Introduction
Esophageal cancer is a particularly lethal
malignancy. The prognosis for esophageal cancer patients is poor,
despite attempts at aggressive multimodality treatment. The 5-year
survival rate of esophageal cancer patients is only 40%, having
been improved by advances in surgical techniques and adjuvant
therapy (1–3).
The absence of a serosal layer allows esophageal
malignancies to invade adjacent tissues easily and to spread
rapidly (4). Approximately half of
the esophageal cancer cases are non-resectable or metastasized
(distant stage) when they are diagnosed. Thus, suppressing the
motility of cancer cells, which is an important process for
invasion and metastasis, is a key to the treatment of esophageal
cancer (5). We focused on Girdin,
an actin-binding Akt substrate, discovered by Enomoto et
al(6). There are numerous
reports of the association between Akt and esophageal cancer as
well as that between Akt and cell motility. It was also reported
that Girdin plays an important role in cell motility of fibroblasts
and a breast cancer cell line (6,7).
Migrating cells form protrusive structures such as
filopodia and lamellipodia. Lamellipodia are two dimensional,
sheet-like structures containing a cross-linked mesh of actin
filaments (8). Girdin forms dimers
through the NH2 terminal domain. Moreover, Girdin, in
which the serine at position 1416 is phosphorylated by Akt binds
actin filaments via the COOH-terminal domain (6). Based on these results and
immunofluorescent staining of Vero fibroblasts and a breast cancer
cell line, it is considered that Girdin cross-links actin filaments
and is involved in the formation of lamellipodia in fibroblasts and
breast cancer (6,7).
Esophageal cancer is divided mainly into two
histological subtypes, ESCC and adenocarcinoma. Although
adenocarcinoma is the most prevalent subtype (>50%) of
esophageal cancer in the USA (9),
>90% of esophageal cancer cases are ESCC in Japan (10,11).
Thus, in the present study we investigated whether Girdin is also
involved in the motility of ESCC cells. We used a human ESCC cell
line, KYSE series. Furthermore, we examined whether there was a
correlation between Girdin expression and the prognosis of ESCC
patients, using specimens resected from ESCC patients. To the best
of our knowledge, this is the first report to describe the inverse
correlation of Girdin expression with the survival of ESCC
patients.
Materials and methods
Antibodies
Rabbit anti-Girdin polyclonal antibodies and rabbit
anti-p-Girdin (phospho-S1417) antibody were purchased from Abcam
(Cambridge, MA, USA). Other antibodies used in this study included
polyclonal goat anti-rabbit immunoglobulins/HRP (Dako, Glostrup,
Denmark) and Alexa Fluor® 488 goat anti-rabbit IgG (green) (Life
Technologies Corp., Carlsbad, CA, USA).
Cells
KYSE (human ESCC) cell lines were obtained from the
Japanese Collection of Research Bioresources (Tokyo, Japan). Het1A,
a normal human esophageal mucosal cell line, was purchased from
ATCC (Manassas, VA, USA). They were cultured in RPMI-1640
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS) (Gibco®, Life Technologies Corp.),
100 U/ml penicillin G, 100 μg/ml streptomycin and 250 ng/ml
amphotericin B.
Real-time polymerase chain reaction
(RT-PCR)
Total RNA was extracted from KYSE cells or specimens
resected from ESCC patients, using RNeasy® Plus Mini kit
(Qiagen, Hilden, Germany) according to the manufacturer’s
instructions. The reverse transcription reaction was subsequently
carried out using SuperScript™ III First-Strand Synthesis SuperMix
for qRT-PCR (Life Technologies) according to the manufacturer’s
instructions. PCR amplification of the cDNA template corresponding
to 20 ng total RNA was performed using TaqMan Universal PCR Master
Mix (Applied Biosystems, Foster City, CA, USA) in a Chromo4™System
(Bio-Rad Laboratories, Hercules, CA, USA). Girdin primer was
purchased from Applied Biosystems (Foster City, CA, USA). PCR
conditions were 50°C for 2 min and 95°C for 10 min followed by 40
cycles of 95°C for 15 sec, and 60°C for 1 min. The expression
levels of Girdin were normalized against glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) (Human GAPDH Endogenous
Control, Applied Biosystems®).
Immunofluorescent staining
KYSE270 cells were seeded on poly-L-lysine
(PLL)-coated cover glass in base dishes and incubated for 21 h.
Then, cells were serum-starved for 3 h and incubated for 1 h in a
medium with or without EGF. The cells were fixed with 3.7%
formaldehyde. Subsequently, immunofluorescent staining was
performed using anti-Girdin antibody, Alexa Fluor® 488
goat anti-rabbit IgG (green) (Life Technologies), Alexa
Fluor® 555 Phalloidin (red) (Life Technologies) and
4′,6-diamidino-2-phenylindole (DAPI) (blue) (Dojindo Laboratories,
Kumamoto, Japan). Fluorescence was observed using a confocal
laser-scanning microscope (Carl Zeiss, Oberkochen, Germany).
Small interfering RNA (siRNA)-mediated
Girdin knockdown
siRNA-mediated knockdown of Girdin was performed.
Referring to RNA interference which Enomoto et al (6) performed, the sequences coding the
siRNA targeted on Girdin were (only the sense sequence is shown):
5′-AACCAGGTCATGCTCCAAATT-3′ (nucleotides 145–165, Girdin siRNA1)
and 5′-AAGAAGGCTTAGGCAGGAATT-3′ (nucleotides 780–800, Girdin
siRNA2). The 21 nucleotide synthetic duplexes mentioned above were
prepared by Qiagen. Cells were transfected with Girdin siRNA or
negative control siRNA (AllStars Negative siRNA Alexa Fluor 488,
Qiagen), using Lipofectamine™ 2000 (Life Technologies) according to
the manufacturer’s instructions.
Western blot analysis
We extracted proteins from KYSE cells that had been
lysed in IP kinase buffer with 1% Halt™Protease Inhibitor
Single-Use Cocktail (Thermo Fisher Scientific, Waltham, MA, USA)
and 1% Halt™ Phosphatase Inhibitor Cocktail (Thermo Fisher
Scientific), using a sonicator, Bioruptor® (Cosmo Bio,
Tokyo, Japan).
In western blot analysis, the sample proteins were
separated on 8% SDS-PAGE. Proteins were transferred to
nitrocellulose membranes, blocked in 3% non-fat skin milk in
phosphate-buffered saline (PBS), incubated with primary antibodies,
rabbit polyclonal anti-Girdin (Abcam) and detected with horseradish
peroxidase (HRP)-conjugated secondary antibodies (polyclonal goat
anti-rabbit immunoglobulins, Dako). For visualization,
SuperSignal® West Pico Chemiluminescent Substrate
(Thermo Fisher Scientific) with 10% SuperSignal® West
Femto Trial kit (Thermo Fisher Scientific) was used. Imaging of
western blots was performed using ImageQuant LAS 4000 mini
(Fujifilm, Tokyo, Japan) according to the manufacturer’s
instruction.
Migration assay
Migration assays were performed using growth factor
reduced Matrigel™ invasion chambers (BD Biosciences, Bedford, MA,
USA) according to the manufacturer’s instructions. KYSE cells
(2×105) transfected with control siRNA or Girdin siRNA
were seeded in Matrigel-coated invasion chambers. The chambers were
then placed into 24-well plates, into which basal medium only or
basal medium containing 1.0 ng/ml epidermal growth factor (EGF) (BD
Biosciences) was added. Following incubation of KYSE cells for 12
h, the upper surface of the Transwell chambers was wiped with a
cotton swab. The migrating cells were fixed, stained with
Diff-Quick (Sysmex, Kobe, Japan), and counted in 5 random
microscopic fields (x200). The experiment was performed three times
with each sample in triplicate. The results were normalized and
expressed as an invasion index, in which the lowest number of
migrating cells in this experiment was designated as 1.
Patients and tumor samples
Patients who had been diagnosed with ESCC and had
undergone esophagectomy at Nagoya City University were selected for
this study. All patients were followed up for at least 5 years from
the time of operation, unless they died or the data were censored.
This study was approved by the Institutional Review Board, and
written consent was obtained from each patient.
Immunohistochemistry
Paraffin-embedded specimens that had been resected
from ESCC patients were analyzed for Girdin expression by
immunohistochemistry (IHC). For fluorescence staining of the ESCC
tissues, the sections were stained with anti-Girdin polyclonal
antibody, followed by the secondary antibody, and were observed
with a light microscope. All processes of IHC were performed using
BOND-MAX (Leica, Wetzlar, Germany).
Results of each IHC staining were evaluated as
follows; the stained cells were counted in five random microscopic
fields (x200); the intensity of tissue staining was graded
semi-quantitatively on a four-point scale (−, +, ++ and +++), and
the proportion of the stained cells was assessed on a four-tier
scale (1, 0–15%; 2, 15–50%; 3, 50–85%; and 4, 85–100% cells
stained). Staining was scored by 3 independent observers blinded to
the disease stage and the patient outcome with >95% agreement.
The cases were classified into a strong staining group and a weak
staining group by the intensity and proportion of immunostained
cancer cells. The cases where the immunostaining intensity was more
than ++ and the proportion was >3 were defined as a strong
staining group.
Statistical analysis
The biostatistical analyses were performed with
StatView software (Abacus Concepts, Berkeley, CA, USA).
Kaplan-Meier estimates of overall survival time were compared using
the log-rank test. P<0.05 was considered to indicate a
statistically significant difference. We used unpaired Student’s
t-test to compare mean age, Fisher’s exact test to compare gender
ratio, and Yates’s chi-squared test to compare other patient
characteristics.
Results
Girdin expression is detected in Het1A
and KYSE cell lines
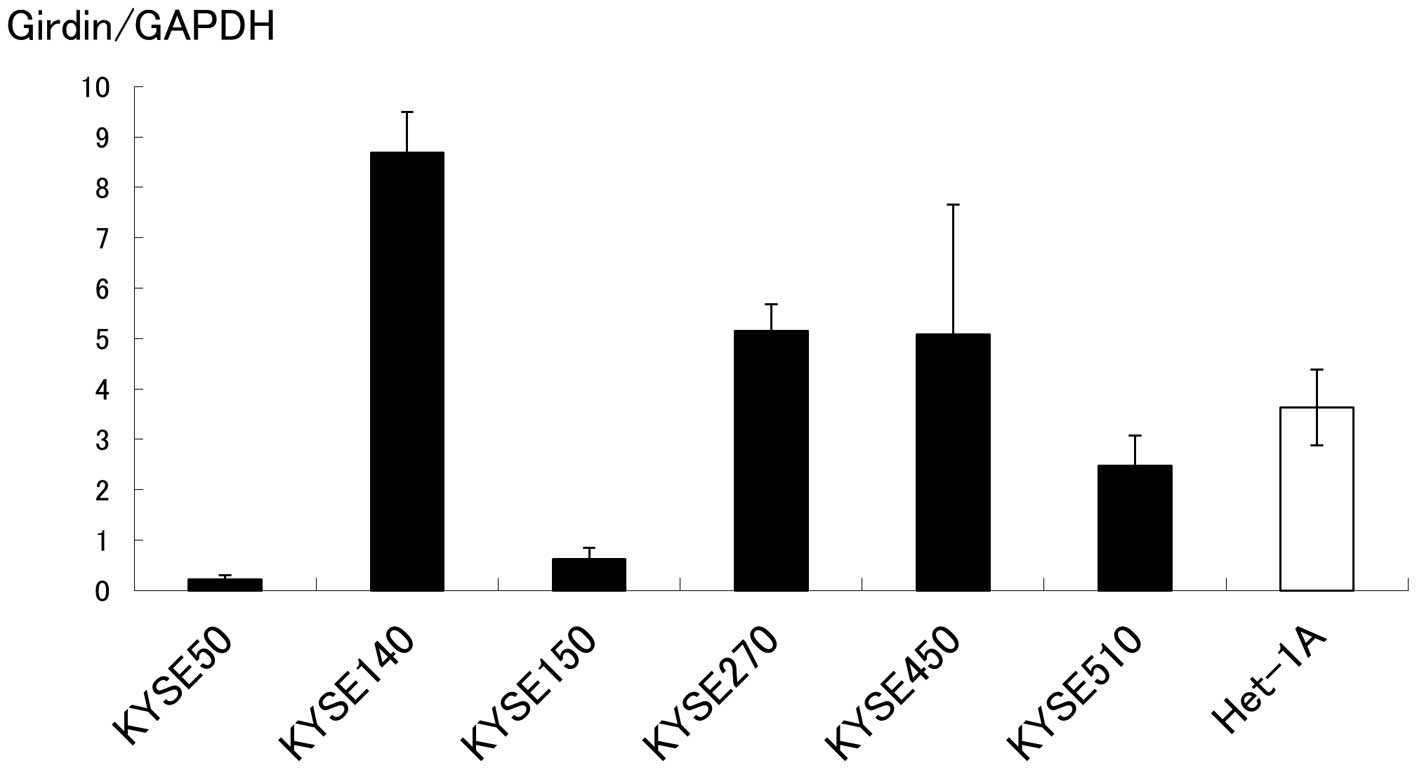
Using RT-PCR, expression of Girdin mRNA in KYSE cell
lines and Het1A was detected in all cell lines examined (Fig. 1).
Girdin is involved in the formation of
lamellipodia in KYSE cell lines stimulated by EGF
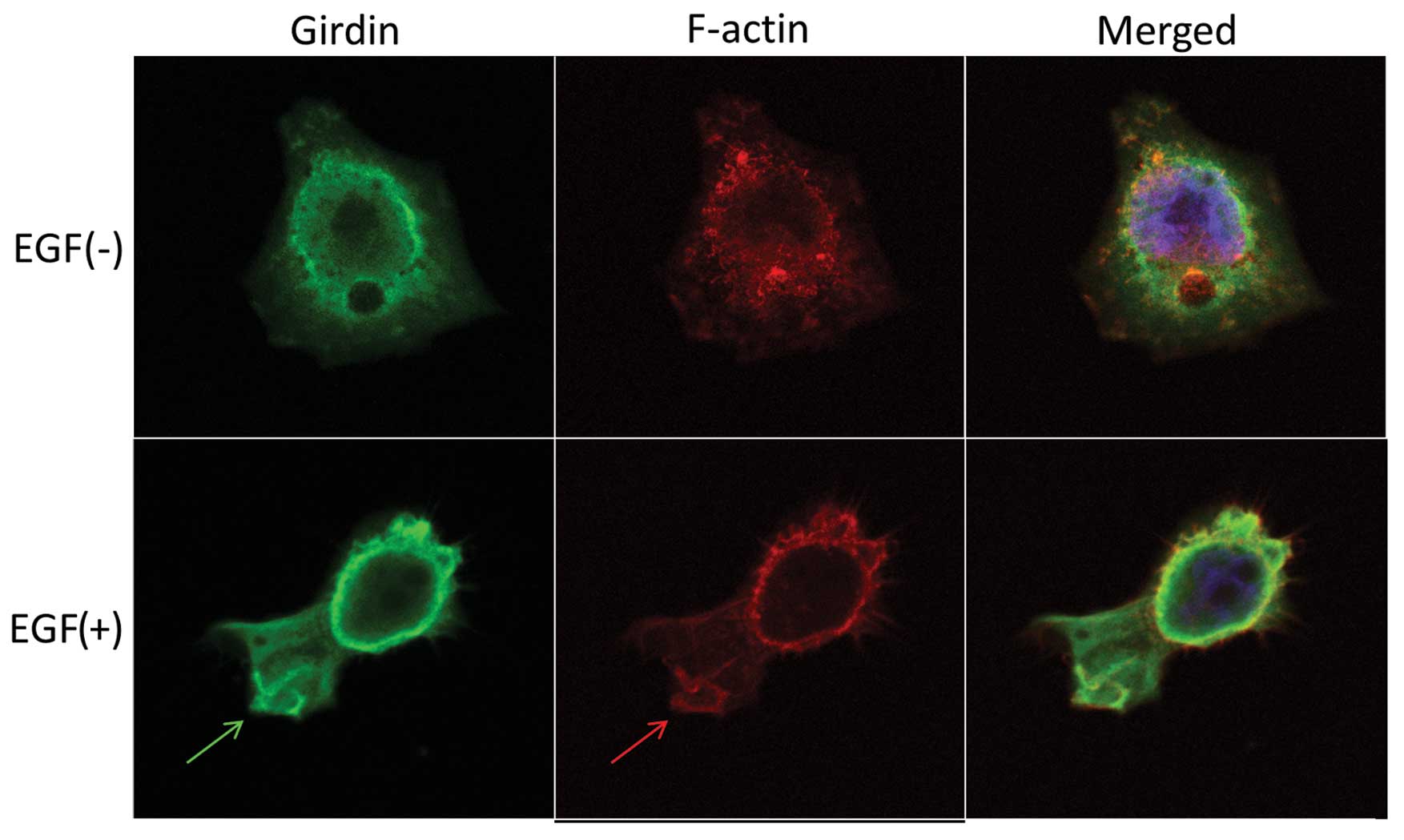
The subcellular localization of Girdin was
investigated using immunofluorescent staining. Girdin and F-actin
were localized diffusely throughout the cytoplasm in quiescent
cells (Fig. 2, upper panel). By
contrast, EGF-stimulated KYSE270 cells extended lamellipodia, where
Girdin was preferentially colocalized with F-actin (Fig. 2, bottom panel). Girdin may play an
important role in cell motility by reconstruction of the actin
cytoskeleton at the leading edge of ESCC cells.
Cell motility is reduced in KYSE cells in
which Girdin is depleted by RNA interference
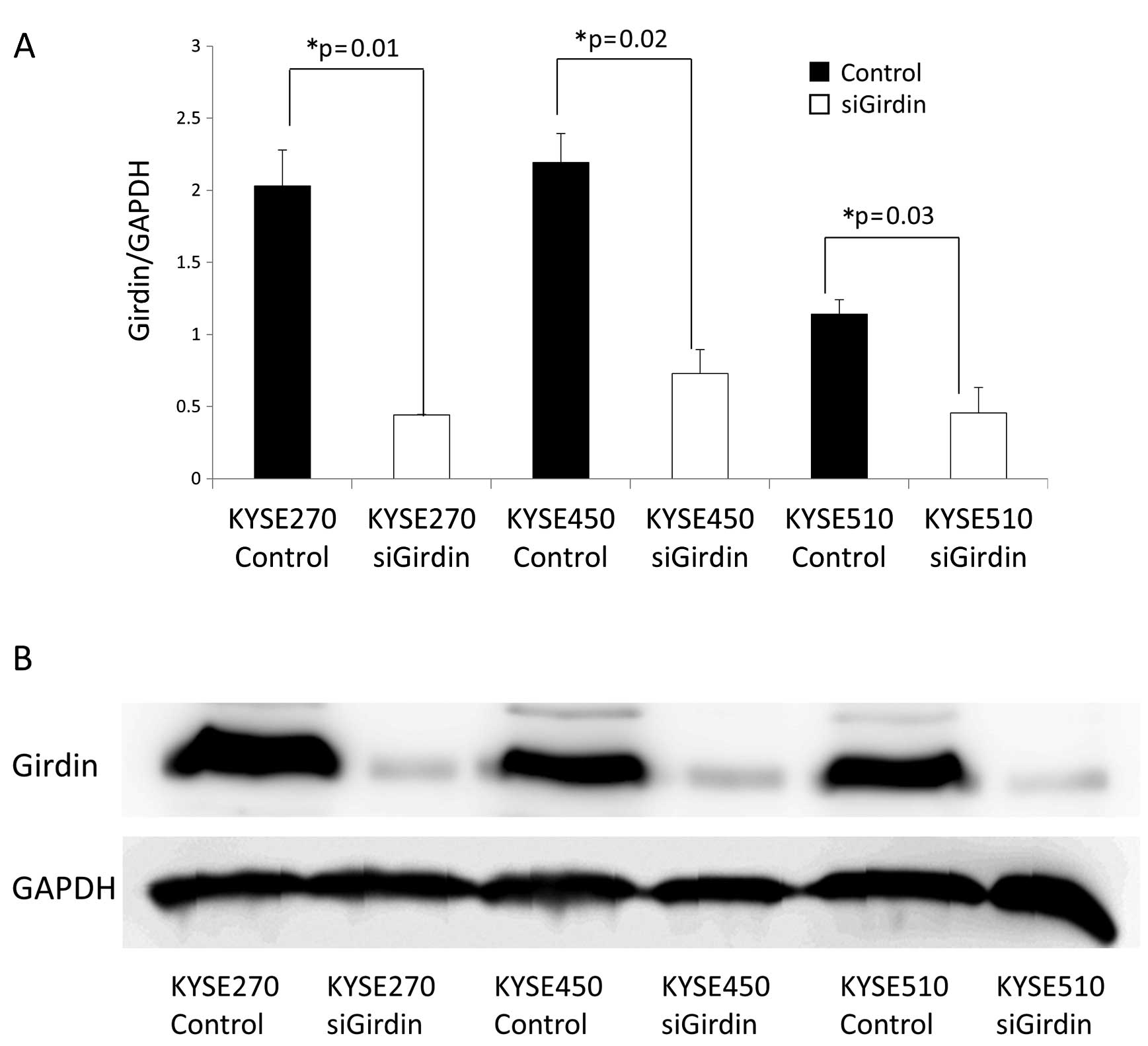
KYSE270, KYSE450 and KYSE510 cell lines were
transfected with either control siRNA or Girdin siRNA. The
knockdown of Girdin was confirmed by RT-PCR and western blot
analyses. Both the Girdin mRNA level (Fig. 3A) and the Girdin protein level
(Fig. 3B) were significantly lower
in the cell lines transfected with Girdin siRNA than in the cell
lines transfected with control siRNA.
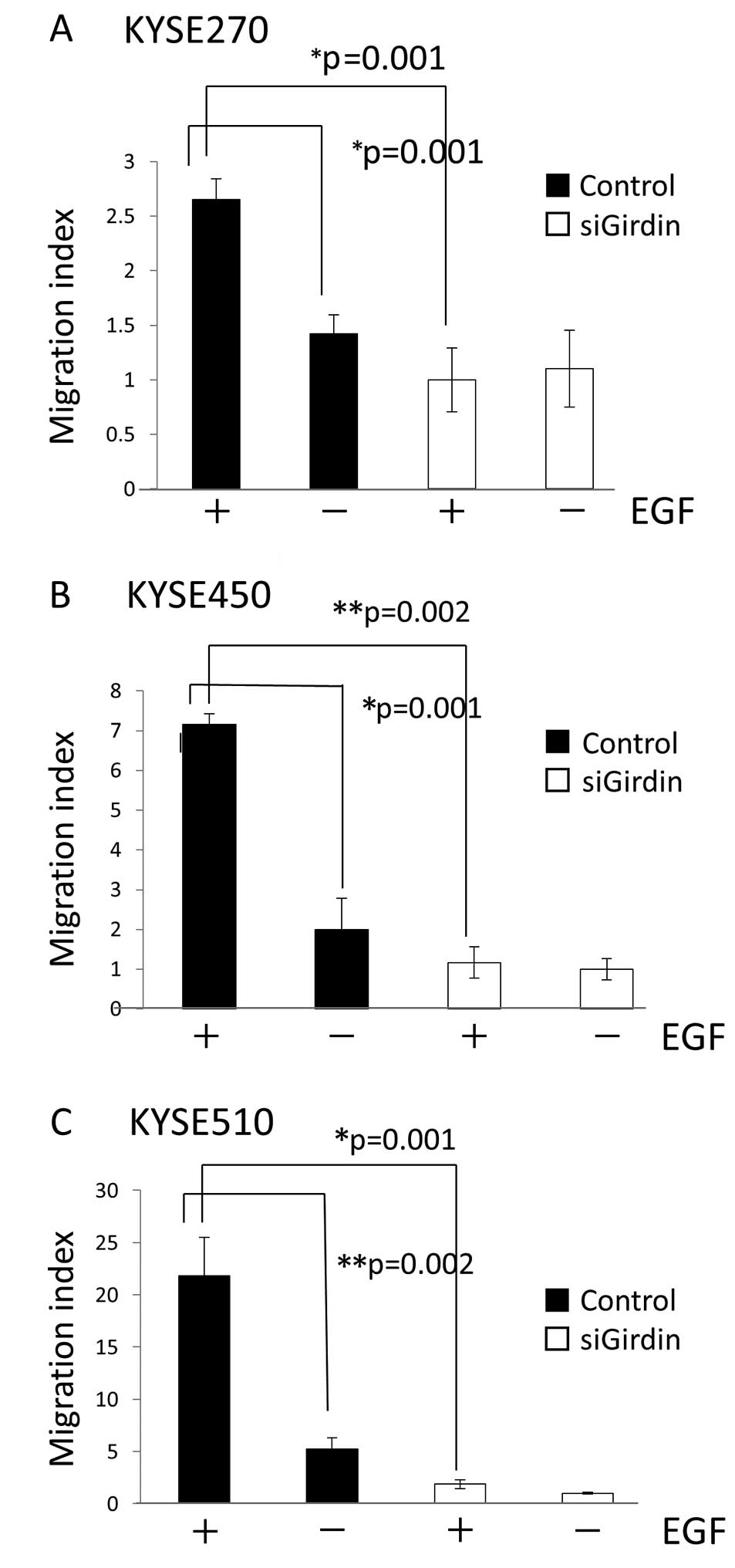
Boyden chamber assays were used to assess cell
migration in vitro. In KYSE cell lines transfected with
control siRNA, the migration index was higher in cells incubated in
medium with EGF compared to cells incubated in medium without EGF.
The migration index was also significantly higher in cells
transfected with control siRNA than in cells transfected with
Girdin siRNA (Fig. 4).
Girdin expression is significantly higher
in tumor tissue than in paired normal esophageal squamous mucosa in
ESCC
We compared Girdin mRNA levels in tumor tissues with
those of paired adjacent normal mucosa, using specimens resected
from 62 ESCC patients who had undergone esophagectomy at the Nagoya
City University from 1996 to 2001. Total RNA was extracted from
esophageal cancer tissues and from paired normal esophageal mucosa
using RNeasy® Plus Mini Kit (Qiagen) and subsequently
subjected to RT-PCR. Data showed that Girdin mRNA from tumor tissue
was significantly higher than that from paired adjacent normal
mucosa in ESCC (Fig. 5).
High Girdin expression correlates with
poor prognosis in ESCC
We next investigated whether expression levels of
Girdin were associated with patient 5-year survival rate following
surgery. We performed IHC using specimens resected from 73 ESCC
patients who had undergone esophagectomy at the Nagoya City
Hospital from 1996 to 2006. Although neoadjuvant chemotherapy for
stage II or III (Japanese classification of esophageal cancer)
cases is standard in Japan, patients who had undergone chemotherapy
and/or irradiation prior to surgery were excluded from this study,
considering the influence of these therapies on Girdin expression
of the tumor tissue.
The invasion of primary tumors was significantly
deeper in the strongly staining group than in the weakly staining
group (T1 vs. T2–T4, P=0.024). There was no significant difference
with regard to the other patient characteristics (Table I). Kaplan-Meier curves show that
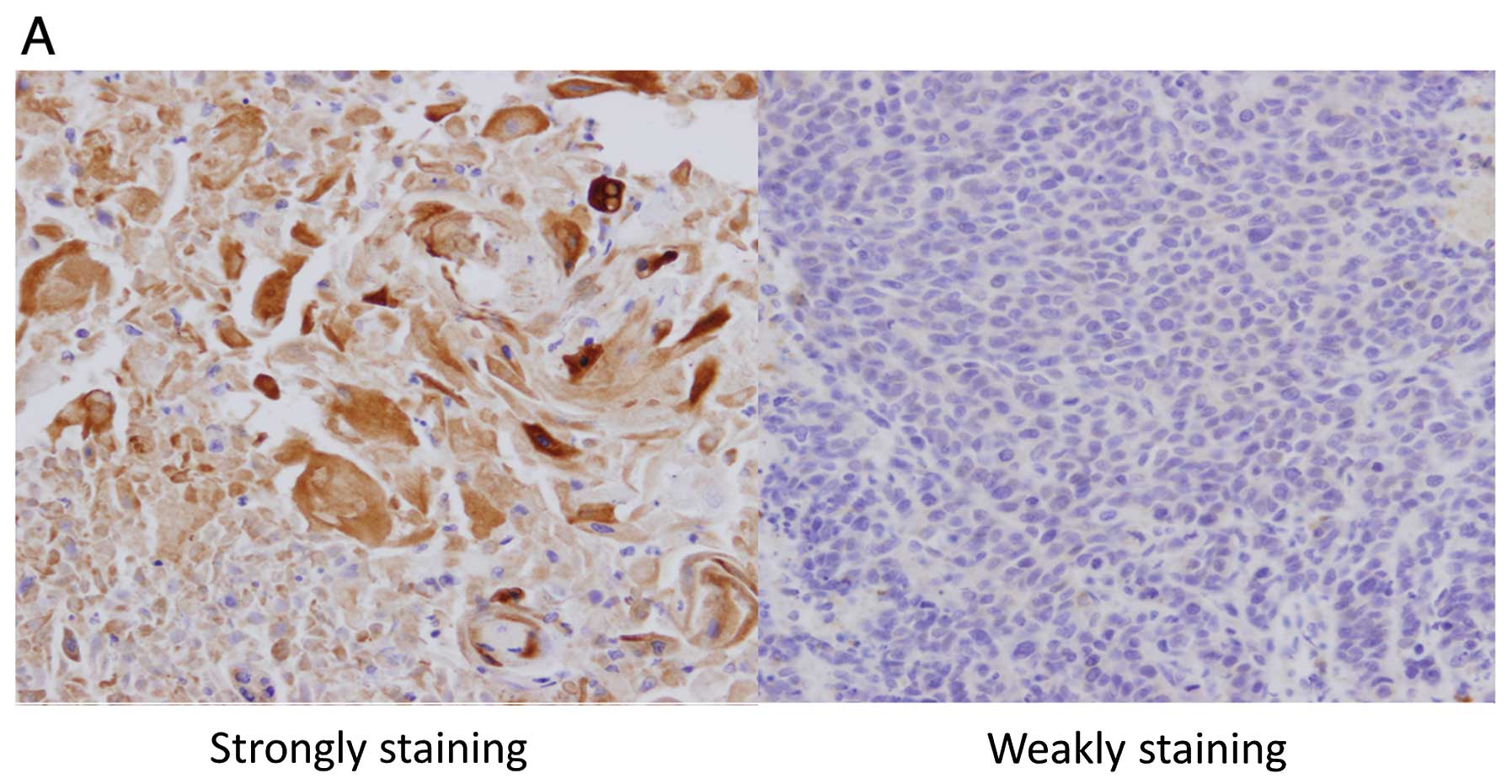
patients whose tumor tissue was stained weakly (Fig. 6A, right panel) had prolonged
survival compared to patients whose tumor tissue was stained
strongly (Fig. 6A, left panel)
(two-tailed P=0.042, log-rank test) (Fig. 6B).
 | Table IMean age, gender ratio and the
histological characteristics of the two patient groups whose tumors
stained strongly and weakly in immunohistochemistry for Girdin. |
Table I
Mean age, gender ratio and the
histological characteristics of the two patient groups whose tumors
stained strongly and weakly in immunohistochemistry for Girdin.
| Girdin
expression | |
|---|
|
| |
|---|
| Characteristics | Strongly staining
(n=50) | Weakly staining
(n=23) | P-value |
|---|
| Mean age (±SD) | 63.6±8.3 | 62.2±8.6 | NS |
| Gender | | | |
| Male | 41 | 18 | NS |
| Female | 9 | 5 | |
| Primary tumor | | | |
| T1 | 11 | 11 | 0.024 |
| T2 | 9 | 2 | (T1 vs. T2–4) |
| T3 | 21 | 6 | |
| T4 | 9 | 4 | |
| Lymph node
metastasis | | | |
| Positive | 33 | 8 | NS |
| Negative | 17 | 15 | |
| Remote
metastasis | | | |
| Positive | 5 | 1 | NS |
| Negative | 45 | 22 | |
| TNM stage | | | |
| 0 | 0 | 1 | NS |
| 1 | 12 | 6 | (stage 0–1 vs.
2–4) |
| 2 | 9 | 3 | (stage 0–2 vs.
3–4) |
| 3 | 24 | 12 | |
| 4 | 5 | 1 | |
| Lymphatic
invasion | | | |
| Positive | 38a | 12b | NS |
| Negative | 10a | 8b | |
| Venous
invasion | | | |
| Positive | 31a | 8a | NS |
| Negative | 17a | 13a | |
Discussion
Cell motility is considered to be an important
process for invasion and metastasis of invasive cancer cells. In
animals, almost all cells move by crawling (12). Cell crawling is composed of three
distinctive phases, protrusion, attachment and traction (13). In protrusion, different cell types
generate a variety of protrusive structures, such as filopodia,
lamellipodia and pseudopodia (14).
Epithelial cells, some types of fibroblasts and some neurons form
lamellipodia, which are two dimensional, sheet-like structures
containing a cross-linked mesh of actin filaments (8). Activation of Rac, a member of the Rho
protein family, by extracellular signals leads to actin nucleation
by the ARP complex and actin cross-linking by filamin (15–17).
Reconstruction of actin filaments forms actin networks in
lamellipodia.
Akt, serine/threonine kinase, has multiple effects
on cells, including survival, proliferation and growth (18). Moreover, it is involved in cell
migration (19). However, the
underlying mechanism is not well understood. Enomoto et
al(6) identified an Akt
substrate using a yeast two-hybrid screen of a human fetal brain
cDNA library, and designated it Girdin. Akt phosphorylates serine
at position 1416 in Girdin. Based on studies of Vero fibroblasts
and the MDA-MB231 breast cancer cell line, it is likely that
phosphorylated Girdin colocalizes with actin filaments in the
lamellipodia and plays a crucial role in the motility of those
cells (6,7). In the present study, we examined
whether Girdin was also involved in the motility of ESCC cells.
Girdin is reportedly highly expressed in a variety
of human malignant tissues including breast, colon, lung, thyroid
and uterine cervical carcinomas (7). We analyzed Girdin mRNA expression in
KYSE cell lines and Het1A by RT-PCR. Expression of mRNA was
confirmed in all cell lines that we examined. For the following
experiments we selected KYSE270, KYSE450 and KYSE510 in which
expression of Girdin mRNA was high.
To visualize the involvement of Girdin in the
formation of lamellipodia, we performed immunofluorescent staining.
Colocalization of Girdin and F-actin was observed in the
lamellipodia in response to EGF stimulation. In migration
experiments, cell motility was reduced in KYSE cell lines compared
to controls when Girdin was depleted by Girdin siRNA.
Collectively, the results of this study suggest that
Girdin plays an important role in the motility of ESCC cells. We
investigated the relationship between Girdin expression and
clinical data in ESCC cases. In RT-PCR analyses of specimens
resected from ESCC patients, Girdin mRNA extracted from tumor
tissue was significantly higher than that from paired normal
esophageal epithelium. Girdin may be expressed in normal cells of
several tissues, as it is involved not only in the motility of
cancer cells but also in functions such as angiogenesis and
neurogenesis. This result suggests that increased Girdin expression
in esophageal squamous cells enhances their ability to invade
surrounding tissues when they become cancerous. In IHC, the
invasion of primary tumors was significantly deeper in the strong
staining group than in the weak staining group, which may reflect
Girdin’s function in cell motility. Garcia-Marcos et
al(20) reported that there was
a significant inverse correlation between Girdin expression and
disease-free survival of stage II colorectal cancer patients. In
the present study, it was shown that higher expression levels of
Girdin were also associated with shorter postoperative survival in
ESCC.
In esophageal cancer, prognostic markers such as
E-cadherin, MDM2 and HGF have been reported (21–23).
We previously showed that expression of DNA fragmentation factor
45, excision repair cross complementing 3, and PABPC1 may be
prognostic markers for ESCC (24–26).
Girdin may now be added to this list. Our data indicate that Girdin
may be a suitable candidate for a molecular prognosis marker for
ESCC. Girdin may also become a therapeutic target in ESCC.
We analyzed the relationship between Girdin and ESCC
for the first time. Girdin appeared to be involved in the motility
of ESCC cells, and the level of its expression correlated inversely
with the survival rate of ESCC patients. In ESCC, Girdin may be a
prognostic marker and it may serve as a therapeutic target as
well.
Acknowledgements
The authors thank Hisashi Takino (Department of
Anatomic Pathology and Molecular Diagnostics, Nagoya City
University) for immunostaining for resected specimens of ESCC.
References
|
1
|
Kuwano H, Nakajima M, Miyazaki T and Kato
H: Distinctive clinicopathological characteristics in esophageal
squamous cell carcinoma. Ann Thorac Cardiovasc Surg. 9:6–13.
2003.PubMed/NCBI
|
|
2
|
Ando N, Ozawa S, Kitagawa Y, Shinozawa Y
and Kitajima M: Improvement in the results of surgical treatment of
advanced squamous esophageal carcinoma during 15 consecutive years.
Ann Surg. 232:225–232. 2000.PubMed/NCBI
|
|
3
|
Hofstetter W, Swisher SG, Correa AM, et
al: Treatment outcomes of resected esophageal cancer. Ann Surg.
236:376–384. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boyce GA and Boyce BH: Esophagus. Text of
Gastro-enterology. Yamada TY, Alpers DH and Laine L: Lippincott
Williams and Wilkins; Philadelphia, PA: pp. 1180–1195. 2003
|
|
5
|
Yamazaki D, Kurisu S and Takenawa T:
Regulation of cancer cell motility through actin reorganization.
Cancer Sci. 96:379–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Enomoto A, Murakami H, Asai N, et al:
Akt/PKB regulates actin organization and cell motility via
Girdin/APE. Dev Cell. 9:389–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang P, Enomoto A, Jijiwa M, et al: An
actin-binding protein Girdin regulates the motility of breast
cancer cells. Cancer Res. 68:1310–1318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rivas RJ and Hatten ME: Motility and
cytoskeletal organization of migrating cerebellar granule neurons.
J Neurosci. 15:981–989. 1995.PubMed/NCBI
|
|
9
|
Trivers KF, Sabatino SA and Stewart SL:
Trends in esophageal cancer incidence by histology, United States,
1998–2003. Int J Cancer. 123:1422–1428. 2008.
|
|
10
|
Ide H, Udagawa H, Ozawa S, et al: The
Japanese Society for Esophageal Diseases: Comprehensive registry of
esophageal cancer in Japan (1998,1999). The Japanese Society for
Esophageal Diseases; Tokyo: 2002
|
|
11
|
Ozawa S, Tachimori Y, Baba H, et al:
Comprehensive registry of esophageal cancer in Japan, 2002.
Esophagus. 7:7–22. 2010. View Article : Google Scholar
|
|
12
|
Harris AK: A dozen questions about how
tissue cells crawl. Biochem Soc Symp. 65:315–341. 1999.PubMed/NCBI
|
|
13
|
Roy S, Miao F and Qi HJ: Cell crawling
assisted by contractile stress induced retraction. J Biomech Eng.
132:0610052010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olson MF and Sahai E: The actin
cytoskeleton in cancer cell motility. Clin Exp Metastasis.
26:273–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Machesky LM and Hall A: Role of actin
polymerization and adhesion to extracellular matrix in Rac- and
Rho-induced cytoskeletal reorganization. J Cell Biol. 138:913–926.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Welch MD: The world according to Arp:
regulation of actin nucleation by the Arp2/3 complex. Trends Cell
Biol. 9:423–427. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohta Y, Suzuki N, Nakamura S, Hartwig JH
and Stossel TP: The small GTPase RalA targets filamin to induce
filopodia. Proc Natl Acad Sci USA. 96:2122–2128. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altomare DA and Khaled AR: Homeostasis and
the importance for a balance between AKT/mTOR activity and
intracellular signaling. Curr Med Chem. 19:3748–3762. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian Y, Corum L, Meng Q, et al: PI3K
induced actin filament remodeling through Akt and p70S6K1:
implication of essential role in cell migration. Am J Physiol Cell
Physiol. 286:C153–C163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garcia-Marcos M, Jung BH, Ear J, Cabrera
B, Carethers JM and Ghosh P: Expression of GIV/Girdin, a
metastasis-related protein, predicts patient survival in colon
cancer. FASEB J. 25:590–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin YC, Wu MY, Li DR, Wu XY and Zheng RM:
Prognostic and clinicopathological features of E-cadherin,
alpha-catenin, beta-catenin, gamma-catenin and cyclin D1 expression
in human esophageal squamous cell carcinoma. World J Gastroenterol.
10:3235–3239. 2004.PubMed/NCBI
|
|
22
|
Shimada Y, Imamura M, Shibagaki I, et al:
Genetic alterations in patients with esophageal cancer with short-
and long-term survival rates after curative esophagectomy. Ann
Surg. 226:162–168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren Y, Cao B, Law S, et al: Hepatocyte
growth factor promotes cancer cell migration and angiogenic factors
expression: a prognostic marker of human esophageal squamous cell
carcinomas. Clin Cancer Res. 11:6190–6197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Konishi S, Ishiguro H, Shibata Y, et al:
Decreased expression of DFF45/ICAD is correlated with a poor
prognosis in patients with esophageal carcinoma. Cancer.
95:2473–2478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Terashita Y, Ishiguro H, Haruki N, et al:
Excision repair cross complementing 3 expression is involved in
patient prognosis and tumor progression in esophageal cancer. Oncol
Rep. 12:827–831. 2004.PubMed/NCBI
|
|
26
|
Takashima N, Ishiguro H, Kuwabara Y, et
al: Expression and prognostic roles of PABPC1 in esophageal cancer:
correlation with tumor progression and postoperative survival.
Oncol Rep. 15:667–671. 2006.PubMed/NCBI
|