Introduction
Although advances have been made in the surgical and
non-surgical therapy of head and neck squamous carcinoma (HNSCC),
the mortality rate from this disease has remained nearly constant
over the last few years. This is mainly due to the development of
therapy-resistant local and regional recurrences (1). Strategies of treatment apart from
surgery, such as chemotherapy and radiation, eradicate a majority
of proliferating cells in malignant tumors, but there is increasing
evidence that there is a subpopulation of resistant tumor cells
that cannot be reached by these regimens called cancer stem cells
(CSCs) (2). As a result, it is
imaginable that these cells are essential and responsible for
initiation, but also maintenance and recurrence of malignant
disease. CSCs have features of somatic stem cells such as
self-renewal, differentiation and extensive proliferation ability.
In recent years, the CSC hypothesis has also been assigned to HNSCC
(3,4). Prince et al showed that
CD44+ cells that were isolated out of the bulk of an
HNSCC tumor, but not the CD44− cancer cells, gave rise
to new tumors in a mouse model. These cells typically comprise
<10% of the cells in an HNSCC tumor (3). Other markers have been evaluated as
cancer stem cell markers in HNSCC as well, such as ALDH1 (5) and CD133 (6). Current research aims to discover a
combination of including and excluding surface markers to finally
isolate the ‘real CSC in HNSCC’.
CD44 is an integral cell membrane glycoprotein. It
comprises different isoforms that arise from alternative splicing
of a region of variable exons. Its apparent molecular mass ranges
from 85 to 250 kDa, as its isoforms differ in the primary amino
acid sequence and the amount of N- and O-glycosylation (7). At least 20 variants of CD44 have been
reported due to the alternative splicing of 10 exons that encode
the membrane's proximal portion of the extracellular domain
(8–10).
In 1991, Günthert et al(11) and Hofmann et al(12) showed that the expression of CD44
gave metastatic potential to a non-metastatic cell line in a rat
carcinoma model. Several analyses have indicated that there is a
correlation between the expression of CD44 and progression,
metastasis and prognosis of malignant disease and this has been
shown in different types of epithelial malignancies, e.g. gastric
cancer (13), hepatocellular
carcinoma (14) or gynecological
malignancies such as breast (15)
or ovarian cancer (16). The
in-depth analysis of expression markers such as CD44 in tissue
samples of HNSCC patients may reveal their role as potential
prognostic biomarkers or therapeutic targets, e.g. for
antigen-directed immunotherapy.
The analysis of the cancer stem cell niche theory,
where CSCs come into contact with their supportive cells
surrounding them, may provide information concerning cell
trafficking and underlying mechanisms, such as tumor expansion,
recurrence and metastatic progression. The interaction between
SDF-1α and its receptor CXCR4 may play an important role in this
field. SDF-1α is a multifunctional cytokine that is expressed and
secreted by several tissues, e.g. endothelium and stromal cells
(17,18), and is one component of the bulk of
an HNSCC tumor. SDF-1 has a single open reading frame of 282
nucleotides encoding a polypeptide of 93 amino acids. It arises in
two forms, SDF-1α (amino acids 24–88) and SDF-1β (amino acids
24–93) by alternative splicing (18–20).
SDF-1α is the only proven chemoattractant for hematopoietic
progenitor cells (HPCs) to date (18,21–23).
Thus, SDF-1α is considered to be one of the key regulators for HPC
trafficking between the peripheral circulation and the bone marrow
(17,18,23,24).
Faber et al and others have demonstrated that SDF-1α induces
polarization and podia formation in HPCs and leukemic cells
(18,25), two properties that represent
prerequisites for directed locomotion. SDF-1α alone showed a
moderate effect on cell proliferation in CD34+ cells
(18,26), and its effect on survival or
apoptosis of HPCs remains controversial (18,26–28).
Furthermore, the SDF-1-CXCR4 axis plays a crucial role in the
regulation of homing and adhesion to the supportive cellular
microenvironment in the hematopoietic stem cell niche (29). It remains unclear whether this
interaction is also important in the cancer stem cell niche of
malignant epithelial tumors such as HNSCC.
Signal transduction pathways initiated by the
binding of SDF-1α to CXCR4 are not fully understood. Mechanisms
involved in CXCR4 signaling and downstream are multi-faceted and
include Gi-protein-mediated activation of intracellular components
(30).
Herein, we monitored the expression of CD44 as a
cancer stem cell marker and of CXCR4 as a potential target of
interaction between CSCs and their supportive cells in the cancer
stem cell niche in human HNSCC tissue samples. Accordingly, we
evaluated SDF-1 serum levels in the peripheral blood of HNSCC
patients compared to healthy donors to find evidence whether
soluble interactions in the cancer stem cell niche of an HNSCC
tumor can be detected in the periphery of human blood circulation.
These findings may facilitate the development of therapeutic
strategies particularly aimed at CSCs or particularly with regard
to the SDF-1-CXCR4 axis aimed at the interaction of CSCs with their
cellular cancer stem cell niche. Differences in the concentration
of SDF-1 in the peripheral blood of HNSCC patients compared to
healthy humans might lead to new strategies of tumor detection and
the role of SDF-1 as a tumor marker.
Materials and methods
Tissue and peripheral blood sample
collection
A total of 9 HNSCC tissue samples from tumor
patients was selected from a tissue database collected from 1997 to
2010 at the Department of Otorhinolaryngology, Head and Neck
Surgery at the University of Mannheim. Samples were fixated
immediately after excision by freezing in liquid nitrogen. All
samples were confirmed by pathology after H&E staining. The
histological and clinical characteristics of all tumor samples are
summarized in Table I.
 | Table IHNSCC tissue collection. |
Table I
HNSCC tissue collection.
| Patient | Gender | Age (yrs.) | Tumor location | TNM status |
|---|
| 1 | M | 71 | Larynx | T4N0 |
| 2 | M | 68 | Larynx | T3N2b |
| 3 | M | 63 | Larynx | T4N0 |
| 4 | M | NA | Oropharynx | T3N2c |
| 5 | M | 67 | Oropharynx | T4N2M0 |
| 6 | F | 50 | Oropharynx | T3N2c |
| 7 | M | 76 | Hypopharynx | NA |
| 8 | M | 59 | Hypopharynx | T4N2b |
| 9 | F | 63 | Hypopharynx | T2N2b |
In addition to the tissue collection, peripheral
blood samples were obtained from HNSCC patients and healthy donors.
Peripheral blood was collected before, during, but never after
tumor surgery. A group of 11 healthy blood donors served as a
control. Blood samples were centrifuged at 2500 rpm for 10 min.
Afterwards, serum samples were harvested and fixated by freezing at
−80°C. Characteristics of the peripheral blood samples are
summarized in Table II.
 | Table IIHNSCC patients enrolled for blood
sample collection. |
Table II
HNSCC patients enrolled for blood
sample collection.
| Patient | Gender | Age (yrs.) | Tumor location | TNM |
|---|
| 1 | M | 54 | Larynx | T4N2xMx |
| 2 | F | 55 | Larynx | T1N0 |
| 3 | M | 62 | Larynx | T4N0 |
| 4 | M | 70 | Larynx | T4N0 |
| 5 | M | 68 | Larynx | T3N2b |
| 6 | M | 68 | Larynx | NA |
| 7 | M | 55 | Larynx | T4N2b |
| 8 | M | 74 | Larynx | T4N0 |
| 9 | M | 59 | Larynx | NA |
| 10 | M | 57 | Larynx | T4N1 |
| 11 | M | 55 | Oral cavity | T4N1 |
| 12 | M | 69 | Oral cavity | T1N2b |
| 13 | F | 54 | Oral cavity | T1N2b |
| 14 | M | 48 | Oral cavity | NA |
| 15 | M | 49 | Oral cavity | T3N1 |
| 16 | M | 51 | Oral cavity | NA |
| 17 | M | 66 | Oral cavity | T2N0 |
| 18 | F | 50 | Oropharynx | T3N2c |
| 19 | M | 64 | Oropharynx | NA |
| 20 | M | 60 | Oropharynx | T4N2c |
| 21 | M | 64 | Oropharynx | T3N2 |
| 22 | F | 55 | Oropharynx | T4N3 |
| 23 | M | 61 | Hypopharynx | T4N2b |
| 24 | M | 66 | Hypopharynx | T2N0 |
| 25 | M | 76 | Hypopharynx | NA |
| 26 | M | 59 | Hypopharynx | T4N2b |
| 27 | F | 62 | Hypopharynx | T2N2b |
| 28 | M | 51 | Hypopharynx | NA |
| 29 | M | 57 | Hypopharynx | T3N2c |
Immunofluorescence labeling
To detect the expression of CD44 and CXCR4 in HNSCC
tissue samples, tumors underwent fixation by freezing in liquid
nitrogen as mentioned above. Specimens were sectioned (5-μm),
air-dried and fixated in acetone for 10 min. Afterwards, the
sections were treated with 4% paraformaldehyde (PFA) for 10 min at
room temperature. After three washing steps with PBS, tumor samples
were treated with 1% serum (goat) for another 10 min. Sections were
then incubated with the CD44/CXCR4 antibody (mouse monoclonal,
1:100; Abcam, Cambridge, UK) for 1 h at 37°C followed by incubation
with a secondary biotinylated antibody (anti-mouse, 1:100) for 30
min. After further washing steps with PBS, the sections were
treated with Streptavidin-Cy3 (1:1000)/Streptavidin-Alexia 488
(1:500) for 30 min at room temperature. Subsequently, sections were
stained with DAPI after washing with PBS. Finally, slices were
covered in FluorSave and dried to be evaluated by fluorescence
microscopy.
Enzyme-linked immunosorbent assay
Serum levels of SDF were measured with a human SDF
ELISA kit (R&D Systems, Wiesbaden, Germany). A monoclonal
antibody against soluble SDF was adsorbed to microwells in 96-well
microtiter plates. Samples, including those with standards with
known SDF concentrations, were pipetted into these wells. During
the first incubation, the SDF antigen was added to the wells. After
washing, a biotinylated monoclonal antibody specific for SDF was
incubated, and the enzyme (streptavidin-peroxidase) was added.
Following incubation and washing to remove all unbound enzymes, a
substrate solution was added, which catalyzed a reaction on the
bound enzyme and induced a colored reaction product. The intensity
of this colored product is directly proportional to the
concentration of SDF present in the samples.
Statistical analysis
All results are plotted as mean ± standard
deviation. To estimate the probability of differences, we adopted
the Student t-test (two-tailed distribution, two-sample equal
variance). A probability value <0.05 was considered to denote a
statistically significant result.
Results
Tissue and peripheral blood sample
collection
A total of 9 HNSCC tissue samples and the 29 blood
serum samples were selected from a database collected from 1997 to
2010 at the Department of Otorhinolaryngology, Head and Neck
Surgery at the Faculty of Medicine Mannheim, University of
Heidelberg. Tissue samples used in the study were derived from 2
female and 7 male patients aged 50–76 years (mean age, 65 years).
Blood samples were derived from 5 female and 24 male patients aged
48–76 years (mean age, 60 years). Sites of the primary tumors were
the larynx, oropharynx, hypopharynx and oral cavity. Patient
characteristics are summarized in Table
I for tissue sample collection and in Table II for the blood sample collection.
A group of 11 healthy blood donors, aged 26–87 years (mean age, 50
years) served as a control for experiments concerning the SDF
concentration in peripheral blood of HNSCC patients.
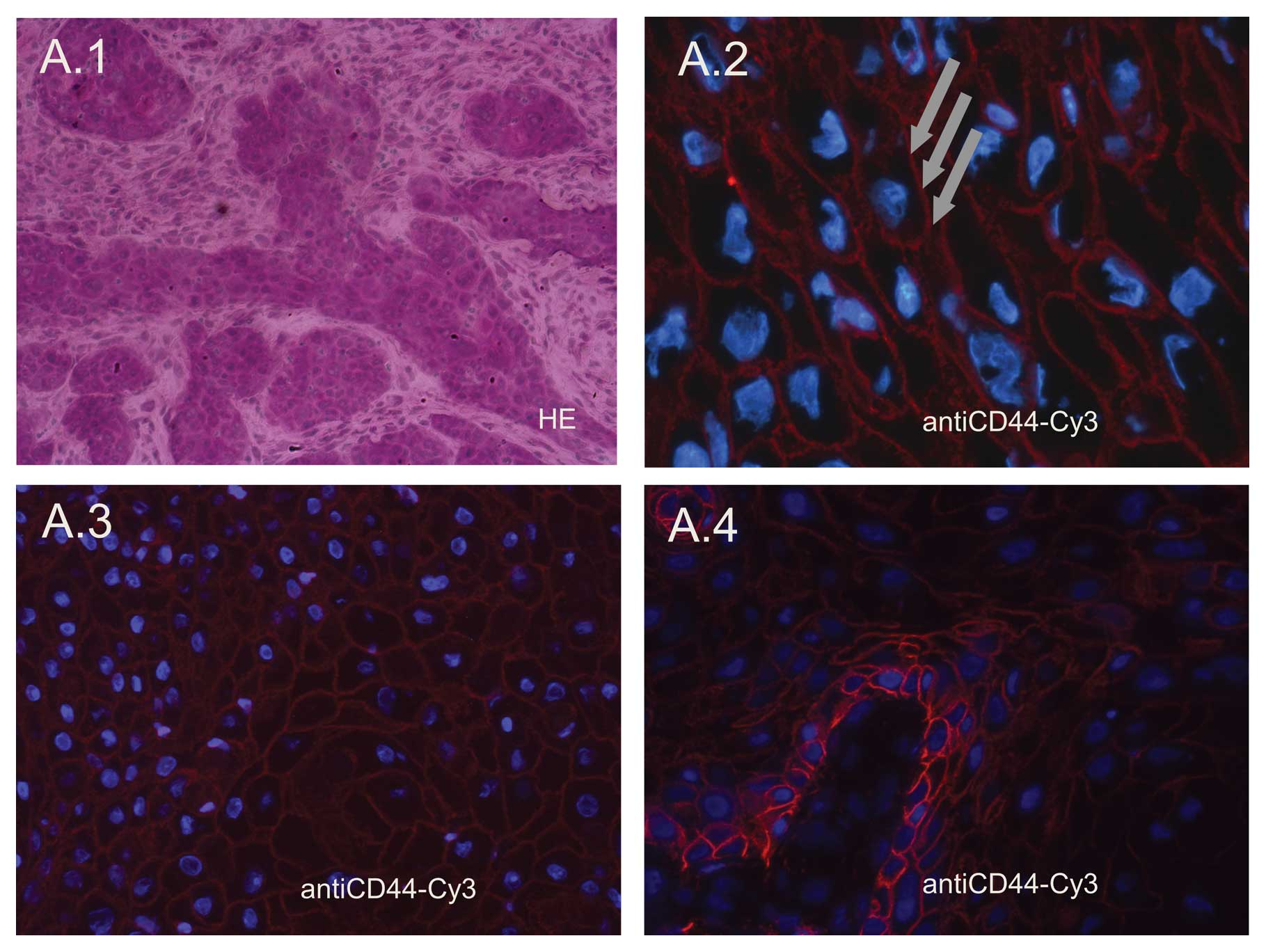
Expression of CD44 in HNSCC tissues
Immunofluorescence labeling was performed to measure
the expression of CD44 in HNSCC tissue samples via staining with
Cy3. In HNSCC tissue samples, expression of CD44 was established in
all cases. Its expression was mainly found in the cells forming the
invasive front of the tumor. This invasive front is in direct
contact with the tumor-surrounding stromal cells, and this
interaction can be postulated as the cancer stem cell niche of
HNSCC (Fig. 1A.4). Tumor samples
stained in our experiments were mainly obtained from the
superficial portions of the HNSCC tumors, although it was noted
that parts located in the center of the tumor had a weaker staining
pattern for CD44 (Fig. 1A.4). In
general CD44 exhibited a membranous staining pattern (Figs. 1A.2 and A.3, and 2B.1). Nuclei were stained with DAPI.
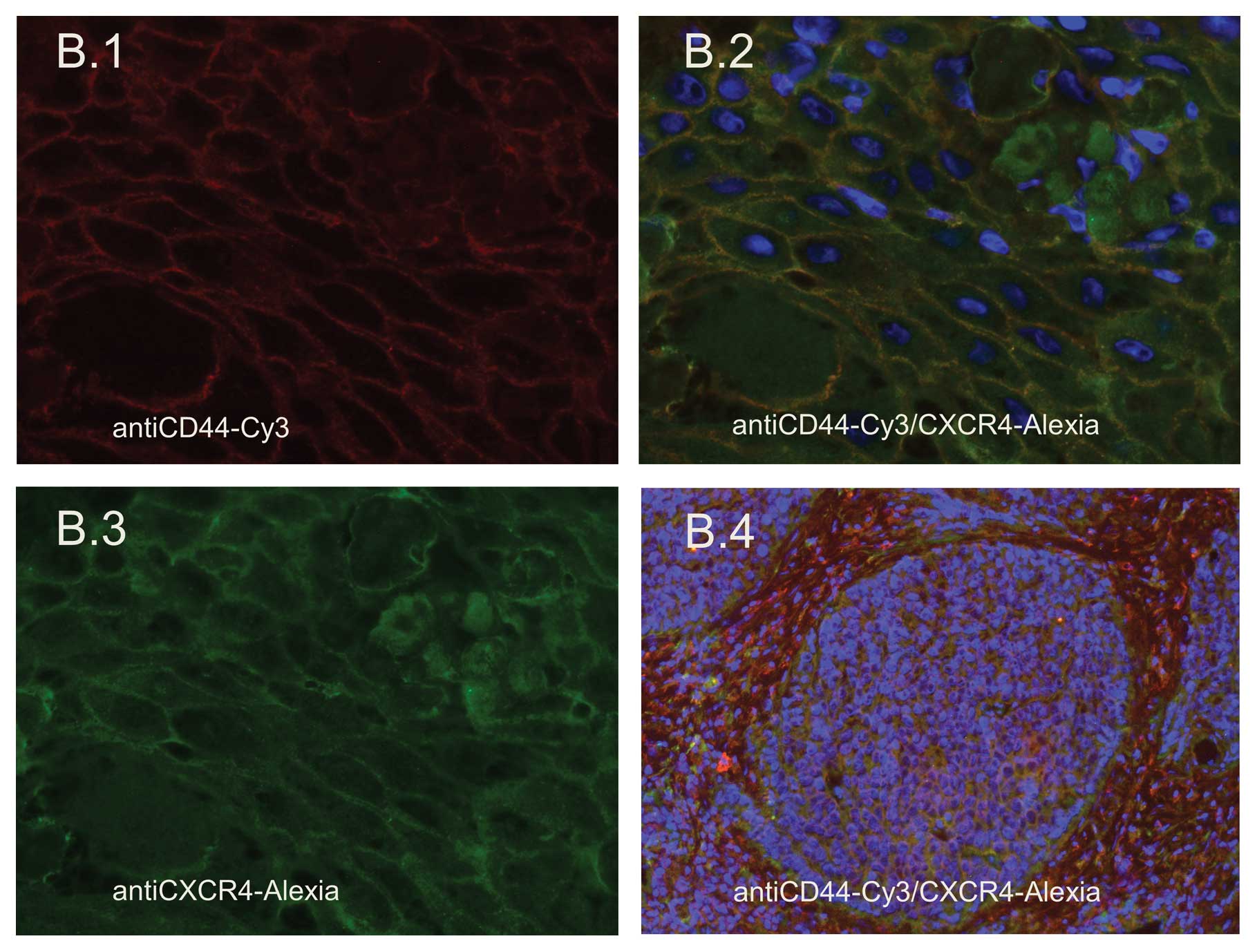
Expression of CXCR4 in HNSCC tissues
Immunofluorescence labeling was performed to measure
the expression of CXCR4 in HNSCC tissue samples by staining with
Alexia 188. CXCR4 was present in all tissue samples stained. CXCR4
exhibited a surface staining pattern as well as cytoplasmic
expression (Fig. 2B.2 and B.3).
Specific expression of CXCR4 at the invasive front of the tumor was
not noted, while CXCR4 expression was high in the tumor nests, but
was not noted in the tumor surrounding stroma (Fig. 2B.4). Thus, it was demonstrated that
the corresponding receptor to SDF, which is transmitted by the
supportive stromal cancer stem cell niche, can be found in the
tumor nest as the counterpart of this interaction. Nuclei were
stained with DAPI.
Concentration of SDF-1 in the peripheral
blood of HNSCC patients
ELISA analysis was performed to evaluate the
concentration of SDF-1 in the peripheral blood of HNSCC patients
according to tumor site compared to a healthy control group. The
was no significant difference in the SDF-1 concentration between
tumor patients and the control group. Values are listed as means:
control, 2057.91 pg/ml; larynx, 2111.50 pg/ml; oral cavity, 1891.43
pg/ml; oropharynx, 2244.2 pg/ml; hypopharynx, 2000.29 pg/ml.
Discussion
In the past few years, the stem cell theory has
become more important in the field of tumor biology, particularly
in issues concerning tumor development, progression and metastasis.
The cancer stem cell theory has generated new ideas and discussion
in the research of malignant disease and in therapeutic options. At
present, the presence of cancer stem cells are not only known to
exist in hematological malignancies but also in solid tumor
entities (3,31). The cancer stem cell theory in solid
tumors such as HNSCC has had dramatic consequences. To date,
therapeutic interventions, e.g. surgery or chemoradiation, are
directed towards the bulk of the tumor without focusing on a small
amount of specialized tumor cells which have the facilities of
self-renewal, differentiation and unlimited proliferation. The
question of which combination of markers should be used to isolate
‘cancer stem cells’ e.g. in HNSCC remains unclear. In addition, the
combination of markers appears to vary in different types of tumors
(3,31,32).
In the hematopoietic system, CD34 has been shown to play an
important role in this context (18), and in acute myeloid leukemia,
CD34+ cells were able to establish a new tumor in a
mouse model (33). Regarding solid
tumor entities, CD133 has been postulated to play an important role
in malignancies of the nervous system, and results showed that
CD133+ cells are relatively radioresistant (34) which can also be assessed as a
feature of cancer stem cells. In contrast, CD133 is a ubiquitous
marker in the hematopoietic and endothelial progenitor cell system
(35), and it has also been shown
to be present in the neurologic system, e.g. in glioblastoma
(36).
In HNSCC, the presence of cancer stem cells has also
been postulated. Prince et al showed that CD44+
cells, compared to CD44− cells, were able to engraft an
entire new HNSCC tumor in a mouse model (3). According to their results, the cell
selection for CD44+ was sufficient to isolate cells with
cancer stem cell properties out of the bulk of an HNSCC tumor
(3). Unfortunately, CD44 is also
expressed in ordinary cells (35).
The prevalent challenge of research is to identify a combination of
surface markers that can identify the subgroup of potent CSCs from
the bulk of a tumor more precisely. CD44 cannot be sufficiently
used as a defining CSC marker alone, since as a cell surface
glycoprotein it takes part in numerous cell-cell interactions
(35,37) and in humans it is present in a
multitude of splice variants (35)
and can be found ubiquitously. Approaches to the investigations of
the ‘real cancer stem cell’ in HNSCC requires research with human
tumor material with isolation of a small subpopulation of cells out
of the bulk of the tumor using complicated cell separation steps
including recurrent washing steps, lysis by DNAses and
FACS-sorting. To concur with the current standard of knowledge, a
combination of lineage markers must be negative to separate CSCs
out of the entire HNSCC tumor (CD3, CD3, CD10, CD18, CD31, CD64,
CD140) (3,35,38).
Other CSC markers (CD44, ALDH1) have been proposed, but only small
amounts of cells carrying these markers are said to be able to
initiate an HNSCC tumor in a mouse model (3,5). The
cell amount remaining after this separation as described above is
often minute and the remaining cells are often not useful for
further experiments. Often it is necessary to revert to cell lines
to perform experiments. ALDH1 is another marker postulated as a
cancer stem cell marker in HNSCC (5). It was also postulated as a cancer stem
cell marker in breast (39) and
lung cancer (40). It was shown
that high expression of ALDH1 in breast cancer is associated with
poor prognosis and early metastasis (41). It is an enzyme that catalyzes the
oxidation of aromatic aldehydes into carboxylic acids and it has
been shown to be responsible for the resistance of progenitor cells
to chemotherapeutic agents by breaking down cytotoxic drugs
(35,42).
In a present study concerning HNSCC, ALDH1 was
declared to be a more specific cancer stem cell marker in
comparison to CD44 (5). In the
present study, only CD44 was used as a cancer stem cell marker, and
we found a high expression of CD44 in all tissue samples tested
especially at the invasive front of the tumor, where HNSCC cells
come into contact with their surrounding cells, e.g. stromal cells.
Our findings corroborate Sterz et al(43) who showed colocalization of CD44,
ALDH1 and CK14 in the basal layer of HNSCC and at the border of the
tumor at the tumor-stroma border. The localization of cancer stem
cell candidates at the border of tumors is feasible as this is
where invasion and the main tumor growth occurs. Highly
differentiated cell layers were negative for these markers, and
this agrees with the supposition that stem cells are localized
close to the basal membrane. Invasiveness and metastasis of tumors
also depend on their capacity to penetrate and rebuild the
extracellular matrix (44).
Malignant cells infiltrate healthy tissue by degradation of the
extracellular matrix components, by breaking down vessel borders
and by promoting metastasis in distant organs. It has been shown
for different tumor entities, that the presence of matrix
metalloproteinases plays an important role in this process and this
has also been shown for HNSCC (43,45).
In HNSCC, MMP-9 and −2 have been shown by Sterz et al to be
located at the invasive front of HNSCC (43).
The SDF-CXCR4 axis is involved in several aspects of
tumor progression, such as angiogenesis, metastasis and survival
(46). The microenvironment of the
bone marrow has been reported to support survival, differentiation
and proliferation of hematopoietic progenitor cells (47), but also malignant progenitor cells
of the hematopoietic system, e.g. B-cell acute lymphoblastic
leukemia (B-ALL) (48). The pathway
which includes the SDF-1-CXCR4 axis has been postulated to be
responsible for retention of lymphoid and myeloid leukemia cells in
the bone marrow (48,49). It becomes obvious that the
importance of the SDF-1-CXCR4 axis in the hematopoietic system is
well-discussed, but this is the first time that the SDF-1-CXCR4
axis has been reported to play a crucial role in the development
and the progression of invasion and metastasis of HNSCC.
In this study, we showed that CXCR4 can be found in
tumor nests of HNSCC, but not in the surrounding stromal region of
the cancer stem cell niche. It has been shown by Clatot et
al that the intratumoral level of SDF-1 is correlated with
survival in HNSCC (50). In
contrast, we showed in this study that the concentration of SDF-1
in the peripheral blood of HNSCC patients does not differ in
comparison to healthy donors. This suggests that the SDF-1-CXCR4
axis plays a role in the cancer stem cell niche within the tumor,
but not in the periphery of the blood system. In previous studies,
we showed that polarization and formation of filopodia and a
prominent uropod were increased in the
CD44+CXCR4+ HNSCC cell line UM-SCC 11A in a
dose-dependent manner by SDF-1α. This effect can probably be
attributed to cytoskeleton rearrangements of actin-containing
protrusions (18,51), and this also might be influenced by
extracellular factors such as matrix metalloproteinases (51,52).
Podia formation was found to occur together with cell adhesion
especially to the microenvironment (18). If evidence can be found that podia
formation and adhesion to the cellular cancer stem cell niche are
also associated with HNSCC CD44+ cells, understanding of
these interactions would offer insight into new strategies of
cancer-directed therapy in HNSCC. For example small-molecule
agonists or antagonists of SDF-1 may be used to interfere with the
cancer stem cell niche resulting in inhibition or ideally blockage
of tumor invasion and metastasis (18). Further experiments using human
material are warranted to expand and specify our insight regarding
the cell-cell interactions in the cancer stem cell niche of solid
tumors. Based on the findings it may be possible to develop
particular therapeutic strategies aimed at CSCs or the SDF-1-CXCR4
axis.
Acknowledgements
We gratefully thank Petra Prohaska for the excellent
technical support.
Abbreviations:
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
CSCs
|
cancer stem cells
|
|
SDF
|
stromal cell-derived factor
|
References
|
1
|
Boehm A, Wichmann G, Mozet C and Dietz A:
Current therapy options in recurrent head and neck cancer. HNO.
58:762–769. 2010.(In German).
|
|
2
|
Mannelli G and Gallo O: Cancer stem cells
hypothesis and stem cells in head and neck cancers. Cancer Treat
Rev. 38:515–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prince ME, Sivanandan R, Kaczorowski A, et
al: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oker N, Kaufmann AM and Albers AE: Biology
and relevance of stem cells in squamous head and neck cancer:
latest insights and review of literature. Laryngorhinootologie.
91:326–332. 2012.(In German).
|
|
5
|
Clay MR, Tabor M, Owen JH, et al:
Single-marker identification of head and neck squamous cell
carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck.
32:1195–1201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen YS, Wu MJ, Huang CY, et al: CD133/Src
axis mediates tumor initiating property and epithelial-mesenchymal
transition of head and neck cancer. PLoS One. 6:e280532011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Franzmann EJ, Reategui EP, Pedroso F, et
al: Soluble CD44 is a potential marker for the early detection of
head and neck cancer. Cancer Epidemiol Biomarkers Prev.
16:1348–1355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ponta H, Wainwright D and Herrlich P: The
CD44 protein family. Int J Biochem Cell Biol. 30:299–305. 1998.
View Article : Google Scholar
|
|
9
|
Screaton GR, Bell MV, Bell JI and Jackson
DG: The identification of a new alternative exon with highly
restricted tissue expression in transcripts encoding the mouse
Pgp-1 (CD44) homing receptor. Comparison of all 10 variable exons
between mouse, human, and rat. J Biol Chem. 268:12235–12238.
1993.
|
|
10
|
Screaton GR, Bell MV, Jackson DG, Cornelis
FB, Gerth U and Bell JI: Genomic structure of DNA encoding the
lymphocyte homing receptor CD44 reveals at least 12 alternatively
spliced exons. Proc Natl Acad Sci USA. 89:12160–12164. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Günthert U, Hofmann M, Rudy W, et al: A
new variant of glycoprotein CD44 confers metastatic potential to
rat carcinoma cells. Cell. 65:13–24. 1991.PubMed/NCBI
|
|
12
|
Hofmann M, Rudy W, Zoller M, et al: CD44
splice variants confer metastatic behavior in rats: homologous
sequences are expressed in human tumor cell lines. Cancer Res.
51:5292–5297. 1991.PubMed/NCBI
|
|
13
|
Zhang H, Xi H, Cai A, et al: Not all side
population cells contain cancer stem-like cells in human gastric
cancer cell lines. Dig Dis Sci. Aug 10–2012.(Epub ahead of
print).
|
|
14
|
Pang RW and Poon RT: Cancer stem cell as a
potential therapeutic target in hepatocellular carcinoma. Curr
Cancer Drug Targets. Aug 2–2012.(Epub ahead of print).
|
|
15
|
Park SY, Lee HE, Li H, Shipitsin M, Gelman
R and Polyak K: Heterogeneity for stem cell-related markers
according to tumor subtype and histologic stage in breast cancer.
Clin Cancer Res. 16:876–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeimet AG, Reimer D, Sopper S, et al:
Ovarian cancer stem cells. Neoplasma. 59:747–755. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dar A, Goichberg P, Shinder V, et al:
Chemokine receptor CXCR4-dependent internalization and resecretion
of functional chemokine SDF-1 by bone marrow endothelial and
stromal cells. Nat Immunol. 6:1038–1046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Faber A, Roderburg C, Wein F, et al: The
many facets of SDF-1alpha, CXCR4 agonists and antagonists on
hematopoietic progenitor cells. J Biomed Biotechnol.
2007:260652007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De La Luz Sierra M, Yang F, Narazaki M, et
al: Differential processing of stromal-derived factor-1alpha and
stromal-derived factor-1beta explains functional diversity. Blood.
103:2452–2459. 2004.PubMed/NCBI
|
|
20
|
Shirozu M, Nakano T, Inazawa J, et al:
Structure and chromosomal localization of the human stromal
cell-derived factor 1 (SDF1) gene. Genomics. 28:495–500. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim CH and Broxmeyer HE: In vitro behavior
of hematopoietic progenitor cells under the influence of
chemoattractants: stromal cell-derived factor-1, steel factor, and
the bone marrow environment. Blood. 91:100–110. 1998.PubMed/NCBI
|
|
22
|
Mohle R, Bautz F, Rafii S, Moore MA,
Brugger W and Kanz L: The chemokine receptor CXCR-4 is expressed on
CD34+ hematopoietic progenitors and leukemic cells and
mediates transendothelial migration induced by stromal cell-derived
factor-1. Blood. 91:4523–4530. 1998.PubMed/NCBI
|
|
23
|
Petit I, Goichberg P, Spiegel A, et al:
Atypical PKC-zeta regulates SDF-1-mediated migration and
development of human CD34+ progenitor cells. J Clin
Invest. 115:168–176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chute JP: Stem cell homing. Curr Opin
Hematol. 13:399–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fruehauf S, Srbic K, Seggewiss R, Topaly J
and Ho AD: Functional characterization of podia formation in normal
and malignant hematopoietic cells. J Leukoc Biol. 71:425–432.
2002.PubMed/NCBI
|
|
26
|
Lataillade JJ, Clay D, Dupuy C, et al:
Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in
synergy with cytokines: possible role in progenitor survival.
Blood. 95:756–768. 2000.
|
|
27
|
Broxmeyer HE, Kohli L, Kim CH, et al:
Stromal cell-derived factor-1/CXCL12 directly enhances
survival/antiapoptosis of myeloid progenitor cells through CXCR4
and G(alpha)i proteins and enhances engraftment of competitive,
repopulating stem cells. J Leukoc Biol. 73:630–638. 2003.
View Article : Google Scholar
|
|
28
|
Kijowski J, Baj-Krzyworzeka M, Majka M, et
al: The SDF-1-CXCR4 axis stimulates VEGF secretion and activates
integrins but does not affect proliferation and survival in
lymphohematopoietic cells. Stem Cells. 19:453–466. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hattori K, Heissig B, Tashiro K, et al:
Plasma elevation of stromal cell-derived factor-1 induces
mobilization of mature and immature hematopoietic progenitor and
stem cells. Blood. 97:3354–3360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kremer KN, Clift IC, Miamen AG, et al:
Stromal cell-derived factor-1 signaling via the CXCR4-TCR
heterodimer requires phospholipase C-β3 and phospholipase C-γ1 for
distinct cellular responses. J Immunol. 187:1440–1447.
2011.PubMed/NCBI
|
|
31
|
La Porta CA: Thoughts about cancer stem
cells in solid tumors. World J Stem Cells. 4:17–20. 2012.PubMed/NCBI
|
|
32
|
Grosse-Gehling P, Fargeas CA, Dittfeld C,
et al: CD133 as a biomarker for putative cancer stem cells in solid
tumours: limitations, problems and challenges. J Pathol. Aug
16–2012.(Epub ahead of print).
|
|
33
|
Schubert M, Herbert N, Taubert I, et al:
Differential survival of AML subpopulations in NOD/SCID mice. Exp
Hematol. 39:250–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jamal M, Rath BH, Tsang PS, Camphausen K
and Tofilon PJ: The brain microenvironment preferentially enhances
the radioresistance of CD133(+) glioblastoma stem-like cells.
Neoplasia. 14:150–158. 2012.PubMed/NCBI
|
|
35
|
Wollenberg B: Implication of stem cells in
the biology and therapy of head and neck cancer.
Laryngorhinootologie. 90(Suppl 1): S110–S119. 2011.(In German).
|
|
36
|
Warrier S, Pavanram P, Raina D and Arvind
M: Study of chemoresistant CD133+cancer stem cells from
human glioblastoma cell line U138MG using multiple assays. Cell
Biol Int. Jul 6–2012.(Epub ahead of print).
|
|
37
|
Jaggupilli A and Elkord E: Significance of
CD44 and CD24 as cancer stem cell markers: an enduring ambiguity.
Clin Dev Immunol. 2012:7080362012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pries R, Wittkopf N, Hasselbacher K and
Wollenberg B: Constitutive expression of the potential stem cell
marker CD44 in permanent HNSCC cell lines. HNO. 56:461–466.
2008.(In German).
|
|
39
|
Badve S and Nakshatri H: Breast-cancer
stem cells - beyond semantics. Lancet Oncol. 13:e43–e48. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang F, Qiu Q, Khanna A, et al: Aldehyde
dehydrogenase 1 is a tumor stem cell-associated marker in lung
cancer. Mol Cancer Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Charafe-Jauffret E, Ginestier C, Iovino F,
et al: Aldehyde dehydrogenase 1-positive cancer stem cells mediate
metastasis and poor clinical outcome in inflammatory breast cancer.
Clin Cancer Res. 16:45–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Silva IA, Bai S, McLean K, et al: Aldehyde
dehydrogenase in combination with CD133 defines angiogenic ovarian
cancer stem cells that portend poor patient survival. Cancer Res.
71:3991–4001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sterz CM, Kulle C, Dakic B, et al: A
basal-cell-like compartment in head and neck squamous cell
carcinomas represents the invasive front of the tumor and is
expressing MMP-9. Oral Oncol. 46:116–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakajima M, Gohji K, Fabra A, Fidler IA
and Tsuruo T: Regulation of tumor metastasis and extracellular
matrix degradative enzyme production by microenvironments. Gan To
Kagaku Ryoho. 20:380–386. 1993.(In Japanese).
|
|
45
|
Schultz JD, Rotunno S, Erben P, et al:
Down-regulation of MMP-2 expression due to inhibition of receptor
tyrosine kinases by imatinib and carboplatin in HNSCC. Oncol Rep.
25:1145–1151. 2011.PubMed/NCBI
|
|
46
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Metzen E, Jin F, Brockmeier U and
Otterbach F: New insight into the SDF-1/CXCR4 axis in a breast
carcinoma model: hypoxia-induced endothelial SDF-1 and tumor cell
CXCR4 are required for tumor cell intravasation. Mol Cancer Res.
10:1021–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Purizaca J, Meza I and Pelayo R: Early
lymphoid development and microenvironmental cues in B-cell acute
lymphoblastic leukemia. Arch Med Res. 43:89–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Juarez J, Dela Pena A, Baraz R, et al:
CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia
cells into the peripheral blood and inhibit engraftment. Leukemia.
21:1249–1257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Clatot F, Picquenot JM, Choussy O, et al:
Intratumoural level of SDF-1 correlates with survival in head and
neck squamous cell carcinoma. Oral Oncol. 47:1062–1068. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Geutskens SB, Andrews WD, van Stalborch
AM, et al: Control of human hematopoietic stem/progenitor cell
migration by the extracellular matrix protein Slit3. Lab Invest.
92:1129–1139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ghosh MC, Makena PS, Gorantla V, Sinclair
SE and Waters CM: CXCR4 regulates migration of lung alveolar
epithelial cells through activation of Rac1 and matrix
metalloproteinase-2. Am J Physiol Lung Cell Mol Physiol.
302:L846–L856. 2012. View Article : Google Scholar : PubMed/NCBI
|