Introduction
Matrix metalloproteinases (MMPs) are a family of
zinc-dependent endopeptidases that degrade the extracellular
matrix. These enzymes are produced by many cell types in response
to inflammation or tumor invasion. Among the variety of proteinases
potentially implicated in tumor invasion, the MMPs have attracted
considerable interest because of their ability to collectively
degrade essentially all protein constituents of connective tissues
(1). Substantial evidence indicates
that the process of the degradation of extracellular matrix and
basement membranes is largely mediated by the combined action of
proteolytic enzymes, thus, facilitating tumor invasion and
metastasis (2). To date, ~30
different human MMPs have been cloned and characterized at the
amino acid sequence level (3). The
correlation between MMPs and malignant tumors is well documented
both in vitro and in vivo(4). In general, MMPs levels are abnormally
induced in metastatic tumor cells and are associated with invasive
lesions and poor clinical prognosis in cancer patients (5,6).
However, new findings of the MMP family and their subsequent
functional characterization at both genetic and biochemical levels
indicate new views on the role of these enzymes in tumor
progression (7). Evidence is
accumulating that MMPs are not exclusively involved in the
proteolysis of the tissue barriers for metastatic spread. Actually,
MMPs have been reported to play direct roles in other critical
events for tumor evolution that occur at earlier stages in
carcinogenesis, events such as tumor promotion, modulation of the
growth of the primary tumor and angiogenesis (8).
Recently, the human matrix metalloproteinase
(MMP)-26, also called matrilysin-2 or endometase, has been isolated
as a matrilysin (MMP-7) homolog (3,9–13).
Analysis of the proteolytic specificity showed that MMP-26 is able
to degrade a wide range of proteins present in the extracellular
matrix and basement membrane such as fibronectin, fibrinogen,
vitronectin, α1-antitrypsin, α2-macroglobulin and denatured
collagen (types I-IV). While the biological function of MMP-26 is
not yet fully understood, several reports describe that MMP-26 may
be related to the development of endometrial carcinoma (13,14).
In the present study, we examine the MMP-26 expression level in
normal and malignant endometrial tissue samples.
As estrogen is a potent inducer of endometrial
proliferation in vivo, it may play a role in the regulation
of MMP-26. It is believed that estrogen is related to the
development of endometrial hyperplasia which is the precursor of
endometrial carcinoma (15,16). Li et al(17) described that estrogen-estrogen
receptor (ER) complex activated the MMP-26 expression. However, the
molecular mechanisms by which ER activates MMP-26 have not been
examined in any detail. Interestingly, computer-assisted homology
searches have revealed potential binding sites for ER in the MMP-26
proximal promoter. Therefore, we also examine the correlation
between estrogen-ER complex and MMP-26 expression.
Materials and methods
Samples
The samples were obtained with informed consent from
patients undergoing surgery or biopsy at Tokyo Medical University
Hospital. We used 51 normal endometria, 6 endometrial hyperplasias
and 30 endometrial carcinomas. The tissues were finely minced into
small pieces with scissors, washed in phosphate-buffered saline
(PBS), snap-frozen and stored at −80°C until use for RNA
extraction.
Cell cultures and estrogen treatment
The examined cell lines. EN and Ishikawa, were
derived from endometrial carcinoma. EN does not express ERα
(18). Ishikawa which expressed ERα
was kindly provided by Dr Masato Nishida (Department of Obstetrics
and Gynecology, National Kasumigaura Hospital, Tsuchiura, Japan)
(19). These cells were grown in
DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin
G sodium and 100 μg/ml streptomycin sulfate in the presence of 5%
CO2 at 37°C.
The cells were plated at a density of
2.5×104 cells/well in 24-well plates and allowed to
attach overnight. After serum starvation, these cells were treated
with vehicle, 17β-estradiol (E2; 1–10 nM) or
E2 (1–10 nM) + ICI 182780 (1–10 mM) with 2%
charcoal-stripped serum. E2 and ICI 182780 were obtained
from Sigma Chemical Co. (St. Louis, MO, USA).
Real-time RT-PCR analysis
Total RNA was isolated using Isogen reagent (Nippon
Gene, Tokyo, Japan) and quantified by A260/A280 measurement using
an Ultrospec 3000 (Amersham Pharmacia Biotech). Total RNA (5 μg)
was reverse transcribed into cDNAs. Real-time PCR was performed for
the quantitative estimation. PCR reactions (20 μl )were set up with
final concentrations of 5 mm MgCl2, 2 μl SYBR-Green
Master Mix (Roche Molecular Biochemicals), 5 μl 1:10 diluted cDNA,
and 0.3 μM of both forward and reverse primers which are as
follows: 5′-CATCCGCAGTGAAAGACAGTA-3′ (MMP-26-F)
5′-TTGCAACCAGGAACAGATTAT-3′ (MMP-26-R).
The reactions were then cycled in the LightCycler
(Roche Molecular Biochemicals) with the following parameters:
denaturation for 1 cycle at 95°C for 10 sec, 45 cycles (temperature
transition of 20°C/sec) of 95°C for 0 sec, 50°C for 10 sec, and
72°C for 15 sec and fluorescence reading taken at 72°C, melting
curve analysis with continuous fluorescence reading. If necessary,
optimization of the protocol was achieved by changing
MgCl2 concentrations and/or reading fluorescence at a
higher temperature and/or using LightCycler FastStart DNA Master
SYBR-Green I (Roche Molecular Biochemicals). The LightCycler
software generated a standard curve (measurements taken during the
exponential phase of the amplification) that enabled the amount of
each gene in each test sample to be determined. The amount of each
gene was normalized by that of the GAPDH.
Immunohistological detection
Anti-human MMP-26 peptide polyclonal antibodies were
developed in rabbits as described (14). Immunochistochemical saining was
carried out using Histofine SAB-PO (R) kit (Nichirei Corp., Tokyo,
Japan). Paraffin sections were de-paraffinized and blocked with
methanol containing 3% H2O2, sequentially 10%
goat normal serum. Sections were incubated with a rabbit
anti-MMP-26 (1:1,000 dilution) for 1 h at room temperature.
Sections were then sequentially treated with a biotinylated goat
anti-rabbit antibody and a horseradish peroxidase (HRP) labeled
streptoavidin. Development was done by treating the sections with a
Liquid DAB-Plus Substrate kit (Zymed Laboratories Inc., San
Francisco, CA, USA). After counterstaining with hematoxylin,
immunostaining of MMP-26 on the tissue sections was detected by
light microscope.
Western blot analysis
Cells and tissues were lysed on ice for 30 min in
lysis buffer [10 mM Tris at pH 8.0, 1 mM EDTA, 400 mM NaCl, 10%
glycerol, 0.5% NP-40, 5 mM sodium fluoride, 0.1 mM
phenylmethylsulfonyl fluoride, 1 mM DTT], containing complete
protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN,
USA). The lysate was subjected to centrifugation at 14,000 rpm for
15 min and the soluble fraction collected. Protein concentrations
were measured using a Bio-Rad protein assay kit (Bio-Rad
Laboratories, Hercules, CA, USA). Equal amounts of protein (40 μg)
were loaded onto a 4–12% SDS-polyacrylamide gel and subjected to
electrophoresis at 200 V for 50 min. The protein was transferred
onto a PVDF membrane and probed with anti-MMP-26 antibody (14) and anti-actin antibody (C4)
(Boehringer Mannheim). The same blot was probed after stripping the
membrane with the different antibodies. Each protein was detected
by horseradish peroxidase-conjugated secondary antibody coupled
with enhanced chemiluminescence (ECL) western blotting detection
reagents (Amersham Pharmacia Biotech, Sweden Uppsala). Each western
blot analysis was performed at least twice. Each band intensity was
normalized by the intensity of the actin band.
DNA plasmids
The ER expression vectors were previously described
(20). pMMP-26-luc is an MMP-26
promoter-luciferase reporter plasmid. A 1000-bp sequence upstream
of the translation start site of MMP-26 is cloned into pGL3-Basic
(Promega) at HindIII sites. PCR was performed to amplify
this MMP-26 promoter region using both forward and reverse primers
which are as follows: MMP-26/clon1-U5′-CCCAAG
CTTGACAAATGAGGGTTTGGCAT-3′
MMP-26/clon804-L5′-CCGCTCGAGTTAAGGTATGTCAGATGAAC-3′.
Transfections and luciferase assays
Cells were seeded at 2×104 cells/well in
96-well plate and incubated overnight at 37°C in a 5%
CO2 incubator. For each transfection, 10 ng of empty
vector and/or expression vectors along with 1–100 ng of
promoter-luciferase DNA were mixed in 25 μl of Opti-MEM (Life
Technologies) and a precipitate formed using Lipofectamine 2000
(Life Technologies) according to the manufacturer’s
recommendations. Cells were washed with Opti-MEM and complexes were
applied to the cells with or without 1–100 nM E2.
Twenty-four hours after transfection, cells were harvested and
extracts were prepared with the Glo Lysis Buffer (Promega, Madison,
WI, USA). Luciferase activity was measured in extracts from
triplicate samples using the Bright-Glo Luciferase Assay system
(Promega).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation assays were performed
using the ChIP Assay kit (Upstate Biotechnology Inc., Lake Placid,
NY, USA) according to the manufacturer’s protocol. EN cells
incubated with/without overexpression of ER in 10-cm dishes for 24
h were cross-linked by treatment with formaldehyde (final
concentration, 1%) for 10 min at room temperature. After washing
with PBS, cells were pelleted down and resuspended in SDS lysis
buffer [1% SDS, 10 mM EDTA, 50 mM Tris-HCI (pH 8.1), 1 mM DTT, 1 mM
PMSF). The lysates were then subjected to sonication to break DNA
to the length of 500–1,000 bp, diluted with dilution buffer [0.01%
SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCI (pH 8.1), 167
mM NaCl] and pre-cleared by incubating with Salmon Sperm
DNA/protein A agarose 50% slurry for 60 min at 4°C. The supernatant
was incubated with anti-ERα (sc-786X, Santa Cruz Biotechnology)
antibody at 4°C overnight. Immunocomplex was collected with Salmon
Sperm DNA/protein A Agarose 50% slurry and eluted after extensive
washings, and cross-linkage was reversed by heating at 65°C,
followed by treatment with 40 mg/ml proteinase K at 45°C for 60
min. DNA was recovered by phenol-chloroform/ethanol precipitation,
and was used as a template for nested PCR to amplify the target
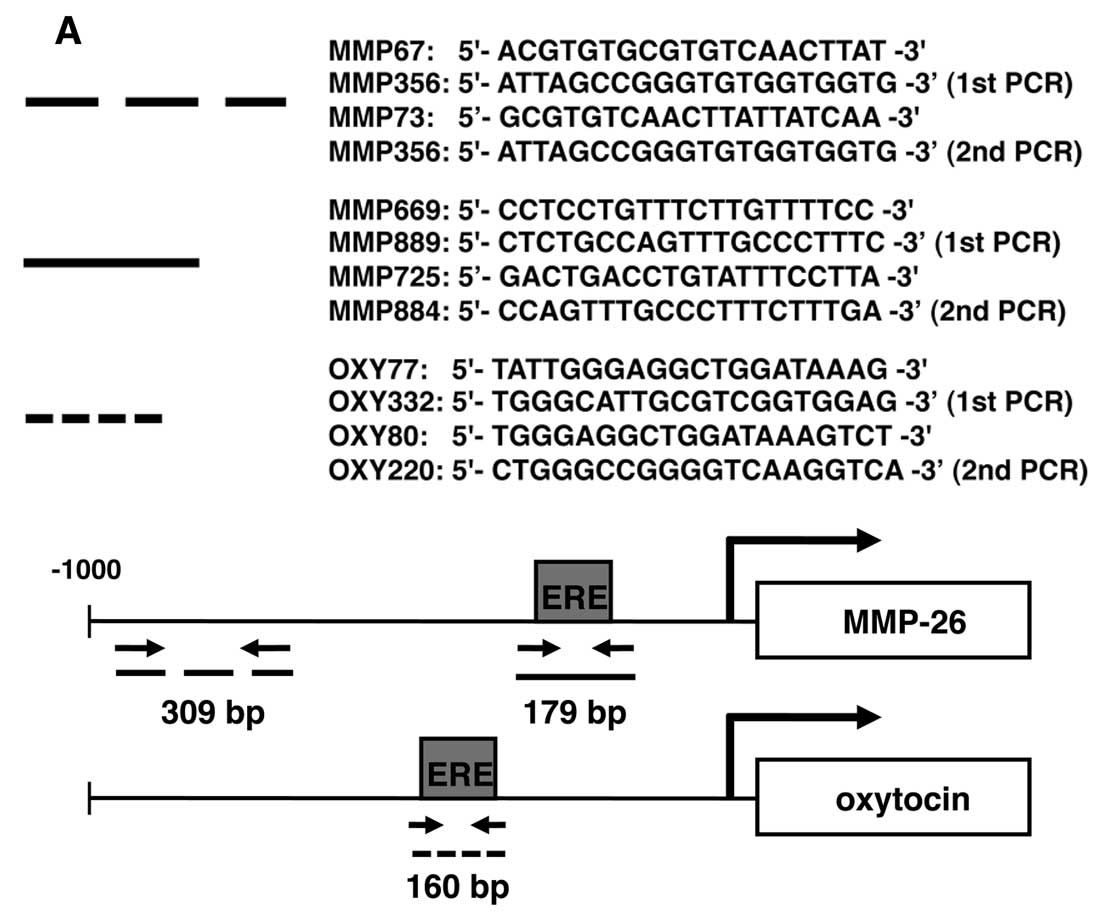
sites in the MMP-26 promoter. The target sites and the
corresponding primer sequences are shown in Fig. 4A. The PCR products were
electrophoresed on a 1.0% agarose gel and visualized by ethidium
bromide staining.
Results
MMP-26 expression is downregulated in
endometrial carcinomas
The expression of MMP-26 mRNA was examined in 30
endometrial carcinoma, 6 endometrial hyperplasia patients and
normal endometrium from 51 patients who suffered from uterine myoma
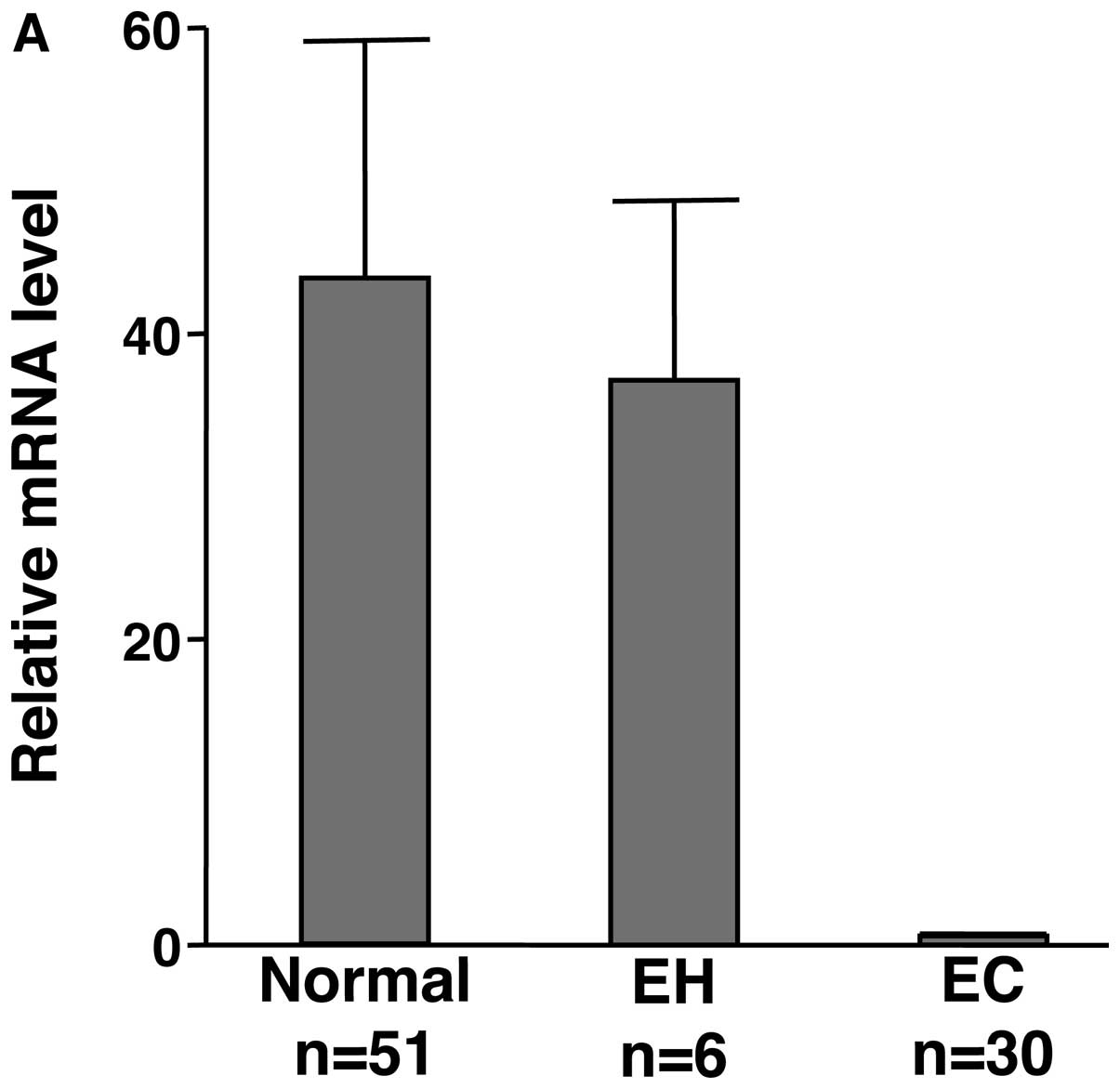
or prolapse of the uterus. Quantitative real-time RT-PCR analysis
revealed that normal endometria expressed significantly higher
levels of MMP-26 mRNA than the endometrial carcinomas tissues
(Fig. 1A). However, endometrial
hyperplasias showed the similar level of MMP-26 as normal
endometria (Fig. 1A).
Furthermore, we examined MMP-26 expression in
protein level by immunohistochemical analysis using anti-MMP-26
antibodies. MMP-26 expression was observed only in epithelial
glandular cells but not in stromal cells (Fig. 1B). No stained or faintly stained
carcinoma cells were observed in endometrial carcinoma tissues
(Fig. 1C).
Estrogen induces the endogenous MMP-26
expression
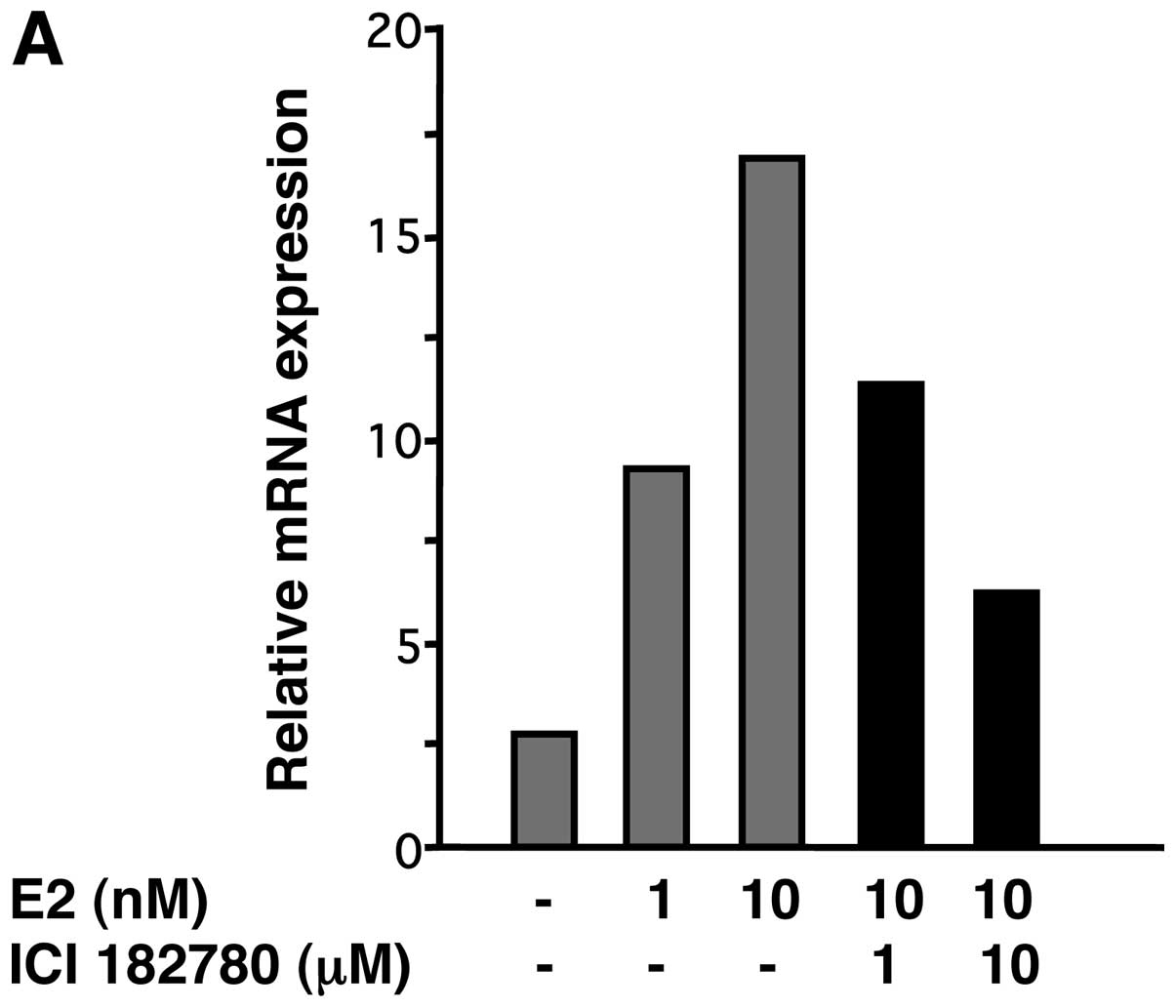
We examined the effect of estrogen on MMP-26 protein
expression in ERα positive endometrial cancer cell line, Ishikawa.
E2 at 1–10 nM dose-dependently increased both MMP-26
mRNA and protein expression levels in Ishikawa cells (Fig. 2). The upregulated expression of
MMP-26 protein was blocked by pretreatment of cells with the
estrogen receptor inhibitor, ICI 182780, dose-dependently,
indicating that the estrogen receptor was involved (Fig. 2).
Estrogen and ER transactivate the MMP-26
promoter
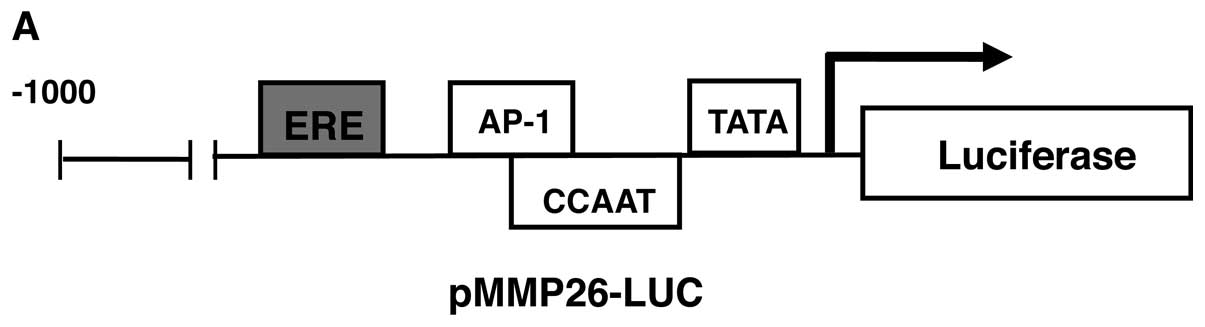
It is probably likely that MMP-26 is regulated by
estrogen through an estrogen-response element (ERE). Bioinformatics
in silico analysis was performed for extensive evaluation of
the MMP-26 gene promoter sequence. We looked for the presence of
ERE like motifs (GGTCANNNTGACC) in the MMP-26 gene and found it in
the promoter region of the MMP-26 gene between nucleotides -129 and
-117 (5′-GGTCACTCTTGCCC-3′). This has the characteristic of the
ERE, a 13-bp palindromic element consisting of two 5-bp arms
separated by a 3-bp spacer. This ERE motif in the MMP-26 promoter
has one arm of the perfect palindromic element sequences and a
second incomplete set. These two arms of the palindrome are
separated by the 3-bp spacing which is essential for ER binding. We
show that the relative positions of the ERE and other binding sites
for transcription factors in the MMP-26 promoter in Fig. 3A.
To examine whether induction of MMP-26 gene is
regulated directly by estrogen through ligand-dependent
transcriptional factors that bind DNA at conserved ERE, EN cells
were transfected with pMMP-26-luc for luciferase assays. ER
overexpression activated the MMP-26 promoter 2-fold (Fig. 3B). Further investigation showed that
1–100 nM E2 significantly enhanced the luciferase
activity by 2.5–5-fold (Fig. 3C).
These results suggest that there may be a functional ERE motif that
mediates estrogen-dependent MMP-26 expression in the promoter
fragment of MMP-26.
ER interacts with the putative ERE in the
MMP-26 promoter
To confirm the existence of an ERE in the MMP-26
promoter, we performed in silico analysis and conventional
ChIP assays. ChIP analysis demonstrated that only fragments
containing the putative ERE were precipitated after administration
of ER transfection (Fig. 4B). No
PCR product was detected from samples precipitated with IgG (data
not shown). These results verify the ERE in the MMP-26 promoter and
indicate that the ER/ERE complex mediates the regulation of MMP-26
expression by estrogen.
Discussion
It is believed that partly estrogen is related to
the development of endometrial carcinoma (21). Actually, estrogen is essential to
develop endometrial hyperplasia, whereas other genes, such as PTEN,
K-ras, hMLH1 and p53 are critical in endometrial carcinogenesis
(22–25). It seems that estrogen is not
directly related to the development of endometrial carcinoma. Our
data suggest estrogen-dependence of MMP-26 expression and
demonstrate the specific association of MMP-26 with endometrial
carcinomas. MMP-26 expression was lost in endometrial carcinoma,
whereas normal endometrium as well as endometrial hyperplasia has
high MMP-26 expression. While the biological function of MMP-26 is
not yet fully understood, if MMP-26 is essential to development of
endometrial carcinoma, estrogen induces endometrial hyperplasia but
possibly not endometrial carcinoma.
Li et al(17)
described that ER activated the MMP-26 expression. However, the
molecular mechanisms by which ER activates MMP-26 have not been
examined in any detail. Interestingly, computer-assisted homology
searches have revealed potential binding sites for ER in the MMP-26
proximal promoter. We performed luciferase assays and ChIP assays
to demonstrate the correlation between ER and MMP-26. The data
demonstrate that the ER transactivates the MMP-26 promoter and
induces MMP-26 expression.
As in a previous study, to define the physiological
role of MMP-26, we have examined the expression pattern of this
enzyme in human endometrial tissues. In marked contrast to the
restricted expression pattern of this enzyme in endometrial
carcinoma, this gene is expressed in normal endometrium and
endometrial hyperplasia. The sensitivity and specificity of
endometrial carcinoma by cytological analysis is ~70–80%, much
lower than those of cervical carcinoma (26,27).
In the present study, we show that there is a significant
difference in MMP-26 expression between normal endometria and
endometrial carcinomas. Combination of cytological analysis and
detection of MMP-26 expression might be a useful examination to
identify endometrial carcinoma. The detectability of combination
examination should be better than that of cytological analysis
only. It is also important to measure the expression level of
MMP-26 mRNA or protein. The expression level might indicate the
malignancy. Additional studies will be necessary to clarify the
clinical significance of the MMP-26 expression in human endometrial
carcinoma (4,6).
Since endometrial hyperplasia tissues, a
precancerous lesion of endometrial carcinomas, did exhibit MMP-26,
downregulation of MMP-26 expression might be a critical event in
endometrial carcinogenesis. MMP-26 expression may affect the
development of endometrial carcinomas. At present, the mechanisms
of MMP-26 downregulation and correlation with other genes are not
clear. Although our data in the present study are insufficient to
make a conclusion, the preliminary data suggest that the MMP-26
gene might play an essential role as a tumor suppressor gene.
Additional studies to explain MMP-26 functions, regulation
mechanisms, and correlation with other genes, are necessary to
fully understand the roles of MMP-26 in tumor biology.
The mechanisms documented in the present study
suggest the involvement of MMP-26 in malignant progression of
endometrial carcinoma and brings us several steps closer to
understanding the functional role of the unconventional MMP-26
enzyme in physiologic and pathologic processes. Further analysis of
MMP-26 regulation in endometrial cells could provide further
insight into the molecular mechanism and the role of MMP-26
expression or activity in human endometrial carcinogenesis.
Acknowledgements
Ishikawa was kindly provided by Dr Masato Nishida
(Department of Obstetrics and Gynecology, National Kasumigaura
Hospital). The present study was supported by JSPS KAKENHI (grant
no. 16790973). The authors thank Ms. Chinatsu Higuma (Department of
Obstetrics and Gynecology, Tokyo Medical University) for invaluable
experimental assistance.
References
|
1
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar
|
|
2
|
Pilka R, Norata GD, Domanski H, Andersson
C, Hansson S, Eriksson P and Casslen B: Matrix metalloproteinase-26
(matrilysin-2) expression is high in endometrial hyperplasia and
decreases with loss of histological differentiation in endometrial
cancer. Gynecol Oncol. 94:661–670. 2004. View Article : Google Scholar
|
|
3
|
Park HI, Ni J, Gerkema FE, Liu D,
Belozerov VE and Sang QX: Identification and characterization of
human endometase (Matrix metalloproteinase-26) from endometrial
tumor. J Biol Chem. 275:20540–20544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stetler-Stevenson WG, Aznavoorian S and
Liotta LA: Tumor cell interactions with the extracellular matrix
during invasion and metastasis. Annu Rev Cell Biol. 9:541–573.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murray GI, Duncan ME, O’Neil P, Melvin WT
and Fothergill JE: Matrix metalloproteinase-1 is associated with
poor prognosis in colorectal cancer. Nat Med. 2:461–462. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000.PubMed/NCBI
|
|
7
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sternlicht MD, Lochter A, Sympson CJ, et
al: The stromal proteinase MMP3/stromelysin-1 promotes mammary
carcinogenesis. Cell. 98:137–146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Velasco G, Pendas AM, Fueyo A, Knauper V,
Murphy G and Lopez-Otin C: Cloning and characterization of human
MMP-23, a new matrix metalloproteinase predominantly expressed in
reproductive tissues and lacking conserved domains in other family
members. J Biol Chem. 274:4570–4576. 1999. View Article : Google Scholar
|
|
10
|
de Coignac AB, Elson G, Delneste Y, et al:
Cloning of MMP-26. A novel matrilysin-like proteinase. Eur J
Biochem. 267:3323–3329. 2000.PubMed/NCBI
|
|
11
|
Marchenko GN, Ratnikov BI, Rozanov DV,
Godzik A, Deryugina EI and Strongin AY: Characterization of matrix
metalloproteinase-26, a novel metalloproteinase widely expressed in
cancer cells of epithelial origin. Biochem J. 356:705–718. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uria JA and Lopez-Otin C: Matrilysin-2, a
new matrix metalloproteinase expressed in human tumors and showing
the minimal domain organization required for secretion, latency,
and activity. Cancer Res. 60:4745–4751. 2000.
|
|
13
|
Trabert B, Wentzensen N, Yang HP, Sherman
ME, Hollenbeck AR, Park Y and Brinton LA: Is estrogen plus
progestin menopausal hormone therapy safe with respect to
endometrial cancer risk? Int J Cancer. 132:417–426. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Isaka K, Nishi H, Nakai H, Nakada T, Feng
Li Y, Ebihara Y and Takayama M: Matrix metalloproteinase-26 is
expressed in human endometrium but not in endometrial carcinoma.
Cancer. 97:79–89. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cooke PS, Buchanan DL, Young P, et al:
Stromal estrogen receptors mediate mitogenic effects of estradiol
on uterine epithelium. Proc Natl Acad Sci USA. 94:6535–6540. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pierro E, Minici F, Alesiani O, et al:
Stromal-epithelial interactions modulate estrogen responsiveness in
normal human endometrium. Biol Reprod. 64:831–838. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, Savinov AY, Rozanov DV, et al:
Title: Matrix metalloproteinase-26 is associated with
estrogen-dependent malignancies and targets α1-antitrypsin serpin.
Cancer Res. 64:8657–8665. 2004.PubMed/NCBI
|
|
18
|
Isaka K, Nishi H, Sagawa Y, et al:
Establishment of a new human cell line (EN) with TP53 mutation
derived from endometrial carcinoma. Cancer Genet Cytogenet.
141:20–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishida M: The Ishikawa cells from birth
to the present. Hum Cell. 15:104–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mak HY and Parker MG: Use of suppressor
mutants to probe the function of estrogen receptor-p160 coactivator
interactions. Mol Cell Biol. 21:4379–4390. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Banno K, Kisu I, Yanokura M, et al:
Progestin therapy for endometrial cancer: the potential of
fourth-generation progestin (Review). Int J Oncol. 40:1755–1762.
2012.PubMed/NCBI
|
|
22
|
Xiong Y, Dowdy SC, Eberhardt NL, Podratz
KC and Jiang SW: hMLH1 promoter methylation and silencing in
primary endometrial cancers are associated with specific
alterations in MBDs occupancy and histone modifications. Gynecol
Oncol. 103:321–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tashiro H, Blazes MS, Wu R, et al:
Mutations in PTEN are frequent in endometrial carcinoma but rare in
other common gynecological malignancies. Cancer Res. 57:3935–3940.
1997.PubMed/NCBI
|
|
24
|
Lax SF, Kendall B, Tashiro H, Slebos RJ
and Hedrick L: The frequency of p53, K-ras mutations, and
microsatellite instability differs in uterine endometrioid and
serous carcinoma: evidence of distinct molecular genetic pathways.
Cancer. 88:814–824. 2000. View Article : Google Scholar
|
|
25
|
Kawaguchi M, Banno K, Yanokura M, et al:
Analysis of candidate target genes for mononucleotide repeat
mutation in microsatellite instability-high (MSI-H) endometrial
cancer. Int J Oncol. 35:977–982. 2009.PubMed/NCBI
|
|
26
|
Suprun HZ, Taendler-Stolero R, Schwartz J
and Ettinger M: Experience with Endopap endometrial sampling in the
cytodiagnosis of endometrial carcinoma and its precursor lesions. I
A correlative cytologic-histologic-hysteroscopic diagnostic pilot
study. Acta Cytol. 38:319–323. 1994.
|
|
27
|
Foulks MJ: The Papanicolaou smear: its
impact on the promotion of women’s health. J Obstet Gynecol
Neonatal Nurs. 27:367–373. 1998.
|