Introduction
Hepatocellular carcinoma (HCC) is a major health
problem worldwide. It is the fifth most common cancer in men and
the seventh in women and the third most common cause of
cancer-related deaths (1–4). In Japan, as well as in other
countries, most cases of HCC are associated with viral infections
such as hepatitis B virus (HBV) and hepatitis C virus (HCV),
although in our country, the number of HCC patients with etiologies
other than HBV and HCV has recently been increasing (2,5). In
general, the prognosis for untreated HCC is poor, and the curative
treatments consist of surgical resection (SR) and liver
transplantation (1,3,4).
However, HCC frequently recurs after curative SR, leading to high
mortality, although recurrence only occurs at intrahepatic sites in
68–96% of patients (6,7). Stringent follow-up of HCC patients
following SR is therefore essential.
Serum antibody to hepatitis B core antigen
(anti-HBc) positivity, which indicates a past history of HBV
infection, has recently been attracting attention as a predictor of
liver carcinogenesis in patients with HCV-related liver diseases
(8–12). HBV DNA may be present in a latent
form, even after seroclearance of HB surface antigen (HBsAg), which
is referred to as occult HBV infection (8,13).
Anti-HBc is reported to be a surrogate marker for such latent
carriers (14). Moreover, previous
studies have indicated that so-called occult HBV infection,
reflected by anti-HBc positivity, is highly prevalent in a number
of patient subgroups, including those with HCV infection and HCC,
and may play an important role in hepatocarcinogenesis through
expression of oncogenic viral protein (10,15).
There have been several reports regarding the effect
of anti-HBc positivity on carcinogenesis in patients with
HCV-related liver disease, and most of these studies have reported
that the presence of anti-HBc is a risk factor for the development
of HCC in individuals with HCV infection (8,10,15,16).
However, it remains unknown whether anti-HBc positivity constitutes
an additional risk factor in terms of survival after curative SR
for HCV-related HCC. The aim of the present study was, therefore,
to examine the relationship between anti-HBc positivity and
survival in HCV-related HCC patients who underwent curative SR.
Patients and methods
Patients
Patients were selected for SR based on assessment of
tumor characteristics, remnant liver volume, and general condition,
through discussion with experienced surgeons, radiologists and
physicians.
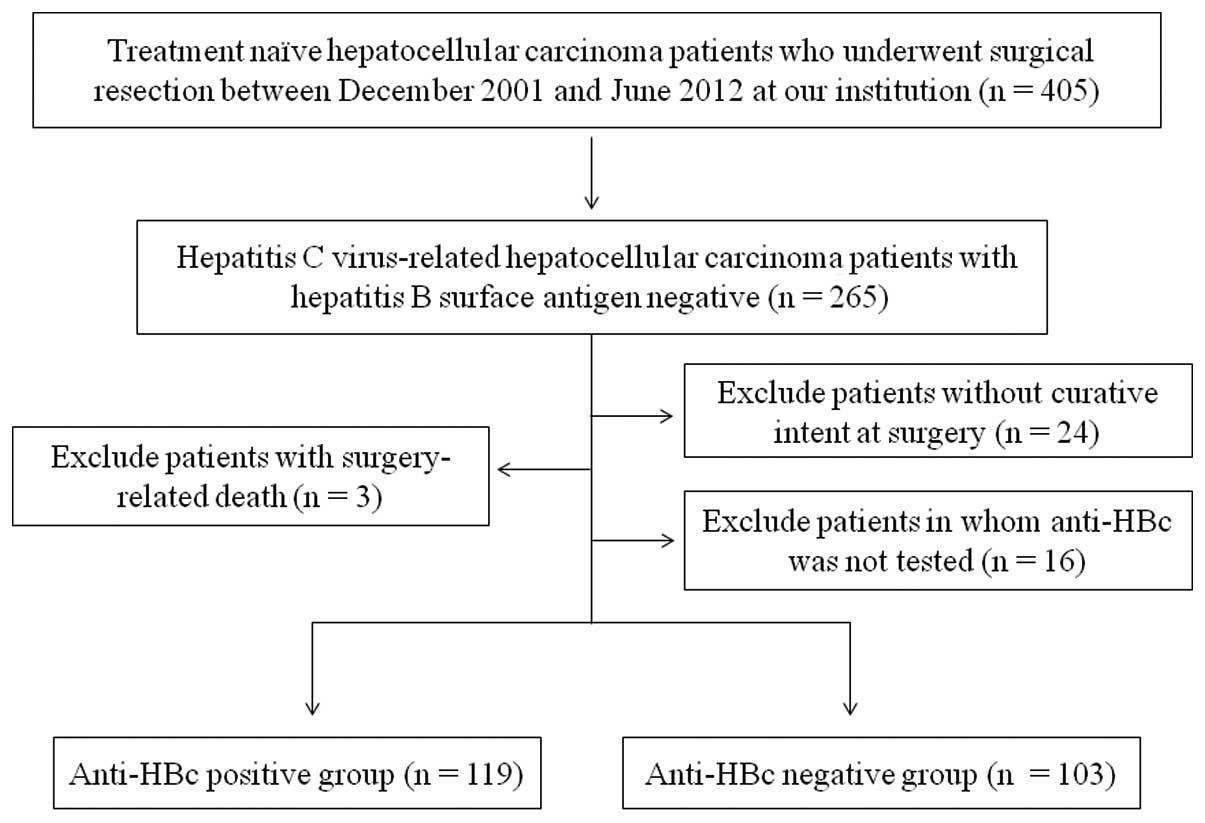
SR was performed on 405 treatment-naïve HCC patients
at the Department of Surgery, Osaka Red Cross Hospital, Japan,
between December 2001 and June 2012. There were 265 HCV-related HCC
patients (64.7%), negative for HBsAg and positive for the HCV
antibody (HCVAb). Of these, we excluded patients operated on
without curative intent (n=24), with surgery-related death (n=3)
and those for whom anti-HBc was not tested (n=16). Curative surgery
was defined as the resection of all tumors detectable using imaging
modalities. A total of 222 HCV-related HCC patients were thus
analyzed in the present study (Fig.
1). Patients were classified into two groups: anti-HBc-positive
(n=119, 53.6%) and anti-HBc-negative (n=103, 46.4%). Overall
survival (OS) and recurrence-free survival (RFS) rates were
compared between the two groups.
All the protocols were approved by the Ethics
Committee of our institution. Written informed consent was obtained
from all patients prior to surgery, and the study protocol complied
with all of the provisions of the Declaration of Helsinki. The
present study comprised a retrospective analysis of patient records
registered in our database and all treatments were conducted in an
open-label manner.
HCC diagnosis
HCC was diagnosed using abdominal ultrasound and
dynamic computed tomography (CT) scans (hyperattenuation during the
arterial phase in all or some part of the tumor and hypoattenuation
in the portal-venous phase) and/or magnetic resonance imaging
(MRI), based mainly on the recommendations of the American
Association for the Study of Liver Diseases (17). Arterial- and portal-phase dynamic CT
images were obtained at ~30 and 120 sec, respectively, after the
injection of the contrast material. HCC stage was determined using
the Liver Cancer Study Group of Japan staging system (18). HCC was confirmed pathologically in
specimens at surgery.
Serological studies
HBsAg and anti-HBc were detected using commercial
enzyme immunoassay kits (Dainabot, Tokyo, Japan) (13). The results of the anti-HBc assays
were expressed as the percentage of inhibition, and the specimens
were considered to be anti-HBc-positive when the percentage of
inhibition was >50% (19). HCVAb
was assessed using second-generation assays (Dainabot) (13). In the present study, serum HCV RNA
levels were tested in 182 (82.0%) out of 222 patients by using a
competitive reverse transcription-polymerase chain reaction
assay.
Follow-up
Follow-up after surgery consisted of periodic blood
tests and monitoring of tumor markers, including α-fetoprotein
(AFP) and des-γ-carboxy prothrombin (DCP), using chemiluminescent
enzyme immunoassays (Lumipulse PIVKA-II Eisai; Eisai Co., Ltd.,
Tokyo, Japan). Dynamic CT scans and/or MRI were obtained every 2–4
months after surgery. Chest CT, whole abdominal CT, brain MRI, and
bone scintigraphy were performed when extrahepatic HCC recurrence
was suspected.
Statistical analysis
Data were analyzed using univariate and multivariate
analyses. Continuous variables were compared using unpaired t-tests
and categorical variables were compared using Fisher’s exact tests.
Time to recurrence was defined as the interval between each therapy
and first confirmed recurrence. For analysis of RFS, follow-up
ended at the time of first recurrence; other patients were censored
at their last follow-up visit or the time of death from any cause
without recurrence. For analysis of OS, follow-up ended at the time
of death from any cause, and the remaining patients were censored
at the last follow-up visit. The cumulative OS and RFS rates were
calculated using the Kaplan-Meier method and tested using the
log-rank test. Factors with a P-value <0.05 in univariate
analysis were subjected to multivariate analysis using the Cox
proportional hazards model. These statistical methods were used to
estimate the interval from initial treatment. Data were analyzed
using SPSS for Windows (SPSS Inc., Chicago, IL, USA). Data are
expressed as means ± standard deviation (SD). Values of P<0.05
were considered to be statistically significant.
Results
Baseline characteristics
The baseline characteristics of the patients in the
two groups are shown in Table I.
The median observation periods were 3.4 years (range, 0.3–10.9
years) in the anti-HBc-positive group and 3.2 years (range,
0.5–10.9 years) in the anti-HBc-negative group. In terms of maximum
tumor size (P=0.046), aspartate aminotransferase (AST) value
(P=0.027) and DCP value (P=0.046), significant differences were
observed in the two groups. The proportion of HCC patients with
cirrhotic liver in the anti-HBc-positive group tended to be higher
than that in the anti-HBc-negative group (P=0.068).
 | Table IBaseline characteristics between the
anti-HBc-postive group and the anti-HBc-negative group. |
Table I
Baseline characteristics between the
anti-HBc-postive group and the anti-HBc-negative group.
| Variables | Anti-HBc positive
group (n=119) | Anti-HBc negative
group (n=103) | P-value |
|---|
| Age (years) | 69.3±8.1 | 69.0±8.3 | 0.798a |
| Gender,
male/female | 85/34 | 66/37 | 0.252b |
| HCC Stage
I/II/III/IVA | 12/70/27/10 | 12/62/25/4 | 0.590b |
| Maximum tumor size
(cm) | 4.3±2.8 | 3.7±1.6 | 0.046a |
| Tumor number,
single/multiple | 79/40 | 74/29 | 0.388b |
| Background liver,
cirrhotic/non-cirrhotic | 83/36 | 59/44 | 0.068b |
| Hepatitis C viral
load, high/low/unknown | 75/24/20 | 69/14/20 | 0.420b |
| IFN therapy after
surgery, yes/no | 11/108 | 5/98 | 0.299b |
| AST (IU/l) | 71.5±41.4 | 60.1±33.9 | 0.027a |
| ALT (IU/l) | 63.7±41.0 | 57.6±44.3 | 0.290a |
| Serum albumin
(g/dl) | 3.78±0.52 | 3.78±0.48 | 0.921a |
| Total bilirubin
(mg/dl) | 0.87±0.44 | 0.82±0.40 | 0.439a |
| Prothrombin time
(%) | 86.1±13.9 | 88.9±12.8 | 0.116a |
| Platelets
(x104/mm3) | 13.1±6.1 | 12.7±4.9 | 0.569a |
| AFP (ng/ml) | 3,089.6±15,640.2 | 789.2±2,708.3 | 0.142a |
| DCP (mAU/ml) |
5,186.4±19,928.0 |
1,207.2±2,845.3 | 0.046a |
| Diabetes mellitus,
yes/no | 31/88 | 32/71 | 0.457b |
| Body mass index
(kg/m2) | 23.0±3.3 | 23.1±4.1 | 0.846a |
Cumulative OS and RFS rates
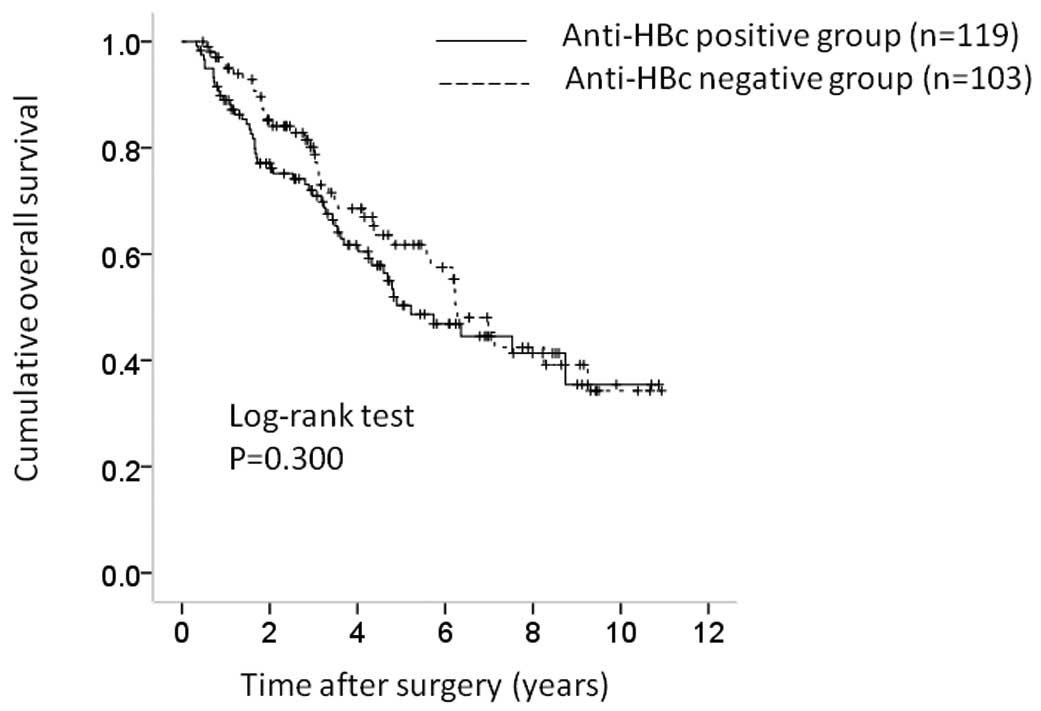
The 1-, 3- and 5-year cumulative OS rates were 88.8,
70.2 and 50.0%, respectively, in the anti-HBc-positive group and
95.8, 77.1 and 61.7% in the anti-HBc-negative group (P=0.300)
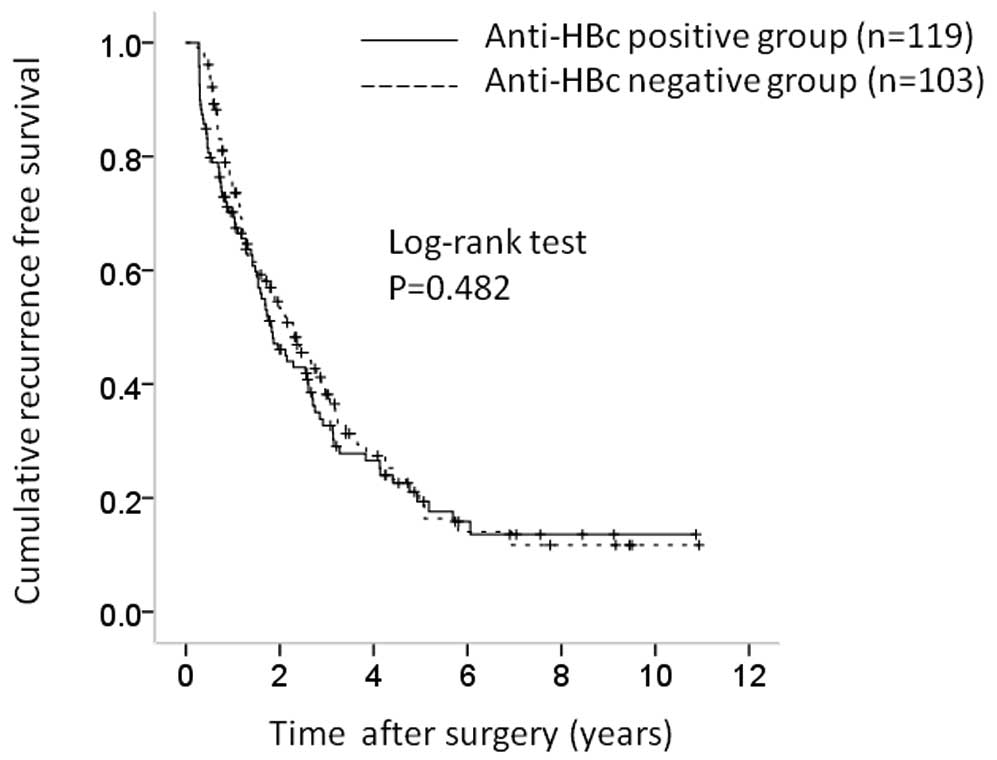
(Fig. 2). The corresponding RFS
rates were 68.7, 33.0 and 20.0%, respectively, in the
anti-HBc-positive group and 74.4, 38.5 and 16.5% in the
anti-HBc-negative group (P=0.482) (Fig.
3).
Univariate and multivariate analyses of
factors contributing to OS
Univariate analysis identified the following factors
as significantly associated with OS for all cases (n=222): HCC
stage (P<0.001); maximum tumor size ≥4 cm (P=0.016); tumor
number (P=0.002); interferon (IFN) therapy after surgery (P=0.044);
serum albumin ≥3.8 g/dl (P=0.023); and microscopic vascular
invasion (P<0.001) (Table II).
The hazard ratios (HRs) and 95% confidence intervals (CIs)
calculated using multivariate analysis for the 6 factors that were
significant in univariate analysis are detailed in Table III. Serum albumin ≥3.8 g/dl
(P=0.005) and microscopic vascular invasion (P<0.001) were found
to be significant predictors linked to OS in multivariate
analysis.
 | Table IIUnivariate analysis contributing to
OS and RFS for all cases (n=222). |
Table II
Univariate analysis contributing to
OS and RFS for all cases (n=222).
| Variables | n | OS P-valuea | RFS P-valuea |
|---|
| Age ≥70
(yes/no) | 115/107 | 0.120 | 0.957 |
| Gender
(male/female) | 151/71 | 0.476 | 0.539 |
| Background liver,
cirrhotic/non-cirrhotic | 142/80 | 0.748 | 0.233 |
| Anti-HBc
(positive/negative) | 119/103 | 0.300 | 0.482 |
| HCC stage (I,
II/III, IV) | 156/66 | <0.001 | <0.001 |
| Maximum tumor size
≥4 cm (yes/no) | 94/128 | 0.016 | 0.023 |
| Tumor number
(single/multiple) | 69/153 | 0.002 | <0.001 |
| IFN therapy after
surgery (yes/no) | 16/206 | 0.044 | 0.019 |
| ICG-R15 ≥14%
(yes/no) | 109/113 | 0.288 | 0.864 |
| Total bilirubin
≥1.0 mg/dl (yes/no) | 61/161 | 0.068 | 0.022 |
| Serum albumin ≥3.8
g/dl (yes/no) | 124/98 | 0.023 | 0.139 |
| AST ≥60 IU/l
(yes/no) | 103/119 | 0.355 | 0.027 |
| ALT ≥50 IU/l
(yes/no) | 109/113 | 0.393 | 0.008 |
| Platelets
≥13×104/mm3 (yes/no) | 103/119 | 0.336 | 0.900 |
| Prothrombin time
≥80% (yes/no) | 151/71 | 0.771 | 0.351 |
| AFP ≥40 ng/ml
(yes/no) | 94/128 | 0.085 | 0.001 |
| DCP ≥100 mAU/ml
(yes/no) | 130/92 | 0.270 | 0.034 |
| Microscopic capsule
(yes/no) | 178/44 | 0.833 | 0.412 |
| Microscopic capsule
invasion (yes/no) | 132/90 | 0.188 | 0.390 |
| Microscopic
vascular invasion (yes/no) | 69/153 | <0.001 | <0.001 |
| Microscopic
surgical margin (yes/no) | 28/194 | 0.475 | 0.912 |
 | Table IIIMultivariate analysis contributing to
OS after surgical resection. |
Table III
Multivariate analysis contributing to
OS after surgical resection.
| Variable | Hazard ratio | 95% confidence
interval | P-valuea |
|---|
| HCC stage |
| I or II | 1.000 | | 0.347 |
| III or IV | 0.621 | 0.230–1.674 | |
| Maximum tumor size
(cm) |
| ≥4 | 0.849 | 0.548–1.316 | 0.465 |
| <4 | 1.000 | | |
| Tumor no. |
| Single | 1.000 | | |
| Multiple | 0.855 | 0.322–2.279 | 0.753 |
| IFN therapy after
surgery |
| Yes | 4.001 | 0.983–16.280 | 0.053 |
| No | 1.000 | | |
| Serum albumin |
| ≥3.8 g/dl | 1.841 | 1.205–2.812 | 0.005 |
| <3.8 g/dl | 1.000 | | |
| Microscopic
vascular invasion |
| Yes | 0.424 | 0.274–0.655 | <0.001 |
| No | 1.000 | | |
Univariate and multivariate analyses of
factors contributing to RFS
Univariate analysis identified the following factors
as significantly associated with RFS for all cases (n=222): HCC
stage (P<0.001); maximum tumor size ≥4 cm (P=0.023); tumor
number (P<0.001); IFN therapy after surgery (P=0.019); total
bilirubin ≥1.0 mg/dl (P=0.022); AST ≥60 IU/l (P=0.027); alanine
aminotransferase ≥50 IU/l (P=0.008); AFP ≥40 ng/ml (P=0.001); DCP
≥100 mAU/ml (P=0.034); and microscopic vascular invasion
(P<0.001) (Table III). The HRs
and 95% CIs calculated using multivariate analysis for the 10
factors that were significant in univariate analysis are detailed
in Table IV. IFN therapy after
surgery (P=0.011), AFP ≥40 ng/ml (P=0.030) and microscopic vascular
invasion (P<0.001) were found to be significant prognostic
factors linked to RFS.
 | Table IVMultivariate analysis contributing to
RFS after surgical resection. |
Table IV
Multivariate analysis contributing to
RFS after surgical resection.
| Variable | Hazard ratio | 95% confidence
interval | P-valuea |
|---|
| HCC stage |
| I or II | 1.000 | | 0.245 |
| III or IV | 0.659 | 0.326–1.331 | |
| Maximum tumor size
(cm) |
| ≥4 | 0.838 | 0.593–1.185 | 0.318 |
| <4 | 1.000 | | |
| Tumor number |
| Single | 1.000 | | 0.283 |
| Multiple | 0.681 | 0.338–1.374 | |
| IFN therapy after
surgery |
| Yes | 2.760 | 1.267–6.012 | 0.011 |
| No | 1.000 | | |
| Total bilirubin
(mg/dl) |
| ≥1 | 0.863 | 0.574–1.298 | 0.480 |
| <1 | 1.000 | | |
| AST (IU/l) |
| ≥60 | 1.000 | | 0.295 |
| <60 | 1.291 | 0.800–2.085 | |
| ALT (IU/l) |
| ≥50 | 1.000 | | 0.796 |
| <50 | 0.942 | 0.600–1.479 | |
| AFP (ng/ml) |
| ≥40 | 0.678 | 0.478–0.962 | 0.030 |
| <40 | 1.000 | | |
| DCP (mAU/ml) |
| ≥100 | 1.000 | | 0.128 |
| <100 | 1.296 | 0.928–1.810 | |
| Microscopic
vascular invasion |
| Yes | 0.480 | 0.335–0.689 | <0.001 |
| No | 1.000 | | |
Causes of death in the two groups
Fifty-two patients in the anti-HBc-positive group
(43.7%) died during the follow-up period. The causes of death were
HCC recurrence in 39 patients, liver failure in 9 and other causes
in 4. Forty patients in the anti-HBc-negative group (38.8%) died
during the follow-up period, and the causes of death were HCC
recurrence in 25 patients, liver failure in 10, and other causes in
5.
HCC recurrence
In the present study, 85 anti-HBc-positive patients
(71.4%) and 68 anti-HBc-negative patients (66.0%) had HCC
recurrence during the follow-up period. The patterns of HCC
recurrence after surgery in the anti-HBc-positive group were:
single HCC recurrence in the liver in 35 patients; multiple HCC
recurrences in the liver in 42 patients; multiple HCC recurrences
in the liver with lung metastases in 3 patients; multiple HCC
recurrences in the liver with lymph node metastases in 2 patients;
multiple HCC recurrences in the liver with peritoneal dissemination
in 1 patient; multiple HCC recurrences in the liver with portal
vein tumor invasion in 1 patient; and single brain metastasis in 1
patient. The patterns of HCC recurrence after surgery in the
anti-HBc-negative group were: single HCC recurrence in the liver in
28 patients; single HCC recurrence in the liver with portal vein
invasion in 1 patient; multiple HCC recurrences in the liver in 36
patients; multiple HCC recurrences in the liver with lymph node
metastases in 1 patient; multiple HCC recurrences in the liver with
inferior vena cava invasion in 1 patient; and multiple HCC
recurrences in the liver with bone metastases in 1 patient.
Treatment methods for HCC recurrence
Treatment methods for the first HCC recurrence in
the anti-HBc-positive group were: SR in 9 patients; radiofrequency
ablation (RFA) in 35; transcatheter arterial chemoembolization
(TACE) in 26; percutaneous ethanol injection (PEI) in 3; systemic
chemotherapy in 3; radiotherapy in 1; and no specific treatment in
8 patients. The treatment methods used in the anti-HBc-negative
group were: SR in 3 patients; RFA in 40; TACE in 20; PEI in 3;
radiotherapy in 1; and no specific treatment in 1 patient.
IFN therapy after surgery
In the present study, 16 patients [11 patients
(9.2%) in the anti-HBc-positive group and 5 (4.9%) in the
anti-HBc-negative group] received IFN therapy after surgery. They
included stage I HCC in 2 patients, stage II in 9, stage III in 4
and stage IV in 1. Whether IFN therapy after surgery was performed
was mainly determined by decision of the attending physicians. Of
these, all patients had high viral load as defined by the
guidelines before IFN therapy (20,21).
Fourteen patients received peginterferon and ribavirin combination
therapy and 2 received long-term low-dose IFN maintenance therapy.
Seven patients (43.8%) achieved sustained virological response as
defined by undetectable HCV RNA 24 weeks after completion of IFN
treatment. Seven patients (43.8%) had HCC recurrence and 2 (12.5%)
died during the follow-up period.
Subgroup analyses according to maximum
tumor size
In terms of maximum tumor size, there was a
significant difference in baseline characteristics between the
anti-HBc-positive and anti-HBc-negative groups. We therefore
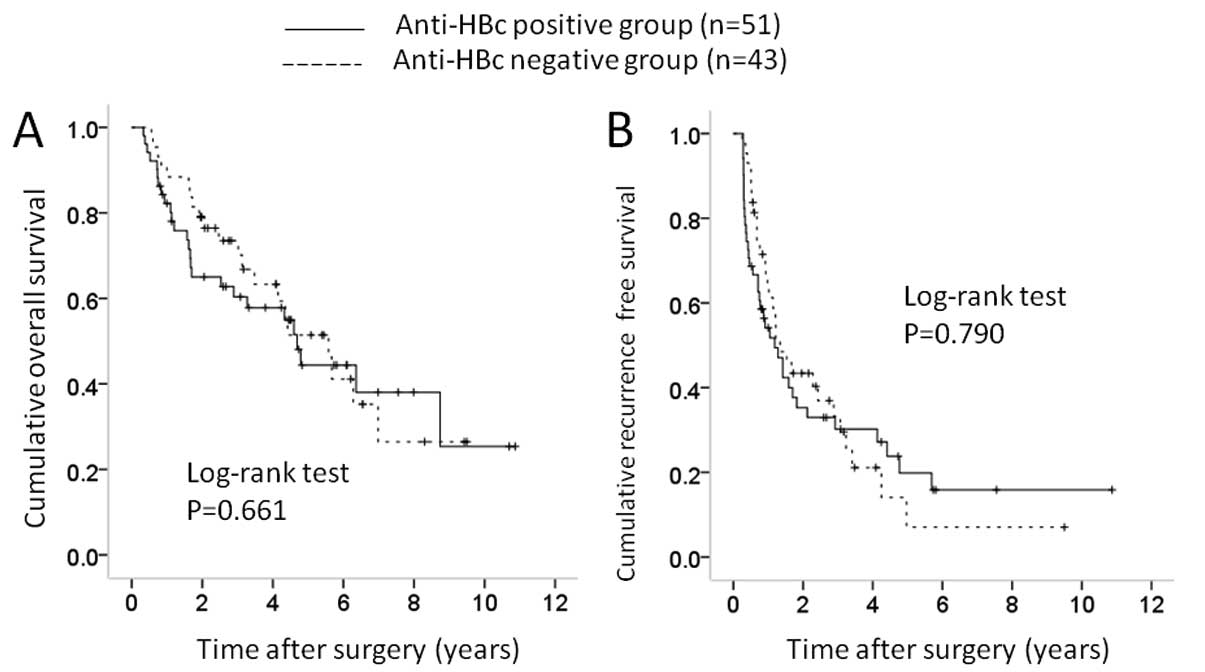
performed subgroup analyses according to maximum tumor size. In
patients with maximum tumor size ≥4 cm [51 (42.9%) in the
anti-HBc-positive group and 43 (41.7%) in the anti-HBc-negative
group], no significant difference was observed in OS (P=0.661) and
RFS (P=0.790) (Fig. 4A and 4B). In
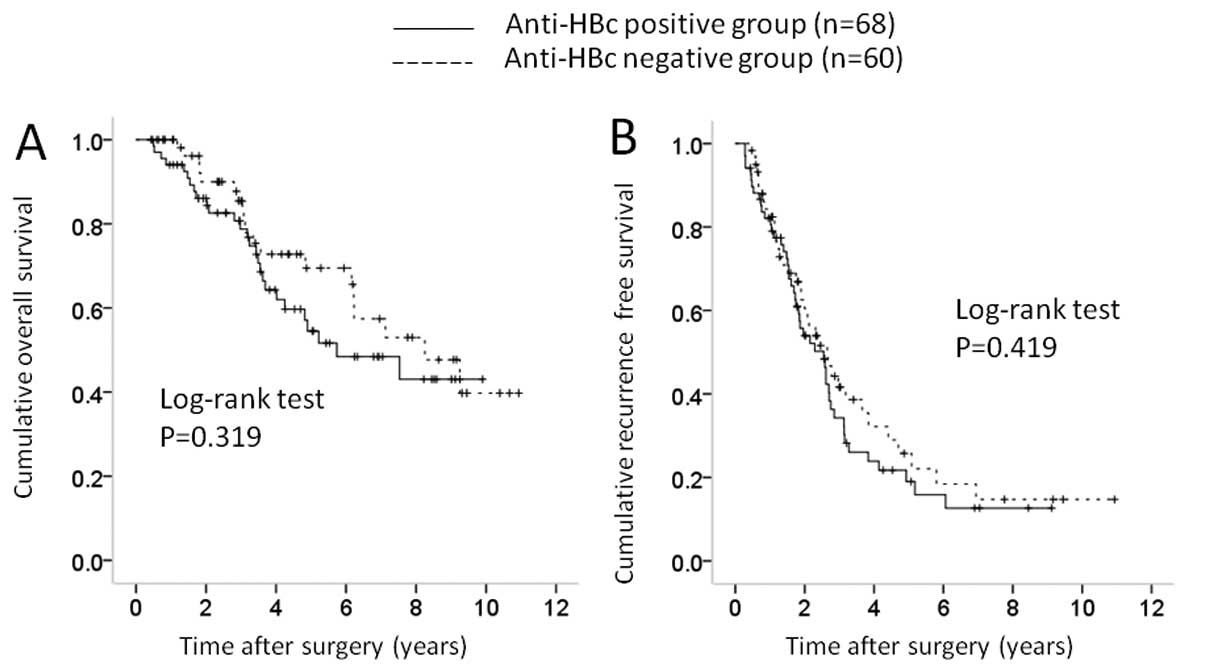
patients with maximum tumor size <4 cm [68 (57.1%) in the
anti-HBc-positive group and 60 (58.3%) in the anti-HBc-negative
group], there was no significant difference in OS (P=0.319) and RFS
(P=0.419) (Fig. 5A and 5B).
Subgroup analyses according to background
liver disease
Marginal significance was observed between the two
groups in terms of background liver disease (P=0.068), and we
therefore performed subgroup analyses accordingly. In patients with
cirrhotic liver [83 (69.7%) in the anti-HBc-positive group and 59
(57.3%) in the anti-HBc-negative group], there was no significant
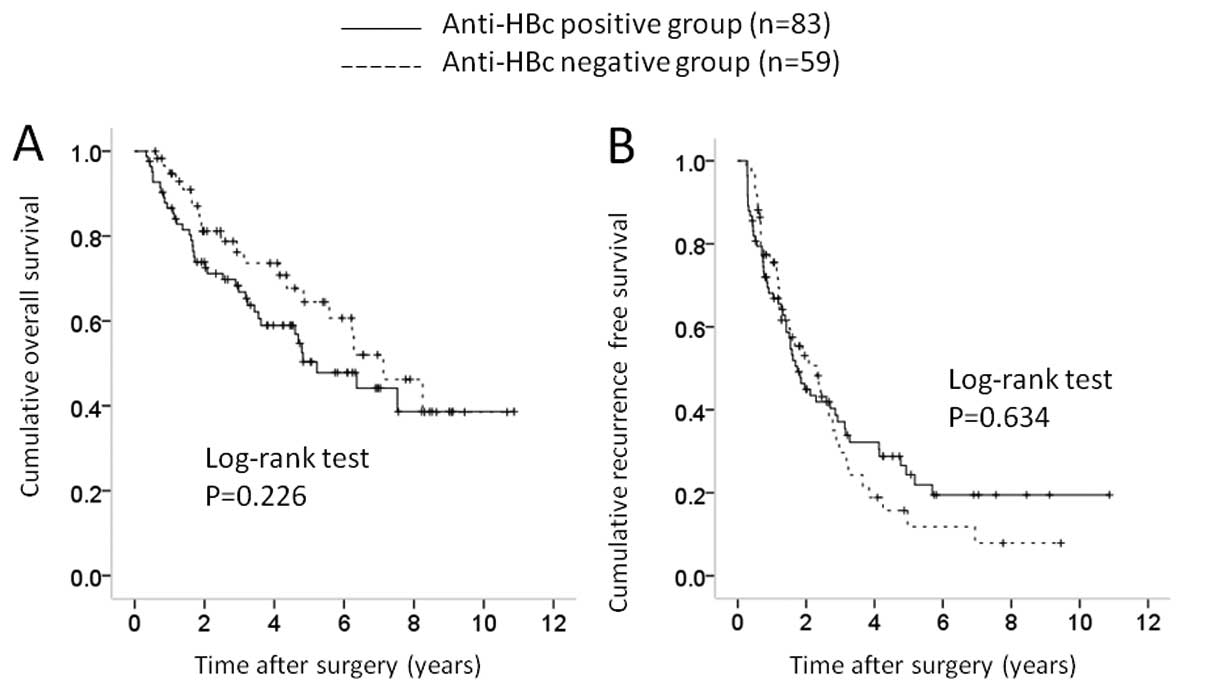
difference in OS (P=0.226) and RFS (P=0.634) (Fig. 6A and 6B). In patients with
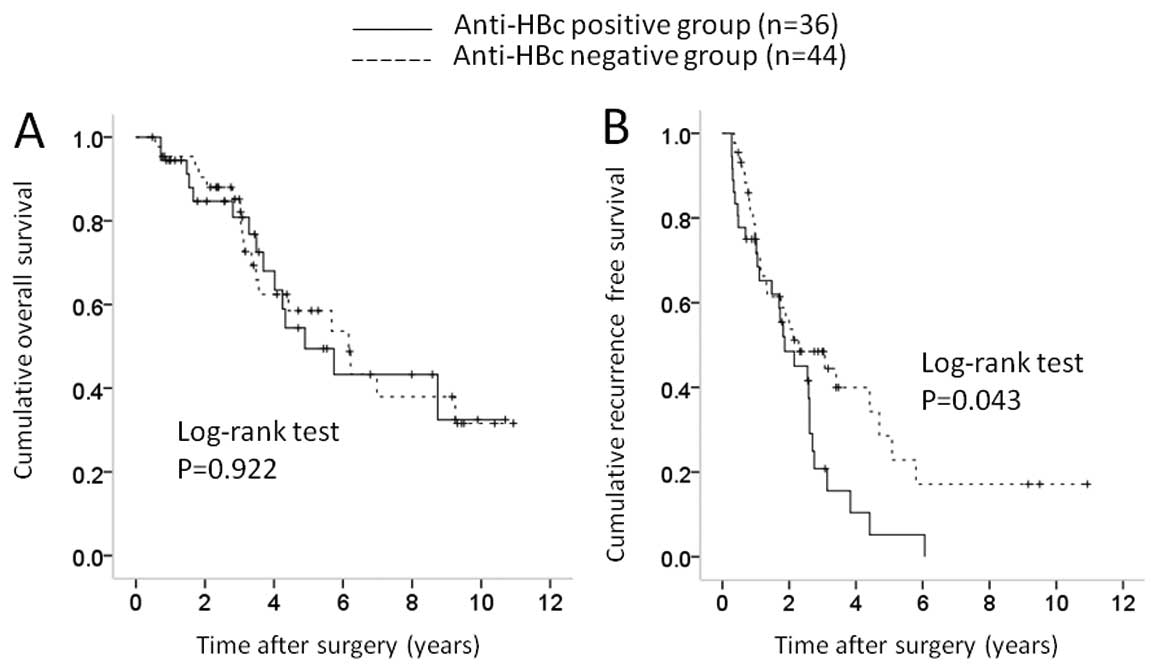
non-cirrhotic liver disease [36 (30.3%) in the anti-HBc-positive
group and 44 (42.7%) in the anti-HBc-negative group], there was no
significant difference in OS (P=0.922), whereas there was a
significant difference in RFS (P=0.043) (Fig. 7A and 7B).
Discussion
To the best of our knowledge, this is the first
reported comparative study to examine the relationship between
anti-HBc positivity and survival in patients with HCV-related HCC
who underwent curative SR, although our study was retrospective in
nature. Several previous studies have reported that anti-HBc
positivity influences carcinogenesis in patients with HCV-related
liver disease; however, as far as we are aware, there has been no
report regarding the effect of anti-HBc positivity on survival
after curative SR for HCC. Hence the reason for the present
study.
In our analyses, anti-HBc positivity was not a
significant factor in terms of OS and RFS. Moreover, in subgroup
analyses according to maximum tumor size and background liver
disease, RFS did not differ significantly in all subgroups except
in patients with non-cirrhotic liver disease. These results suggest
that anti-HBc positivity cannot be a useful predictor for patients
with HCV-related HCC who undergo curative SR, although in those
with non-cirrhotic liver disease, it may be associated with HCC
recurrence after surgery.
There were 119 patients (53.6%) with anti-HBc
positivity in the present study. Marusawa et al(12) reported that 363 (59.4%) out of 611
HCV-related HCC patients had anti-HBc positivity, and have
suggested that HBV infection, including latent infection, plays an
important role in carcinogenesis in patients with HCV-related liver
disease. Our results were similar to that study, although in terms
of HCC recurrence, anti-HBc positivity was not demonstrated to be a
prognostic factor. In contrast, Mazzaferro et al(22) reported that 70 (46.7%) out of 150
patients with HCV-related HCC were anti-HBc positive, which was
slightly lower than the result in our study. Racial and
geographical factors may have been associated with these
results.
In our study, the proportion of patients with
cirrhotic liver in the anti-HBc-positive group tended to be higher
than that in the anti-HBc-negative group. Several studies have
demonstrated that the prevalence of anti-HBc positivity is closely
correlated with the clinical stage of liver disease (9,10,12).
Our results were consistent with these reports, which suggest that
anti-HBc should be closely monitored in patients with advanced
HCV-related liver disease.
The baseline AST level in the anti-HBc-positive
group was significantly higher than that in the anti-HBc-negative
group in our study. Although the reasons for this are unknown, in
patients with HCV-related to liver disease, anti-HBc positivity may
correlate with higher activity of background liver disease.
HCC often recurs after curative surgery, leading to
high mortality (6). Indeed, in the
present study, 85 anti-HBc-positive patients (71.4%) and 68
anti-HBc-negative patients (66.0%) had HCC recurrence during the
follow-up period. Stringent follow-up of HCC patients following SR
is therefore essential. In the present study, microvascular
invasion was the strongest prognostic factor in terms of both OS
and RFS. In the postoperative management of HCC, preoperative
factors such as degree of liver damage, radiological findings, and
tumor markers as well as factors based on postoperative status
should be considered (23).
In the present study, IFN therapy after surgery was
a significant prognostic factor in terms of RFS, and was a
marginally significant factor associated with OS, although the
number of patients who received IFN therapy after surgery was
small. Several investigators have reported that IFN therapy after
curative SR in patients with HCV-related HCC improves clinical
outcome (24–26). Our results are consistent with these
reports; additional IFN therapy should be taken into account in
patients with HCV-related HCC who undergo curative SR.
Serum albumin levels were significantly associated
with OS in our multivariate analysis. Patients with liver cirrhosis
and low serum albumin levels can develop protein-energy
malnutrition with increased catabolism (27). Protein-energy malnutrition is
associated with high morbidity and mortality because of an
increased risk of life-threatening complications, resulting in poor
survival and reduced quality of life (28). In the present study, 142 patients
(64.0%) had cirrhotic liver, indicating that a high proportion of
patients with HCV-related HCC have concurrent liver cirrhosis.
Branched chain amino acid treatment may optimize clinical outcome
in these patients (29,30).
There were several limitations to the present study.
First, it was a single-center retrospective study. Second, the
median observation periods in the two groups were relatively short
for survival analysis. Third, patients in whom anti-HBc was not
tested were excluded from our analysis, leading to bias. Larger
prospective studies with longer observation periods are thus
required to confirm these results. However, the current study
demonstrated that anti-HBc positivity was not associated with
survival in patients with HCV-related HCC who underwent curative
surgery. In conclusion, anti-HBc positivity need not be taken into
account when assessing clinical outcome in patients with
HCV-related HCC after curative surgery.
Acknowledgements
The authors would like to thank Haruko Takada for
data collection.
References
|
1
|
Livraghi T, Mäkisalo H and Line PD:
Treatment options in hepatocellular carcinoma today. Scand J Surg.
100:22–29. 2011.PubMed/NCBI
|
|
2
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Lope CR, Tremosini S, Forner A, et al:
Management of HCC. J Hepatol. 56(Suppl 1): S75–S87. 2012.
|
|
4
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Umemura T and Kiyosawa K: Epidemiology of
hepatocellular carcinoma in Japan. Hepatol Res. 37(Suppl 2):
S95–S100. 2007. View Article : Google Scholar
|
|
6
|
Nishikawa H, Osaki Y, Kita R, et al:
Transcatheter arterial infusion chemotherapy prior to
radiofrequency thermal ablation for single hepatocellular carcinoma
reduces the risk of intrahepatic distant recurrence. Int J Oncol.
41:903–909. 2012.
|
|
7
|
Zhou WP, Lai EC, Li AJ, et al: A
prospective, randomized, controlled trial of preoperative
transarterial chemoembolization for resectable large hepatocellular
carcinoma. Ann Surg. 249:195–202. 2009. View Article : Google Scholar
|
|
8
|
Ohki T, Tateishi R, Goto E, et al:
Influence of anti-HBc seropositivity on the risk of hepatocellular
carcinoma in HCV-infected patients after adjusting for confounding
factors. J Viral Hepat. 17:91–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Squadrito G, Pollicino T, Cacciola I, et
al: Occult hepatitis B virus infection is associated with the
development of hepatocellular carcinoma in chronic hepatitis C
patients. Cancer. 106:1326–1330. 2006. View Article : Google Scholar
|
|
10
|
Ikeda K, Marusawa H, Osaki Y, et al:
Antibody to hepatitis B core antigen and risk for hepatitis
C-related hepatocellular carcinoma: a prospective study. Ann Intern
Med. 146:649–656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lok AS, Everhart JE and Di Bisceglie AM;
HALT-C Trial Group. Occult and previous hepatitis B virus infection
are not associated with hepatocellular carcinoma in United States
patients with chronic hepatitis C. Hepatology. 54:434–442. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marusawa H, Osaki Y, Kimura T, et al: High
prevalence of anti-hepatitis B virus serological markers in
patients with hepatitis C virus related chronic liver disease in
Japan. Gut. 45:284–288. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shiota G, Oyama K, Udagawa A, et al:
Occult hepatitis B virus infection in HBs antigen-negative
hepatocellular carcinoma in a Japanese population: involvement of
HBx and p53. J Med Virol. 62:151–158. 2000. View Article : Google Scholar
|
|
14
|
Jilg W, Sieger E, Zachoval R and Schätzl
H: Individuals with antibodies against hepatitis B core antigen as
the only serological marker for hepatitis B infection: high
percentage of carriers of hepatitis B and C virus. J Hepatol.
23:14–20. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cacciola I, Pollicino T, Squadrito G,
Cerenzia G, Orlando ME and Raimondo G: Occult hepatitis B virus
infection in patients with chronic hepatitis C liver disease. N
Engl J Med. 341:22–26. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka K, Nagao Y, Ide T, Kumashiro R and
Sata M: Antibody to hepatitis B core antigen is associated with the
development of hepatocellular carcinoma in hepatitis C
virus-infected persons: a 12-year prospective study. Int J Mol Med.
17:827–832. 2006.
|
|
17
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
18
|
Liver Cancer Study Group of Japan. The
general rules for the clinical and pathological study of primary
liver cancer. Jpn J Surg. 19:98–129. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marusawa H, Uemoto S, Hijikata M, et al:
Latent hepatitis B virus infection in healthy individuals with
antibodies to hepatitis B core antigen. Hepatology. 31:488–495.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Institutes of Health. Consensus
Development Conference Statement. Management of hepatitis C: June
10–12, 2002. Hepatology. 36(Suppl 1): S3–S20. 2002.
|
|
21
|
Strader DB, Wright T, Thomas DL and Seeff
LB; American Association for the Study of Liver Diseases.
Diagnosis, management, and treatment of hepatitis C. Hepatology.
39:1147–1171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mazzaferro V, Romito R and Schiavo M; HCC
Italian Task Force. Prevention of hepatocellular carcinoma
recurrence with alpha-interferon after liver resection in HCV
cirrhosis. Hepatology. 44:1543–1554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim KC, Chow PK, Allen JC, et al:
Microvascular invasion is a better predictor of tumor recurrence
and overall survival following surgical resection for
hepatocellular carcinoma compared to the Milan criteria. Ann Surg.
254:108–113. 2011. View Article : Google Scholar
|
|
24
|
Sakae M, Kubo S, Takemura S, et al: Effect
of interferon therapy on first and second recurrence after
resection of hepatitis C virus-related hepatocellular carcinoma.
Hepatol Res. 42:564–573. 2012. View Article : Google Scholar
|
|
25
|
Tanimoto Y, Tashiro H, Aikata H, et al:
Impact of pegylated interferon therapy on outcomes of patients with
hepatitis C virus-related hepatocellular carcinoma after curative
hepatic resection. Ann Surg Oncol. 19:418–425. 2012. View Article : Google Scholar
|
|
26
|
Singal AK, Freeman DH Jr and Anand BS:
Meta-analysis: interferon improves outcomes following ablation or
resection of hepatocellular carcinoma. Aliment Pharmacol Ther.
32:851–858. 2010. View Article : Google Scholar
|
|
27
|
Lautz HU, Selberg O, Körber J, Bürger M
and Müller MJ: Protein-calorie malnutrition in liver cirrhosis.
Clin Investig. 70:478–486. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Italian Multicentre Cooperative Project on
Nutrition in Liver Cirrhosis. Nutritional status in cirrhosis. J
Hepatology. 21:317–335. 1994. View Article : Google Scholar
|
|
29
|
Nishikawa H, Osaki Y, Inuzuka T, et al:
Branched-chain amino acid treatment before transcatheter arterial
chemoembolization for hepatocellular carcinoma. World J
Gastroenterol. 18:1379–1384. 2012. View Article : Google Scholar
|
|
30
|
Nishikawa H, Osaki Y, Iguchi E, et al: The
effect of long-term supplementation with branched-chain amino acid
granules in patients with hepatitis C virus-related hepatocellular
carcinoma after radiofrequency thermal ablation. J Clin
Gastroenterol. 47:359–366. 2012. View Article : Google Scholar
|