Introduction
Colorectal cancer is the third most common cancer in
the United States with a significant risk of recurrence by
peritoneal seeding following potentially curative surgery (1,2). In
general, the survival for patients with colorectal carcinomatosis
is poor (3). Traditional systemic
chemotherapy has a limited potential for survival prolongation or
cure. The 2-year survival rate for patients with colorectal
carcinomatosis treated with systemic chemotherapy alone is a dismal
10% (4).
The peritoneum may be the only site of metastatic
disease in ~25% of recurrent colorectal cancer patients (1). Therefore, improved survival or even
‘cure’ might be achieved by eradicating disease from all peritoneal
surfaces (5). As a regional
therapy, removal of all visible tumor deposits within the
peritoneal cavity, termed cytoreduction, has been associated with
improved survival (6,7). Clinically, this may also require the
stripping of parietal and/or visceral peritoneum and solid organ
resections (8). As an adjunct to
cytoreduction, the intraoperative administration of hyperthermic,
high-dose chemotherapy to the peritoneal cavity is thought to
eliminate microscopic residual disease. The best studied
chemotherapy agent used for hyperthermic intraperitoneal
chemoperfusion (HIPEC) is mitomycin C (MMC), however, other agents
have also been employed (9,10).
A randomized phase III clinical trial has shown that
long-term survival for colorectal cancer patients undergoing
cytoreduction/HIPEC is significantly improved as compared to
systemic chemotherapy alone, 20 vs. 5%, respectively, at 6 years
(7) and cytoreduction/HIPEC has
continually gained acceptance within the oncology community.
Interestingly, the concept of cytoreduction/HIPEC was immediately
used for clinical patient care; it was not developed, translated,
or refined from an animal model.
Animal models, especially murine models, present an
attractive modality to study and test future therapies for humans.
A recent rat model of cytoreduction/HIPEC has been developed that
allows for surgical cytoreduction and treatment with
intraperitoneal MMC (11). A study
using this rat model showed that the addition of HIPEC to
cytoreduction improved survival. However, the central issue of
completeness of cytoreduction in animal models, similar to
cytoreduction/HIPEC in humans, may be a confounding variable,
making the study of the independent effects of intraperitoneal
chemotherapy difficult. Taking a reductionist approach, we
developed a mouse model that mimics the post-cytoreductive state
and allows for the contributions of intraperitoneal chemotherapy to
be examined in the absence of cytoreduction and hyperthermia. Using
this model, the dose and exposure time of intraperitoneal
chemotherapy appeared to significantly influence the clinical
outcome in a beneficial manner.
Materials and methods
Mice
Age-matched BALB/c female mice (10 weeks of age)
were purchased from the National Cancer Institute (Frederick, MD,
USA). Mice were maintained in pathogen-free barrier conditions. The
Roswell Park Cancer Institutional Animal Care and Use Committee
approved all animal protocols.
Tumor cell line
The murine tumor cell line colon 26 (CT26), a weakly
immunogenic colon cancer cell line derived from BALB/c mice, was
obtained from the American Type Culture Collection (ATCC,
Rockville, MD, USA). Cells were maintained in a monolayer culture
with RPMI-1640 media (Gibco, Invitrogen Inc., Grand Island, NY,
USA) containing 10% fetal bovine serum, 2 mmol/l L-glutamine, 0.15%
sodium bicarbonate and 1% penicillin/streptomycin at 37°C in a
humidified atmosphere containing 5% CO2.
Establishment of carcinomatosis
model
Establishment of diffuse carcinomatosis was
performed by injecting BALB/c mice intraperitoneally with
5×104 live CT26 cells in 2 ml of buffered saline using a
27-gauge needle and 6-ml syringe. Necropsy was performed at
different time points to determine the optimal time point for
intraperitoneal chemotherapy that would mimic the
post-cytoreductive microscopic disease-state. Serosal and
mesenteric tumor implantation was assessed and digitally
photographed with the aid of a dissecting microscope (VWR
International, Radnor, PA, USA) at ×8–10 magnification. Frozen
section of representative day 2 tumor implant was performed with
hematoxylin solution (Sigma-Aldrich, St. Louis, MO, USA)
counterstain. All mice underwent intravenous or intraperitoneal
treatment 2 days after tumor injection to allow for tumor
dissemination and implantation onto the peritoneal surfaces.
Chemotherapy
Mitomycin C (MMC; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) was suspended in sterile normal saline
immediately prior to use at concentrations of 6 μg/ml (low dose)
and 8 μg/ml (high dose), corresponding to 15 and 20 μg per mouse,
respectively. Total volume administered intraperitoneally was 2.5
ml. For intravenous administration, a more concentrated solution of
15 or 20 μg in 200 μl of sterile saline was used. All solutions
were kept at room temperature.
Intravenous chemotherapy
administration
To simulate systemic chemotherapy administration
provided clinically, a single bolus of MMC was provided via tail
vein injection using a 29-gauge needle. Low dose was provided as 15
μg in 200 μl sterile saline, and high dose at 20 μg in 200 μl
sterile saline, which was equivalent to the total low and high dose
provided intraperitoneally.
Mouse intraperitoneal chemotherapy
Induction of anesthesia was achieved by inhalation
of 4% isofluorane (Abbott Laboratories, Chicago, IL, USA) in a
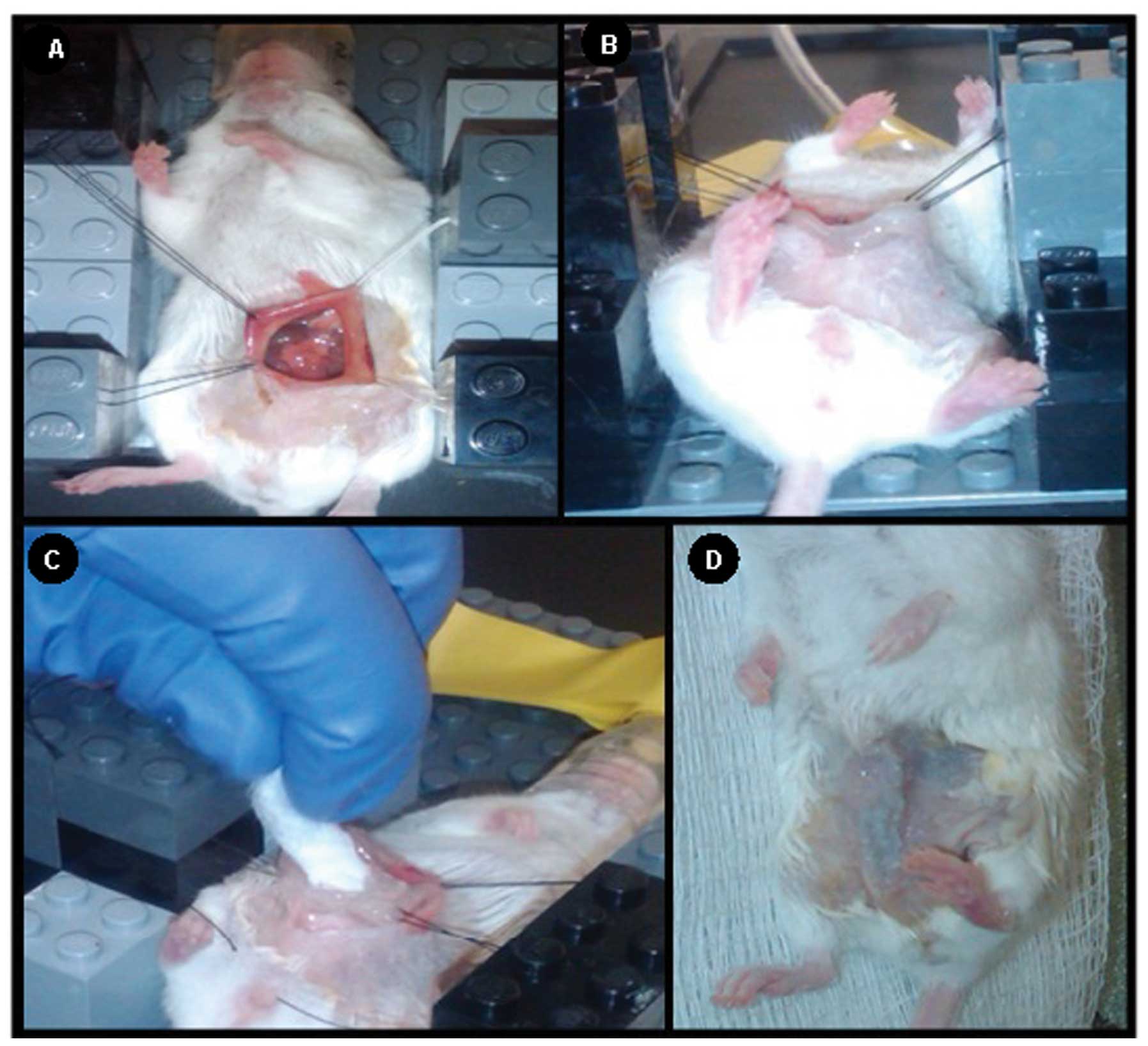
sealed chamber. Mice were then placed on a Lego®
(Billund, Denmark) platform directly over a water circulating
heating pad to maintain normothermia. Anesthesia was maintained
during intraperitoneal chemotherapy through controlled regulation
of ~2% isofluorane by means of an inlet tube and individual
anesthetic nose-cones. The abdominal wall was prepped by clipping
overlying hair and applying alcohol to the skin. The abdominal wall
and peritoneal cavity were opened with scissors and retracted in a
fixed, coliseum fashion using 4-0 Vicryl® suture
(Ethicon, Inc., Somerville, NJ, USA) anchored to the platform
constructed from building blocks (Fig.
1A). A layer of petroleum jelly was applied to the retracted
skin edges to create a barrier to prevent perfusate loss and ensure
chemotherapy contact with all peritoneal surfaces (Fig. 1B). Exactly 2.5 ml of MMC or vehicle
control (saline) was pipetted into the peritoneal cavity and
distributed intraperitoneally for either 60 or 90 min with constant
agitation on a programmable shaker (160 rpm) at room temperature.
All solutions were evacuated prior to closure of the abdomen by
wicking the perfusate with sterile 4 × 4 gauze and washing with
sterile saline (Fig. 1C). The
peritoneum was closed with a 4-0 absorbable monofilament suture and
the skin with Vetbond™ Tissue Adhesive (3M Company, St. Paul, MN,
USA) (Fig. 1D). All mice were
recovered on a warming blanket and given buprenorphine (0.2 mg/kg
body weight) subcutaneously for post-operative pain control.
In vitro assays of tumor cell
viability
CT26 cells were cultured in 25-cm flasks (Costar,
Corning Inc., Corning, NY, USA) to 3×106 cell density in
5 ml of media. To confirm the cytotoxic activity of the MMC doses
used in vivo, 1 ml of high dose MMC (8 μg/ml) was added to
individual flasks. Cell death was assessed after the addition of
MMC by light microscopy, and dead cells were identified by their
non-adherence and cellular debris.
Magnetic resonance imaging
Preclinical magnetic resonance imaging (MRI)
examinations were carried out using a 4.7 T/33-cm horizontal bore
magnet (GE NMR Instruments, Fremont, CA, USA) incorporating Avance
digital electronics (Bruker BioSpec, ParaVision 3.1.; Bruker
Medical, Billerica, MA, USA), a removable gradient coil insert
(G060; Bruker Medical) generating a maximum field strength of 950
mT/m, and a custom designed 35-mm radiofrequency transmit-receive
coil. Anesthetized animals (achieved by 2–3% isoflurane inhalation)
were placed in a form-fitted MR-compatible ‘mouse sled’ (Dazai
Research Instruments, Toronto, Canada) within a carrier tube and
positioned in the scanner. The body temperature of animals during
image acquisition was maintained using an air heater system (SA
Instruments Inc., Stony Brook, NY, USA) connected to a thermocouple
embedded within the sled that provided feedback for automated
temperature control. Animals were imaged 21 days post
intraperitoneal inoculation of CT26 tumor cells (19 days post
laparotomy/intraperitoneal chemotherapy).
Preliminary localizer images were acquired to enable
positioning of slices for T2-weighted scans. T2-weighted spin echo
images incorporating RARE (rapid acquisition with relaxation
enhancement) encoding were acquired on the coronal plane for each
mouse using the following parameters: TE/TR = 41/2500 msec, slice
thickness 1 mm, 21 slices, field-of-view (FOV): 4.8 × 3.2 cm
(coronal). Post processing of datasets was performed using the
medical imaging software, Analyze PC (Version 8.0; AnalyzeDirect,
Overland Park, KS, USA). A region of interest was manually traced
over the total extent of the tumor on the coronal T2-weighted
image. Tumor volume was calculated by measuring the cross-sectional
area on each slice and multiplying their sum by the slice
thickness. Values are reported as means ± standard error for each
experimental group.
Survival measurements
The mice were followed until tumor growth caused a
moribund status and/or any of the criteria for humane euthanasia
per approved protocols were observed. Survival was assessed and
recorded as the time to euthanasia.
Whole blood and peritoneum concentrations
of MMC
To determine the distribution of MMC in the mouse,
administered via tail vein bolus injection vs. peritoneal
administration, ultra-high performance liquid chromatography (UPLC)
was used. Mice underwent tail vein injection of 20 μg MMC in 200 μl
sterile saline or treatment with 20 μg (8 μg/ml) MMC
intraperitoneally. For mice treated intraperitoneally, chemotherapy
solution was wicked out and irrigated with sterile saline prior to
peritoneum specimen collection. Intraperitoneal treatment with MMC
occurred to a maximum of 90 min. After this time point, wicking and
washout occurred and mice at the 120- and 180-min time points did
not have any further intraperitoneal chemotherapy administered. At
time points 5, 15, 30, 60, 90, 120 and 180 min, mice were
sacrificed and whole blood was collected via 2.5-ml collection
tubes containing EDTA (Greiner Bio-One, Monroe, NC, USA) and the
entire parietal peritoneum surgically dissected and placed into
15-ml conical tubes (BD Biosciences, San Jose, CA, USA) for
immediate freezing.
Sample preparation for UPLC
Whole blood samples were thawed and centrifuged
briefly to pull sample to the bottom of the tube. Samples were
sonicated for 1 min in a Branson 2510 sonicator water bath (Branson
Ultrasonic Corp., Danbury, CT, USA). Sonicated samples were
vortexed and extracted using 200 μl aliquots. Peritoneum samples
were pre-weighed and homogenized in 1 ml 75% 0.01 M
NaH2PO4 pH 7.0:25% MeOH using a Polytron PT
2100 homogenizer (Kinematica, Inc., Bohemia, NY, USA). Homogenized
samples were extracted using 200 μl aliquots.
Extraction method
Samples (200 μl) were extracted with 1 ml of HPLC
grade acetonitrile (VWR), vortexed for 1 min and centrifuged at
14,000 rpm for 15 min at 4°C. Supernatants (1.1 ml) were
transferred to 13 × 100 mm glass tubes and dried under nitrogen at
37°C. Samples were reconstituted in 80 μl of 10 mM
NaH2PO4 at pH 7.0 and vortexed for 15 sec
twice. Contents were transferred to microcentrifuge tubes and
centrifuged at 14,000 rpm for 15 min at 4°C. Supernatants were
transferred to a 0.45 μm 96-well filter plate (VWR) and centrifuged
at 3,400 rpm for 6 min at 4°C. Filtrates were transferred to amber
autosampler vials for injection.
UPLC conditions
Samples (40 μl) were injected onto a 100 × 2.1 mm
internal diameter Acquity UPLC BEH C18 column, particle size 1.7 μm
(Waters, Milford, MA, USA) with a column temperature of 40°C.
Mitomycin C was eluted by a two-solvent gradient using a Waters
Acquity UPLC system. Solvent A contained 0.01 M
NaH2PO4, pH 7.0 buffer. Solvent B contained
HPLC grade MeOH (VWR). The gradient began at 0.2 ml/min with a 4
min hold at 75% solvent A. At 4 min the flow rate changed to 0.3
ml/min and progressed linearly to 10% solvent A over 1 min. Column
was held at 10% solvent A for 5 min before returning to initial
conditions. Mitomycin C was detected at a UV wavelength of 365 nm.
Data were collected and analyzed using Waters Empower Pro
chromatography software (version 6.21; Waters).
Statistical analysis
Tumor volume was reported as the mean ± one standard
deviation for each group. The association between tumor volume and
group was assessed using a one-way ANOVA test, with post-hoc
pair-wise comparisons conducted using Scheffe’s test. The
assumptions of the ANOVA test were assessed graphically and
indicated the need for a log transformation. Survival data were
analyzed using standard Kaplan-Meier methods, with comparisons made
using the log-rank test. All analyses were conducted in SAS v9.3
(SAS Institute Inc., Cary, NC, USA) at a nominal significance level
of 0.05. All experiments were performed in triplicate to verify
reproducibility and tumor volume and survival data were pooled for
analyses.
Results
Intraperitoneal injection of CT26 mimics
human colorectal carcinomatosis
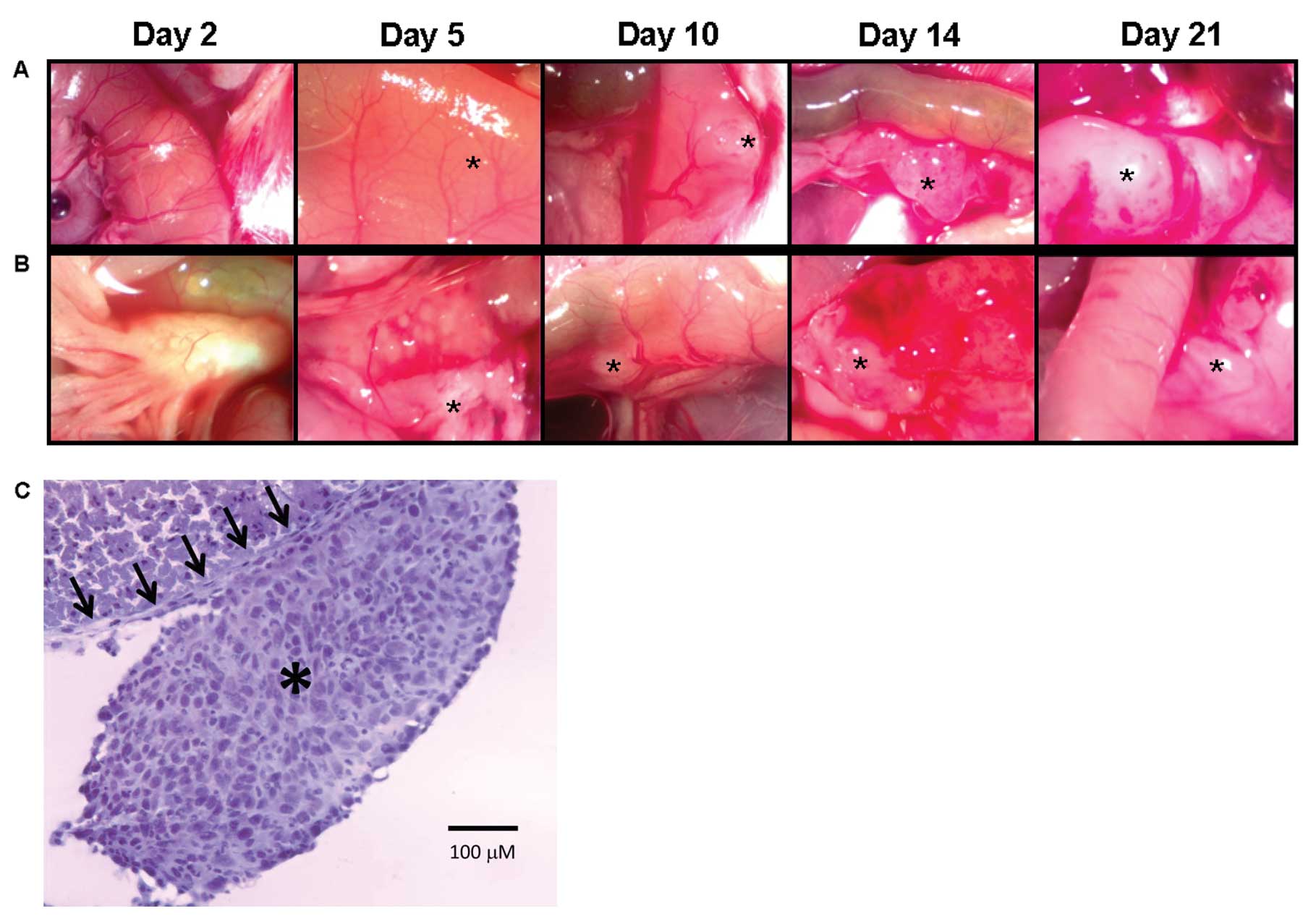
To determine the optimal time for the establishment
of microscopic intraperitoneal tumor similar to the clinical
post-cytoreductive state, necropsy was performed on mice at various
time points following CT26 tumor inoculation. Tumor implants became
visible (indicated by asterisk on the images) within 5 days of
tumor injection both on the small bowel serosa (Fig. 2A) and small bowel mesentery
(Fig. 2B). Carcinomatosis developed
in 100% of the mice and was progressive resulting in rapid
dissemination and growth on all peritoneal surfaces. If left
untreated, 100% mortality was noted by day 30. On day 2 following
tumor injection, no visible lesions were noted, but diffuse
microscopic nodules were present on the peritoneal surfaces
(Fig. 2C) and this time point was
chosen as it best represented microscopic disease similar to the
clinical post-cytoreductive state.
Murine MMC dosing based upon clinical
HIPEC protocols is well tolerated and efficacious
Confirming the in vivo antitumor effects and
potency of the MMC used for intraperitoneal chemotherapy,
verification of MMC activity in vitro demonstrated 100% cell
death between 36 and 120 h for high-dose and low-dose MMC,
respectively. Mice underwent intravenous bolus injection or
intraperitoneal administration of chemotherapy with either low dose
(6 μg/ml; 15 μg/mouse) or high dose MMC (8 μg/ml; 20 μg/mouse)
based upon a MMC/body weight ratio equivalent to the clinical dose
of 30 mg of MMC used during HIPEC for a 70 kg individual (the
average mouse weight ranged from 25 to 35 g). The standard dose
reduction seen in clinical HIPEC from 40 to 30 mg is reflected in
the murine doses tested. Mice tolerated the high or low dose MMC
and the short or long-duration treatments equally well and did not
demonstrate any perioperative morbidity or mortality.
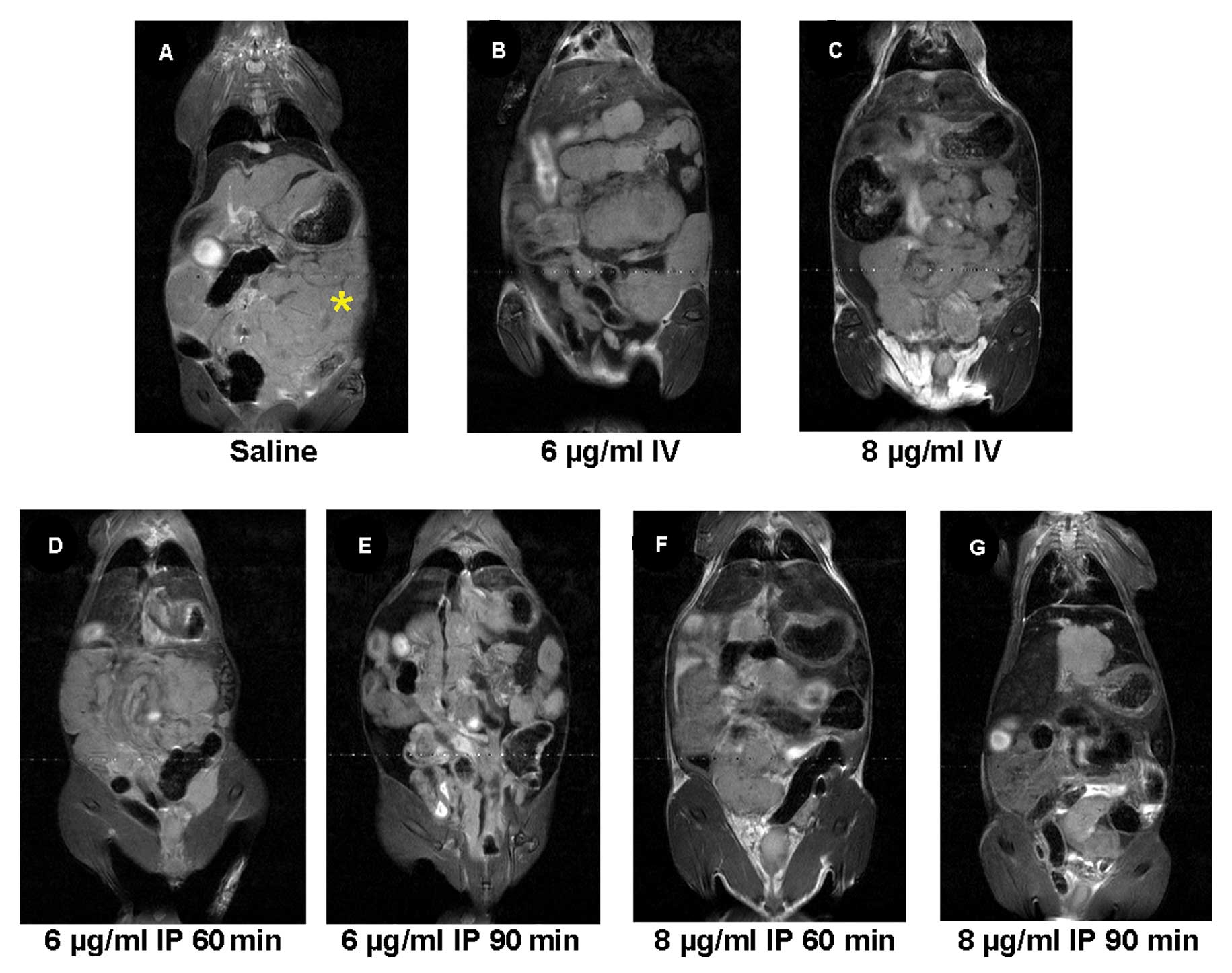
The panel of images shown in Fig. 3 represents slices from coronal
T2-weighted MRI for all experimental groups. Corresponding tumor
volume measurements are shown in Fig.
4. A diffuse pattern of tumor growth in the peritoneum was
visualized on the MR images mimicking clinical colorectal
carcinomatosis (Fig. 3).
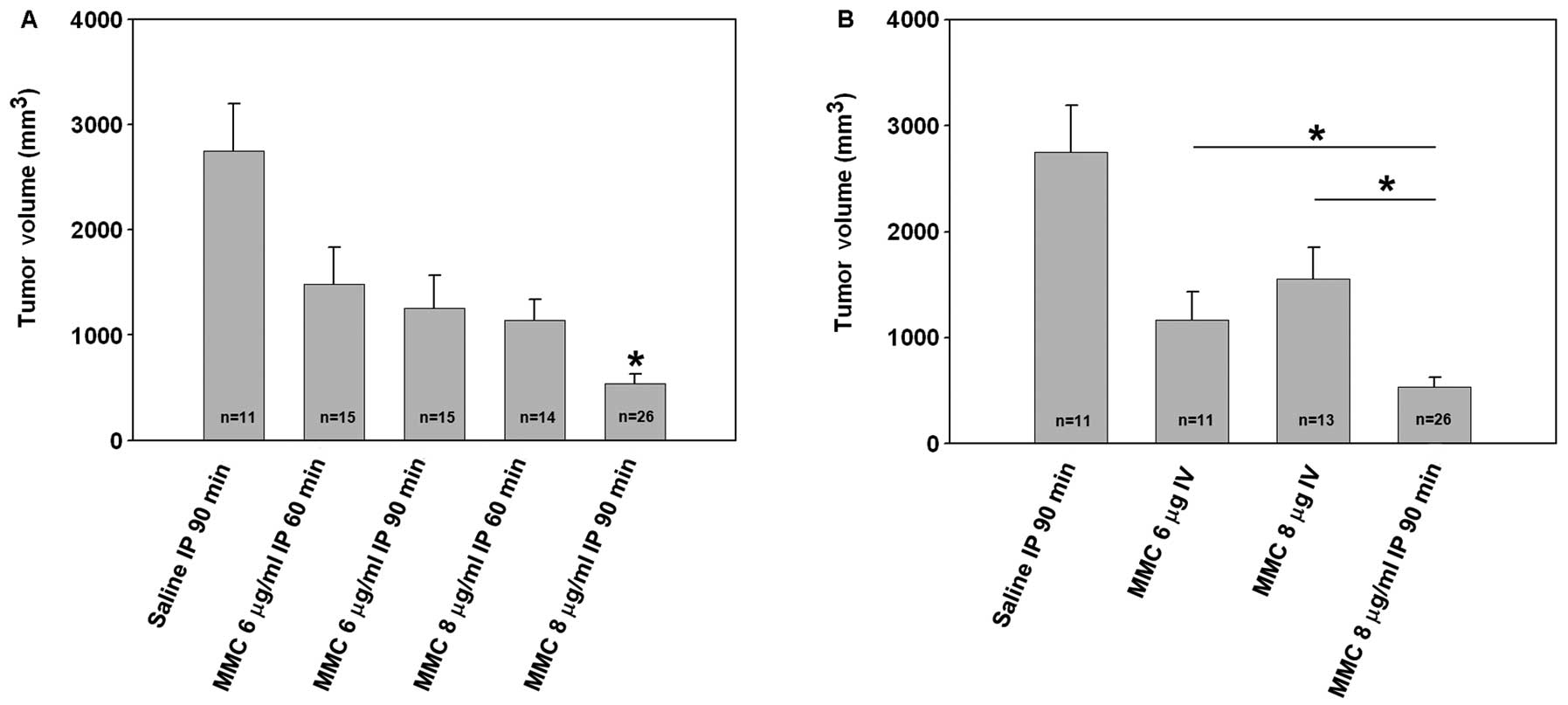
Quantitative estimates of tumor volume demonstrated a time and
dose-dependent intraperitoneal chemotherapeutic effect. Extensive
tumor-burden was visualized in saline-treated control animals
(2748±446 mm3, n=11) on day 21 post tumor cell
inoculation. Mice treated with low dose MMC (for 60 or 90 min)
showed a trend to a reduction in tumor volume compared to saline
controls (60 min; 1480±352 mm3, n=15; 90 min; 1252±318
mm3, n=15). Treatment with high dose MMC for 60 min
resulted in a comparable trend to reduction in tumor volume
(1141±193 mm3, n=14). Intravenous MMC at low or high
dose also resulted in a non-statistically significant reduction in
tumor burden compared to controls. However, the greatest reduction
in tumor burden following treatment was seen with high dose MMC for
90 min (538±89 mm3, n=26) which was statistically
significant as compared to controls (Fig. 4A, P<0.001) and intravenous MMC
treated mice (Fig. 4B,
P<0.05).
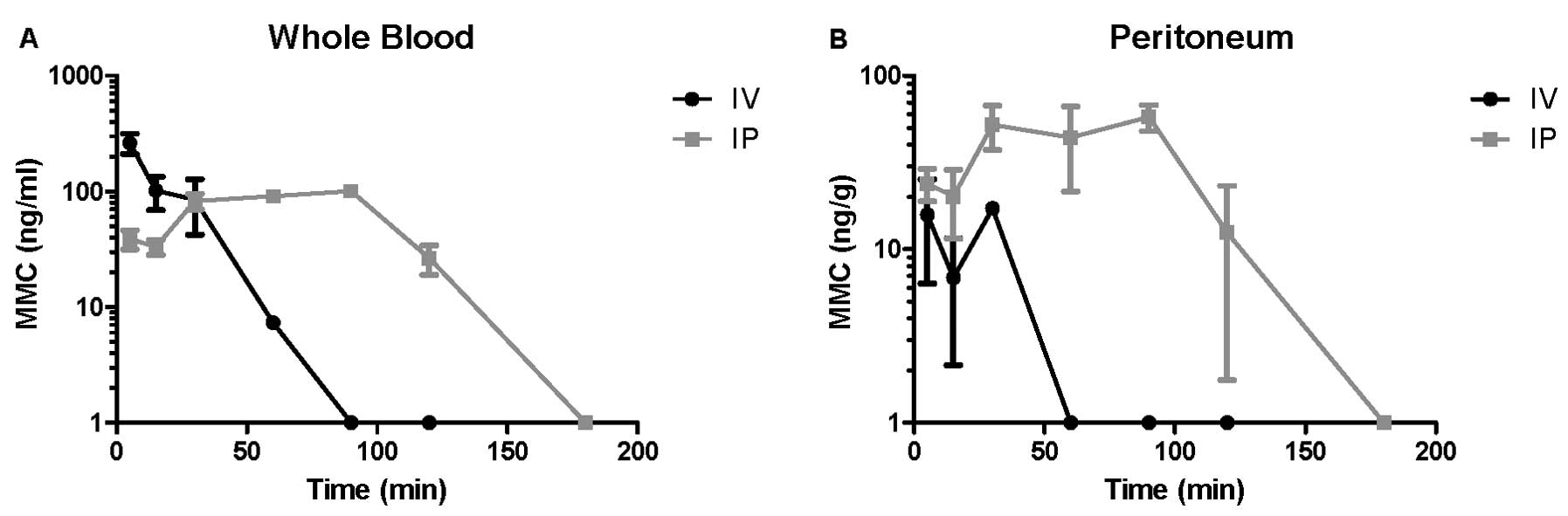
Tissue concentrations and rate of
clearance of MMC vary by the route of delivery
Tissue distribution of MMC after intravenous or
intraperitoneal administration, with respect to whole blood or
parietal peritoneum was determined by UPLC. In the whole blood, an
immediate peak in MMC and a gradual decline to below detectable
limits by 90 min was observed for the intravenous groups. In
contrast, the whole blood samples of intraperitoneally administered
MMC peaked with a plateau from 30 to 90 min, and gradual decline
extending to 180 min (Fig. 5A and
B). Peritoneal tissue concentrations of intravenously
administered MMC demonstrated a peak from 5 to 30 min with a steep
decline that reached levels below quantification at 60 min. On the
contrary, intraperitoneal administration of MMC resulted in a
plateau in the peritoneal tissue from 30 to 90 min with a slow
decline to 180 min (Fig. 5A and B).
Overall, intravenous administration led to a rapid peak and
subsequently quick elimination of MMC in the tissues studied,
whereas intraperitoneal administration led to a more prolonged
whole blood concentration and a higher peritoneal concentration
that was persistent from 30 to 120 min. It should be noted that the
intraperitoneal chemotherapy was evacuated from the peritoneum and
the peritoneal cavity lavaged with saline at the completion of 60
or 90 min, similar to clinical protocols, yet, peritoneal tissue
concentrations of MMC persisted well beyond this point of
evacuation.
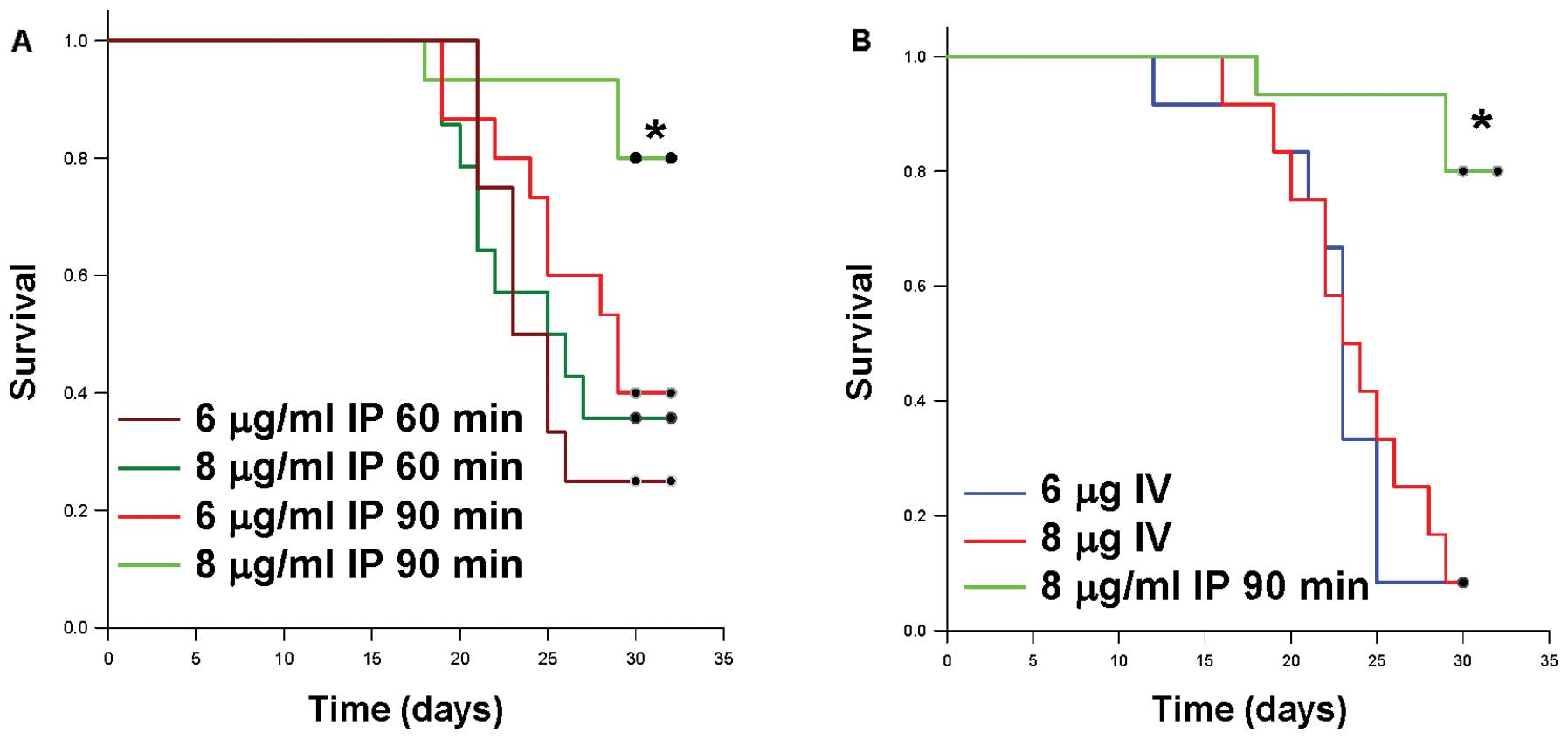
Intraperitoneal treatments associated
with lower tumor burden also demonstrate improved survival
Survival appeared to be correlated to the tumor
burden results, as the high dose intraperitoneal MMC-treated mice
had improved survival compared with low dose intraperitoneal MMC
treatment, intravenous treatment or saline control. Mice receiving
a lower dose of MMC (6 μg/ml IP) or mice receiving decreased
exposure time to the MMC lavage (i.e. 60 min), had a significantly
decreased survival (Fig. 6A). In
agreement with the tumor growth inhibition results, mice receiving
intravenous MMC had a significantly decreased survival as compared
to the optimal intraperitoneal treatment (Fig. 6B). Overall, the majority of mice in
the saline, intravenous, and low dose intraperitoneal MMC groups
died or met the criteria for euthanasia between 25 and 30 days
post-implantation, while the mice treated with high dose MMC for 90
min demonstrated improved survival.
Discussion
The overall objective of the present study was to
examine the effects of varying the dose and exposure time of
intraperitoneal chemotherapy on treatment outcome in a clinically
relevant animal model. The present study was designed to eliminate
the variability of both cytoreduction and hyperthermia in order to
focus solely on the contribution of the intraperitoneal
chemotherapy component of HIPEC. The model is unique as it
replicated the prolonged anesthesia, laparotomy, intraperitoneal
chemotherapy dwell times with agitation, and chemotherapy wash out
performed clinically in patients. Importantly, mice subjected to
the intraperitoneal chemotherapy component of HIPEC in this fashion
tolerated the procedure well with no perioperative morbidity or
mortality. The dosing range of MMC in the present study
corresponded with the clinically accepted dose used for human HIPEC
and demonstrated significant time- and dose-dependent tumor growth
inhibition. The high dose of MMC for 90 min displayed the greatest
tumor growth inhibition and was statistically associated with
improved survival. These results indicate that the intraperitoneal
chemotherapy component of HIPEC contributes to the observed
clinical outcomes independent of cytoreduction and hyperthermia.
Furthermore, intraperitoneal administration of MMC was shown in the
present study, through UPLC, to result in an overall higher
peritoneal concentration of chemotherapy, longer tissue retention
time, and a more prolonged whole blood level, which may explain the
improved tumor control and prolonged survival seen with the
intraperitoneally treated mice.
A potential survival benefit and reduction in tumor
volume associated with intraperitoneal chemotherapy has been
reported in other murine models (11–13).
However, many of these preclinical studies have administered a
single intraperitoneal injection of chemotherapy agent without
prolonged anesthesia, laparotomy, or the washout that occurs during
clinical HIPEC (12,14,15).
In studies that attempt to mimic clinical HIPEC, the variability in
cytoreduction (11,16,17) or
the method of tumor establishment (13,17)
may be confounding variables. The mouse model used for the
currently reported study differs from other models in that the
tumor volume was constant and recapitulated the microscopic disease
burden that remains after the completion of cytoreduction. To
determine tumor volumes, previous studies have utilized necropsy
evaluation or imaging methods such as PET in HIPEC models (11,13,18).
In the present study, assessment of intraperitoneal tumor burden
was performed non-invasively using MRI, given its ability to
provide high soft tissue contrast without the use of ionizing
radiation or need for radioactive tracers. The results demonstrate
that tumor growth inhibition following intraperitoneal chemotherapy
is dependent on the dose and duration of exposure. Collectively,
our results are the first to relate improved tumor control,
prolonged chemotherapy tissue concentrations, and better overall
survival during intraperitoneal chemotherapy that was delivered in
a manner designed to precisely mimic clinical conditions.
By purposefully eliminating the variable of
cytoreduction, a shortcoming of this model may be its inability to
address potential synergy between surgical cytoreduction and
intraperitoneal chemotherapy. Theoretically, a local inflammatory
state may exist following cytoreduction that may potentiate the
tumoricidal activity of HIPEC (19,20).
Additionally, this mouse model mimics an open or ‘coliseum’
technique of HIPEC and may underestimate the contributions of
volume, pressure, and flow parameters on drug kinetics and
antitumor effect (19). In the
present study, no cures were generated, and only survival
prolongation was observed. All mice, regardless of treatment,
required euthanasia for progressive peritoneal disease. While there
may be a clinical benefit in terms of survival prolongation, the
absolute impact of the various components of cytoreductive
surgery/HIPEC (cytoreduction, intraperitoneal chemotherapy and
hyperthermia) in patients who are ultimately cured cannot be
determined from this study. It is possible that the clinical
benefit from cytoreduction alone may outweigh any contribution
derived from intraperitoneal chemotherapy. However, these results
suggest that intraperitoneal chemotherapy, as an independent
variable, may have a positive influence on clinical outcome,
particularly in the background of residual microscopic disease.
The elucidation of HIPEC variables and their
clinical significance in this mouse model may direct future
clinical investigation and generate preclinical data as a rational
basis for human clinical trials, which has been lacking. Future
experiments utilizing this mouse model will interrogate the role of
hyperthermia as an independent variable of HIPEC. The ability to
study novel agents and immune mediated responses following HIPEC is
also possible with this model. Preliminary studies from our
laboratory have begun to address these issues with the ultimate
goal of understanding and optimizing clinical protocols.
Acknowledgements
We would like to acknowledge Daniel Fisher and Jason
Muhitch for technical support and manuscript review. The present
study was funded by the Rowswell Park Cancer Institute and National
Cancer Institute (P30 CA016056).
References
|
1
|
Koppe MJ, Boerman OC, Oyen WJ and
Bleichrodt RP: Peritoneal carcinomatosis of colorectal origin:
incidence and current treatment strategies. Ann Surg. 243:212–222.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, DeSantis C, Virgo K, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chu DZ, Lang NP, Thompson C, Osteen PK and
Westbrook KC: Peritoneal carcinomatosis in nongynecologic
malignancy. A prospective study of prognostic factors. Cancer.
63:364–367. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elias D, Delperro JR, Sideris L, et al:
Treatment of peritoneal carcinomatosis from colorectal cancer:
impact of complete cytoreductive surgery and difficulties in
conducting randomized trials. Ann Surg Oncol. 11:518–521. 2004.
View Article : Google Scholar
|
|
5
|
Sugarbaker PH: A curative approach to
peritoneal carcinomatosis from colorectal cancer. Semin Oncol.
32:S68–S73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glehen O, Kwiatkowski F, Sugarbaker PH, et
al: Cytoreductive surgery combined with perioperative
intraperitoneal chemotherapy for the management of peritoneal
carcinomatosis from colorectal cancer: a multi-institutional study.
J Clin Oncol. 22:3284–3292. 2004. View Article : Google Scholar
|
|
7
|
Verwaal VJ: Long-term results of
cytoreduction and HIPEC followed by systemic chemotherapy. Cancer
J. 15:212–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugarbaker PH: Peritonectomy procedures.
Surg Oncol Clin N Am. 12:703–727. xiii2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rossi CR, Mocellin S, Pilati P, et al:
Pharmacokinetics of intraperitoneal cisplatin and doxorubicin. Surg
Oncol Clin N Am. 12:781–794. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Ruth S, Verwaal VJ and Zoetmulder FA:
Pharmacokinetics of intraperitoneal mitomycin C. Surg Oncol Clin N
Am. 12:771–780. 2003.
|
|
11
|
Klaver YL, Hendriks T, Lomme RM, Rutten
HJ, Bleichrodt RP and de Hingh IH: Hyperthermia and intraperitoneal
chemotherapy for the treatment of peritoneal carcinomatosis: an
experimental study. Ann Surg. 254:125–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen MS, Al-Kasspooles MF, Williamson SK,
Henry D, Broward M and Roby KF: Combination intraperitoneal
chemotherapy is superior to mitomycin C or oxaliplatin for
colorectal carcinomatosis in vivo. Ann Surg Oncol. 17:296–303.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pelz JO, Doerfer J, Hohenberger W and
Meyer T: A new survival model for hyperthermic intraperitoneal
chemotherapy (HIPEC) in tumor-bearing rats in the treatment of
peritoneal carcinomatosis. BMC Cancer. 5:562005. View Article : Google Scholar
|
|
14
|
Bevanda M, Orsolic N, Basic I, et al:
Prevention of peritoneal carcinomatosis in mice with combination
hyperthermal intraperitoneal chemotherapy and IL-2. Int J
Hyperthermia. 25:132–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zahedi P, De Souza R, Piquette-Miller M
and Allen C: Docetaxel distribution following intraperitoneal
administration in mice. J Pharm Pharm Sci. 14:90–99.
2011.PubMed/NCBI
|
|
16
|
Aarts F, Hendriks T, Boerman OC, Oyen WJ
and Bleichrodt RP: Hyperthermia and fibrinolytic therapy do not
improve the beneficial effect of radioimmunotherapy following
cytoreductive surgery in rats with peritoneal carcinomatosis of
colorectal origin. Cancer Biother Radiopharm. 23:301–309. 2008.
View Article : Google Scholar
|
|
17
|
Hartmann J, Kilian M, Atanassov V,
Braumann C, Ordemann J and Jacobi CA: First surgical tumour
reduction of peritoneal surface malignancy in a rat’s model. Clin
Exp Metastasis. 25:445–449. 2008.PubMed/NCBI
|
|
18
|
Bouquet W, Deleye S, Staelens S, et al:
Antitumour efficacy of two paclitaxel formulations for hyperthermic
intraperitoneal chemotherapy (HIPEC) in an in vivo rat model. Pharm
Res. 28:1653–1660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gesson-Paute A, Ferron G, Thomas F, de
Lara EC, Chatelut E and Querleu D: Pharmacokinetics of oxaliplatin
during open versus laparoscopically assisted heated intraoperative
intraperitoneal chemotherapy (HIPEC): an experimental study. Ann
Surg Oncol. 15:339–344. 2008. View Article : Google Scholar
|
|
20
|
Muller H, Hahn M, Weller L and Simsa J:
Strategies to reduce perioperative morbidity in cytoreductive
surgery. Hepatogastroenterology. 55:1523–1529. 2008.PubMed/NCBI
|