Introduction
Gastric cancer is one of the most common
malignancies and the second leading cause of cancer-related
mortality worldwide. Surgery and systemic treatment regimens in
combination with radiotherapy and chemotherapy are able to cure
this malignancy in the majority of cases. Unfortunately, the young
age of typical gastric cancer patients further enhances the time at
risk for the side effects of these therapies. Delayed toxicity and
the development of secondary malignancies are a serious concern for
the current anti-neoplastic armamentarium (1,2).
Therefore, it is urgent to further characterize the entity of
gastric tumors to better understand and predict their biological
behaviors and to identify novel high efficient and low toxic
therapeutic agents for the treatment of gastric cancer in clinical
practice (3).
Modern pharmacologic study proves that C-21
steroidal glycosides are important biological active compounds,
which are widely found in the plants of the Asclepiadaceae
family and exhibit extensive pharmacological effects such as
inhibition of proliferation, induction of apoptosis and inhibition
of the invasion of tumor cells (4,5).
Caudatin, a C-21 steroidal glycoside, is mainly isolated from the
root of Cynanchum bungei Decne in China. Recent studies
demonstrated that caudatin induced the apoptosis of HepG2 cells or
the SMMC772 cell line (6,7). Wang et al(8) found that caudatin had an inhibitory
activity on the secretion of HBsAg and HBV DNA replication.
Furthermore, caudatin as a prospective anti-HCC drug with the
mechanism of inhibiting cell proliferation and inducing cell
apoptosis has been reported (9).
Yet, there is no report concerning the effects of caudatin on
gastric adenocarcinoma, and the underlying mechanisms are not well
documented.
The Wnt/β-catenin signaling pathway is involved in
multiple developmental events during embryogenesis and is also
implicated in tumorigenesis, cell-fate decisions, proliferation and
apoptosis (10,11). Wnt ligands trigger at least three
different intracellular signaling cascades: the canonical Wnt
pathway, which results in transcriptional regulation of target
genes via β-catenin; the planar cell polarity (PCP) pathway; and
the Wnt-dependent calcium/protein kinase C (PKC) pathway. The
protein β-catenin is the central player in one major arm of the
canonical Wnt pathway (12,13). Hyperactivation of β-catenin
signaling has been implicated as a driver of various types of
cancers, including human gastric cancer (14–16).
Thus, there is a great interest in identifying inhibitors of the
Wnt/β-catenin signaling pathway.
miRNAs are small non-coding RNAs consisting of
~17–24 nucleotides, each of which is predicted to regulate hundreds
of genes (both coding and non-coding) by post-transcriptional
silencing (17). Many have been
shown to function in cell proliferation, apoptosis, migration and
development (18,19). Certain miRNAs have been reported to
be associated with the initiation and progression of gastric
cancer. For example, aberrant expression of miR-106a was detected
in gastric carcinoma tissue and was found to promote gastric
carcinogenesis (20). miR-21 has
been found to be correlated with H. pylori infection and
gastric cancer progression (21).
Recently, Belair et al(22)
studied the miRNA expression profile of AGS cells and found that
miR-372 had the highest expression of the total miRNAs with the
percentage of miR-372 reaching 11%. We previously reported that
miR-372 maintains oncogene characteristics by targeting
TNFAIP1-regulated AGS cell growth (23). Moreover, recent studies have
demonstrated a connection between miRNAs and the Wnt/β-catenin
signaling pathway. Zhou et al(24) reported that β-catenin transactivates
the microRNA-371–373 cluster in colorectal cancer (CRC) cell lines.
In contrast, the let-7 microRNA family was found to be
downregulated in human gastric cancer (25,26).
These findings demonstrate that miRNAs are involved in gastric
carcinogenesis via varied patterns and pathways.
In the present study, we evaluated the effects of
caudatin on gastric cancer cells and elucidated the mechanism
mediated through the Wnt/β-catenin signaling pathway.
Materials and methods
Reagents
Caudatin was purchased from the Shenzhen Medherb
Biotechnology Co., Ltd. (Shenzhen, China) and dissolved with 100%
dimethyl sulfoxide (DMSO) (concentration of the stock solution, 10
mM).
Human tissue samples
All human gastric adenocarcinoma tissue samples and
adjacent normal tissues were obtained from the Department of
Pathology, Xiangya Hospital, Changsha, China. The samples were
classified according to World Health Organization criteria
published in 2000. All patients signed consent forms and the study
protocol was approved by the Committee on Human Rights in Research
of the Ethics Committee of the College of Life Science, Hunan
Normal University, Changsha, China.
Cells and cell culture
The human gastric carcinoma cell lines (AGS, HGC-27,
GES-1) were obtained from the Cell Bank of the Chinese Academy of
Sciences (China). Cells were cultured at 37°C in 5% CO2
in F12 or Dulbecco’s modified Eagle’s medium (DMEM; both from
Gibco-BRL) supplemented with 10% fetal bovine serum (Gibco-BRL),
100 U/ml penicillin and 100 μg/ml streptomycin.
MTT assay
Briefly, cells were seeded in 24-well tissue culture
plates in a final volume of 500 μl. After attachment for 24 h,
cells were treated with 25–100 μM caudatin or DMSO as a blank.
After drug exposure, 50 μl of MTT [5 mg/ml in phosphate-buffered
saline (PBS)] solution was added to each well for an additional 4
h. The reaction was stopped by removal of MTT, and the formazan
crystals were solubilized in 100 μl DMSO (Sigma, St. Louis, MO,
USA) in each well. Absorbance at 570 nm was recorded using a
microplate spectrophotometer. Three wells were assigned to each
caudatin dose.
FACS assays
Cells were harvested at 600 × g for 3 min, washed
twice in PBS at room temperature and resuspended in appropriate
PBS. Then, cells were resuspended in 1X FACS binding buffer
containing Annexin V and propidium iodide and analyzed using a FACS
flow cytometer (BD Biosciences). A total of 20,000 cells was
counted for each sample.
Hoechst 33258 staining
Apoptotic morphological changes in the nuclear
chromatin of cells were detected by Hoechst 33258 (Sigma) staining.
AGS or HGC-27 cells were seeded on sterile cover glasses and washed
with PBS and fixed with 4% paraformaldehyde for 10 min, and then
incubated with 50 μl Hoechst 33258 staining solution for 10 min.
After three washes with PBS, the cells were viewed under a
fluorescence microscope (Zeiss Axioskop; Zeiss, Oberkochen,
Germany).
Western blot analysis
Cell lysates were subjected to polyacrylamide gel
electrophoresis and blotted onto Immobilon-P polyvinylidene
difluoride membranes (Millipore, Bedford, MA, USA). Caspase-3,
caspase-8, caspase-9, β-catenin, cyclinD1 and C-myc antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). β-actin antibody was purchased from GenScript, Inc.
(Piscataway, NJ, USA), and the protein was detected using a
HRP-conjugated secondary antibody and a Chemilucent ECL Detection
System (Millipore, Billerica, MA, USA). The experiments were
repeated at least twice with separately collected protein
extracts.
miRNA analysis
Total RNA was extracted using the
E.Z.N.A.® FFPE RNA Isolation kit (Omega) according to
the manufacturer’s instructions. For miRNA detection, 2 μg of small
RNA was reverse transcribed to cDNA using the miRNA First-Strand
cDNA Synthesis kit (Invitrogen, USA) according to the
manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR)
analysis for miR-372, miR-21 and Let-7a was performed in triplicate
using the SYBR-Green PCR Master Mix (Perkin-Elmer Applied
Biosystems) according to the manufacturer’s instructions. U6 RNA
was used to normalize expression. Primers used were: U6 forward,
5′-CTCGCTTCGGCA GCACA-3′; Let-7a forward, 5′-GAGGTAGTAGGTTGT
ATAGTTTAGAA-3′; miR-21 forward, 5′-GCTTATCAGAC TGATGTTGACTG-3′,
miR-372 forward, 5′-GCCCGCAA AGTGCTGCGAACAT-3′. All reverse primers
were Uni-miR qPCR Primers obtained from the First-Strand cDNA
Synthesis kit. Data analysis was performed using the
2−ΔΔCt method (27).
Immunofluorescence staining
The cells were plated overnight on coverslips on
12-well plates. AGS cells were pre-incubated with the proteosome
inhibitor MG132 (100 μM) for 5 h or the lysosome inhibitor
NH4Cl (100 μM) for 5 h, prior to caudatin (50 μM)
treatment for 24 h. Cells were then fixed with paraformaldehyde for
15 min, rinsed with PBS and incubated with 2% bovine serum albumin
in PBS for 30 min. The cells were then incubated overnight with the
anti-β-catenin antibody. After washing with PBS, the cells were
incubated with the Alexa Red-conjugated anti-mouse IgG secondary
antibody (Molecular Probes). Hoechst 33258 was used to stain the
cell nuclei. Cell images were observed under a fluorescence
microscope.
Statistical analysis
All data are presented as means ± standard deviation
from at least three separate experiments. The differences among
groups were analyzed using the double-sided Student’s t-test, and
statistical significance was determined at a P-value of
<0.05.
Results
Caudatin inhibits gastric cancer cell
growth
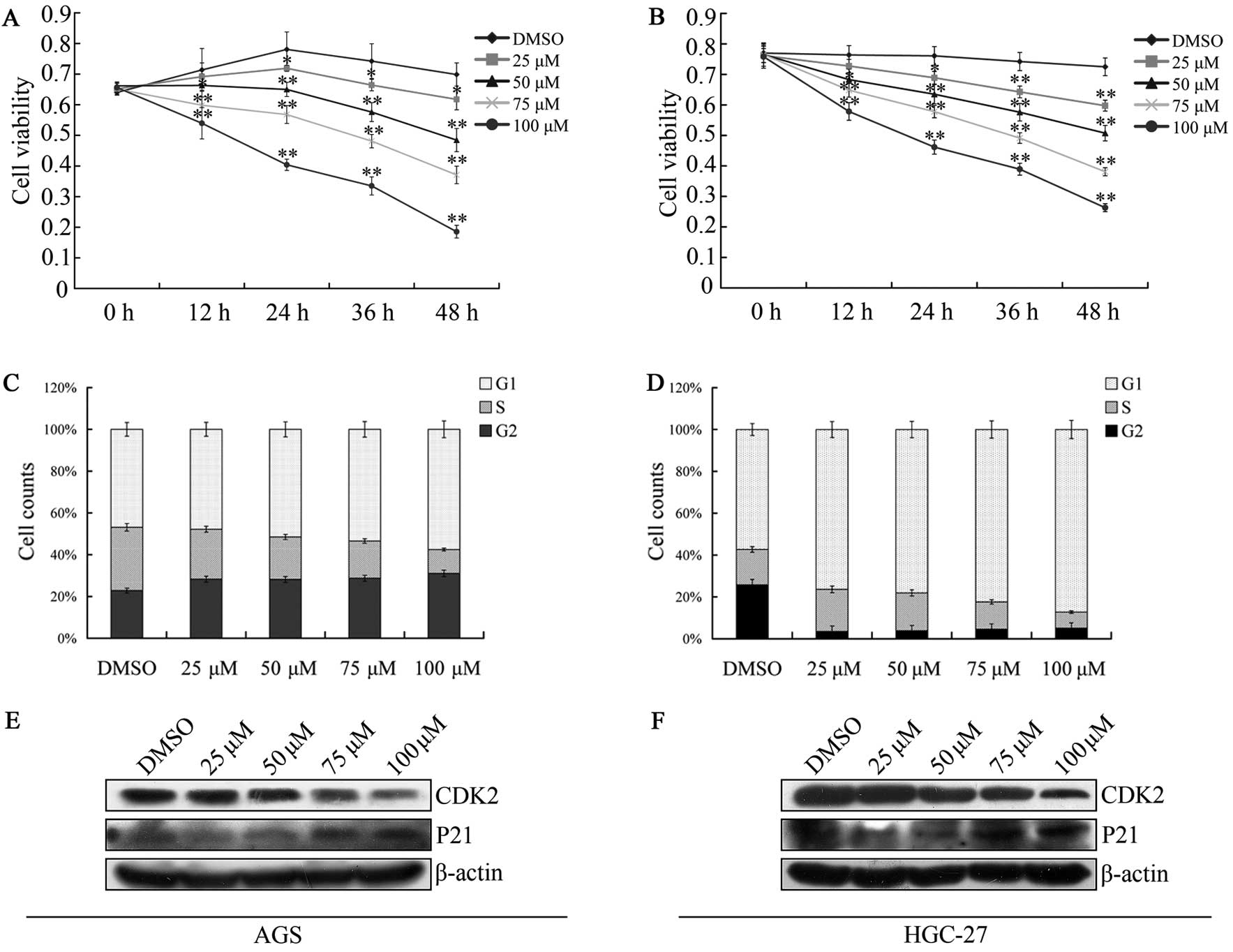
We determined the effect of caudatin on gastric
cancer cell proliferation in the AGS and HGC-27 cells. Caudatin
significantly suppressed the proliferation of the gastric cancer
cell lines in a dose- and time-dependent manner. This
anti-proliferative effect on tumor cells was observed for every
12-h period following 5 treatments (DMSO or caudatin at 25, 50, 75,
100 μM) (Fig. 1A and B). The
IC50 values for caudatin in AGS cells at 12, 24, 36 and
48 h were 180.31, 109.97, 86.45 and 54.92 μmol/l, respectively. The
IC50 values for caudatin in HGC-27 cells at 12, 24, 36
and 48 h were 200.06, 129.24, 102.88 and 65.98 μmol/l,
respectively. Of note, the MTT assay that is used to determine cell
proliferation does not directly distinguish between induction of
cell death or prevention of cell division; however, the result is
clear that caudatin significantly suppressed the proliferation of
the gastric cancer cell lines. Cell proliferation is also
controlled by the progression of the cell cycle. Our results showed
the treatment with caudatin distinctly inhibited the cell cycle of
AGS and HGC-27 cells at the G1/S-phase transition (Fig. 1C and D). CDK2 is one of the cell
cycle regulatory protein that regulates the G1 to S-phase
transition of the cell cycle and functions as a cofactor for
several transcription factors in numerous cell lines (28). Contrarily, P21 can suppress CDK2 and
block the G1 to S-phase transition. In agreement with this theory,
western blotting demonstrated that caudatin significantly
downregulated CDK2 protein levels in a dose-dependent manner in the
AGS and HGC-27 cells. In contrast, the expression of the P21
protein was upregulated distinctly after caudatin treatment
(Fig. 1E and F). These results
demonstrated that caudatin inhibited the growth of gastric cancer
cells in a dose- and time-dependent manner.
Caudatin induces gastric cancer cell
death by apoptosis
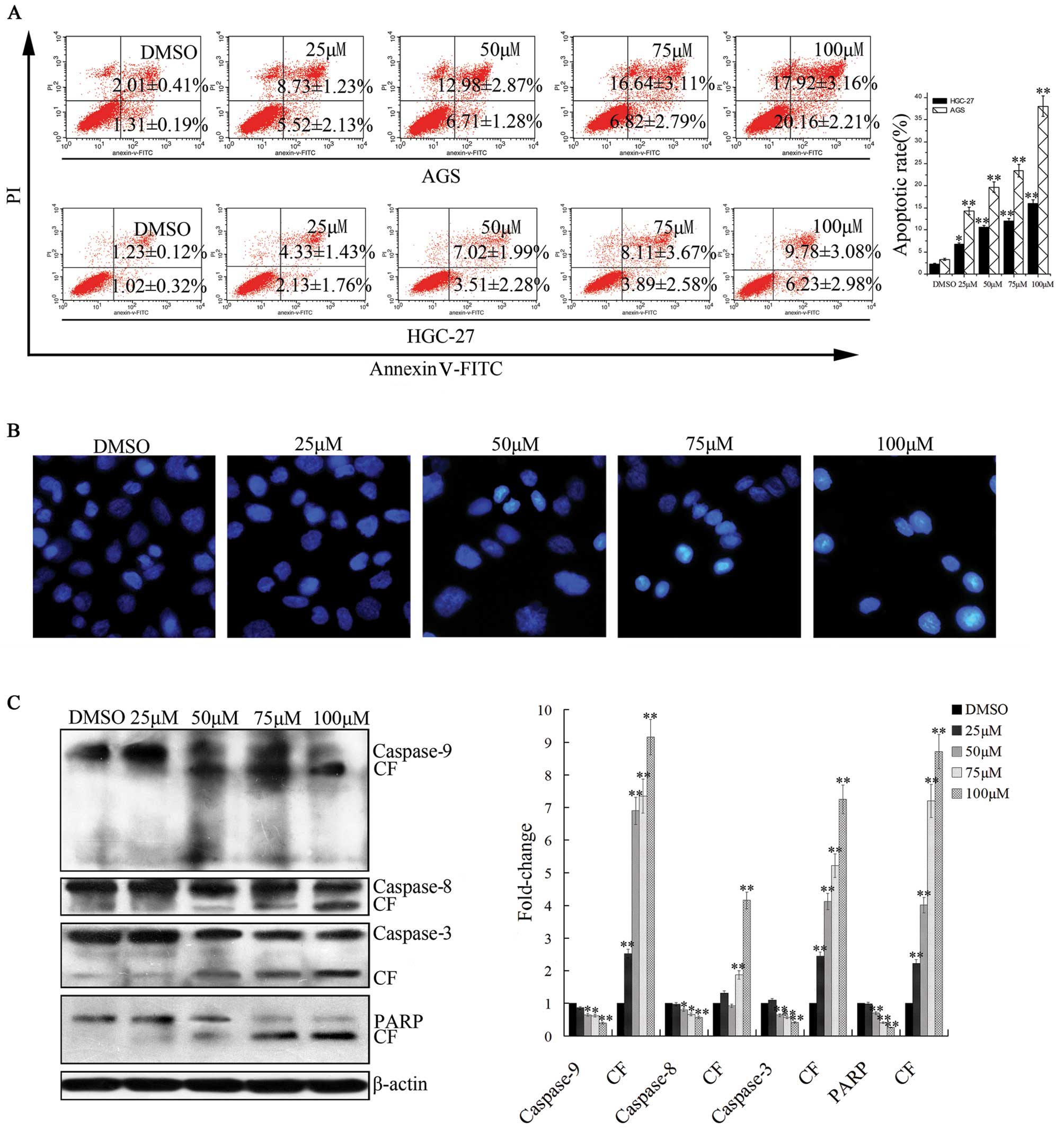
Flow cytometric analysis indicated the induction of
apoptosis by caudatin occurred in AGS and HGC-27 cells. Caudatin
treatment resulted in a substantial increase in Annexin V-positive
cells (Fig. 2A), indicating
induction of cell death via an apoptotic pathway. Notably, the AGS
cells presented a higher apoptosis rate than the HGC-27 cells.
Hochest 33258 staining indicated that typical apoptotic cell
morphology, such as the formation of apoptotic bodies, appeared
after the AGS cells were treated for 24 h with different
concentrations (25–100 μM) of caudatin, while the control cells did
not obviously show evident apoptotic morphological changes
(Fig. 2B). We confirmed this
observation by evaluating the expression of PARP and caspase-3, -8
and -9, as proteolytic cleavage and subsequent activation of these
molecules activate apoptotic pathways. The expression level of
full-length caspase-3, -8 and -9 was decreased, suggesting cleavage
and activation of the caspase pathway. Moreover, cleaved products
of caspase-3, -8 or -9 were also detectable. Furthermore, PARP,
which is the prime marker for caspase-dependent apoptosis, showed
cleavage in a dose-dependent manner (Fig. 2C). These data suggest that caudatin
is a potent inducer of apoptosis in gastric cancer cells, and the
apoptosis involves the caspase-related pathway.
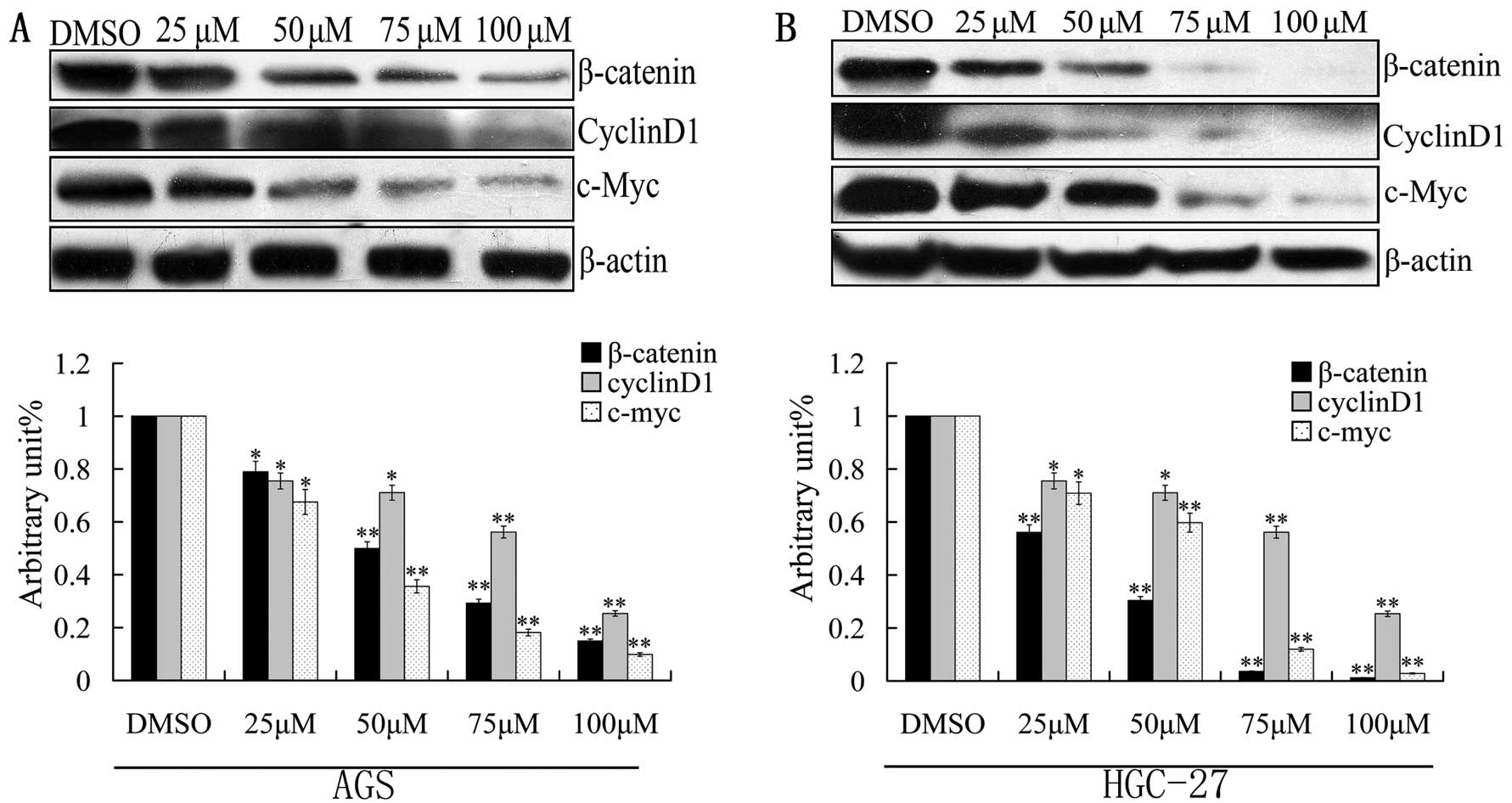
Caudatin reduces β-catenin expression in
a dose-dependent manner
Deregulation of the Wnt/β-catenin pathway has been
shown in gastric cancer. As a modulator of the Wnt signaling
pathway, β-catenin functions as a transcription factor that is
translocated into the nucleus where it binds with the TCF
transcription factor. To assess whether caudatin affects the
expression of β-catenin, AGS and HGC-27 cells were treated with
various concentrations of caudatin for 24 h. Western blotting
results indicated that caudatin significantly downregulated the
expression of β-catenin in the AGS and HGC-27 cells. Inhibition of
the level of cyclinD1, a downstream protein of β-catenin (29), was also observed following treatment
with caudatin. This result supports the above conclusion that
caudatin suppresses G1 to S-phase transition of the cell cycle in
AGS and HGC-27 cells. In addition, the protein level of c-MYC
(30), which is an important
downstream regulatory gene of β-catenin, also was decreased
(Fig. 3). These results suggest
that caudatin blocks the Wnt/β-catenin pathway by reducing the
β-catenin protein level.
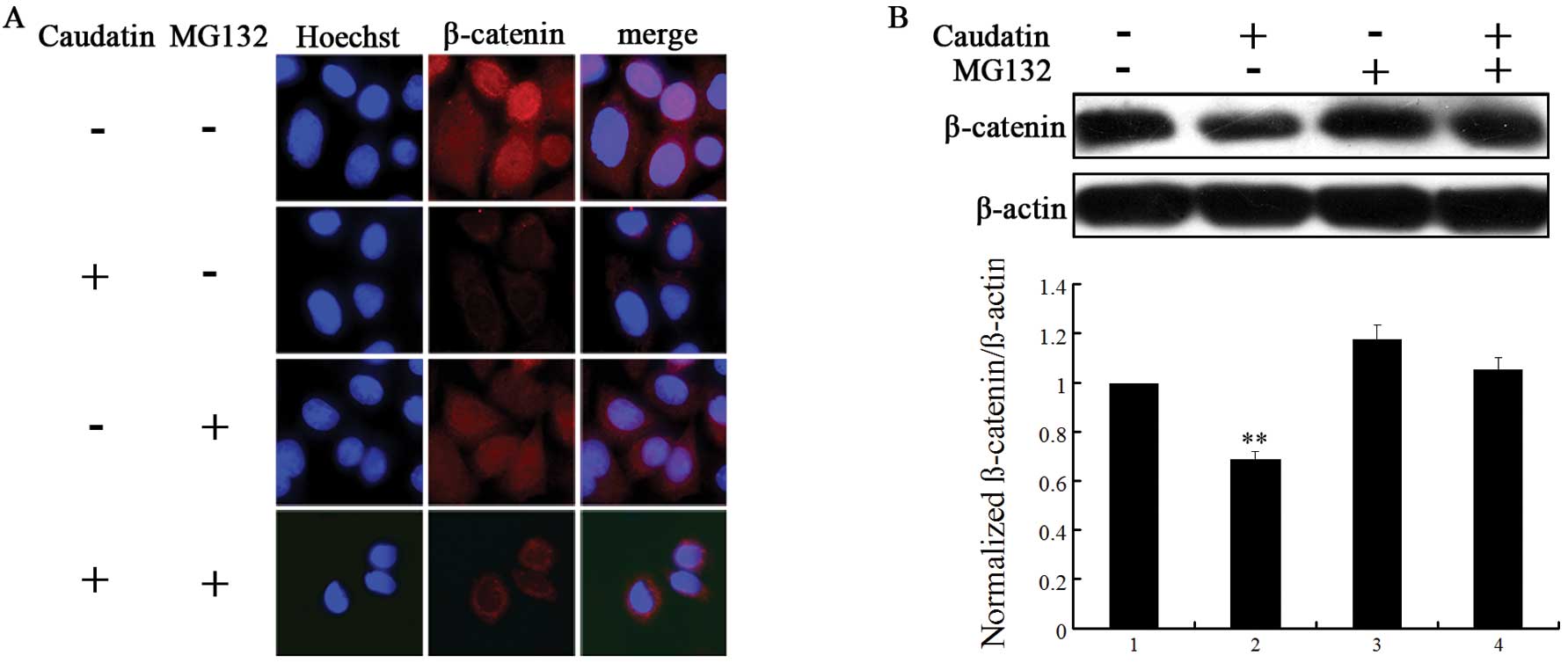
Caudatin induces proteasome-mediated
degradation of β-catenin
The proteosome and lysosome pathways are two main
degradation mechanisms of protein (31). To verify which pattern drives
β-catenin into degradation, we pre-incubated AGS cells with the
proteosome inhibitor MG132 (100 μM) for 5 h or the lysosome
inhibitor NH4Cl (100 μM) for 5 h, prior to caudatin (50
μM) treatment for 24 h. Whole cell protein extracts were prepared
and evaluated for β-catenin level by western blotting. As shown in
Fig. 4B, the protein level of
β-catenin was blocked in the presence of MG132. However, when AGS
cells were pre-treated with NH4Cl, there was no change
in β-catenin protein level (data not shown). This result was
confirmed using immunocytochemical staining in AGS cells (Fig. 4A). Notably, AGS cells treated with
DMSO revealed nuclear location of β-catenin. Following treatment
with 50 μM of caudatin, β-catenin was mainly located under the
plasma membrane. These data suggest that caudatin treatment
attenuates nuclear β-catenin signaling, which is known to play a
significant role in cancer cell proliferation.
Caudatin inhibits oncomir miRNA and
increases tumor suppressor miRNA in gastric cancer cells
Recently, we demonstrated that miRNA-372 maintains
oncogene characteristics in AGS cells and may become a biomarker
for gastric cancer progression, while Zhou et al(24) found that β-catenin transactivates
the microRNA-371–373 cluster and modulates the Wnt/β-catenin
signaling pathway. To confirm the expression level of miR-372 in
gastric adenocarcinoma tissue, total RNA was extracted from 15
gastric adenocarcinoma samples, consisting of 5 grade I, 5 grade II
and 5 grade III gastric adenocarcinoma tissues, 15 matched normal
tissues and 3 human gastric carcinoma cell lines. qRT-PCR was
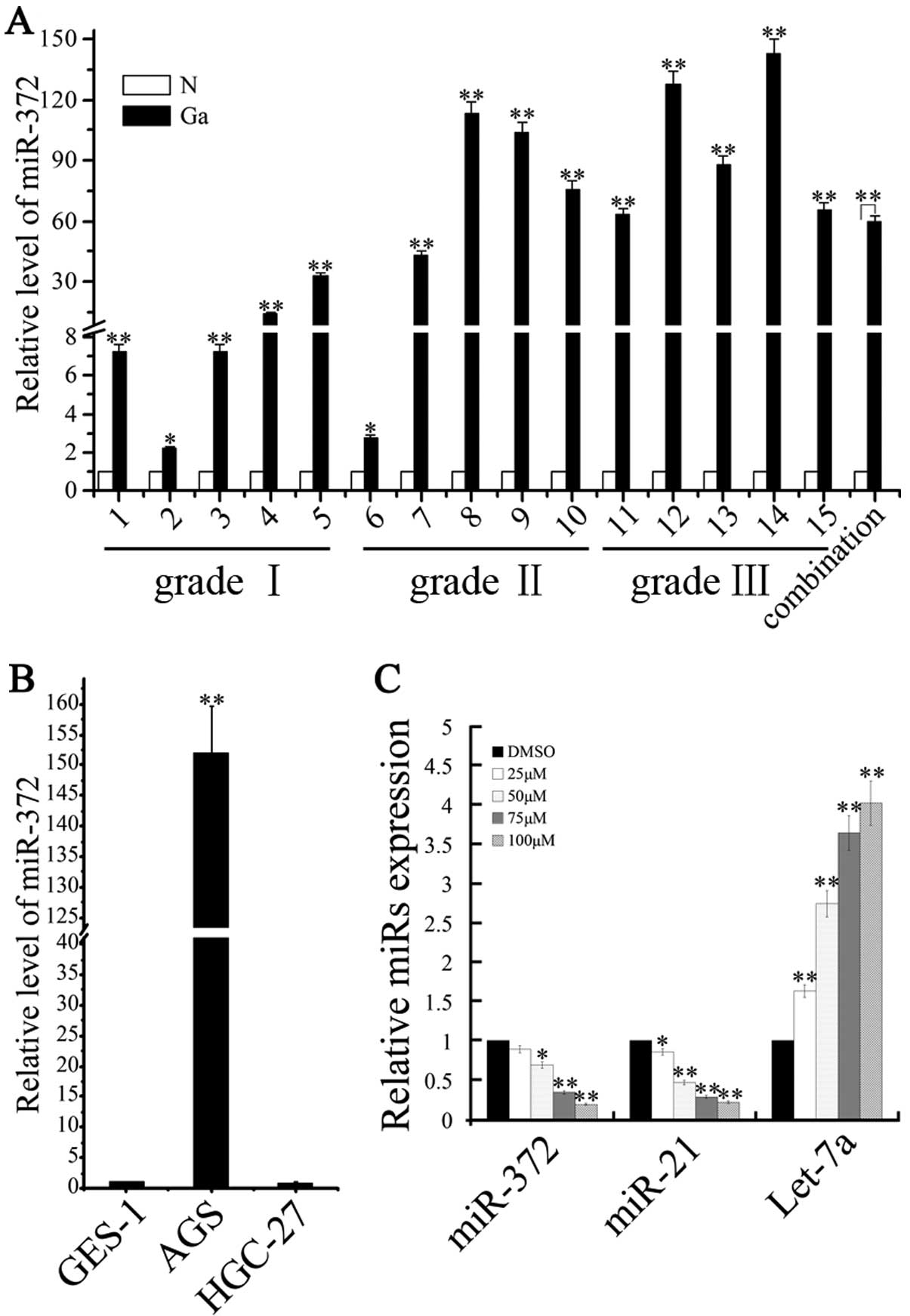
performed to analyze the expression profile. As shown in Fig. 5A, we found that miR-372 had higher
expression levels in the gastric adenocarcinoma tissue when
compared with the matched normal tissue. Moreover, miR-372 also
showed abnormal upregulation in the adenocarcinoma cancer cells
(AGS cells) (Fig. 5B). We next
determined the effects of caudatin on oncomir miRNA and tumor
suppressor miRNA in the gastric cancer cells. Caudatin treatment
significantly downregulated miR-372 and miR-21 expression while
upregulating let-7a miRNA expression in a dose-dependent manner
(Fig. 5C). Therefore, these
findings reveal that the expression of miRNAs were altered after
caudatin treated.
Discussion
Gastric cancer is increasing in incidence in many
countries, and is among the top two causes of cancer-related
mortality worldwide. It is estimated that 360,000 individuals
succumb to gastric cancer each year in China. In the present study,
we used gastric cancer cells as experimental material to confirm
the antitumor effect of caudatin and to illustrate the underlying
mechanisms of its anticancer activity. MTT assay was performed to
quantify the effects of caudatin on AGS and HGC-27 cell growth
inhibition. The data presented here showed that caudatin treatment
resulted in a dose- and time-dependent inhibition of proliferation
in gastric carcinoma cell lines. The IC50 values for
caudatin in AGS cells at 12, 24, 36 and 48 h were 180.31, 109.97,
86.45 and 54.92 μmol/l, respectively. The IC50 values
for caudatin in HGC-27 cells at 12, 24, 36 and 48 h were 200.06,
129.24, 102.88 and 65.98 μmol/l, respectively. The cell growth
inhibition induced by many cancer chemopreventive agents is
correlated with perturbations in the cell cycle progression. Thus,
we determined the effect of caudatin on cell cycle distribution to
gain insights into the mechanism of its cell growth suppression.
Exposure of AGS and HGC-27 cells to caudatin resulted in the
accumulation of the G0/G1 cell fraction in a
concentration-dependent manner, which was accompanied by a decrease
in the percentage of S-phase cells. CDK2 is one the cell cycle
regulatory proteins that regulates the G1 to S-phase transition of
the cell cycle and functions as a cofactor for several
transcription factors in numerous cell lines. Contrarily, P21 can
suppress CDK2 and block G1 to S-phase transition. In agreement with
this theory (32), western blot
analyses demonstrated that caudatin significantly downregulated
CDK2 protein levels in a dose-dependent manner in the AGS and
HGC-27 cells. In addition, p21, which plays a crucial role in the
regulation of G1 to S-phase transition by a p53-independent
pathway, was also triggered by caudatin in gastric cancer cells.
The expression of P21 protein was distinctly upregulated after
caudatin treatment in the gastric carcinoma cell lines. Similar
caudatin-induced G0/G1 arrest has also been shown to occur in other
cancer cell types, including hepatic and lung cancers.
In addition to inappropriate growth signals, many
cancer cells lose their ability to undergo apoptosis. Disturbances
in apoptosis are important for the development of cancer.
Therefore, the killing of tumors through induction of apoptosis has
been recognized as a novel strategy for the identification of
anticancer drugs. In order to distinguish that the cell death
caused by caudatin is due to apoptosis or necrosis, Annexin V/PI
double-labeling analysis was performed which enables further
distinction of necrotic/late apoptotic (Annexin
V+/PI+) and early apoptotic (Annexin
V+/PI−) cells. Our results showed that
treatment of AGS and HGC-27 cells with caudatin resulted in a
time-dependent increase in the numbers of both early apoptotic and
late apoptotic/necrotic cells. Hoechst staining showed that the
typical morphological changes attributed to apoptosis, such as
formation of apoptotic bodies, appeared after the cells were
treated for 24 h with 25–100 μM caudatin, whereas the control cells
did not show evident apoptotic morphological changes. Apoptosis may
occur via a mitochondrial-dependent intrinsic pathway or a death
receptor-mediated extrinsic pathway. Caspases, a family of cysteine
proteases, are synthesized as inactive pro-enzymes which are
processed to an active form in cells undergoing apoptosis.
Caspase-3 is situated at a pivotal junction in the apoptotic
pathway and is considered to be the most important of the
executioner caspases. Caspase-3 is expressed in almost all types of
cells as an inactive pro-enzyme and is activated by any of the
initiator caspases (caspase-8, -9, or -10) and subsequently cleaves
various specific substrates. Once activated, initiator caspases
cleave and activate effector caspases, which in turn cleave a
variety of cellular protein substrates, ultimately leading to cell
apoptosis (33). In an attempt to
identify the apoptotic signaling pathway induced by caudatin, we
evaluated the cleavage of caspase-3, -8, -9 and PARP in AGS cells
by western blot assay. Mechanistic studies showed that
caudatin-induced cell apoptosis was accompanied by significant
activation of caspase-3, -8 and -9, and subsequent upregulated
cleavage of PARP (Fig. 2).
The β-catenin pathway is known to be disrupted in a
variety of cancers, including gastric cancer. It has also been
shown that β-catenin activity also inhibits apoptosis in cancer
cells. Activation of the β-catenin signaling pathway leads to
nuclear localization of β-catenin which interacts with the TCF
transcription factor and modulates the expression of a wide range
of proto-oncogenes. Therefore, we hypothesized that caudatin exerts
its effects through the modulation of the expression of β-catenin.
In the present study, we found that caudatin downregulated the
Wnt/β-catenin signaling pathway through the inhibition of β-catenin
in gastric cancer cells. We also found that caudatin treatment led
to an ~50% reduction in β-catenin, cyclinD1 and c-MYC protein
levels (Fig. 3). These data suggest
that caudatin-mediated cell growth inhibition and induction of cell
apoptosis are partly mediated via inactivation of Wnt/β-catenin
activity, which is known to play a significant role in cancer cell
proliferation. To further elucidate the pathway by which caudatin
activates β-catenin degradation, we assessed the effects of the
proteosome inhibitor MG132 or the lysosome pathway inhibitor
NH4Cl in caudatin-treated AGS cells. We found that
caudatin induced a reduction in β-catenin only via the proteosome
pathway. As shown in Fig. 4,
β-catenin was much more rapidly degraded in cells treated with
caudatin than in cells treated with DMSO. However, treatment with
the proteasomal inhibitor MG132 efficiently rescued the protein
level of β-catenin, indicating that β-catenin was degraded in an
ubiquitin-proteasome-dependent degradation manner. These results
suggest that caudatin can increase the proteasome-dependent
degradation of β-catenin. However, Wnt/β-catenin is not the only
pathway active in gastric cancer. It is thus important to determine
whether caudatin is equally potent in inhibiting other signal
transduction pathways.
microRNAs regulate multiple coding genes which are
associated with tumor growth. Thus, assessment of specific miRNA
expression is useful for predicting disease outcome. We recently
demonstrated that miR-372 was abnormally upregulated in AGS cells
and may be a biomarker for gastric adenocarcinoma. miR-372 can also
act as an oncogene and promote the growth of testicular germ cells
or AGS cells by targeting the LATS2 or TNFAIP1 gene (34,35).
Zhou et al(24) demonstrated
that β-catenin transactivates the microRNA-371–373 cluster. In the
present study, this finding was further confirmed by detecting the
miR-372 expression in 15 pairs of gastric adenocarcinoma tissues.
The results showed that expression of miR-372 was upregulated in
both gastric cancer tissues and AGS cells when compared to normal
gastric tissues. Previous studies identified the altered expression
of miR-21 in gastric cancer cells. Interestingly, miR-21 was
previously shown to additionally target the tumor suppressor PTEN,
RECK and PDCD4 and induce tumorgenesis (36), suggesting a general role for miR-21
in tumor progression. miR-21 can also inhibit apoptosis in breast
cancer cells by regulating Bcl-2 expression (37). Let-7a is a tumor suppressor miRNA
and is downregulated in many cancers. Let-7a decreased cell
proliferation and migration of glioblastoma and reduced tumor size
in a xenograft model. A recent study demonstrated that the let-7
family inhibits the proliferation of human gastric cancer cells by
targetting HMGA2 (38). It would be
interesting to determine whether caudatin affects the expression of
these genes in gastric cancer cells. Caudatin inhibits oncomir
miRNA and increases tumor suppressor miRNA in gastric cancer cells.
Our result showed that caudatin treatment significantly reduced
miR-372 and miR-21 expression while inducing let-7a in AGS cells.
There is also an important link between caudatin, microRNAs and
β-catenin regulation.
Taken together, an in vitro model was
successfully established to evaluate the possible mechanism of
caudatin in the therapy of gastric cancer. Our preliminary
observations indicate that caudatin arrests proliferation of human
gastric carcinoma cell lines and induces cell apoptosis, at least
partially, via the caspase-dependent apoptotic pathway and the
Wnt/β-catenin signaling pathway. Thus, targeting β-catenin may be
an effective strategy to control tissue destruction in gastric
cancer patients. Caudatin seems to have multiple molecular targets
and shows enhanced potency in various cancer cell lines. Thus,
caudatin may be a strong candidate for therapeutic applications for
gastric cancer as well as other types of cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81071696, 31071150 and
81071656), the Project of Hunan Provincial Masters Innovation Fund
(CX2012B227), Project of Chang Sha Science and Technology Plan
(K1109006-31 and K1207014-31) and Hunan Normal University Student
Innovation (2012042).
References
|
1
|
Catalano V, Labianca R, Beretta GD, Gatta
G, de Braud F and Van Cutsem E: Gastric cancer. Crit Rev Oncol
Hematol. 71:127–164. 2009. View Article : Google Scholar
|
|
2
|
Nakajima T: Gastric cancer treatment
guidelines in Japan. Gastric Cancer. 5:1–5. 2002. View Article : Google Scholar
|
|
3
|
Han J, Jiao D, Cao J, Fing P, Liu X and
Zhuang Y: Effects of the extract from semen viticis negundo with
acetoactate on human gastric carcinoma SGC-7901 cells in vitro and
in vivo. Chinese Pharmacological Bulletin. 12:0292008.
|
|
4
|
Yang NI and Yi-ping YE: Distribution of
C_(21) steroidal glycosides in plants of Asclepiadaceae and their
pharmacological activities. Chinese Traditional and Herbal Drugs.
1:0472010.(In Chinese).
|
|
5
|
Yao N, Gu X and Li Y: Effects of three C21
steroidal saponins from Cynanchum auriculatum on cell growth
and cell cycle of human lung cancer A549 cells. Zhongguo Zhong Yao
Za Zhi. 34:1418–1421. 2009.(In Chinese).
|
|
6
|
Fei HR, Chen HL, Xiao T, Chen G and Wang
FZ: Caudatin induces cell cycle arrest and caspase-dependent
apoptosis in HepG2 cell. Mol Biol Rep. 39:131–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng YR, Li YB, Liu XD, Zhang JF and Duan
JA: Apoptosis induced by caudatin in human hepatoma cell line
SMMC7721. Chin J Nat Med. 6:210–213. 2008. View Article : Google Scholar
|
|
8
|
Wang LJ, Geng CA, Ma YB, et al: Design,
synthesis, and molecular hybrids of caudatin and cinnamic acids as
novel anti-hepatitis B virus agents. Eur J Med Chem. 54:352–365.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng YR, Ding YF, Wei YJ, Shu B, Li YB and
Liu XD: Caudatin-2,6-dideoxy-3-O-methy-β-D-cymaropyranoside 1
induced apoptosis through caspase 3-dependent pathway in human
hepatoma cell line SMMC7721. Phytother Res. 25:631–637. 2011.
|
|
10
|
MacDonald BT, Tamai K and He X:
Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev
Cell. 17:9–26. 2009.
|
|
11
|
Naito AT, Shiojima I and Komuro I: Wnt
signaling and aging-related heart disorders. Circ Res.
107:1295–1303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee E, Salic A, Krüger R, Heinrich R and
Kirschner MW: The roles of APC and Axin derived from experimental
and theoretical analysis of the Wnt pathway. PLos Biol. 1:E102003.
View Article : Google Scholar
|
|
13
|
Liu C, Li Y, Semenov M, et al: Control of
β-catenin phosphorylation/degradation by a dual-kinase mechanism.
Cell. 108:837–847. 2002.
|
|
14
|
Clements WM, Wang J, Sarnaik A, et al:
β-Catenin mutation is a frequent cause of Wnt pathway activation in
gastric cancer. Cancer Res. 62:3503–3506. 2002.
|
|
15
|
Huiping C, Kristjansdottir S, Jonasson JG,
Magnusson J, Egilsson V and Ingvarsson S: Alterations of E-cadherin
and β-catenin in gastric cancer. BMC Cancer. 1:162001.
|
|
16
|
Luu HH, Zhang R, Haydon RC, et al:
Wnt/β-catenin signaling pathway as novel cancer drug targets. Curr
Cancer Drug Targets. 4:653–671. 2004.
|
|
17
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carleton M, Cleary MA and Linsley PS:
MicroRNAs and cell cycle regulation. Cell Cycle. 6:2127–2132. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wienholds E and Plasterk RH: MicroRNA
function in animal development. FEBS Lett. 579:5911–5922. 2005.
View Article : Google Scholar
|
|
20
|
Xiao B, Guo J, Miao Y, et al: Detection of
miR-106a in gastric carcinoma and its clinical significance. Clin
Chim Acta. 400:97–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Li Z, Gao C, et al: miR-21 plays
a pivotal role in gastric cancer pathogenesis and progression. Lab
Invest. 88:1358–1366. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Belair C, Baud J, Chabas S, et al:
Helicobacter pylori interferes with an embryonic stem cell
microRNA cluster to block cell cycle progression. Silence. 2:72011.
View Article : Google Scholar
|
|
23
|
Zhou C, Li X, Zhang X, et al: microRNA-372
maintains oncogene characteristics by targeting TNFAIP1 and affects
NFκB signaling in human gastric carcinoma cells. Int J Oncol.
42:635–642. 2012.PubMed/NCBI
|
|
24
|
Zhou AD, Diao LT, Xu H, et al:
β-Catenin/LEF1 transactivates the microRNA-371–373 cluster that
modulates the Wnt/β-catenin-signaling pathway. Oncogene.
31:2968–2978. 2011.
|
|
25
|
Lee ST, Chu K, Oh HJ, et al: Let-7
microRNA inhibits the proliferation of human glioblastoma cells. J
Neurooncol. 102:19–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Motoyama K, Inoue H, Nakamura Y, Uetake H,
Sugihara K and Mori M: Clinical significance of high mobility group
A2 in human gastric cancer and its relationship to let-7 microRNA
family. Clin Cancer Res. 14:2334–2340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsai LH, Lees E, Faha B, Harlow E and
Riabowol K: The cdk2 kinase is required for the G1-to-S transition
in mammalian cells. Oncogene. 8:1593–1602. 1993.PubMed/NCBI
|
|
29
|
Tetsu O and McCormick F: β-Catenin
regulates expression of cyclinD1 in colon carcinoma cells. Nature.
398:422–426. 1999.
|
|
30
|
He TC, Sparks AB, Rago C, et al:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ciechanover A: Proteolysis: from the
lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol.
6:79–87. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harper JW, Elledge SJ, Keyomarsi K, et al:
Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell.
6:387–400. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cohen GM: Caspases: the executioners of
apoptosis. Biochem J. 326:1–16. 1997.
|
|
34
|
Cho WJ, Shin JM, Kim JS, et al: miR-372
regulates cell cycle and apoptosis of ags human gastric cancer cell
line through direct regulation of LATS2. Mol Cells. 28:521–527.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Voorhoeve PM, le Sage C, Schrier M, et al:
A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in
testicular germ cell tumors. Cell. 124:1169–1181. 2006. View Article : Google Scholar
|
|
36
|
Asangani IA, Rasheed SA, Nikolova DA, et
al: MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor
suppressor Pdcd4 and stimulates invasion, intravasation and
metastasis in colorectal cancer. Oncogene. 27:2128–2136. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Si M, Zhu S, Wu H, Lu Z, Wu F and Mo YY:
miR-21-mediated tumor growth. Oncogene. 26:2799–2803. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee YS and Dutta A: The tumor suppressor
microRNA let-7 represses the HMGA2 oncogene. Genes Dev.
21:1025–1030. 2007. View Article : Google Scholar : PubMed/NCBI
|