Introduction
Breast cancer is one of the leading causes of
cancer-related mortality among women worldwide. Recent studies have
shown that an increasing number of multiple signaling pathways and
genes are involved in breast cancer tumorigenesis (1–3).
However, the precise mechanisms determining breast cancer cell
aberrant proliferation, differentiation and sensitivity to
chemotherapy remain unknown. Bone morphogenetic proteins (BMPs),
belonging to the transforming growth factor (TGF)-β superfamily,
have been widely considered as crucial molecules involved in cell
proliferation, differentiation, apoptosis and migration in various
tissues (4,5). Currently, they are well known to play
critical roles in tumorigenesis and progression of breast cancer,
prostate cancer, multiple myeloma, and ovarian cancer (6). BMP6 has been detected in various types
of cancer and cell lines, and is associated with cancer cell
growth, migration and drug resistance (7). In breast cancer, BMP6 was found to
function as an inhibitor of cancer cell growth and migration
(7). A recent study found that the
BMP inhibitor Coco was also able to reactivate breast cancer cells
at lung metastatic sites (2). These
findings indicate that BMP6 is involved in breast cancer
development and progression. However, the mechanism underlying the
role of BMP6 in breast cancer cell proliferation, differentiation
and chemoresistance remains unclear. Therefore, a further
understanding of the molecular mechanism of BMP6 in breast cancer
is urgently needed.
In the present study, we first investigated the
expression pattern of BMP6 to determine its potential role in
breast cancer tumorigenesis. We showed that BMP6 was downregulated
in breast cancer and was closely related to cancer cell
proliferation and tumor grade. Therefore, we evaluated the function
of BMP6 in proliferation and the chemoresistance of breast cancer
cells. Our study revealed that suppression of BMP6 expression in
breast cancer cell line MCF7 increased cell proliferation and
chemoresistance via activation of the ERK signaling pathway. Based
on the data from these experiments, BMP6 plays an important role in
breast cancer and may serve as a diagnostic biomarker and potential
therapeutic target for breast cancer.
Materials and methods
Patients and tissue specimens
Thirty-six fresh breast cancer tissues and
patient-matched adjacent normal breast tissues were obtained from
patients who underwent surgery at West China Hospital of Sichuan
University. All tumor tissues were reviewed by an experienced
pathologist using World Health Organization recommendations on
histopathologic typing. Parts of the fresh specimens were frozen in
liquid nitrogen and stored at −80°C for total RNA and protein
isolation. Other parts were fixed in 4% paraformaldehyde and
embedded in paraffin for histological sections and
immunohistochemical staining. In addition, 80 paraffin-embedded
breast cancer tissues were obtained from the Cancer Hospital of
Sichuan Province for immunohistochemical staining. None of the
patients received chemotherapy or radiotherapy prior to surgery.
The study protocol conformed to the local ethical standards of the
institutional review board.
Immunohistochemistry
Immunostaining was performed on paraffin-embedded
sections. The sections were dewaxed in xylene and immersed in
graded ethanol and distilled water. Immunohistochemical staining
was performed using the avidin-biotin peroxidase complex (ABC)
method, according to the manufacturer’s instructions. The primary
antibody for BMP6 (Abcam) was diluted to 1:200. The primary
antibody was omitted as a negative control for the immunostaining.
The tissue specimens were viewed separately by two pathologists
under double-blind conditions. The immunoreactivity score system
was in accordance with a previously described system (8).
RNA isolation and quantitative
RT-PCR
Total RNA from 36 cancer and adjacent normal breast
tissues was extracted using TRIzol (Invitrogen) according to the
manufacturer’s instructions. Total RNA was retrotranscribed using
the RevertAid™ First Strand cDNA Synthesis kit (Fermentas)
according to the manufacturer’s instructions. PCR amplification and
detection of the PCR amplified gene products were performed with
SYBR-Green PCR Master Mix (Tiangen). Real time-PCR was performed on
Mastercycler® ep realplex (Eppendorf). The reaction
cycle consisted of a hot start at 95°C for 10 min, then 30 cycles
of denaturation at 95°C for 30 sec and extension at 65°C for 30
sec. Levels of mRNA expression were quantified after normalization
with endogenous control GAPDH using the 2−ΔCT method
(9). The primer sequences used for
PCR were as follows: GAPDH, 5′-ctttggtatcgtggaaggactc-3′ and
5′-gtagaggcagggatgatgttct-3′ [product size, 132 base pairs (bp)];
bmp6, 5′-gtcttacaggagcatcagcacag-3′ and 5′-ggagtcacaacccacagattg-3′
(product size, 128 bp); mdr-1, 5′-gctcctgactatgccaaagc-3′ and
5′-tcttcacctccaggctcagt-3′ (product size, 202 bp). Experiments were
performed independently for each sample and at least 3 technical
replicates were run for each treated sample and controls.
Restriction fragment differential display
(RFDD)-PCR
Total-RNA of the cancer and adjacent normal breast
tissues was extracted respectively by TRIzol according to the
manufacturer’s instructions. RFDD-PCR for each sample was
accomplished by Display PROfile™ kits (cat. nos. 600–100, 615–100,
616–100, 617–100, 618–100) provided by Qbiogene, Inc. and MP
Biomedicals, Inc. (Carlsbad, CA, USA) as previously described and
used in our laboratory (10,11).
After RFDD-PCR, 15 μl loading buffer was added, and the samples
were heated to 85°C for 5 min and placed directly on ice. Each
sample (5 μl) was loaded on a 7% urea polyacrylamide gel that had
been pre-run for 30 min at 60 W in a high-voltage vertical
electrophoresis system (Bio-Rad, Hercules, CA, USA) and 0.6X
Tris-borate (TBE)-ethylenediaminetetraacetic acid (EDTA) was used
as the electrophoresis buffer. Gels were scanned with Typhoon 9200
variable mode imager (GE Healthcare Biosciences). The scanning
parameters consisted of a wavelength of 33/670 nm, PMT voltage of
625 V and scanning precision of 200 μm. Based on the linear
correlation between the molecule weight (bp) of DNA and its space
of shift in gel, the standard curve was obtained with UTHSCSA Image
Tool and Excel 2000 for each gel resulting in acquisition of the
size (bp) of each fragment of DNA. According to the number of bp,
genes were determined by searching in the corresponding database
provided by Qbiogene, Inc. and MP Biomedicals, Inc. (http://www.qbio-gene.com/displayft/).
Cell culture
The MCF7 cell line was purchase from American Type
Culture Collection (ATCC), and doxorubicin resistant subline
(MCF7/Adr) was generated by continuously culturing the
drug-sensitive parental cell line (MCF7) in medium containing
incrementally increasing concentrations of doxorubicin
(Sigma-Aldrich). The cells were cultured in RPMI-1640 (Gibco)
supplemented with 10% heat inactivated fetal calf serum, 100 U/ml
penicillin and 100 U/ml streptomycin at 37°C and 5% CO2.
The drug resistant cell line was cultured in drug-free medium for
over 2 weeks before subsequent experiments to avoid the influence
of the drug.
BMP6 small hairpin (sh)RNA transfection
and isolation of clones stably expressing BMP6 shRNA
BMP6-specific shRNA (pGPU6/GFP/NEO-BMP6) and a
control shRNA vector (pGPU6/GFP/NEO-shNC) were purchased from
Genepharma (Shanghai, China). Aiming at the sequence of BMP6, two
DNA chains with the following sense and antisense sequences were
synthesized: shRNA-1, 5′-GCT AAATGCCATCTCGGTTCT-3′ and shRNA-2,
5′-GGT TGTGACTCCACAGCATAA-3′. The target sequence of the negative
control group which was named pGPU6/GFP/NEO-shNC was
5′-GTTCTCCGAACGTGTCAC GT-3′, which has no homology with that of
human beings or mice. MCF7 cells grown on 24-well plates were
transfected either with pGPU6/GFP/NEO-BMP6 or the negative control
plasmid. Transfection was performed using Lipofectamine 2000. The
cells were incubated at 37°C in 5% CO2 overnight, and
then DMEM plus 10% FCS was added. After another 12-h incubation,
the cells were re-plated at a 1/10–1/40 dilution onto 6-well
plates. Selection with G418 (500 μg/ml) was initiated on the next
day and the process lasted for 2 weeks. MCF7 cell clones with
stably decreased expression of BMP6 as well as the control clones
were obtained for further study. BMP6 knockdown was evaluated by
real-time PCR and western blotting with antibodies to BMP6.
Cell proliferation assay
Proliferation of cells stably transfected with
shRNAs for BMP6 or the negative control vector was determined with
the Cell Counting Kit-8 (CCK-8) assay and BrdU labeling. Briefly,
cells of each group (1,000 cells/well) were plated in 96-well
plates. After culturing for 1–5 days, the supernatant was removed
and cell growth was detected using the CCK-8 Kit (Dojindo
Laboratories, Kumamoto, Japan) according to the manufacturer’s
instructions. Absorbance was measured at 450 nm using a microplate
reader (Thermo Fisher Scientific). All experiments were performed
in triplicate and repeated at least three times. As for BrdU
labeling, the cells were treated with BrdU for 2 h. Cells were
fixed and acid-treated, followed by mouse anti-BrdU antibody
(diluted 1:200; Millipore) incubation for 2 h at 37°C. The cells
were then immunostained with FITC-conjugated secondary antibody.
Quantitative studies were based on four or more replicates.
Growth inhibition assay
Cells from each group were seeded at
4×103 cells/well in 96-well tissue culture plates. After
24 h, cells were exposed to increasing concentrations of
doxorubicin (Sigma-Aldrich) in the absence or presence of the
inhibitor of MAPK/ERK kinase U0126 (20 μM). After 24 or 48 h of
incubation, CCK-8 reagent (10 μl) was added to each well of a
96-well flat-bottomed microplate containing 100 μl of culture
medium, and the plate was incubated for 1 h at 37°C. Viable cells
were evaluated by absorbance measurements at 450 nm using an auto
microplate reader. The OD450 value was proportional to the degree
of cell survival. All results are representative of three
independent experiments.
Western blot analysis
For protein extraction, each sample was made into
powder and placed in liquid nitrogen with a pre-cooled mortar and
pestle. The tissue powder was constructed using lysis buffer [50 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.25% sodium
deoxycholate, 1 mmol/l EDTA, 1 mmol/l phenylmethlsulfonyl, 1 mmol/l
Na3VO4 and 1 mmol/l NaF] and incubated at 4°C
for 30 min, followed by centrifugation at 10,000 × g at 4°C for 20
min. Protein concentrations were then measured using protein assay
kits (Bio-Rad). The protein lysates were then resolved by SDS-PAGE
and then transferred onto polyvinylidene difluoride membranes (GE
Healthcare Biosciences), blocked with PBS containing 0.2% Tween-20
and 5% non-fat dry milk and incubated with the primary antibody
against BMP6 (1:1,000), P-gp (1:1,000) and actin (1:1,000) from
Abcam. Antibody binding was revealed by incubation with horseradish
peroxidase-conjugated secondary antibodies and the ECL Plus
immunoblotting detection system (GE Healthcare Biosciences).
Signals were quantified using NIH ImageJ 1.63 software.
Statistical analyses
Data are expressed as the means ± SD. The paired
t-test, one-way ANOVA and Mann Whitney U test and Kruskal-Wallis
test were applied for statistical analysis using SPSS software
(13.0 version). Statistical significance was set at P<0.05.
Results
Expression of BMP6 is downregulated and
correlates with the clinicopathological parameters in the breast
cancer tissues
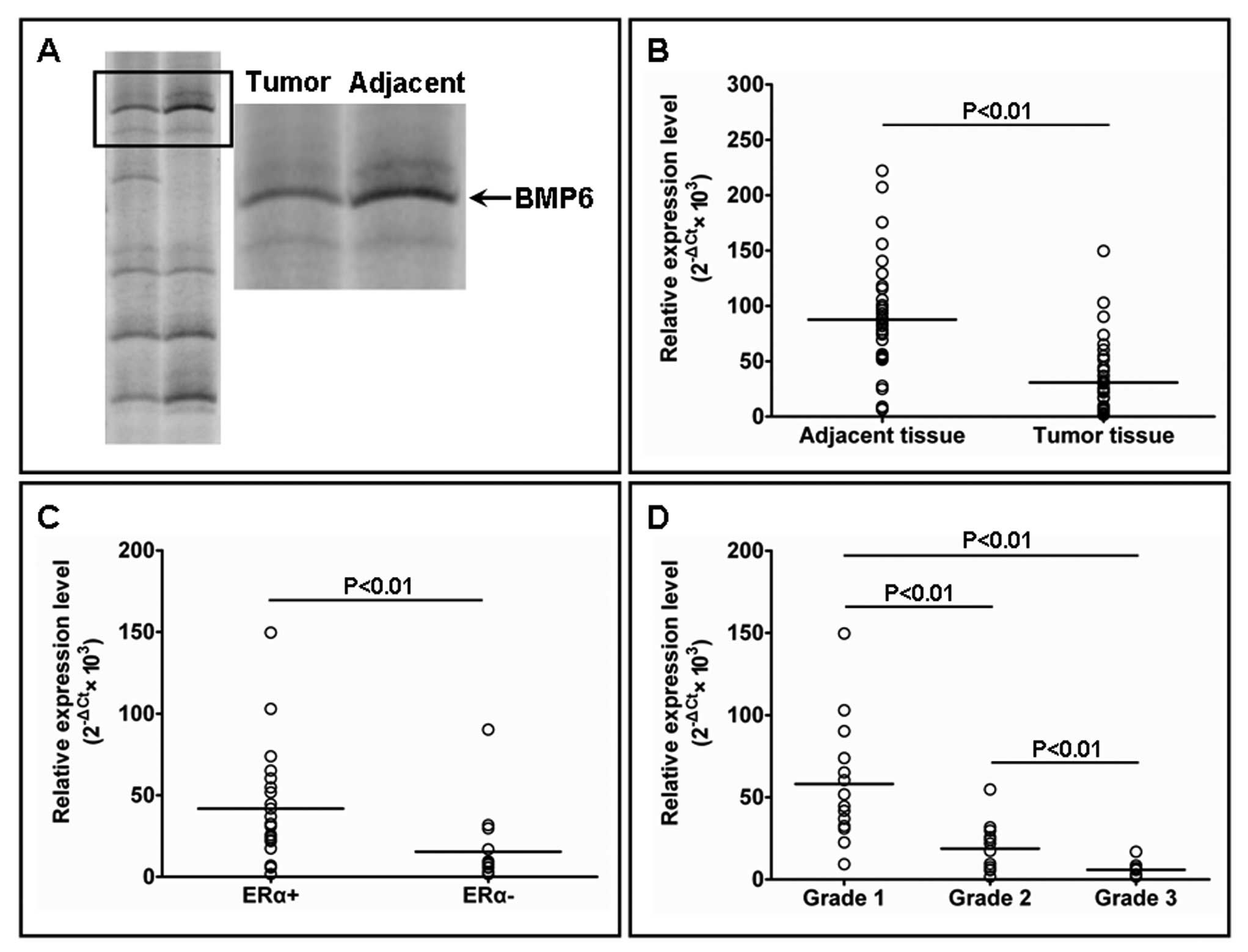
The RFDD-PCR analyses of mRNA expression profiles
between tumor and adjacent tissues identified several gene
fragments. These were isolated and subjected to bioinformatic
analysis. Among them, BMP6 was identified as being significantly
downregulated in the breast cancer tissues (Fig. 1A). Subsequently, real time-PCR and
immunohistochemical straining confirmed the expression patterns in
the breast cancer and adjacent normal breast tissues. Real-time PCR
showed that the BMP6 mRNA expression level was significantly higher
in adjacent normal breast tissues when compared to the expression
level in the matched breast cancer tissues (Fig. 1B). In tumor tissues, the BMP6 mRNA
expression level was significantly higher in estrogen receptor
(ER)-positive breast cancers than in ER-negative breast cancers
(Fig. 1C). With increasing tumor
histologic grade, the expression of BMP6 mRNA was significantly
downregulated (Fig. 1D). In
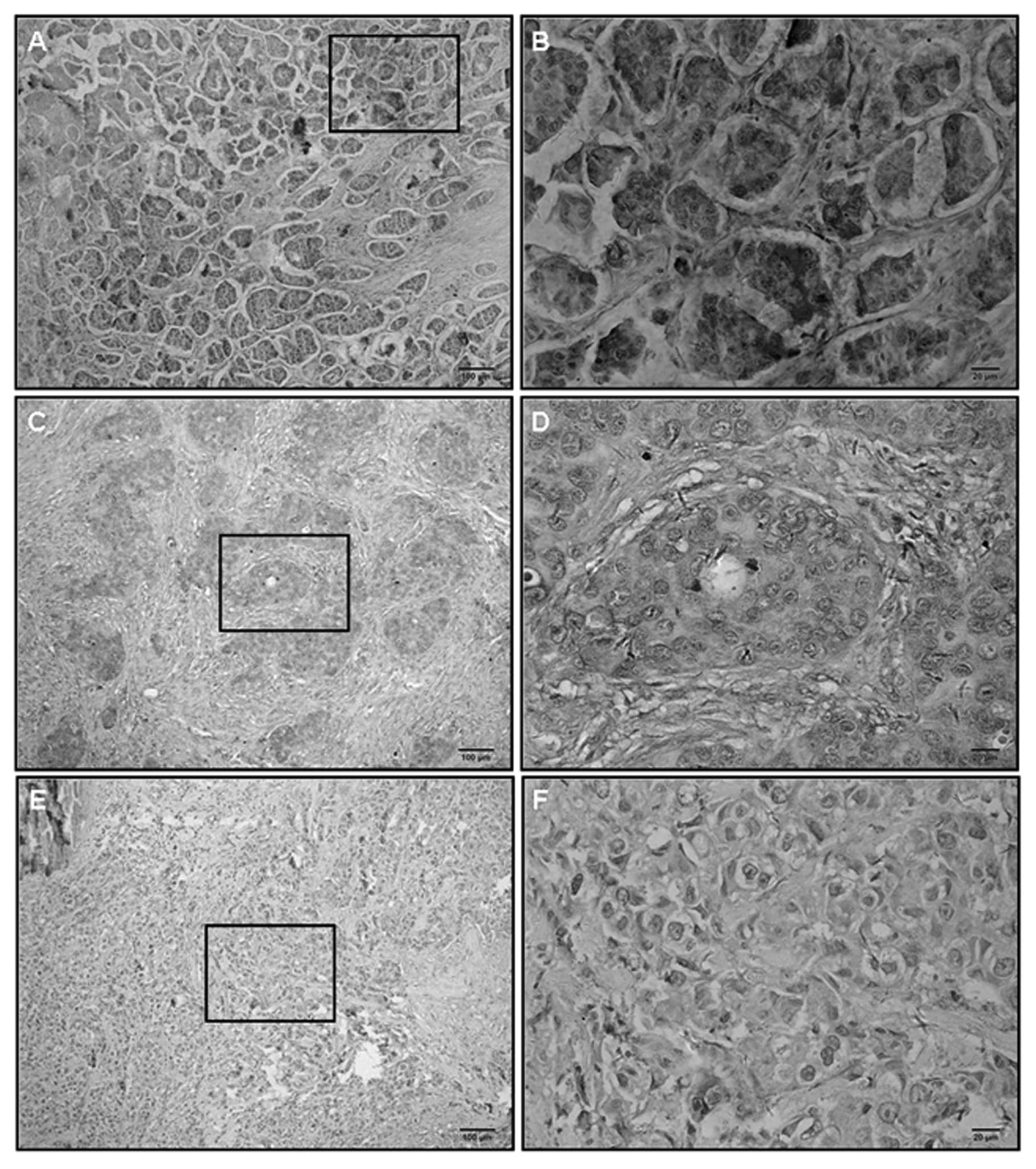
addition, we performed immunostaining of 80 breast cancer samples.
Cellular staining for BMP6 was detected in both the membrane and
the cytoplasm (Fig. 2). Based on
the scoring system of immunostaining, the expression of BMP6 was
closely related with tumor ER, and progesterone receptor (PR)
expression and tumor histologic grade (Table I). Most importantly, the significant
association between BMP6 expression and cancer tissue Ki67 status
was observed (Table I).
 | Table ICorrelation between BMP6 expression
and the clinical characteristics of the breast cancer cases. |
Table I
Correlation between BMP6 expression
and the clinical characteristics of the breast cancer cases.
| | BMP6 expression | |
|---|
| |
| |
|---|
| Characteristics | n | − | + | ++ | +++ | P-value |
|---|
| Total | 80 | 37 | 14 | 16 | 13 | |
| Age (years) | | | | | | 0.262 |
| ≤40 | 22 | 8 | 6 | 4 | 4 | |
| 41–49 | 20 | 9 | 5 | 4 | 2 | |
| 50–60 | 18 | 11 | 2 | 2 | 3 | |
| >60 | 20 | 9 | 1 | 6 | 4 | |
| Histology | | | | | | 0.302 |
| Ductal | 34 | 13 | 7 | 8 | 6 | |
| Lobular | 32 | 15 | 6 | 5 | 6 | |
| Other | 14 | 9 | 1 | 3 | 1 | |
| Grade | | | | | | 0.027 |
| 1 | 33 | 14 | 5 | 8 | 6 | |
| 2 | 22 | 10 | 3 | 3 | 6 | |
| 3 | 25 | 13 | 6 | 5 | 1 | |
| ER status | | | | | | 0.030 |
| Positive | 39 | 15 | 6 | 8 | 10 | |
| Negative | 41 | 22 | 8 | 8 | 3 | |
| PR status | | | | | | 0.004 |
| Positive | 36 | 16 | 6 | 6 | 8 | |
| Negative | 44 | 21 | 8 | 10 | 5 | |
| HER2 status | | | | | | 0.282 |
| Positive | 43 | 18 | 10 | 8 | 7 | |
| Negative | 37 | 19 | 4 | 8 | 6 | |
| Ki67
expression | | | | | | 0.012 |
| 0–20% | 29 | 16 | 3 | 5 | 5 | |
| 21–40% | 23 | 8 | 5 | 5 | 5 | |
| >40% | 28 | 13 | 6 | 6 | 3 | |
Decrease in BMP-6 expression by shRNA
enhances proliferation and chemoresistance in MCF7 cells
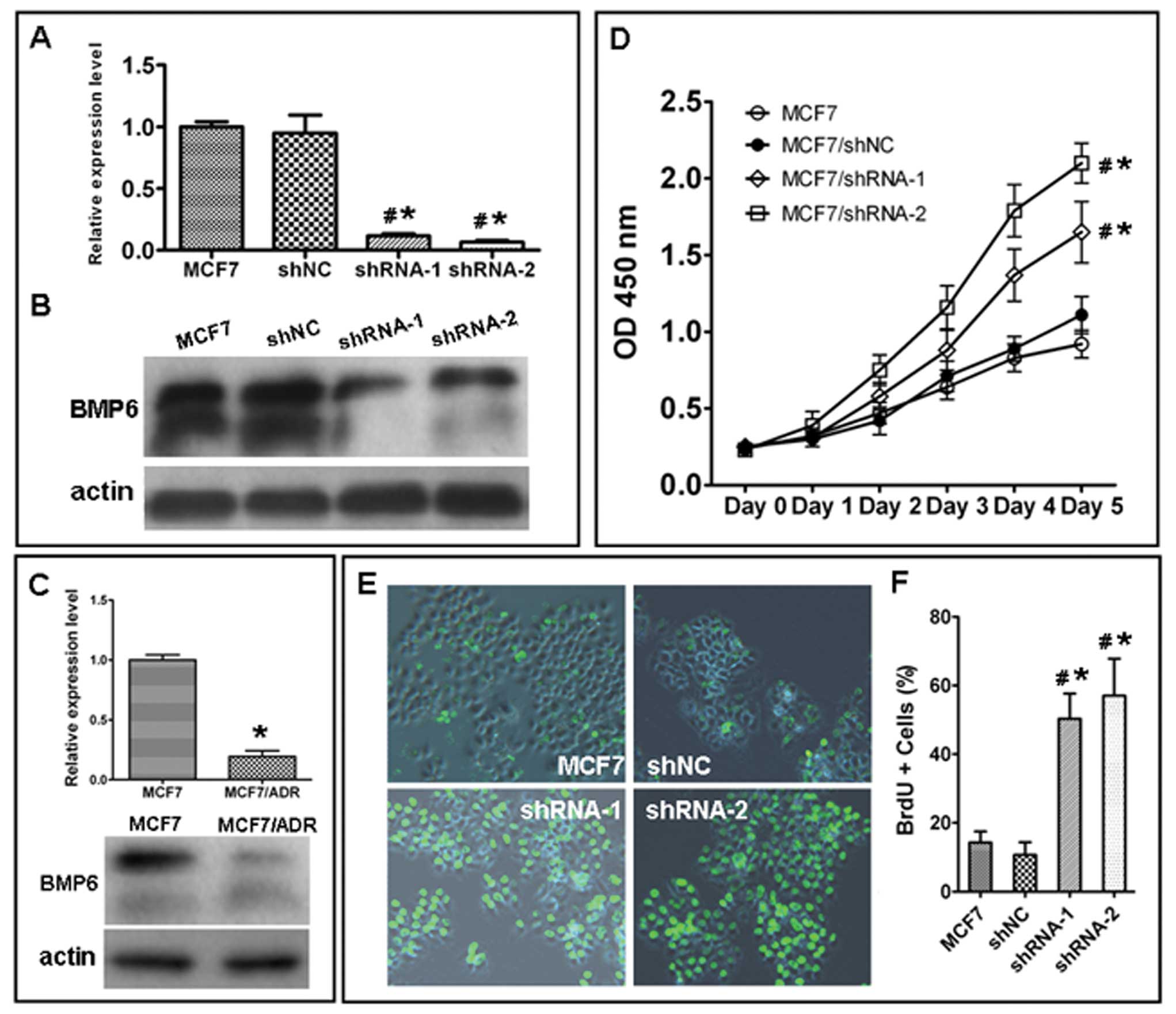
Real-time PCR and western blotting revealed that
BMP6 mRNA and protein were downregulated in multi-drug-resistant
breast cancer cell line MCF7/Adr compared with its parental cell
line MCF7 (Fig. 3C). To evaluate
the effect of the BMP6 expression in MCF7 cells, the negative
control shRNA and BMP6-specific shRNA vectors were transfected into
the MCF7 cells and the efficacy of the downregulation of expression
of the BMP6 gene was detected by RT-PCR and western blot analysis.
The mRNA (Fig. 3A) and protein
(Fig. 3B) levels of BMP6 were
decreased in the BMP6 shRNA transfection group but not in the
control shRNA group.
To evaluate whether silencing the BMP6 gene in MCF7
cells affects cell growth and proliferation, the CCK-8 assay and
BrdU labeling analyses were performed. As shown in Fig. 3D, after 5 days of transfection, a
significant increase in growth was observed between the NC group
cells and the shRNA group cells, respectively. Cell proliferation
was also examined by BrdU labeling analysis. The percentage of
BrdU-positive cells was significantly lower in the NC group than
that in the BMP6 shRNA-transfected cells (Fig. 3E and F).
Furthermore, the effects of BMP6 expression on
doxorubicin resistance in the BMP6-specific shRNA transfected and
parental cells were assessed by growth inhibition analysis using
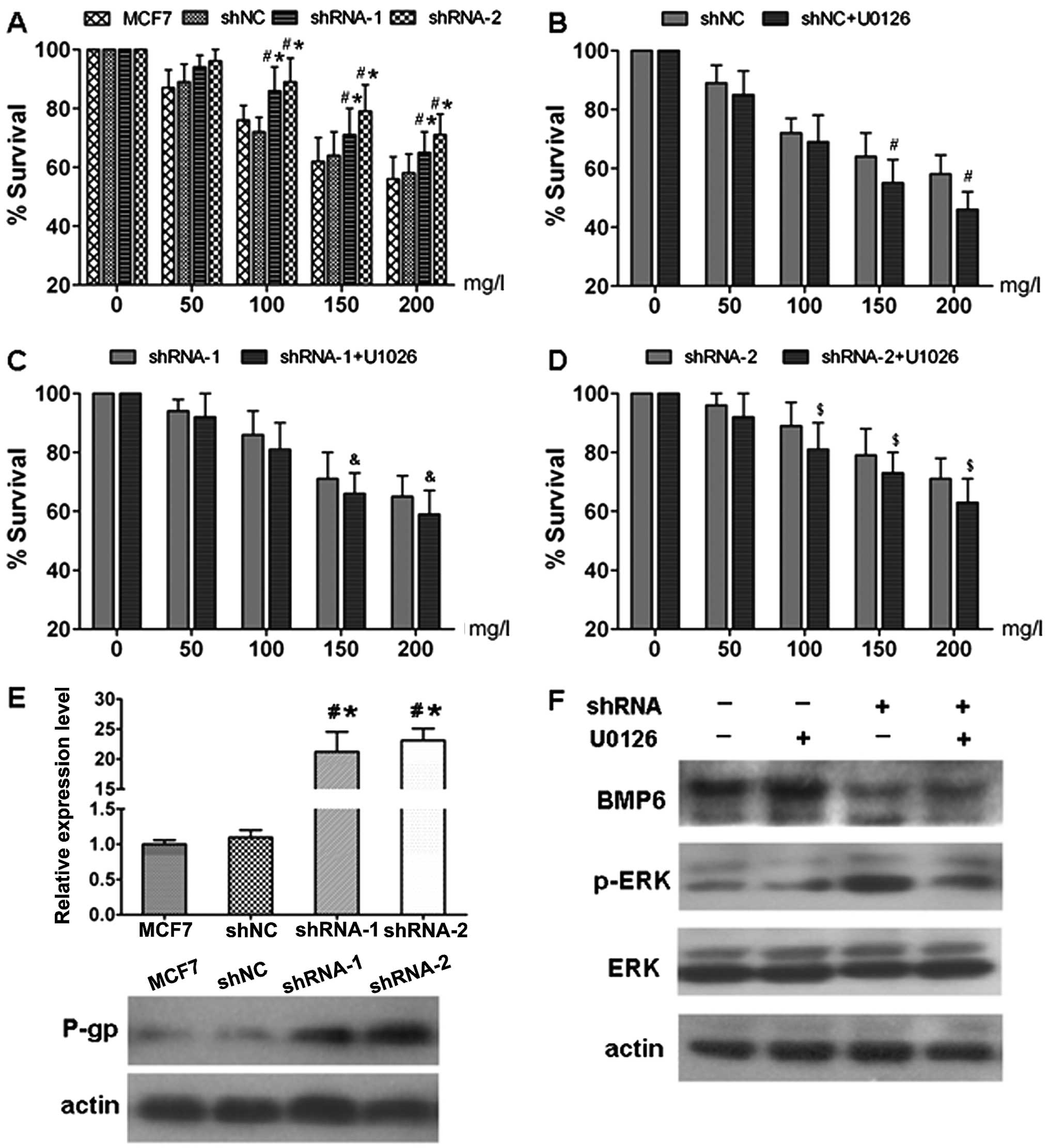
the CCK-8 assay. The survival ratio of MCF7, MCF7/shNC,
MCF7/shRNA-1 and MCF7/shRNA-2 cells was analyzed after treatment
with a range of increasing concentrations of doxorubicin for 24 h
by CCK-8 assay. As shown in Fig.
4A, following treatment with doxorubicin for 24 h, number of
surviving MCF7/shRNA-1 and MCF-7/shRNA-2 cells was significantly
increased compared to the number of cells in the MCF7 and MCF7/shNC
cells. There was no significant difference in doxorubicin
resistance between the MCF7 and MCF7/shNC cells. These results
suggest that inhibition of BMP6 expression in MCF7 cells decreased
the sensitivity to the cytotoxic effects of doxorubicin.
BMP6 knockdown increases mdr-1/P-gp
expression and activates the ERK signaling pathway
As shown in Fig. 4E,
expression of the multi-drug resistance gene mdr-1 and protein P-gp
were significantly downregulated after BMP6 knockdown in the MCF7
cell lines. Furthermore, the activation of the ERK signaling
pathway was observed in MCF7 cells transfected with BMP6-specific
shRNA. The increased level of p-ERK was attenuated by the MAPK/ERK
inhibitor U0126 (Fig. 4F). In
addition, U0126 also reduced the survival of MCF7 cells transfected
with the BMP6-specific shRNA following treatment with doxorubicin
(Fig. 4B-D). These results indicate
that enhancement of MCF7 cell chemoresistance induced by BMP6
knockdown involves ERK signaling pathway activation.
Discussion
BMPs have been found to be associated with various
types of cancers such as breast cancer, prostate cancer, multiple
myeloma and ovarian cancer (6).
Recently studies of various cancer models have indicated the
critical role of the BMP signaling pathway in tumor cell
carcinogenesis, through autocrine and paracrine mechanisms,
resulting in the modulation of tumor cell growth, invasion and
metastasis (12–14). Regarding BMP6, previous studies have
shown that it is expressed in primary breast cancer samples and
cell lines and functions as an inhibitor of breast cancer cell
growth and migration (7). However,
the relationship between BMP6 expression and histopathological
characteristics of breast cancer and the function of BMP6 in breast
cancer chemoresistance are yet unclear. In the present study, we
showed that BMP6 expression was significantly downregulated in the
majority of primary breast cancer specimens when compared with that
in the adjacent normal tissues by RFDD-PCR, real-time PCR and
immunohistochemistry. In the tumor tissues, the BMP6 mRNA or
protein expression was significantly correlated with breast cancer
tumor grade and ER and PR statuses. BMP6 expression in ER-negative
breast cancer was obviously lower than that in ER-positive breast
cancer. Previous studies have shown that promoter hypermethylation
contributes to the downregulation of BMP6 expression in ER-negative
breast cancer patients (15,16).
Our results were in agreement with a previous observation (7) and moreover, further analysis also
revealed a significant association between tumor cell grade and
BMP6 expression in tumor tissues. More importantly, we found that
the expression of BMP6 was closely related to the Ki67 expression
of tumor cells, which is recognized as a marker for tumor cell
proliferation. In order to confirm the function of BMP6 in breast
cancer cell proliferation, an shRNA expression vector targeting the
BMP6 gene was constructed and transfected into MCF7 cells. The
results showed that inhibition of the expression of BMP6
significantly enhanced cell proliferation. Notably, BMP6 was found
to inhibit cell proliferation in various cancer types including
breast cancer, multiple myeloma, prostate cancer and skin tumors
via multiple mechanisms including suppression of the p38-MAPK
signaling pathway, cell cycle arrest and downregulation of AP-1
expression (17–20). These data suggest that BMP6 may be a
new diagnostic marker of breast cancer, and deregulation of BMP6 is
involved in loss of proliferation control and failure to undergo
cellular differentiation during tumorigenesis.
Cancer cell chemoresistance is a major obstacle to
cancer treatment. Chemoresistance accounts for most of the failure
in adjuvant chemotherapy in breast cancer. To date, several
mechanisms have been proven to play an important role in the
chemoresistance phenotype (21–23).
In the present study, we first noted that BMP6 expression was
associated with drug resistance. Previous studies have shown that
BMPs are involved in metastasis, epithelial to mesenchymal
transformation, altered cellular behavior and angiogenesis in human
cancer, and are closely related to drug resistance (24–26).
In addition, we also found that BMP6 mRNA and protein were
downregulated in the multi-drug-resistant cell line MCF7/ADR.
Therefore, we hypothesized that BMP6 expression is involved in
breast cancer drug resistance. In the present study, BMP6 knockdown
by BMP6-specific shRNAs in MCF7 cells resulted in a significant
upregulation of P-glycoprotein, one of the well characterized
factors of breast cancer multi-drug resistance encoded by the mdr-1
gene. As known, BMPs regulate the gene expression through the
smad-dependent and smad-independent signaling pathway (27,28).
Here, we showed that BMP6 knockdown activated the expression of the
ERK signaling pathway and elevated the resistance of MCF7 cells to
the chemotherapy drug doxorubicin. Moreover, the selective MAPK/ERK
signaling pathway inhibitor U0126 attenuated the chemoresistance of
MCF7 cells resulting from BMP6 knockdown. In fact, our previous and
other various studies have also confirmed that ERK signaling
pathway activation is involved in cancer cell chemoresistance
(23,29). ERK signaling pathway-modulated mdr-1
gene expression was also confirmed in various studies (30,31).
Thus, these findings suggest that downregulation of BMP6
upregulates mdr-1/p-gp expression by activating the ERK signaling
pathway. Further analysis of the related mechanisms is needed to
fully assess the function of BMP6 in breast cancer cell
chemoresistance.
In summary, we demonstrated the expression pattern
of BMP6 in breast cancer and revealed that the downregulation of
BMP6 was associated with breast cancer grade, enhanced cancer cell
proliferation and chemoresistance. Furthermore, BMP6 knockdown
enhanced breast cancer cell chemoresistance via activation of the
ERK signaling pathway. Our results provide evidence suggesting a
critical role of BMP6 in breast cancer, and BMP6 may serve as a
novel diagnostic biomarker or therapeutic target for breast
cancer.
References
|
1
|
Cancer Genome Atlas Network. Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao H, Chakraborty G, Lee-Lim AP, et al:
The BMP inhibitor Coco reactivates breast cancer cells at lung
metastatic sites. Cell. 150:764–779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cellurale C, Girnius N, Jiang F, et al:
Role of JNK in mammary gland development and breast cancer. Cancer
Res. 72:472–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perrimon N, Pitsouli C and Shilo BZ:
Signaling mechanisms controlling cell fate and embryonic
patterning. Cold Spring Harb Perspect Biol. 4:a0059752012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo J and Wu G: The signaling and
functions of heterodimeric bone morphogenetic proteins. Cytokine
Growth Factor Rev. 23:61–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh A and Morris RJ: The Yin and Yang of
bone morphogenetic proteins in cancer. Cytokine Growth Factor Rev.
21:299–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alarmo EL and Kallioniemi A: Bone
morphogenetic proteins in breast cancer: dual role in
tumourigenesis? Endocr Relat Cancer. 17:R123–R139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rhodes A, Jasani B, Barnes DM, et al:
Reliability of immunohistochemical demonstration of oestrogen
receptors in routine practice: interlaboratory variance in the
sensitivity of detection and evaluation of scoring systems. J Clin
Pathol. 53:125–130. 2000. View Article : Google Scholar
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
10
|
Deng S, Zhou H, Xiong R, et al:
Over-expression of genes and proteins of ubiquitin specific
peptidases (USPs) and proteasome subunits (PSs) in breast cancer
tissue observed by the methods of RFDD-PCR and proteomics. Breast
Cancer Res Treat. 104:21–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou HY, Mei Y, Lu YG, et al: Application
of restriction fragment differential display-polymerase chain
reaction in study on differential expression profiles of human
diseases. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 22:294–297.
2005.
|
|
12
|
Piccirillo SG and Vescovi AL: Bone
morphogenetic proteins regulate tumorigenicity in human
glioblastoma stem cells. Ernst Schering Found Symp Proc. 5:59–81.
2006.PubMed/NCBI
|
|
13
|
Bunyaratavej P, Hullinger TG and Somerman
MJ: Bone morphogenetic proteins secreted by breast cancer cells
upregulate bone sialoprotein expression in preosteoblast cells. Exp
Cell Res. 260:324–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shepherd TG, Thériault BL and Nachtigal
MW: Autocrine BMP4 signalling regulates ID3 proto-oncogene
expression in human ovarian cancer cells. Gene. 414:95–105. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barekati Z, Radpour R, Lu Q, et al:
Methylation signature of lymph node metastases in breast cancer
patients. BMC Cancer. 12:2442012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang M, Wang Q, Yuan W, et al: Epigenetic
regulation of bone morphogenetic protein-6 gene expression in
breast cancer cells. J Steroid Biochem Mol Biol. 105:91–97. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seckinger A, Meissner T, Moreaux J,
Goldschmidt H, Fuhler GM, Benner A, et al: Bone morphogenic protein
6: a member of a novel class of prognostic factors expressed by
normal and malignant plasma cells inhibiting proliferation and
angiogenesis. Oncogene. 28:3866–3879. 2009. View Article : Google Scholar
|
|
18
|
Takahashi M, Otsuka F, Miyoshi T, et al:
Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit
estrogen-induced proliferation of breast cancer cells by
suppressing p38 mitogen-activated protein kinase activation. J
Endocrinol. 199:445–455. 2008. View Article : Google Scholar
|
|
19
|
Haudenschild DR, Palmer SM, Moseley TA,
You Z and Reddi AH: Bone morphogenetic protein (BMP)-6 signaling
and BMP antagonist noggin in prostate cancer. Cancer Res.
64:8276–8284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wach S, Schirmacher P, Protschka M and
Blessing M: Overexpression of bone morphogenetic protein-6 (BMP-6)
in murine epidermis suppresses skin tumor formation by induction of
apoptosis and downregulation of fos/jun family members. Oncogene.
20:7761–7769. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coley HM: Mechanisms and consequences of
chemotherapy resistance in breast cancer. Eur J Cancer. (Suppl 7):
3–7. 2009. View Article : Google Scholar
|
|
22
|
Drasin DJ, Robin TP and Ford HL: Breast
cancer epithelial-to-mesenchymal transition: examining the
functional consequences of plasticity. Breast Cancer Res.
13:2262011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen GQ, Zhao ZW, Zhou HY, Liu YJ and Yang
HJ: Systematic analysis of microRNA involved in resistance of the
MCF-7 human breast cancer cell to doxorubicin. Med Oncol.
27:406–415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang S, Du J, Wang Z, et al: Dual
mechanism of deltaEF1 expression regulated by bone morphogenetic
protein-6 in breast cancer. Int J Biochem Cell Biol. 41:853–861.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang S, Du J, Wang Z, et al: BMP-6
promotes E-cadherin expression through repressing deltaEF1 in
breast cancer cells. BMC Cancer. 7:2112007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YE: Non-Smad pathways in TGF-beta
signaling. Cell Res. 19:128–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li B: Bone morphogenetic protein-Smad
pathway as drug targets for osteoporosis and cancer therapy. Endocr
Metab Immune Disord Drug Targets. 8:208–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karam M, Legay C, Auclair C and Ricort JM:
Protein kinase D1 stimulates proliferation and enhances
tumorigenesis of MCF-7 human breast cancer cells through a
MEK/ERK-dependent signaling pathway. Exp Cell Res. 318:558–569.
2012. View Article : Google Scholar
|
|
30
|
Shen H, Xu W, Luo W, et al: Upregulation
of mdr1 gene is related to activation of the MAPK/ERK signal
transduction pathway and YB-1 nuclear translocation in B-cell
lymphoma. Exp Hematol. 39:558–569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guan J, Chen XP, Zhu H, Luo SF, Cao B and
Ding L: Involvement of extracellular signal-regulated
kinase/mitogen-activated protein kinase pathway in multidrug
resistance induced by HBx in hepatoma cell line. World J
Gastroenterol. 10:3522–3527. 2004.PubMed/NCBI
|