Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common and the third most lethal cancer worldwide, accounting for
550,000 deaths annually. At present, Southern Italy has the highest
rates of HCC in Europe (1). HCC is
unique among cancers, occurring mostly in patients with a known
risk factor; 90% of HCCs develop in the context of chronic liver
inflammation and cirrhosis. Major risk factors for HCC include
infection with hepatitis B (HBV) and C (HCV) viruses, alcoholic
liver disease, and possibly non-alcoholic fatty liver disease. Less
common causes include hereditary hemochromatosis,
α1-antitrypsin deficiency, autoimmune hepatitis, certain
types of porphyria and Wilson’s disease (2). HBV and HCV viruses are the major cause
of liver disease worldwide. Fortunately, the HBV vaccine has led to
a substantial decline in the number of new cases of acute hepatitis
B among children, adolescents and adults in wWestern countries
since the mid-1980s (3). This
success is not yet duplicable for HCV, where active or passive
vaccination is not available.
Approximately 80% of newly infected patients develop
a chronic infection, of which an estimated 10–20% develop cirrhosis
and 1–5% advance to end-stage liver cancer over a period of 20–30
years (4). The management of
patients at risk for developing HCC remains challenging. The
detection and diagnosis of liver cancer at an early stage may
improve the prognosis for such patients. An increased understanding
of cancer biology and technological advances have enabled the
identification of a multitude of pathological, genetic and
molecular events that drive hepatocarcinogenesis, leading to the
discovery of numerous potential biomarkers in this disease
(5).
Tumor-induced angiogenesis is a pathophysiological
condition that results from the aberrant deployment of normal
angiogenesis. HCC is generally characterized as a hypervascular
tumor of rapid growth with the pathological formation of new blood
vessels (angiogenesis), a feature that has implications for
investigative procedures applied for its detection (6). It is unclear whether or not
angiogenesis merely represents a homeostatic mechanism aimed at
ensuring an adequate oxygen and nutrient supply or one that exerts
an additional pathogenetic role contributing to liver damage
(7). Among features of the
vasculature in the liver not found in other solid tissues, are the
hepatic sinusoids, the characteristics of which include the
presence of liver sinusoidal endothelial cells (LSECs) that possess
distinctive fenestrations and pericytes, or hepatic stellate cells
(HSCs). Angiogenesis in HCC depends on the same fundamental
principles of activation, proliferation and migration of
endothelial cells that occur in other tumors and diseases in which
enhanced angiogenesis occurs (8).
In general, it is known that tumor angiogenesis is
influenced by the microenvironment and is modulated by several pro-
and anti-angiogenic factors released from tumor cells,
tumor-associated inflammatory cells, and/or from the extracellular
matrix and by different signaling pathways. Mechanistically driven
by tumor progression, these factors may be present in serum,
reflecting the overall angiogenic activity of tumors (9). Since tumor progression and patient
survival correlate with the serum levels of angiogenic factors
(10) in several types of cancer
such as HCC, they are thus ideal prognostic biomarker candidates of
the chronic HCV infection process leading to cirrhosis and HCC.
In the present study, we discovered prognostic
biomarkers for the chronic hepatitis C (CHC) virus infection
process leading to liver cirrhosis (LC) and HCC by composite
profiling of serum angiogenic factors using the Bio-Plex Pro™ Human
Cancer Biomarker Panel 1, a 16-plex unique blend of magnetic
bead-based assays. The advantages of this multiplex approach
include specimen conservation, limited sample handling, increased
throughput and reduced labor costs.
Patients and methods
Patients
In the current study, we enrolled 30 CHC patients
(15 females and 15 males), 30 HCV-related LC patients (16 females
and 14 males), 26 HCC patients (8 females and 18 males) and 20
healthy control subjects (11 females and 9 males). This was based
upon our interest in studying the progression from chronic liver
damage to cirrhosis and cancer. In particular, the severity of
cirrhosis was defined by clinical diagnosis in the presence of
liver functional insufficiency, ascites and encephalopathy
(Child-Pugh score B and C) or by liver biopsies in the case of
initial chronic hepatopathy without clear liver laboratory function
tests of cirrhotic evolution (Child-Pugh score A). However, HCC
patients had HCV-related cirrhosis, and a potentially curative
resection with tumor-free margins, macro- and microscopically.
The clinical characteristics of all the study
participants are listed in Table I.
The patients with CHC, LC and HCC had higher serum transaminase
(alanine aminotransferase (ALT) and aspartate aminotransferase
(AST) levels compared to the control patients, as evaluated in the
healthy donors. Moreover, patients with LC and cancer presented
higher bilirubin and lower albumin levels and platelet counts (PLT)
compared to the control patients. LC and HCC patients had normal
α-fetoprotein (AFP) levels and no clinical manifestations of
cancer. This study was approved by the Ethics Committee of the
National Cancer Institute ‘G. Pascale Foundation’ - Oncology
Research Center of Mercogliano (CROM), Mercogliano, Italy and
written informed consent was obtained from all participants.
 | Table IClinical characteristics of patients
with chronic hepatitis C virus (CHC), liver cirrhosis (LC) and
hepatocellular carcinoma (HCC) with CHC-related hepatitis and
LC. |
Table I
Clinical characteristics of patients
with chronic hepatitis C virus (CHC), liver cirrhosis (LC) and
hepatocellular carcinoma (HCC) with CHC-related hepatitis and
LC.
| Characteristics | CHC | LC | HCC | Control range |
|---|
| Age (years) | 63.86 | 67.96 | 70 | 60.92 |
| Gender (M/F) | 15 M/15 F | 14 M/16 F | 18 M/8 F | 9 M/11 F |
| AST (IU/l) | 70.69 | 80.54 | 92.1 | 5–40 |
| ALT (IU/l) | 120.90 | 71.96 | 106.7 | 7–56 |
| Total bilirubin
(mg/dl) | 0.91 | 1.70 | 1.68 | 0.20–1.30 |
| Albumin (g/dl) | 4.11 | 2.61 | 3.0 | 3.5–5 |
| PLT (ml) | 187,413 | 113,875 | 144,712 | 150,000–400,000 |
| HCV-PCR RNA | Positive | Positive | Positive | |
| HCV genotype (no. of
patients) | 1 (18), 2 (12) | 1 (22), 2 (8) | 1 (15), 2 (11) | |
| AFP (ng/ml) | <10 | <20 | >20 | |
| Child-Pugh score (no.
of patients) | | A (12), B (10), C
(8) | A (10), B (10), C
(6) | |
| Tumor number (no. of
patients) | | | 1 (17), 2 (4), 3 (1)
>3 (4) | |
| Tumor invasion (no.
of patients) | | | T1 (8), T2 (9), T3
(9) | |
| Tumor size (no. of
patients) | | | <2 (3), 2–5 (5),
>5 (18) | |
Bio-Plex assay
Blood samples were collected from a peripheral vein
and kept on ice. Serum was collected using centrifugation (3,000
rpm for 10 min at 4°C), aliquoted and stored at −80°C until
analysis. A multiplex biometric enzyme-linked immunosorbent assay
(ELISA)-based immunoassay containing dyed microspheres conjugated
with a target protein-specific monoclonal antibody was used,
according to the manufacturer’s instructions (Bio-Plex; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The following soluble
molecules were measured using the Bio-Plex Pro Human Cancer
Biomarker Panel 1, a 16-plex multiplex immunoassay: sEGFR,
fibroblast growth factor (FGF)-basic, follistatin,
granulocyte-colony stimulating factor (G-CSF), soluble human
epidermal growth factor receptor-2 (sHER-2/neu; ErbB-2), hepatocyte
growth factor (HGF), sIL-6Ra, leptin (LEP), osteopontin,
platelet-derived growth factor (PDGF)-AB/BB, platelet endothelial
cell adhesion molecule-1 (PECAM-1), prolactin (PRL), stem cell
factor (SCF), sTIE-2, soluble vascular endothelial growth factor
receptor (sVEGFR)-1 and sVEGFR-2.
Each experiment was performed in duplicate using the
procedure described in our previous studies (11–13).
Serum levels of the proteins were determined using a Bio-Plex array
reader (Luminex, Austin, TX, USA) that quantifies multiplex
immunoassays in a 96-well plate with extremely small fluid volumes.
The analyte concentration was calculated using a standard curve,
with the software provided by the manufacturer (Bio-Plex Manager
Software).
Data analysis and statistics
The non-parametric Mann- Whitney U test was used to
evaluate differences between protein ratios in the patients and
healthy controls. The T-test was used to compare the serum levels
of these proteins evaluated in the different groups of patients.
The correlations between the molecule concentrations and
clinical/biochemical data were determined using the Pearson’s
correlation co-efficient. P<0.05 was considered to indicate a
statistically significant difference. The statistical program Prism
4 (GraphPad Software, San Diego, CA, USA) was employed.
Results and Discussion
Comparison between patients and healthy
donors
The varying levels of the proteins present in the
serum of CHC, LC and HCC patients compared to the healthy controls
are presented in Table II (data
not statistically significant, not shown). Higher levels of
sHER-2/neu (ERbB-2), sIL-6Ra, LEP and PECAM-1 were secreted by all
the patients, whereas higher levels of HGF and PRL were secreted
only by LC and HCC patients.
 | Table IIVarying levels of proteins present in
the serum of CHC, LC and HCC patients compared to the healthy
controls. |
Table II
Varying levels of proteins present in
the serum of CHC, LC and HCC patients compared to the healthy
controls.
| Molecules | CHC vs. controls | LC vs. controls | HCC vs. controls |
|---|
| sHER-2/neu | 0.0002c | 0.00015c | 0.0002c |
| HGF | 0.0655 | 0.0057b | 0.0004c |
| sIL-6Ra | 0.0011b | 0.0001c | 0.0006c |
| Leptin | 0.0130a | 0.0039b | 0.0078b |
| PECAM-1 | 0.0001c | 0.0003c | 0.0008c |
| Prolactin | 0.0622 | 0.0430a | 0.00199b |
HER-2/neu, also termed ErbB-2, is encoded by the
ERBB2 gene. HER-2/neu is expressed in a variety of tissues of
epithelial origin and plays a fundamental role in cellular
proliferation and differentiation during fetal development.
Previous studies have shown that the overexpression of this protein
in HCC tissues plays a role in tumor invasion, metastasis and
progression (9), underling that its
amplification is not the primary mechanism in the development of
liver tumors and contributes to one of the steps of multistage
carcinogenesis (14).
sIL-6Ra is a soluble form of the interleukin-6
receptor and is a ligand-binding protein that constitutes the
extracellular part of the IL-6 receptor. It markedly prolongs the
IL-6 plasma half-life, and, after having bound IL-6, can interact
with membrane-bound gp130, thereby leading to the activation of the
intracellular signaling pathway (15), acting as an agonist and stimulating
a variety of cellular responses, including proliferation,
differentiation and the activation of inflammatory processes
(16). Additionally, through this
mechanism, primary unresponsive cells expressing only gp130 and no
gp80 can be activated through the sIL-6R/IL-6 complex. This process
has been termed ‘trans-signaling’ (17). Recently, we reported that IL-6
levels were elevated in CHC, LC and HCC patients (11,12).
In the literature, sIL-6R overexpression has also been observed in
various pathological conditions such as liver diseases and HCC,
indicating that serum IL-6 and its soluble receptor levels
correlate with liver function impairment as well as the degree of
liver fibrosis in patients with HCV infection (18). Studies using animal models have
shown that transgenic mice expressing high levels of IL-6 and
sIL-6R develop hepatic nodular hyperplasia and signs of sustained
hepatocyte proliferation, suggesting that IL-6 and sIL-6R may
provide the primary stimulus to cell proliferation and are involved
in the development of HCC (19).
LEP is mainly produced by adipose tissues, detected
in activated hepatic stellate cells and, although it serves as a
regulatory mediator between the brain and the periphery through
modulating the hypothalamic-pituitary-adrenal (HPA) axis, its
circulating level is also regulated by hormones secreted by the HPA
system, including corticosteroids, PRL, and insulin (20). Recently, we found elevated LEP
levels in CHC and LC patients, suggesting that this protein is part
of the immune response and host defense (13) during infection and inflammation, and
acts as a paracrine modulator of hepatic fibrogenesis (21,22).
PECAM-1 is constitutively expressed in platelets,
monocytes, neutrophils, natural killer (NK) and CD8 T cells. It is
highly expressed in continuous endothelial cells at cell-cell
borders, whereas its expression is weak in sinusoidal endothelial
cells (SEC) (23). PECAM-1 plays a
putative role in the inflammatory process and leukocyte-endothelial
interaction, particularly in the transmigration of leukocytes
through intercellular junctions, and has been implicated in cell
survival and angiogenesis (24).
Significantly higher PECAM-1 concentrations in patients with more
advanced hepatitis and varying fibrotic levels suggest that this
protein may reflect liver disease progression (23) and that serum PECAM-1 measurement may
be useful in distinguishing between patients with or without
fibrosis (25).
PRL is a polypeptide hormone secreted by the
anterior pituitary gland, and plays a physiological role in breast
development and lactation. When produced in excess it may lead to
sterility, menorrhea and loss of libido (26). LC is associated with elevated levels
of PRL. In fact, this is commonly attributed to an impaired hepatic
metabolism of estrogens. In particular, LC is associated with
profound endocrinological disturbances and this may explain why
higher levels of this protein are found in LC, but not in CHC
patients. Until recently, elevated PRL levels in LC patients were
considered to be induced mainly by the ineffective elimination of
hormones by the diseased liver. At present, the pathogenesis of
disturbed hormonal function in LC is known to be more complex,
since it usually involves disturbed secretion and feedback
mechanisms (27). However, high PRL
levels have also been found to correlate with the severity of liver
disease, particularly in patients with ascites and hepatic
encephalopathy (28).
Finally, in our previous studies (11–13),
HGF was found significantly upregulated in LC and HCC patients but
not in patients with CHC. This protein is a multifunctional growth
factor that regulates growth and cell motility. It exerts mitogenic
effects on hepatocytes and epithelial cells, and plays diverse
roles in organ development, tissue regeneration and tumor
progression (29). Moreover, it has
been implicated, along with IL-6, IL-8 and IL-1, in the hepatic
stellate cell-activation pathway. Hence, we suggested that this
growth factor could be used as an index of cellular growth and of
the development of HCC in LC patients (11–13).
Comparison between patients with CHC, LC
and HCC
We compared the mean concentrations of these 6
molecules in 3 patient groups using the Student’s t-test. As shown
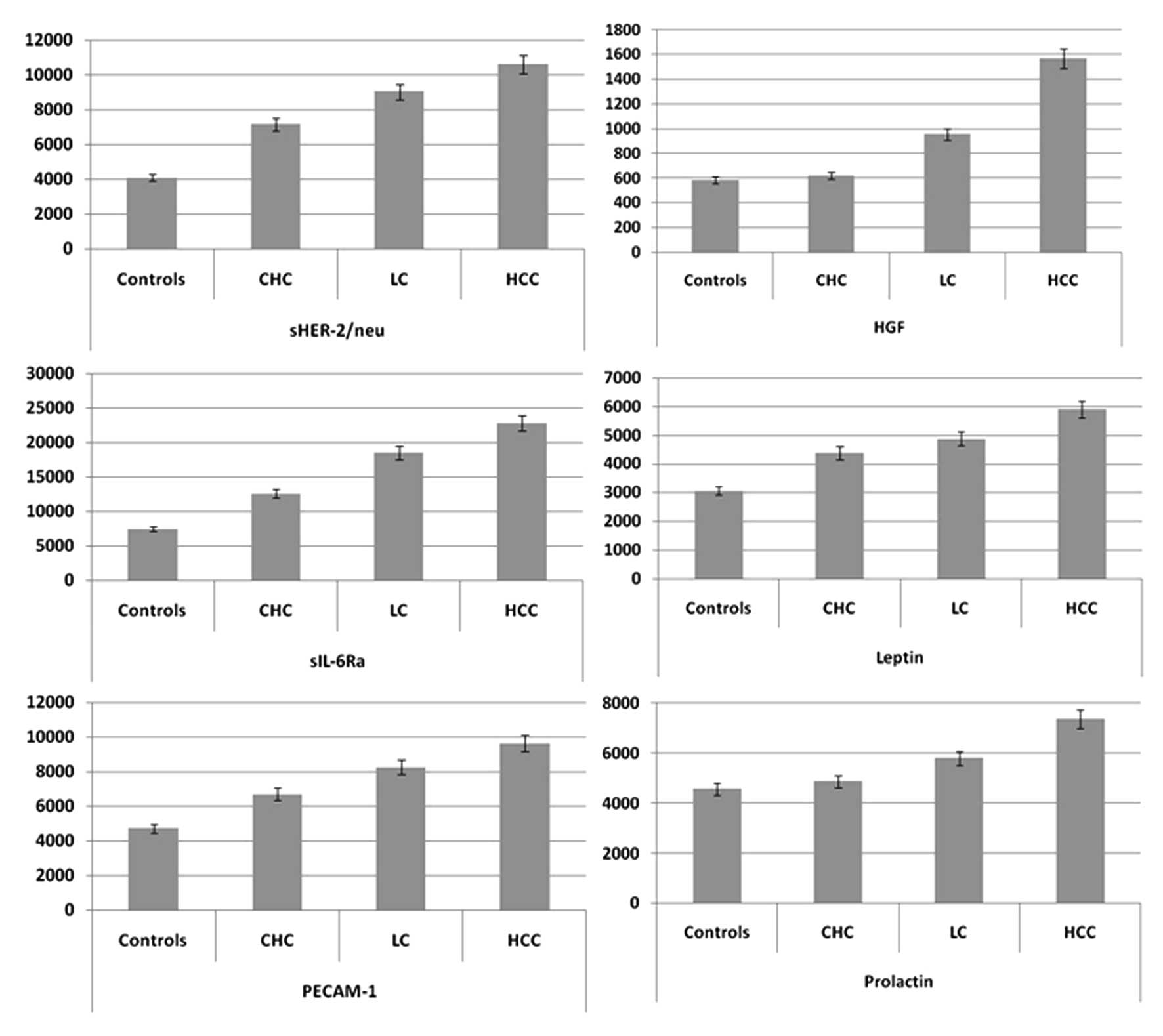
in Fig. 1, the concentrations of
sHER-2/neu, sIL-6Ra, LEP and PECAM-1 were higher (P<0.05) in
patients with LC compared to those with CHC, while the
concentrations of sHER-2/neu, HGF, sIL-6Ra, LEP, PECAM-1 and PRL
were higher in HCC patients compared to those with LC. Hence, the
expression of these 4 molecules, i.e., sHER-2/neu, sIL-6Ra, LEP and
PECAM-1, tends to increase in the progression of chronic
inflammation leading to LC and HCC. Consequently, their evaluation
may be used for prognostic studies and therapy guidance.
Of note, HGF and PRL levels varied significantly in
LC patients compared to the healthy controls and CHC patients,
while their concentrations in HCC patients were higher compared to
patients with LC. This suggests that the levels of HGF and PRL may
have increased during the progression of chronic inflammation to LC
and cancer. Therefore, we suggest that HGF and PRL may represent
the degree of the carcinogenic state in the liver of chronic
inflammation and LC patients, and may potentially be used for
predicting the progression to HCC in patients with chronic
HCV-related liver disease.
The serum levels of the significant proteins in CHC,
LC and HCC patients were then compared with clinical/biochemical
data using Pearson’s correlation co-efficient. sIL-6Ra showed a
significant correlation (P<0.01) with the fibrotic stage in CHC
patients, with Child-Pugh score in patients with LC and with tumor
size in patients with HCC. These results confirm that sIL-6Ra may
be used as a predictor of liver damage and of inflammatory
processes leading to fibrosis, cirrhosis and subsequently, to
cancer.
Interactomic analysis
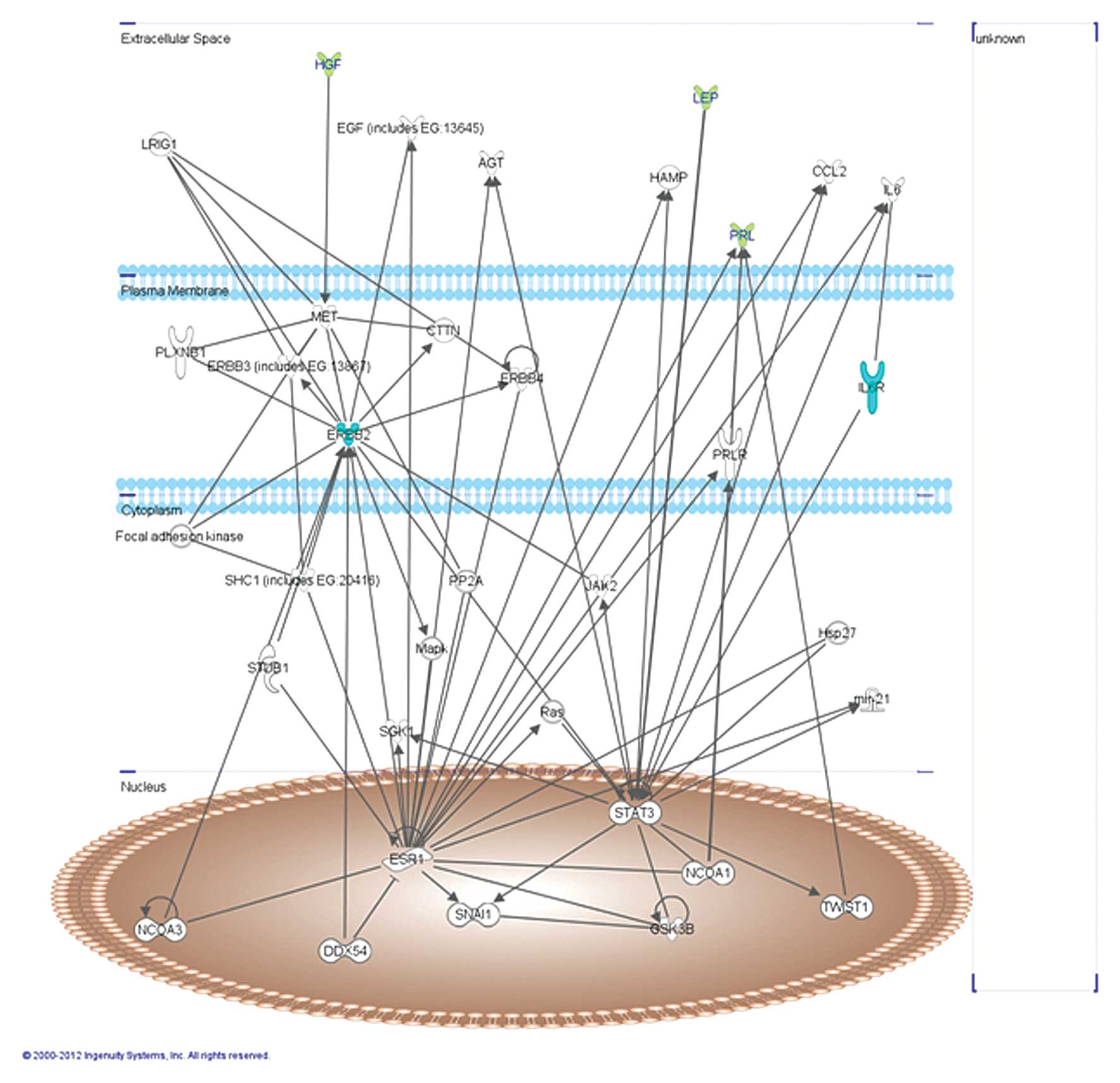
The abovementioned molecules were analyzed using the
Ingenuity Pathway Analysis (IPA) software version 7.1 (Ingenuity
Systems, Inc., Redwood City, CA, USA) that has created a network on
the basis of associated functions and data mining from experimental
studies reported in the literature (Fig. 2). This graph presents 2 hub genes,
signal transducer and activator of transcription (STAT3) and
estrogen receptor 1 (ESR1).
In particular, STAT3 is activated in response to
various cytokines and growth factors, including IL-6 and LEP. In
fact, the binding of LEP or IL-6 to their receptors triggers the
signal transduction through the stimulation of JAK2-STAT3 pathway
(30,31) and induces the phosphorylation of its
tyrosine 705. However, phosphorylated STAT3 dimerizes and
translocates to the nucleus, where it regulates gene transcription.
Thus, it can be hypothesized that in these patients, the higher
level of LEP, IL-6 and its receptor may induce increased STAT3
expression and consequently, an increase in angiotensinogen (AGT)
and chemokine (C-C motif) ligand 2 (CCL2) levels.
ESR1 is associated with ERbB-2 and PRL receptor
(PRLR) at the plasma membrane (32). Notwithstanding, ERbB-2 is frequently
co-activated with EGF and c-Met that binds HGF. In particular, this
growth factor regulates cell growth, cell motility and
morphogenesis by activating a tyrosine kinase signaling cascade
after binding to the proto-oncogenic c-Met receptor that is highly
expressed in the liver (33). These
data prove the existence of a correlation between 5 statistically
significant proteins, ErbB-2, sIL-6Ra, PRL, HGF and LEP.
In conclusion, in the present study, we evaluated
the analytical performance of the Bio-Plex Pro Human Cancer
Biomarker Panel 1, a 16-plex unique blend of magnetic bead-based
assays, in the serum of CHC, LC and HCC patients for the composite
profiling of angiogenic factors. Our results demonstrate that: i)
high levels of HGF and PRL in LC and HCC patients represent the
degree of the carcinogenic state in the liver of chronic
inflammation, ii) high levels of sHER-2/neu, sIL-6Ra, LEP and
PECAM-1 in CHC, LC and HCC patients suggest that these 4 proteins
are markers of the chronic inflammation progression that leads to
LC and HCC and iii) sIL-6Ra correlates with the fibrosis stage in
CHC patients, with the Child-Pugh score in patients with LC, and
with tumor size in patients with HCC, confirming that this protein
may be used as a predictor of liver damage and inflammatory process
leading to liver fibrosis and cirrhosis and subsequently, to
cancer.
References
|
1
|
Fusco M, Girardi E, Piselli P, Palombino
R, Polesel J, Maione C, Scognamiglio P, Pisanti FA, Solmone M, Di
Cicco P, Ippolito G, Franceschi S and Serraino D: Epidemiology of
viral hepatitis infections in an area of southern Italy with high
incidence rates of liver cancer. Eur J Cancer. 44:847–853. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castello G, Scala S, Palmieri G, Curley SA
and Izzo F: HCV-related hepatocellular carcinoma: From chronic
inflammation to cancer. Clin Immunol. 134:237–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueno Y, Sollano JD and Farrell GC:
Prevention of hepatocellular carcinoma complicating chronic
hepatitis C. J Gastroenterol Hepatol. 24:531–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Behne T and Copur MS: Biomarkers for
hepatocellular carcinoma. Int J Hepatol. 2012:8590762012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forner A, Vilana R, Ayuso C, Bianchi L,
Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM, Brú C and
Bruix J: Diagnosis of hepatic nodules 20 mm or smaller in
cirrhosis: prospective validation of the noninvasive diagnostic
criteria for hepatocellular carcinoma. Hepatology. 47:97–104. 2008.
View Article : Google Scholar
|
|
7
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Semela D and Dufour JF: Angiogenesis and
hepatocellular carcinoma. J Hepatol. 41:864–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang JK, Pan PL, Wu YM, Xiao JJ and Peng
JW: Expression of HER-2/neu oncogene in hepatocellular carcinoma
and the clinical implications. Nan Fang Yi Ke Da Xue Xue Bao.
30:326–328. 2010.(In Chinese).
|
|
10
|
Li D, Chiu H, Gupta V and Chan DW:
Validation of a multiplex immunoassay for serum angiogenic factors
as biomarkers for aggressive prostate cancer. Clin Chim Acta.
413:1506–1511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Capone F, Costantini S, Guerriero E,
Calemma R, Napolitano M, Scala S, Izzo F and Castello G: Serum
cytokine levels in patients with hepatocellular carcinoma. Eur
Cytokine Netw. 21:99–104. 2010.PubMed/NCBI
|
|
12
|
Costantini S, Capone F, Guerriero E, Maio
P, Colonna G and Castello G: Serum cytokine levels as putative
prognostic markers in the progression of chronic HCV hepatitis
leading to cirrhosis. Eur Cytokine Netw. 21:251–256.
2010.PubMed/NCBI
|
|
13
|
Costantini S, Capone F, Guerriero E,
Marfella R, Sorice A, Maio P, Di Stasio M, Paolisso G, Castello G
and Colonna G: Cytokinome profile of patients with type 2 diabetes
and/or chronic hepatitis C infection. PLoS One. 7:e394862012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bacaksiz A, Sahin FI, Bilezikci B and
Yilmaz Z: Determination of HER-2/Neu status in hepatocellular
carcinoma cases. Genet Test. 12:211–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rose-John S: Interleukin-6 biology is
coordinated by membrane bound and soluble receptors. Acta Biochim
Pol. 50:603–611. 2003.PubMed/NCBI
|
|
16
|
Rose-John S and Heinrich PC: Soluble
receptors for cytokines and growth factors: generation and
biological function. Biochem J. 300:281–290. 1994.PubMed/NCBI
|
|
17
|
Montero-Julian FA: The soluble IL-6
receptors: serum levels and biological function. Cell Mol Biol.
47:583–597. 2001.PubMed/NCBI
|
|
18
|
Migita K, Abiru S, Maeda Y, Daikoku M,
Ohata K, Nakamura M, Komori A, Yano K, Yatsuhashi H, Eguchi K and
Ishibashi H: Serum levels of interleukin-6 and its soluble
receptors in patients with hepatitis C virus infection. Hum
Immunol. 67:27–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maione D, Di Carlo E, Li W, Musiani P,
Modesti A, Peters M, Rose-John S, Della Rocca C, Tripodi M, Lazzaro
D, Taub R, Savino R and Ciliberto G: Coexpression of IL-6 and
soluble IL-6R causes nodular regenerative hyperplasia and adenomas
of the liver. EMBO J. 17:5588–5597. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balci H, Akgun-Dar K, Gazioglu N, Kapucu
A, Bolayirli M and Oz B: The relationship between prolactin (PRL),
leptin, nitric oxide (NO), and cytokines in patients with
hyperprolactinemia. Pituitary. 12:170–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reeves HL and Friedman SL: Activation of
hepatic stellate cells - a key issue in liver fibrosis. Front
Biosci. 7:d808–d826. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Otte C, Otte JM, Strodthoff D, Bornstein
SR, Fölsch UR, Mönig H and Kloehn S: Expression of leptin and
leptin receptor during the development of liver fibrosis and
cirrhosis. Exp Clin Endocrinol Diabetes. 112:10–17. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katz SC, Pillarisetty VG, Bleier JI, Shah
AB and DeMatteo RP: Liver sinusoidal endothelial cells are
insufficient to activate T cells. J Immunol. 173:230–235. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Newman PJ and Newman DK: Signal
transduction pathways mediated by PECAM-1. New roles for an old
molecule in platelet and vascular biology. Arterioscler Thromb Vasc
Biol. 23:953–964. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kukla M, Zwirska-Korczala K, Gabriel A,
Janczewska-Kazek E, Berdowska A, Wiczkowski A, Rybus-Kalinowska B,
Kalinowski M, Ziolkowski A, Wozniak-Grygiel E, Waluga M and Nowak
B: sPECAM-1 and sVCAM-1: role in pathogenesis and diagnosis of
chronic hepatitis C and association with response to antiviral
therapy. Therap Adv Gastroenterol. 2:79–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kollerová J, Koller T and Payer J:
Endocrine changes in liver disease. Vnitr Lek. 58:24–30. 2012.(In
Slovak).
|
|
27
|
Simon-Holtorf J, Mönig H, Klomp HJ,
Reinecke-Lüthge A, Fölsch UR and Kloehn S: Expression and
distribution of prolactin receptor in normal, fibrotic, and
cirrhotic human liver. Exp Clin Endocrinol Diabetes. 114:584–589.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Payer J, Koller T, Baqi L and Kollerova J:
Prolactin levels in patients with cirrhosis increase with severity
of liver disease. Endocrine Abstracts. 16:P4362008.
|
|
29
|
Gentile A, Trusolino L and Comoglio PM:
The Met tyrosine kinase receptor in development and cancer. Cancer
Metastasis Rev. 27:85–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sahu A: Leptin signaling in the
hypothalamus: emphasis on energy homeostasis and leptin resistance.
Front Neuroendocrinol. 24:225–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
15:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pancholi S, Lykkesfeldt AE, Hilmi C,
Banerjee S, Leary A, Drury S, Johnston S, Dowsett M and Martin L-A:
ERBB2 influences the subcellular localization of the estrogen
receptor in tamoxifen-resistant MCF-7 cells leading to the
activation of AKT and RPS6KA2. Endocr Relat Cancer. 15:985–1002.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naldini L, Vigna E, Narsimhan RP, Gaudino
G, Zarnegar R, Michalopoulos GK and Comoglio PM: Hepatocyte growth
factor (HGF) stimulates the tyrosine kinase activity of the
receptor encoded by the proto-oncogene c-MET. Oncogene. 6:501–504.
1991.PubMed/NCBI
|