Introduction
The p21Waf1/Cip1 protein (p21) and the
c-Jun N-terminal kinase (JNK) are two well-characterized cell
modulators that play a crucial role in cell differentiation,
senescence and apoptosis. p21 is a cyclin kinase inhibitor (CKI)
that, among other things, directly inhibits the activity of cyclin
E/CDK2 and cyclin D/CDK4/6 complexes and functions as a regulator
of cell cycle progression at the S phase (1,2).
Results indicate that p21 expression is controlled by the
tumor-suppressor protein p53 and that the expression of p21 is
mainly dependent on two factors: i) the stimulus provided and ii)
the type of the cell.
The JNK pathway is one of three principal
mitogen-activated protein kinase (MAPK) pathways involved in signal
transduction of extracellular receptor-mediated stimuli to the
nucleus of the cell (3,4). The JNK pathway is rapidly and strongly
activated by numerous pro-inflammatory cytokines and by many
non-receptor-mediated events including DNA damaging agents such as
UV light and numerous genotoxic chemotherapeutic agents (5). Once activated, JNK-1 can upregulate
gene expression by phosphorylating the activating motif of
transcription factors such as ATF-2 (6,7), c-Jun
and Jun D (8) and the Ets domain of
transcription factor Elk-1 and Sap-1 (9). JNK kinases are believed to participate
in most aspects of cellular function, including replication,
growth, metabolism, differentiation and apoptosis (10,11).
Moreover, the role of JNK in apoptosis is unclear as these proteins
have been assigned both pro- and anti-apoptotic properties
(10–14).
In contrast, the early growth response protein 1
(Egr-1) is a C2H2-zinc finger-containing transcriptional regulator
involved in the control of cell proliferation and apoptosis
(15,16). Egr-1 is rapidly induced by growth
factors to transduce the proliferative signal. The induction of
Egr-1 by external stimuli is generally transient but appears to be
sustained in some prostate tumor cell lines and tumors, suggesting
that Egr-1 stimulates tumor cell growth and could have an important
function as its expression level increases with the degree of
malignancy as measured by the Gleason grade of the tumor (17). In addition, overexpression of Egr-1
is correlated with the loss of its co-repressor NAB2 in primary
prostate carcinoma (18,19). This disruption in the balance
between Egr-1 and NAB2 expression results in a high Egr-1
transcriptional activity in prostate carcinoma cells (20). In contradiction, in breast, lung and
brain tumors, Egr-1 expression is often absent or reduced and its
re-expression results in growth suppression (18,21).
Egr-1 also plays a role in tumor progression, through the hypoxic
signal generated in growing tumors. Egr-1 is highly induced under
these conditions and its activities stimulate angiogenesis
(22,23). However, one of the major concerns
about Egr-1 overexpression in carcinoma tumors is that it is
associated with a loss in functionality of the prostate cells
and/or the development of autonomous growth.
In this study, we showed that blocking of Egr-1
expression by a small interfering RNA (siRNA-Egr-1) increased the
activity of p21Waf1/Cip1 and decreased the expression of
JNK protein. In contrast, siRNA silencing of p21 or JNK-1 with
siRNAs was unable to decrease the expression of Egr-1.
Materials and methods
The protease inhibitors, phenylmethylsulfonyl
fluoride (PMSF), leupeptin, pepstatin, aprotinin and bestatin, were
purchased from Roche (USA); T4 polynucleotide kinase and
poly(dI-dC)2 were obtained from Amersham Pharmacia Biotech
(Piscataway, NJ, USA). Tris-borate-EDTA buffer and
acrylamide-bisacrylamide (29:1) were obtained from Bio-Rad
(Richmond, CA, USA). Antibodies against JNK, p21, Egr-1 and β-actin
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). MBP was purchased from Stratagene (La Jolla, CA, USA).
Phorbol 12-myristate 13-acetate (TPA), tumor necrosis factor-α
(TNF-α), arsenite and fetal bovine serum (FBS) were purchase from
Sigma-Aldrich, Inc. (USA).
Cell lines and culture
Human prostate carcinoma cell lines LNCaP and PC-3
were a gift from Dr Dan Mercola (SKCC, La Jolla, CA, USA). The
cells were cultured in RPMI-1640 medium supplemented with 100 ml/l
FBS, 8×105 U/l penicillin and 0.1 g/l streptomycin in a
humidified incubator containing 50 ml/l CO2 at 37°C
(24). The antibodies used for
western blotting included those against protein kinase JNK-1 and
JNK-2, Egr-1 and p21. Western blotting was performed as previously
described (12).
siRNA preparation and transfection of
short interfering RNAs
The siRNAs for Egr-1 and JNK-1 were obtained as
ready-annealed, purified duplex probes, and the scrambled control
siRNAs were purchased from Shanghai Genechem Co. siRNAs for Egr-1
were: sense, 5′-CAGCAGCAGCAGCAG CAGCTT-3′ and antisense,
5′-AAGCTGCTGCTGCTGCT GCTG-3′. The siRNA oligonucleotides for JNK-1
were: sense, 5′-AAGCCCAGTAATATAGTAGTA-3′ and antisense, 5′-TAC
TACTATATTACTGGGCTT-3′; JNK-2 sense, 5′-CATGAT GTTATCATATCTTAT-3′
and antisense 5′-ATAAGATAT GATAACATCATG-3′. The
p21WAF1/CIP1-targeted siRNAs were obtained from Santa
Cruz Biotechnology, Inc. Cells were treated in parallel with
scrambled siRNA sense, 5′-AATTC TCCGAACGTGTCACGT-3′ and antisense,
5′-ACGTGACA CGTTCGGAGAATT-3′. The cells were cultured in medium
without antibiotics, and 24 h before transfection resulting in a
confluence of the cell monolayer by 50–70%. Specific Egr-1, p21,
JNK-1/2 siRNAs or non-silencing siRNA (70 nmol) were mixed with
Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s
recommendation and added to the cells. After 6 h at 37°C, the
medium was replaced, and the cells were cultivated in RPMI-1640
supplemented with 10% heat-inactivated FBS.
Apoptotic cell death assay
The cells were treated with different siRNAs. At
indicated time points, cells were collected and washed with
phosphate-buffered saline (PBS). After fixation with 70% ethanol,
cells were washed twice with PBS and stained with a solution
containing 20 mg/ml propidium iodide (PI) and 50 mg/ml RNase A.
Cells were incubated for 30 min at room temperature and the cell
cycle profiles were determined by flow cytometry using a FACScan
(Becton-Dickinson, San Jose, CA, USA).
Western blot analysis of protein
expression
Cells were chilled on ice and washed twice with
ice-cold PBS (43 mM K2HPO4, 9 mM
Na2HPO4, 120 mM NaCl; pH 7.4). They were
solubilized on ice in lysis buffer containing 50 mM HEPES pH 7.5,
150 mM NaCl, 100 mM NaF, 10 mM EDTA, 10 mM
Na4P2O7, 1% (v/v) Triton X-100,
0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS) and a
protease inhibitor cocktail (Sigma-Aldrich, Inc., St. Louis, MO,
USA). Lysates were then clarified by centrifugation at 13,000 × g
for 10 min at 4°C. The protein concentration was determined using
the BCA™ protein assay reagent (Pierce, Rockford, IL, USA). Cleared
lysates were resuspended in sample buffer containing 70 mM
Tris-HCl, 10% (v/v) glycerol, 2% (w/v) SDS, 0.01% (w/v) bromophenol
blue and 1.5% (v/v) 2-mercaptoethanol. Samples were subjected to
electrophoresis on a 12% acrylamide gel and transferred to
Immobilon-P membranes (Millipore, Bedford, MA, USA) using standard
procedures. Membranes were blocked in saline buffer [25 mM
Tris-HCl, pH 7.4, 140 mM NaCl, 0.1% (v/v) Tween-20] containing 5%
(w/v) non-fat milk for 2 h at 22°C before the addition of the
antibodies for an overnight incubation at 41°C. Several washes were
performed in saline buffer, and peroxidase-conjugated antibodies
against mouse or rabbit immunoglobulins (Amersham Biosciences,
Piscataway, NJ, USA) were added at a dilution of 1/6,000 for 45 min
at 22°C. After washing, the membranes were soaked in western
blotting luminol reagent (Santa Cruz Biotechnology, Inc.) followed
by autoradiography. When appropriate, membranes were stripped using
Restore™ stripping buffer (Pierce) for 15 min at 22°C and reprobed
with the indicated antibodies.
Antibodies
Antibodies to Egr-1 (sc-189),
p21Waf1/Cip1 (sc-6264), JNK (FL) (sc-571), JNK-1 (C-17)
(sc-474), and β-actin (sc-1616) were used at a concentration of
0.07 and 0.1 mg/ml, respectively (in a total volume of 12 ml).
Antibodies to β-actin (clone AC-15) were from Sigma-Aldrich, Inc.
and were used at a concentration of 0.22 mg/ml in a total volume of
12 ml.
Results
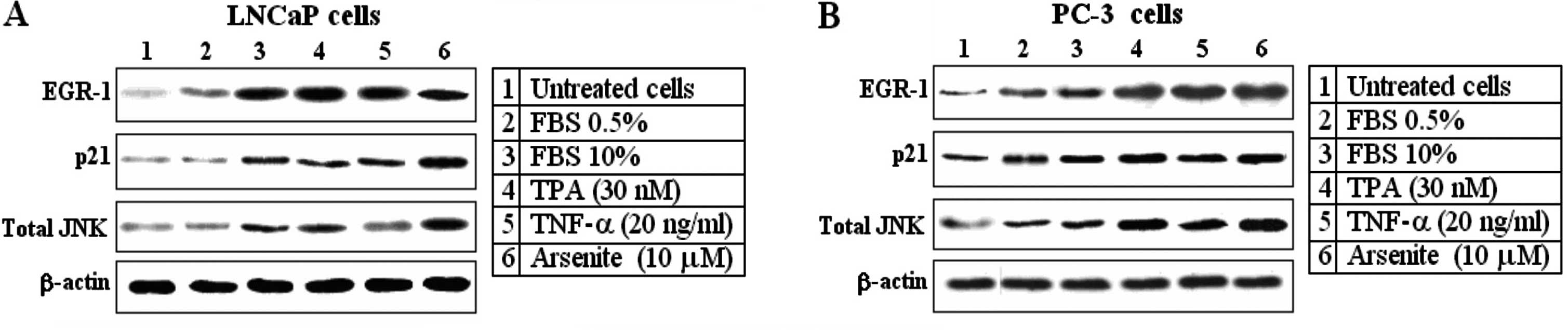
Egr-1, p21 and JNK are strongly induced
by several different stimuli in LNCaP prostate cancer cells
In order to test the transforming role of Egr-1, JNK
and p21Waf1/Cip1 in prostate carcinoma cell lines, we
collected and examined two prostate cell lines. Both, LNCaP and
PC-3 cell lines exhibited serum inducible growth (FBS 10%). Egr-1,
p21 and total JNK activities were assessed under a similar
condition, i.e. 48 h following plating in low serum followed by 3 h
in complete serum-containing growth medium. Both cell lines
exhibited readily detectable EGR-1, p21 and JNK activity, even in
low serum (FBS 0.5%). However, all three proteins were strongly
induced by FBS 10%, TPA (30 nM), TNF-α (20 ng/ml) and arsenite (10
μM) (Fig. 1). β-actin was used as a
loading control. The response of Egr-1 to all the stimuli was
highly efficient and remained elevated when compared with the
response of the other proteins.
Knockdown of Egr-1 expression by siRNA
strongly decreases the activity of p21 and JNK and reverses the
increasing effect of TPA (30 nM)
LNCaP cells were transfected with a siRNA against
Egr-1 or with a nonspecific siRNA (control). At 48 h after
transfection, the cells were treated with TPA (30 mM) and cultured
for an additional 12 h. At the indicated time point, the cells were
harvested and analyzed for expression of EGR-1, p21 and JNK-1
proteins by western blot analysis (Fig.
2A). As shown in Fig. 2A,
siRNA-Egr-1 strongly decreased p21 protein expression and reversed
the effect of TPA. At the same time a high specificity to
completely block the expression of EGR-1 was noted (Fig. 2A). To determine whether blocking the
expression of p21 by an siRNA decreases the expression of EGR-1 and
JNK-1, LNCaP cells were transfected with an siRNA against p21 or
with a nonspecific siRNA (control). At 48 h after transfection,
cell were treated with TPA (30 mM) and cultured for an additional
12 h. At the indicated time point, the cells were harvested and
analyzed for expression of EGR-1, p21 and JNK-1 proteins by western
blotting. As shown in Fig. 2B,
p21-siRNA strongly decreased p21 protein expression but was unable
to reverse the effect of TPA. Similar to the above experiments, we
investigated the effect of the blockage of JNK-1 by an siRNA on the
expression of EGR-1 and p21 proteins. As shown in Fig. 2C, siRNA-JNK-1 moderately decreased
p21 protein expression but was unable to substantially decrease the
expression of EGR-1.
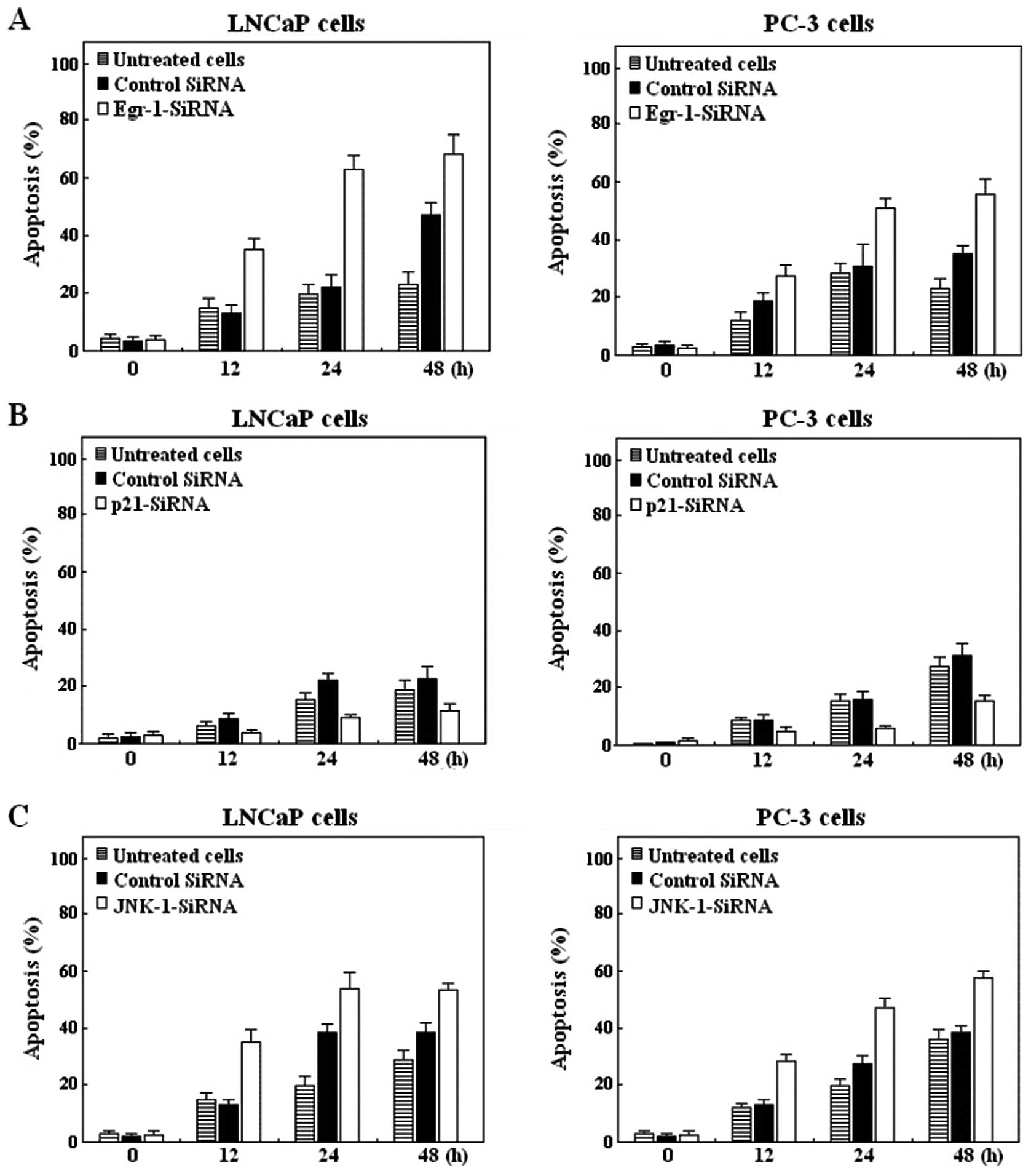
Knockdown of Egr-1 or JNK-1 but not p21
increased apoptosis in LNCaP and PC-3 cells treated with siRNAs
against these proteins
Next, we sought to determine whether blocking EGR-1,
p21 or JNK-1 has an apoptotic effect in LNCaP or PC-3 cells treated
with siRNAs against these proteins. To this effect, LNCaP and PC-3
cells were transfected with an siRNA against Egr-1 (Fig. 3A), against p21 (Fig. 3B) or against JNK-1 (Fig. 3C) and with a nonspecific siRNA as
control. After the indicated times of the culture, LNCaP cells were
harvested and analyzed for induction of apoptosis. As shown by flow
cytometric analysis, knockdown of Egr-1 using siRNA significantly
induced apoptosis in LNCaP cells (Fig.
3A). However, when LNCaP cells were transfected with p21-siRNA
and analyzed for induction of apoptosis, the effect was only
marginal (Fig. 3B) compared with
the effect induced by siRNA-Egr-1 (Fig.
3A), and was decreased after 72 h transfection (data not
shown). We then proceeded to analyze the effects of blocking JNK
expression on the apoptosis process. As shown in Fig. 3C, blocking JNK-1, moderately induced
apoptosis after 48 h transfection, compared with untreated and
control cells’. The results suggest that the differential
expression of Egr-1 was responsible for the differences in the
apoptotic response demonstrated in the LNCaP and PC-3 cell
lines.
Discussion
Egr-1 is a member of the immediate early gene
family and encodes a nuclear phosphoprotein involved in the
regulation of cell growth and differentiation in response to
signals such as mitogens, growth factors and stress stimuli
(15,16,18,25).
However, in other circumstances, Egr-1 is induced very early
in the apoptotic process (26),
where it mediates the activation of downstream regulatory genes
(27,28). It has been previously demonstrated
that Egr-1 is required for tumor formation by a variety of human
cancers (15,16,21,25).
As we know, degradation of mRNA mediated by siRNA is a powerful
means of specifically knocking down the expression of a target gene
(29,30). We previously used small interference
RNAs to treat cells expressing the nuclear factor Egr-1 (12,14,20,24,31).
In this study, we showed that blocking Egr-1 by specific siRNA,
strongly decreased the activity and expression of p21 and JNK
proteins. Similar to Egr-1, both LNCaP (wild-type p53) and PC-3
(p53-deficient) were able to strongly express JNK and
p21WAF-1/CIP1 upon exposure to several stimuli,
suggesting that the expression of these protein may be initiated
through intracellular signaling through the stress-activated
kinases or other pathways (32,33).
As expected, the response of PC-3 and LNCaP cells to the different
stimuli was followed by high expression of EGR-1 and JNK, while the
expression of p21 was only moderate, suggesting that the activation
of p21 requires the participation of several factors (32,33).
We simultaneously examined the effect of siRNAs on the protein
expression of Egr-1, p21 and JNK. We found that the expression of
all three proteins was decreased when cells were treated with
specific Egr-1-siRNA or p21-siRNA or with JNK-1-siRNA. Both
p21-siRNA and JNK-1-siRNA were unable to decrease the expression of
EGR-1 in the PC-3 and LNCaP cells, suggesting a regulatory role of
Egr-1 in controlling the expression of these proteins (34). As we know, JNK activation
participates in DNA repair pathways that induce cell cycle arrest
and has been implicated in the regulation of the cell cycle
regulators p21waf-1 and cyclin D1. Studies have shown
that other MAPK pathways participate in cell cycle control
(32,33,35).
ERK induces G1/S transition while p38 inhibits G2/M transition
(32,33). Other studies have shown that JNK is
required for efficient induction of apoptosis in response to
ionizing radiation (36). Based on
these findings, it is reasonable to hypothesize that activation of
JNK-1 induces apoptosis and cell cycle arrest (37). Although Egr-1, but not JNK-1
(38) activation, is associated
with the induction of both cyclin D1 and p21, several studies have
shown that the cellular decision to induce p21 in the G1 phase of
the cell cycle could be dictated by the magnitude of the ERK1/2
signal (39). In this respect it is
interesting to note that in both cell lines we found a moderate
expression of p21 expression associated with reduced cell apoptosis
when compared with the scramble-transfected cells. Because we were
able to show that Egr-1 inhibition strongly blocks JNK-1 and
moderately blocks p21, it is tempting to speculate that the strong
inactivation of Egr-1 by an siRNA in these cells may lead to a
strong and persistent p21WAF1/Cip1 expression favoring
apoptosis.
In conclusion, we showed that inhibition of Egr-1
activity by Egr-1-siRNA was associated with a marked reduction in
p21 and JNK activities. However, siRNAs against p21 and JNK-1 were
unable to decrease the activity of Egr-1. We found that
siRNA-mediated Egr-1 inhibition also resulted in the cell death of
>60% cancer cells at 48 h, when compared with this percentage in
the cells treated with either p21-siRNA or JNK-1-siRNA.
Acknowledgements
We would like to thank Dr Dan Mercola (Laboratory of
Gene Therapy, Sidney Kimmel Cancer Center, La Jolla, CA, USA) for
providing the LNCaP and PC-3 prostate cancer cell lines. This study
was supported by the Intramural Regular Research Grant from the
University of Tarapaca-Chile, UTA-4710-12.
References
|
1
|
Stewart AZ, Leach DS and Pietenpol AJ:
p21(Waf1/Cip1) inhibition of cyclin E/Cdk2 activity prevents
endoreduplication after mitotic spindle disruption. Mol Cell Biol.
19:205–215. 1999.PubMed/NCBI
|
|
2
|
Satyanarayana A, Hilton MB and Kaldis P:
p21 inhibits Cdk1 in the absence of Cdk2 to maintain the G1/S phase
DNA damage checkpoint. Mol Biol Cell. 19:65–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heasley LE and Han SY: JNK regulation of
oncogenesis. Mol Cells. 21:167–173. 2006.
|
|
4
|
Matsukawa J, Matsuzawa A, Takeda K and
Ichijo H: The ASK1-MAP kinase cascades in mammalian stress
response. J Biochem. 136:261–265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Potapova O, Haghighi A, Bost F, Liu C,
Birrer M, Gjerset R and Mercola D: The Jun kinase/stress-activated
protein kinase pathway functions to regulate DNA repair and
inhibition of the pathway sensitizes tumor cells to cisplatin. J
Biol Chem. 272:14041–14044. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta S, Campbell D, Dérijard B and Davis
RJ: Transcription factor ATF2 regulation by the JNK signal
transduction pathway. Science. 267:389–393. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bocco JL, Bahr A, Goetz J, Hauss C,
Kallunki T, Kedinger C and Chatton B: In vivo association of ATFa
with JNK/SAP kinase activities. Oncogene. 12:1971–1980.
1996.PubMed/NCBI
|
|
8
|
Hibi M, Lin A, Smeal T, Minden A and Karin
M: Identification of an oncoprotein- and UV-responsive protein
kinase that binds and potentiates the c-Jun activation domain.
Genes Dev. 7:2135–2148. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Janknecht R and Hunter T: Convergence of
MAP kinase pathways on the ternary complex factor Sap-1a. EMBO J.
16:1620–1627. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J and Lin A: Role of JNK activation in
apoptosis: a double-edged sword. Cell Res. 15:36–42. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parra E and Ferreira J: Knockdown of the
c-Jun-N-terminal kinase expression by siRNA inhibits MCF-7 breast
carcinoma cell lines growth. Oncol Rep. 24:1339–1345. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parra E: Activation of MAP kinase family
members triggered by TPA or ionomycin occurs via the protein
phosphatase 4 pathway in Jurkat leukemia T cells. Mol Med Rep.
5:773–778. 2012.PubMed/NCBI
|
|
14
|
Parra E: Inhibition of JNK-1 by small
interference RNA induces apoptotic signalling in PC-3 prostate
cancer cells. Int J Mol Med. 30:923–930. 2012.PubMed/NCBI
|
|
15
|
Beckmann AM and Wilce PA: EGR
transcription factors in the nervous system. Neurochem Int.
31:477–510. 1997. View Article : Google Scholar
|
|
16
|
Whitlock NC, Bahn JH, Lee SH, Eling TE and
Baek SJ: Resveratrol-induced apoptosis is mediated by early growth
response-1, Krüppel-like factor 4, and activating transcription
factor 3. Cancer Prev Res. 4:116–127. 2011.PubMed/NCBI
|
|
17
|
Eichelberger L, Koch MO, Eble JN, Ulbright
TM, Juliar BE and Cheng L: Maximum tumor diameter is an independent
predictor of prostate-specific antigen recurrence in prostate
cancer. Mod Pathol. 18:886–890. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Houston P, Campbell CJ, Svaren J,
Milbrandt J and Braddock M: The transcriptional corepressor NAB2
blocks EGR-1-mediated growth factor activation and agiogenesis.
Biochem Biophys Res Commun. 283:480–486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Collins S, Lutz MA, Zank PE, Anders RA,
Kersh GJ and Powell JD: Opposing regulation of T cell function by
EGR-1/NAB2 and EGR-2/EGR-3. Eur J Immunol. 38:528–536. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parra E, Ortega A and Saenz L:
Down-regulation of EGR-1 by siRNA inhibits growth of human prostate
carcinoma cell line PC-3. Oncol Rep. 22:1513–1518. 2009.PubMed/NCBI
|
|
21
|
Pignatelli M, Luna-Medina R, Pérez-Rendón
A, Santos A and Perez-Castillo A: The transcription factor early
growth response factor-1 (EGR-1) promotes apoptosis of
neuroblastoma cells. Biochem J. 373:739–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Banks MF, Gerasimovskaya EV, Tucker DA,
Frid MG, Carpenter TC and Stenmark KR: EGR-1 antisense
oligonucleotides inhibit hypoxia-induced proliferation of pulmonary
artery adventitial fibroblasts. J Appl Physiol. 98:732–738. 2005.
View Article : Google Scholar
|
|
23
|
Sperandio S, Fortin J, Sasik R, Robitaille
L, Corbeil J and de Belle I: The transcription factor EGR1
regulates the HIF-1alpha gene during hypoxia. Mol Carcinog.
48:38–44. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parra E and Ferreira J: The Effect of
siRNA-EGR-1 and camptothecin on growth and chemosensitivity of
breast cancer cell lines. Oncol Rep. 22:1159–1165. 2010.PubMed/NCBI
|
|
25
|
Adamson ED and Mercola D: EGR1
transcription factor: multiple roles in prostate tumor cell growth
and survival. Tumour Biol. 23:93–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Catania MV, Copani A, Calogero A, Ragonese
GI, Condorelli DF and Nicoletti F: An enhanced expression of the
immediate early gene, EGR-1, is associated with neuronal apoptosis
in culture. Neuroscience. 91:1529–1538. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nair P, Muthukkumar S, Sells SF, Han S,
Sukhatme VP and Rangnekar VM: Early growth response-1-dependent
apoptosis is mediated by p53. J Biol Chem. 272:20131–20138. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calogero A, Arcella A, De Gregorio G,
Porcellini A, Mercola D, Liu C, Lombari V, Zani M, Giannini G, et
al: The early growth response gene EGR-1 behaves as a suppressor
gene that is down-regulated independent of ARF/Mdm2 but not p53
alterations in fresh human gliomas. Clin Cancer Res. 7:2788–2796.
2001.PubMed/NCBI
|
|
29
|
Stevenson M: Therapeutic potential of RNA
interference. N Engl J Med. 351:1772–1777. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sui G, Soohoo C, Affar el B, Gay F and Shi
Y, Forrester WC and Shi Y: A DNA vector-based RNAi technology to
suppress gene expression in mammalian cells. Proc Natl Acad Sci
USA. 99:5515–5520. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parra E, Ferreira J and Saenz L:
Inhibition of Egr-1 by siRNA in prostate carcinoma cell lines is
associated with decreased expression of AP-1 and NF-κB. Int J Mol
Med. 28:847–853. 2011.PubMed/NCBI
|
|
32
|
Ciccarelli C, Marampon F, Scoglio A, Mauro
A, Giacinti C, De Cesaris P and Zani BM: p21WAF1
expression induced by MEK/ERK pathway activation or inhibition
correlates with growth arrest, myogenic differentiation and
onco-phenotype reversal in rhabdomyosarcoma cells. Mol Cancer.
4:412005. View Article : Google Scholar
|
|
33
|
Todd DE, Densham RM, Molton SA, Balmanno
K, Newson C, Weston CR, Garner AP, Scott L and Cook SJ: ERK1/2 and
p38 cooperate to induce a p21CIP1-dependent G1 cell
cycle arrest. Oncogene. 23:3284–3295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ragione FD, Cucciolla V, Criniti V, Indaco
S, Borriello A and Zappia V: p21Cip1 gene expression is
modulated by EGR1: a novel regulatory mechanism involved in the
resveratrol antiproliferative effect. J Bio Chem. 278:23360–23368.
2003.
|
|
35
|
Boutros T, Chevet E and Metrakos P:
Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase
regulation: roles in cell growth, death, and cancer. Pharmacol Rev.
60:261–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim MJ, Choi SY, Park IC, Hwang SG, Kim C,
Choi YH, Kim H, Lee KH and Lee SJ: Opposing roles of c-Jun
NH2-terminal kinase and p38 mitogen-activated protein kinase in the
cellular response to ionizing radiation in human cervical cancer
cells. Mol Cancer Res. 6:1718–1731. 2008. View Article : Google Scholar
|
|
37
|
Wu J, Sun J and Xue Y: Involvement of JNK
and P53 activation in G2/M cell cycle arrest and apoptosis induced
by titanium dioxide nanoparticles in neuron cells. Toxicol Lett.
199:269–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi BH, Kim CG, Bae YS, Lim Y, Lee YH and
Shin SY: p21Waf1/Cip1 expression by curcumin in U-87MG
human glioma cells: role of early growth response-1 expression.
Cancer Res. 68:1369–1377. 2008.
|
|
39
|
Clark JA, Black AR, Leontieva OV, Frey MR,
Pysz MA, Kunneva L, Woloszynska-Read A, Roy D and Black JD:
Involvement of the ERK signaling cascade in protein kinase
C-mediated cell cycle arrest in intestinal epithelial cells. J Biol
Chem. 279:9233–9247. 2004. View Article : Google Scholar : PubMed/NCBI
|