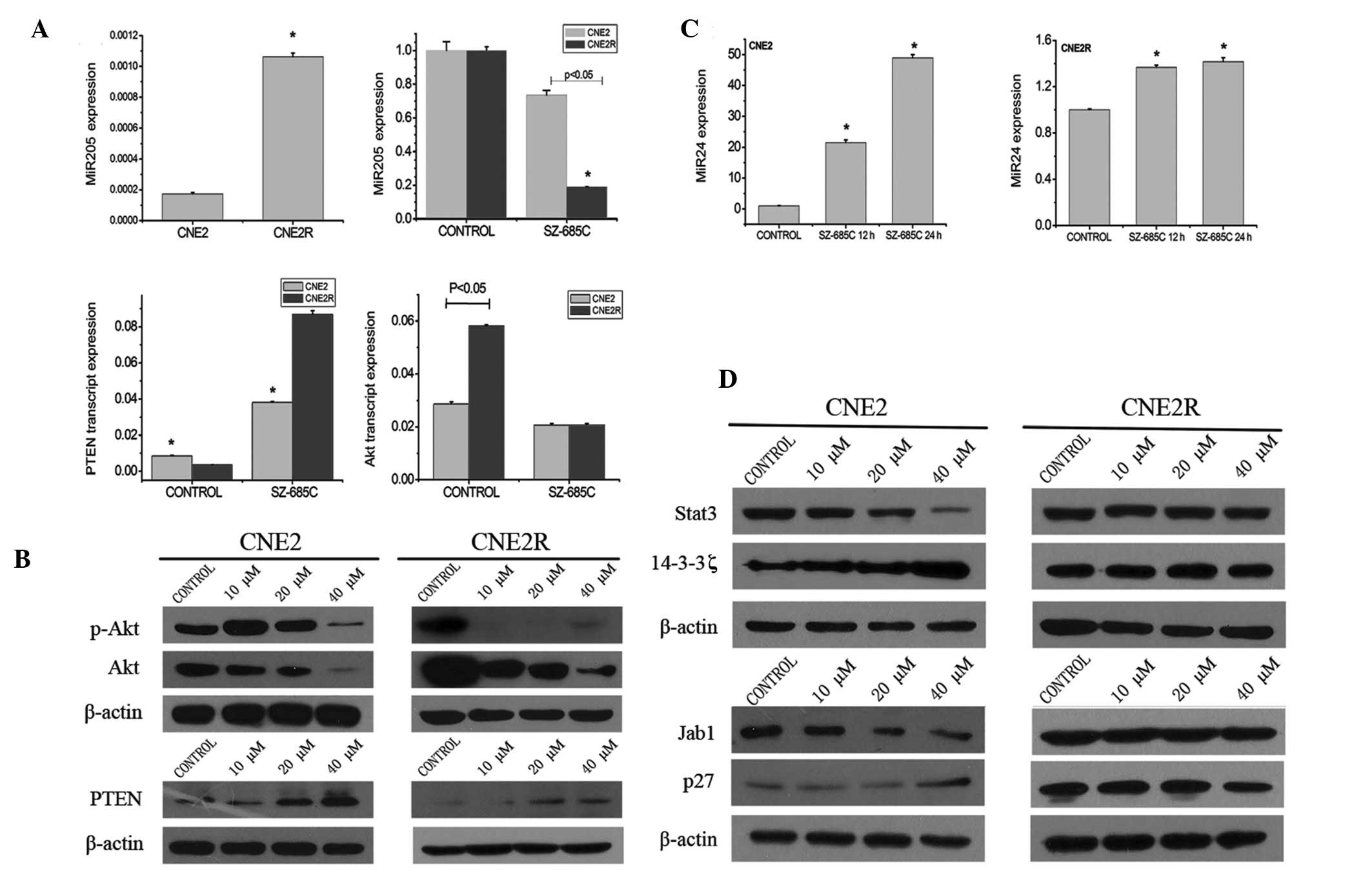
To elucidate the anticancer mechanisms involved in
the pro-apoptotic effect of SZ-685C on NPC cells which are mediated
through the Akt pathway, the expression of PTEN and Akt and the
phosphorylation status of Akt were assessed (Fig. 4B). SZ-685C treatment for 48 h
resulted in a decrease in Akt and phosphor-Ser473-Akt and an
increase in PTEN in the CNE2 and CNE2R cell lines. We found that
after treatment with SZ-685C at the concentration of 40 μM for 48 h
phosphor-Ser473-Akt decreased in the CNE2 cells; however, in CNE2R
cells it was altered at 10 μM. The mRNA levels of Akt1 in the two
cell lines were not identical; the level was higher in the CNE2R
cells when compared with that in the CNE2 cells. Following
treatment with SZ-685C for 48 h, the mRNA level of Akt1 was
decreased more significantly in CNE2R cells than that in CNE2 cells
(Fig. 4A).
We subsequently detected the mRNA expression of PTEN
and miR-205 (Fig. 4A). As
predicted, the tendency was similar, while the difference between
the two cell lines was noted. The initial mRNA level of PTEN in the
CNE2R cells without SZ-685C was much lower when compared with that
in CNE2 cells, while the level of miR-205 was much higher in CNE2R
cells than that in the CNE2 cells. After treatment with SZ-685C for
1 h, the level of miR-205 decreased in the CNE2R cells, and the
tendency was significant. However, in CNE2 cells, the decrease in
the level of miR-205 was slight at 4 h following SZ-685C treatment.
A similar trend was determined for PTEN. The increased level of
PTEN in CNE2R cells was higher than that in CNE2 cells upon SZ-685C
treatment for 4 h.
SZ-685C induces changes in the
Stat3-Jab1-p27 pathway in CNE2 cells but not in CNE2R cells
We screened the proteins involved in apoptosis and
survival. We found different changes in p27, Stat3 and 14-3-3ζ in
the two NPC cell lines. We discovered that in CNE2 cells treated
with SZ-685C for 12 h, the level of Stat3 decreased and the level
of 14-3-3ζ increased in a dose-dependent manner, and the level of
p27 increased after treatment with SZ-685C for 48 h (Fig. 4D). In an opposing manner, the levels
of the three proteins did not change in CNE2R cells under the same
conditions.
SZ-685C regulates miR-24 expression
After establishment of the radioresistant NPC cell
line, CNE2R, we screened for different miRNAs between CNE2 and
CNE2R. miR-205 was one of the 9 miRNAs with differential expression
and it was the most significant one. Thus, we aimed to ascertain
whether expression of other miRNAs could be regulated by SZ-685C.
We found that miR-24 expression was obviously altered after
treatment with SZ-685C for 12 and 24 h, implying that miR-24 may
participate in the pro-apoptotic effect of SZ-685C on both cell
lines. We also found that the change was much more significant in
CNE2 cells than that in CNE2R cells (Fig. 4C).
Discussion
Radiotherapy is a major treatment option for NPC;
yet, radioresistance is the main obstacle to the treatment of such
patients. Therefore, understanding the mechanisms of
radioresistance and developing compounds to reverse this resistance
are challenging for improving the prognosis of NPC patients. Based
on the radioresistant cell line CNE2R, we previously reported that
miR-205 targets tumor-suppressor PTEN and consequently activates
the PI3K/Akt pathway leading to increase radioresistance of NPC,
indicating that the miR-205-PTEN pathway may determine the
threshold for overcoming radioresistance in NPC cells (9). It has been demonstrated that estrogen
receptor (ER)-negative, invasive MD-MB-435 cells are highly
sensitive to SZ-685C treatment. In addition, SZ-685C also
represented a promising approach to the treatment of
adriamycin-resistant breast cancer (13).
In the present study, we found that SZ-685C
inhibited the viability of CNE2 and CNE2R cells with mild
cytotoxicity. The IC50 value of CNE2 was higher than
that of CNE2R for SZ-685C treatment for 24 h. However, the results
did not achieve a significant difference. The IC50
values in the two cell lines were almost the same at 72 h. Our data
indicated that SZ-685C exhibited a cytotoxic effect in both
radioresistant and radiosensitive NPC cells. As we all know, the
survival of cancer cells involves many factors, such as avoiding
apoptosis and promoting cell cycle progression. We concluded that
the anticancer activity of SZ-685C was mainly based on inducing
apoptosis, partially resulting in cell cycle arrest (G2/M). The
apoptotic pathways have been classified into two main types:
extrinsic and intrinsic pathways. The extrinsic pathways include
death receptor and survival factor withdrawal pathways, and the
intrinsic pathways are caused either by DNA damage or stress to
endoplasmic reticulum (ERS). Both pathways subsequently activate
certain caspases. Activation of caspase-8 and caspase-4 seems to be
involved in extrinsic pathways, while caspase-4 is also linked with
ERS-induced apoptosis. Activation of these specific capases
ultimately leads to cell death through their effect on
mitochondrial membrane stability and activation of caspase-9
(14,15). In order to fully understand the
pro-apoptotic mechanisms of SZ-685C, we analyzed the changes in
various capsases. We found the activation of caspase-3, -4 and -8
in both cell lines. But the activation of caspase-9 was only found
in CNE2R cells. We assumed that both extrinsic and intrinsic
apoptotic pathways were activated after exposure to SZ-685C, but
there was a slight difference between the two cell lines concerning
the caspase-apoptotic pathways.
Numerous studies have demonstrated that Akt plays a
pivotal role in chemotherapeutic resistance in many cancer types
(16). Activation of Akt has been
described in different tumors which are resistant to irradiation
therapy (17), and PTEN is a
negative factor for activation of Akt (18). Notably, Li et al reported
that latent membrane protein 1 (LMP1) mediates the drug resistance
of NPC cells to apoptosis by activation of the PI3K/Akt signaling
pathway (19). Suppressing Akt
activity may sensitize cancer cells to chemotherapy and irradiation
(20). Importantly, research
indicated that SZ-685C displayed marked pro-apoptotic activity by
suppression of the Akt/FOXO pathway, which was related closely with
cancer resistance. Therefore, these results provided reasonable
evidence that SZ-685C induced apoptosis in NPC cells, particularly
in radioresistant cells, through inhibition of the Akt pathway. Our
data indicated that SZ-685C exhibited pro-apoptotic activity via
inactivation of Akt and phosphorylation of Akt, and demonstrated
that the inactivation of p-Akt was negatively regulated by PTEN. Qu
et al(9) screened the
differential expression of miRs between CNE2 and CNE2R cells and
found that miR-205 was highly expressed in CNE2R cells. They also
elucidated that miR-205 significantly upregulated Akt through
targeting PTEN, resulting in the radioresistance of NPC cells.
Based on our findings, we concluded that SZ-685C induced apoptosis
by inactivating Akt through a decrease in miR-205 expression level
which targeted PTEN, demonstrating that SZ-685C had an impact on
the miR-205-PTEN-Akt pathway in both NPC cell lines. Yet, we found
that the change in this pathway was more significant in CNE2R than
that in CNE2 cells.
It was rational to declare that SZ-685C induced
apoptosis through the miR-205-PTEN-Akt signaling pathway in CNE2R
cells as the expression level of miR-205 was high. But we
questioned why SZ-685C also showed a similar pro-apoptotic effect
in CNE2 cells, in which the level of miR-205 was much lower than
that of CNE2R cells, Was this the only pathway? To further
elucidate the anticancer mechanisms in CNE2 cells, we assessed
other probable anticancer pathway proteins. Notably, we found that
various proteins related to apoptosis including 14-3-3ζ, Stat3,
Jab1 and p27 were altered in the CNE2 cells but not in the CNE2R
cells. It has been confirmed that Jab1 plays a crucial role in the
inactivation of several key tumor suppressors, such as p27, p53 and
Smad 417 (21–23). Importantly, Jab1 and p27 protein
levels were found to be inversely correlated in NPC cells (24). Upstream of Jab1, Stat3 acts as a
positive regulator of Jab1. Its phosphorylation aids in its
translocation to the nucleus, resulting in activation of Jab1
(25). Our findings suggest that
SZ-685C downregulated the activation of Stat3 after SZ-685C
treatment for 12 h, and the level of p27 increased after treatment
with SZ-685C for 48 h. There is evidence that 14-3-3ζ as a
chaperonin has a high affinity to Stat3. It is essential for
activation of p-Stat3, binding to Ser727 position of Stat3
(26). However, we found that the
level of 14-3-3ζ increased in CNE2 cells treated with SZ-685C for
12 h. The helping role of 14-3-3ζ in the Stat3-Jab1-p27 pathway
should be subsequently explored. A few studies have observed that
the activation of Stat3 and Akt concern parallel signaling pathways
involved in tumor resistance, or carcinogenesis, migration and
invasion. One study pointed out that the Akt signaling pathway is
related to apoptosis, and the Stat3 signaling pathway may be
concerned with migration and invasion (27). There is also evidence to suggest
that activation of Stat3 may facilitate the activation of Akt
(28). After treatment with SZ-685C
for 12 h in CNE2 cells, the level of Stat3 was significantly
decreased in a dose-dependent manner, and at the same time the
level of total Akt maintained the same level (data not shown). We
conjectured that activation of Stat3 may occur before inactivation
of Akt, but the mechanism between these two pathways should be
explored next. Collectively, our data strongly suggest that SZ-685C
regulates the Stat3-Jab1-p27 pathway, which is important to its
anticancer effect in CNE2 cells.
We further studied changes in the expression of
various miRs between the two cell lines after treatment with
SZ-685C. Surprisingly, we found that expression of miR-24 was
increased to nearly 50-fold upon SZ-685C treatment for 24 h in CNE2
cells. We proposed that miR-24 may be a key factor in the
pro-apoptotic effect of SZ-685C on CNE2 cells. The relationship
between miR-24 and Stat3-Jab1-p27 should be explored. We supposed
that SZ-685C exhibited the pro-apoptotic activity in both
radiosensitive and radioresistant NPC cells by regulating miRs
leading to a series of changes in cell signaling pathways.
Taken together, we conclude that SZ-685C induced
apoptosis in both radiosensitive and radioresistant NPC cell lines.
We demonstrated that the pro-apoptotic mechanism of SZ-685C was
associated with multi-signaling pathways, not only miR-205-PTEN-Akt
but also Stat3-Jab1-p27, and that miR-24 may participate in the
anticancer effect, but the exact mechanism still needs to be
explored. Although various issues need to be further investigated,
SZ-685C shows promise to become an effective candidate anticancer
drug for NPC treatment.
Acknowledgements
This study was supported by the Guangdong Science
and Technology Program (no. 2008A030201009). We thank Professor Z.
She for providing the drug.
References
|
1
|
Her C: Nasopharyngeal cancer and the
Southeast Asian patient. Am Fam Physician. 63:1776–1782.
2001.PubMed/NCBI
|
|
2
|
Lo KW, To KF and Huang DP: Focus on
nasopharyngeal carcinoma. Cancer Cell. 5:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qin L, Zhang X, Zhang L, et al:
Downregulation of BMI-1 enhances 5-fluorouracil-induced apoptosis
in nasopharyngeal carcinoma cells. Biochem Biophys Res Commun.
371:531–535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schoof CR, Botelho EL, Izzotti A and dos
Vasques LR: MicroRNAs in cancer treatment and prognosis. Am J
Cancer Res. 2:414–433. 2012.PubMed/NCBI
|
|
5
|
Lee KM, Choi EJ and Kim IA: microRNA-7
increases radiosensitivity of human cancer cells with activated
EGFR-associated signaling. Radiother Oncol. 101:171–176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeong SH, Wu HG and Park WY: LIN28B
confers radio-resistance through the posttranscriptional control of
KRAS. Exp Mol Med. 41:912–918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian W, Chen J, He H and Deng Y: MicroRNAs
and drug resistance of breast cancer: basic evidence and clinical
applications. Clin Transl Oncol. Aug 23–2012.(Epub ahead of
print).
|
|
8
|
Kanakkanthara A and Miller JH: MicroRNAs:
novel mediators of resistance to microtubule-targeting agents.
Cancer Treat Rev. 39:161–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qu C, Liang Z, Huang J, et al: MiR-205
determines the radio-resistance of human nasopharyngeal carcinoma
by directly targeting PTEN. Cell Cycle. 11:785–796. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
She Z, Chen S, Lin Y, Yuan J, Pang J and
Li M: SZ-685C preparation method and antitumor application.
Application No: 00810028628.3, Application Date. 2008.6:6
|
|
11
|
Xie G, Zhu X, Li Q, et al: SZ-685C, a
marine anthraquinone, is a potent inducer of apoptosis with
anticancer activity by suppression of the Akt/FOXO pathway. Br J
Pharmacol. 159:689–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Gan B, Liu D and Paik JH: FoxO
family members in cancer. Cancer Biol Ther. 12:253–259. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu X, He Z, Wu J, et al: A marine
anthraquinone SZ-685C overrides adriamycin-resistance in breast
cancer cells through suppressing Akt signaling. Mar Drugs.
10:694–711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hitomi J, Katayama T, Eguchi Y, et al:
Involvement of caspase-4 in endoplasmic reticulum stress-induced
apoptosis and Abeta-induced cell death. J Cell Biol. 165:347–356.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tadros SF, D’Souza M, Zhu X and Frisina
RD: Apoptosis-related genes change their expression with age and
hearing loss in the mouse cochlea. Apoptosis. 13:1303–1321. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
LoPiccolo J, Granville CA, Gills JJ and
Dennis PA: Targeting Akt in cancer therapy. Anticancer Drugs.
18:861–874. 2007.
|
|
17
|
Eke I, Koch U, Hehlgans S, et al: PINCH1
regulates Akt1 activation and enhances radio-resistance by
inhibiting PP1alpha. J Clin Invest. 120:2516–2527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012.PubMed/NCBI
|
|
19
|
Li SS, Yang S, Wang S, Yang XM, Tang QL
and Wang SH: Latent membrane protein 1 mediates the resistance of
nasopharyngeal carcinoma cells to TRAIL-induced apoptosis by
activation of the PI3K/Akt signaling pathway. Oncol Rep.
26:1573–1579. 2011.PubMed/NCBI
|
|
20
|
Kraus AC, Ferber I, Bachmann SO, et al: In
vitro chemo- and radio-resistance in small cell lung cancer
correlates with cell adhesion and constitutive activation of AKT
and MAP kinase pathways. Oncogene. 21:8683–8695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomoda K, Kubota Y and Kato J: Degradation
of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by
Jab1. Nature. 398:160–165. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oh W, Lee EW, Sung YH, et al: Jab1 induces
the cytoplasmic localization and degradation of p53 in coordination
with Hdm2. J Biol Chem. 281:17457–17465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wan M, Cao X, Wu Y, et al: Jab1
antagonizes TGF-beta signaling by inducing Smad4 degradation. EMBO
Rep. 3:171–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan Y, Zhang Q, Tian L, et al: Jab1/CSN5
negatively regulates p27 and plays a role in the pathogenesis of
nasopharyngeal carcinoma. Cancer Res. 72:1890–1900. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shackleford TJ, Zhang Q, Tian L, et al:
Stat3 and CCAAT/enhancer binding protein beta (C/EBP-beta) regulate
Jab1/CSN5 expression in mammary carcinoma cells. Breast Cancer Res.
13:R652011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Chen F, Li W, et al: 14-3-3ζ
interacts with stat3 and regulates its constitutive activation in
multiple myeloma cells. PLoS One. 7:e295542012.
|
|
27
|
Azijli K, Yuvaraj S, Peppelenbosch MP, et
al: Kinome profiling of non-canonical TRAIL signaling reveals
RIP1-Src-STAT3 dependent invasion in resistant non-small cell lung
cancer cells. J Cell Sci. 125:4651–4661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen F: JNK dependent Stat3
phosphorylation contributes to Akt activation in response to
arsenic exposure. Toxicol Sci. 129:363–371. 2012. View Article : Google Scholar : PubMed/NCBI
|