Introduction
Metastasis, which is the spread of cancer to distant
locations in the body, is a complex process. Approximately 90% of
deaths associated with cancer are due to the metastasis of the
original tumor cells to sites distant from the primary tumor.
Metastasis occurs through a series of sequential steps including
invasion of adjacent tissues, intravasation, transport of cancer
cells through the circulatory system, arrest at a secondary site,
extravasation and growth in a secondary organ (1). For most cancer cell types, the ability
to metastasize leads to clinically incurable disease (2). However, the mechanisms underlying a
cell’s ability to extravasate from the primary tumor, circulate and
invade new tissue remain to be established (3). The organized breakdown of the
extracellular matrix (ECM) by matrix metalloproteinases (MMPs) is
involved in the complex cascade of events, such as invasion and
extravasation. The human MMP family is divided into secreted and
membrane-type MMPs (MT-MMPs). MT-MMPs are membrane-tethered
proteolytic enzymes and constitute the largest family of MMPs
identified thus far (4). The type I
transmembrane structural class of MT-MMPs contains MMP-14
(MT1-MMP), MMP-15 (MT2-MMP), MMP-16 (MT3-MMP) and MMP-24 (MT5-MMP).
Overexpression of MT-MMPs has been observed in several types of
human cancer (5). The functions of
MT-MMPs are known to include roles in activation of other MMP
family members, pericellular proteolysis, modulation of cellular
signaling and cellular migration, the angiogenic response and
regulation of cell proliferation, apoptosis and tumor metastasis
(6–8). Among these members, MMP-14 is
overexpressed in pancreatic ductal adenocarcinoma (PDAC) compared
with normal pancreatic tissue, which is induced by TGF-β1 (9). The overexpression of MMP-14 is also
observed in non-small cell lung carcinoma (NSCLC) (10), breast cancer (11), malignant mesothelioma (12), and supraglottic cancer (13). The activity of these proteases is
tightly regulated by specific inhibitors, known as tissue
inhibitors of MMPs (TIMPs) (14). A
selective MMP-14 inhibitor reduces cancer cell motility and tumor
growth (15). Therefore, to uncover
the regulation mechanisms of MMP-14, particularly in cancer
metastasis, is of great importance for understanding cancer biology
and improving treatment.
microRNAs (miRNAs) are 20–23-nucleotide long
single-stranded RNAs that are encoded by eukaryotic nuclear DNA and
function in the post-transcriptional regulation of gene expression
via base-pairing with complementary sequences within 3′-UTR of
target mRNAs (16). Mature miRNAs,
Argonaute (Ago) and several other associated proteins form the
RNA-induced silencing complex (RISC) mediating gene silencing at
post-transcriptional and translational levels (17). miRNAs have been shown to be involved
in a wide variety of biological processes such as apoptosis,
development, aging and cancer. Previous studies suggested that
miRNAs contribute to the initiation and development of various
types of cancer (18). There is
also accumulating evidence to suggest that miRNAs are involved in
tumor metastasis. miR-200 family and miR-205 can inhibit
epithelial-mesenchymal transition (EMT), through which
epithelial cancer cells invade and metastasize, by targeting ZEB1,
ZEB1 and SIP1 (19–21). In human breast cancer,
overexpression of miR-21 is significantly correlated with lymph
node metastasis, advanced clinical stage and shortened survival
time (22). miR-10b is also
reported to initiate tumor invasion and metastasis in breast cancer
(23,24). In prostate cancer progenitor cells,
miR-34a acts as a potent inhibitor of metastasis by directly
suppressing CD44 (25). miR-9, an
MYC/MYCN-activated miRNA, is reported to enhance cell motility and
invasiveness by targeting CDH1 (26). Therefore, it is reasonable to assume
that more miRNAs would be discovered to govern metastasis. miR-133a
has previously been reported to play roles in diabetic hearts
(27), myogenic differentiation
(28) and osteoblast lineage
commitment program (29). Recent
findings showed that miR-1 and miR-133a are frequently
downregulated in various types of cancer (30). In the present study, we proved that
MPP-14 is a target of miR-133a by dual luciferase reporter assay,
and overexpression of miR-133a decreases the mRNA and protein
levels of MMP-14 in lung cancer cell lines. In conclusion, the
miR-133a-induced suppression of cell proliferation, migration and
invasion addresses the anti-metastatic role of miR-133a in lung
cancer.
Materials and methods
Cell culture and transfection
NSCLC cell lines A549, NCI-H1299 and human HEK-293T
cell lines were purchased from the American Type Culture Collection
(ATCC, Rockville, MD, USA). The cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS) (S/N:16000-044;
Gibco) and antibiotics (100 U/ml penicillin and streptomycin;
Invitrogen) at 37°C in a humidified 5% CO2 atmosphere.
Cell transfection was performed using Lipofectamine™ 2000
(Invitrogen). Cells were harvested at 48 h post-transfection for
protein analysis or luciferase activity assay. The related
sequences are shown in Table I.
 | Table IPrimers used for reverse transcription
and real-time polymerase chain reaction. |
Table I
Primers used for reverse transcription
and real-time polymerase chain reaction.
| Primer | Sequence |
|---|
|
miR-133a-stem-loop | 5′-GTC GTA TCC AGT
GCA GGG TCC GAG |
| RT primer | GTA TTC GCA CTG GAT
ACG ACT CAG CTC-3′ |
| RNU6-RT | 5′-AAA ATA TGG AAC
GCT-3′ |
| miR-133a mimics | 5′-TTT GGT CCC CTT
CAA CCA GCT G-3′ |
| Mimics control | 5′-AGT GTG AGT TCT
ACC ATT GCC AAA-3′ |
| miR-133a
inhibitor | 5′-CAG CTG GTT GAA
GGG GAC CAA A-3′ (2′Ome) |
| miR-133a-F | 5′-GTG CAT TTG GTC
CCC TTC A-3′ |
| miR-133a-R | 5′-CGG GCT GTC AGT
TTG TCA-3′ |
| RNU6-F | 5′-CTC GCT TCG GCA
GCA CA-3′ |
| RNU6-R | 5′-AAC GCT TCA CGA
ATT TGC GT-3′ |
| GAPDH-F | 5′-AGG GCA TCT TGG
GCT ACA C-3′ |
| GAPDH-R | 5′-TGG TCC AGG GTT
TCT TAC TCC-3′ |
| MMP-14-F | 5′-GCA GAA GTT TTA
CGG CTT GCA-3′ |
| MMP-14-R | 5′-TCG AAC ATT GGC
CTT GAT CTC-3′ |
Plasmid construction
The full length 3′UTR of human MMP-14 was
PCR-amplified from human genomic DNA and cloned into psiCHECK™-2
dual luciferase reporter plasmid (Promega, Madison, WI, USA)
immediately downstream of the stop codon of the Renilla luciferase
gene with Xhol/NotI to generate
psiCHECK™-2-MMP-14-3′UTR-wt. The mutant human MMP-14 3′UTR
reporter, designated as psiCHECK™-2-MMP-14-3′UTR-mut, was created
within the predicted hsa-miR-133a seed sequence binding site
(GGACCAAA to
GCTGGTAA) by
site-directed PCR mutation. The construction was confirmed by DNA
sequencing.
Luciferase reporter assay
For luciferase reporter assay, HEK-293T cells
(4.0×103 cells/well) were plated in a 96-well plate
(Corning, USA) 24 h before transfection. Cells were co-transfected
with 60 ng of either the psiCHECK™-2-MMP-14-3′UTR-wt,
psiCHECK™-2-MMP-14-3′UTR-mut or psiCHECK™-2 vector and 20 nM (final
concentration) of either miRNA mimics negative control or miR-133a
mimics or miR-133a inhibitor, which is antisense oligonucleotides
with 2′O-methyl modification (RiboBio, Guangzhou, China).
Forty-eight hours after transfection, the cells were washed with
PBS twice and lysed in 100 μl Passive Lysis Buffer (Promega) and
the luciferase activities were measured from 20 μl lysate using the
Dual-Luciferase Reporter Assay kit (Promega) following the
manufacturer’s instructions on a luminometer (Lumat LB 9507;
Berthold, Germany). The data were obtained by averaging the results
from six independent repeats. Transfection efficiency was
normalized to thymidine kinase-driven Renilla luciferase
activity.
RNA isolation and real-time PCR
Total RNA was isolated from cultured cells using
TRIzol Reagent (Invitrogen). cDNA was synthesized by reverse
transcription using oligo (dT) as the primer and proceeded to
real-time PCR with gene-specific primers in the presence of 2X
SYBR-Green Master Mix (DBI Bioscience, Shanghai, China). The
relative abundance of mRNA was calculated by normalization to
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Specific
stem-loop RT primers were used for reverse transcription reaction
of miR-133a. Normalization of miR-133a was performed by using RNU6
primers. All reactions were carried out in triplicate and all
experiments were performed three independent times.
Proliferation, migration and Matrigel
invasion assay
For the proliferation assay, 6 h after transfection,
3,000 cells were seeded in triplicate in 6-well plates. Cell
Counting Kit-8 (CCK-8) reagent (C0038; Beyotime, China) was added
at 12, 24, 48, 72 h after seeding and incubated at 37°C for half to
4 h according to the color change. The data of optical density (OD)
value at 450 nm were read by a microplate reader (Synergy 2;
BioTek, USA). Migration and invasion assays were performed
according to the manufacturer's instructions. Cells
(3×104) in 0.5 ml of serum-free medium were seeded onto
the top of each chamber containing BD BioCoat cell culture inserts
(354578) or Matrigel Invasion Chamber (354480; both from BD
Biosciences), and 0.75 ml of complete growth medium containing 10%
FBS was added to each well in the lower chamber. Following
incubation for 48 h at 37°C, non-invasive cells were removed from
the upper chamber, the cells attached to the lower chamber were
fixed with methanol, stained with 0.1% crystal violet and then
counted under a light microscope.
Western blot analysis
Fifty micrograms of total proteins were loaded onto
10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto polyvinylidene difluoride
membranes (PVDF; Millipore, Billerico, MA, USA). After blocking
with 3% bovine serum albumin, the blots were incubated with
antibodies against MMP-14 (Bioworld, Atlanta, GA, USA), GAPDH
(Chemicon International, Temecula, CA, USA). Following incubation
with red flurescence conjugated secondary antibody, protein bands
were scanned using Odyssey bands scanner (S/N ODY-2792 model: 9120)
The intensity of the bands was analyzed using Bandscan
Software.
Statistical analysis
Analysis of variance and Student’s t-test were used
to compare means of two or more different treatment groups.
P<0.05 was considered to indicate a statistically significant
difference, unless otherwise stated. Data are expressed as mean ±
SE.
Results
MMP-14 is a functional target of
miR-133a
To identify potential targets of miR-133a both for
experimental validation and functional studies in lung cancer, we
performed in silico analysis of a range of miRNA target
prediction databases. The target prediction of miR-133a was
performed by using the following databases: TargetScan (http://www.targetscan.org), MicroCosm (http://www.ebi.ac.uk/), miRanda (http://www.microrna.org/microrna/getGeneForm.do) and
miRGen (www.diana.pcbi.upenn.edu/cgi-bin/miRGen/v3/Targets.cgi).
All databases analyzed presented MMP-14 as a converging target of
miR-133a, which plays an essential role in the degradation of
basement membranes and the expression of MMP-14 is correlated with
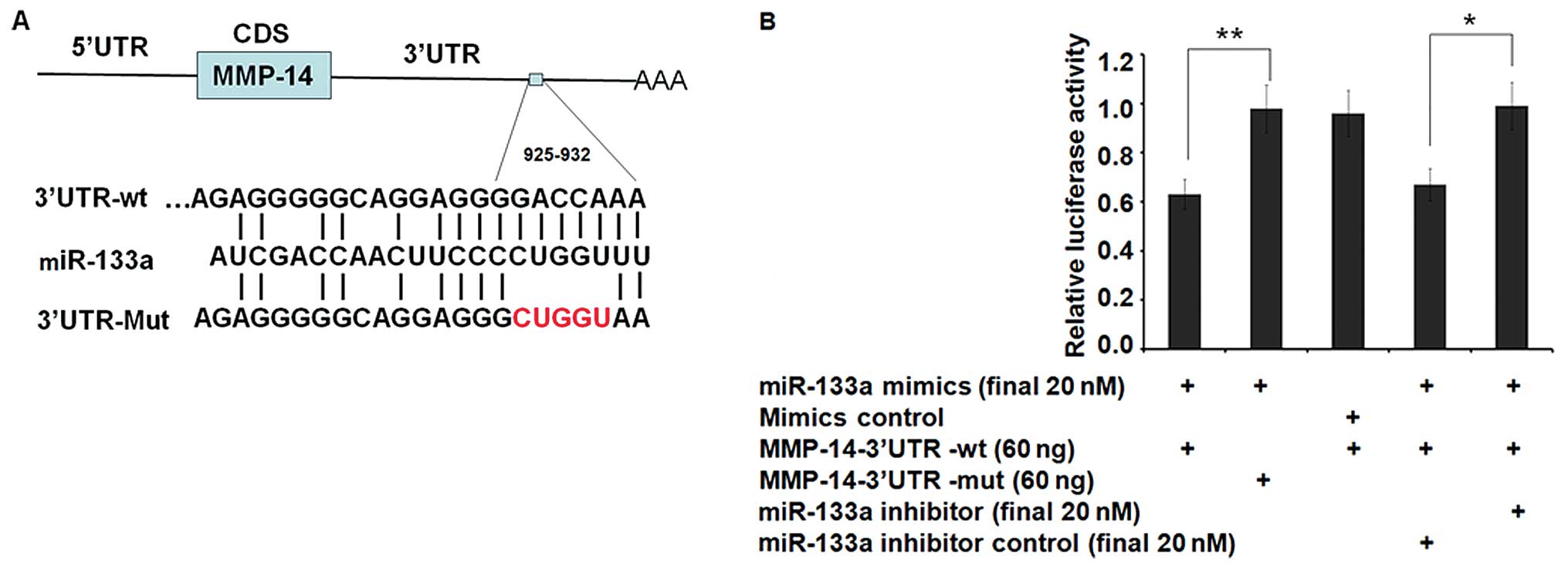
metastasis. To validate MMP-14 as a de novo target of
miR-133a, we constructed a luciferase reporter vector containing
the entire wild-type 3′UTR of MMP-14 (psiCHECK™-2-MMP-14-3′UTR-wt)
or the mutant 3′UTR (psiCHECK™-2-MMP-14-3′UTR-mut) with a
5-nucleotide mutation previously mentioned (Fig. 1A). HEK-293T cells, which exhibit low
levels of miR-133a expression, were used for transient
transfections with psiCHECK™-2-MMP-14-3′UTR-wt or
psiCHECK™2-MMP-14-3′UTR-mut. Co-transfection with miR-133a (a
synthetic miR-133a mimic) resulted in a significant decrease (to
63%) in luciferase gene expression from the reporter vector
containing the wild-type 3′UTR of MMP-14 when compared with a
scrambled control (Fig. 1B),
demonstrating direct targeting by miR-133a (P<0.01). Consistent
with the data, no decrease in luciferase activity was observed when
miR-133a was co-transfected with the mutant 3′UTR of MMP-14
reporter. Furthermore, miR-133a specific inhibitor, which is the
antisense oligonucleotides of miR-133a can almost restore the
inhibition effect (P<0.05) (Fig.
1B). These results indicate that MMP-14 is a direct target of
miR-133a and is responsible for miR-133a targeting in the
3′UTR.
miR-133a suppresses cell proliferation in
lung cancer A549 and NCI-H1299 cell lines
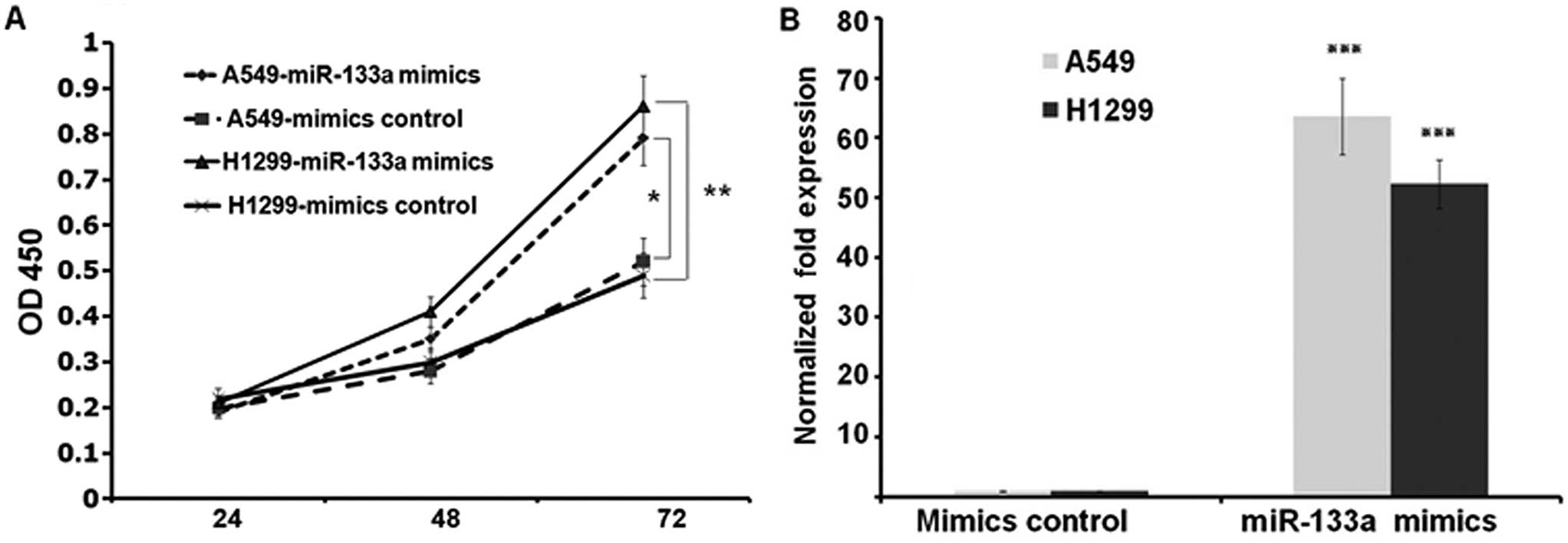
To characterize the functional impact of miR-133a on
lung cancer cell behavior such as growth rate, we transfected A549
and NCI-H1299 cells with either miR-133a mimics or mimics control
and transferred the cells into 96-well plates at 3.0×103
cells/well post transfection. We harvested and detected the OD450
value of each well by CCK-8 kit at indicated times after seeding.
These results showed that the growth rates of the two lung cancer
cell lines were suppressed in miR-133a mimics transfected groups
compared to control groups at 72 h (P<0.01 and P<0.05 at A549
and NCI-H1299 cell lines, respectively) (Fig. 2A). To confirm the transfection, we
assessed the expressions of miR-133a at 72 h post transfection. As
shown in Fig. 2B, miR-133a was
upregulated to 63.6- and 52.3-fold at A549 and NCI-H1299 cells,
respectively (both P<0.001) (Fig.
2B).
Overexpression of miR-133a decreases the
mRNA and protein levels of MMP-14
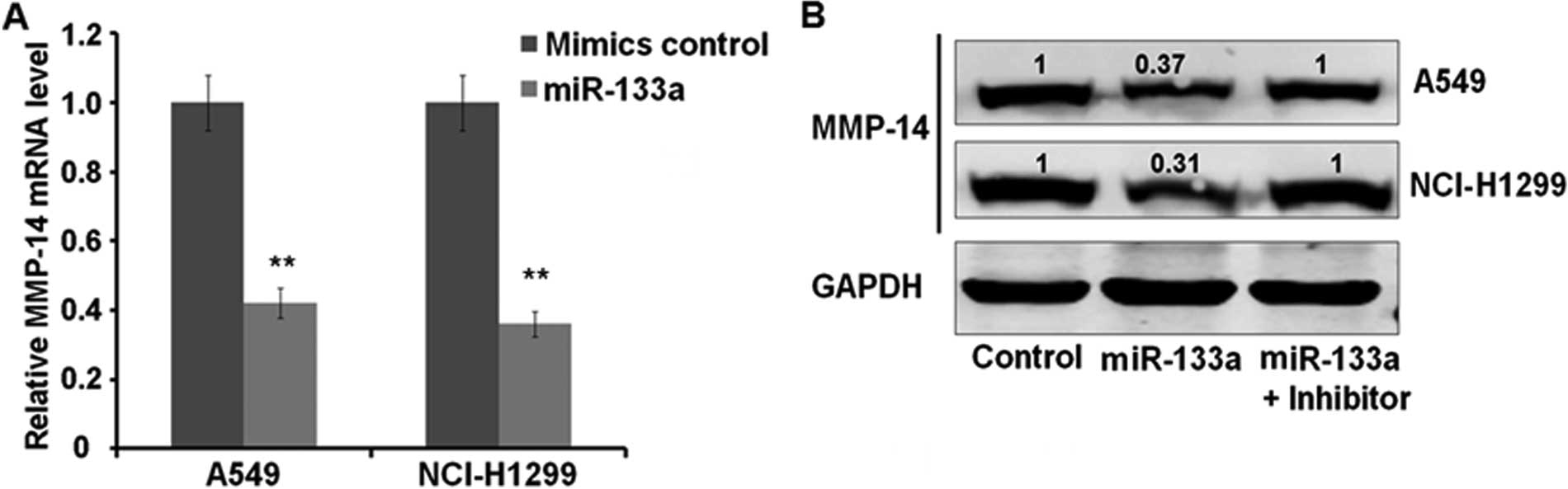
We next assessed the effect of miR-133a
overexpression on MMP-14. Transfection of miR-133a into A549 and
NCI-H1299 cells resulted in a significant decrease of MMP-14 mRNA
levels compared with control-transfected cells. As measured by
qRT-PCR, the MMP-14 mRNA levels were decreased to 41 and 38% in
A549 and NCI-H1299, respectively (both P<0.01) (Fig. 3A). Subsequent western blot analysis
of MMP-14 showed that miR-133a overexpression caused a reduction in
MMP-14 protein levels, which can be reversed by miR-133a specific
inhibitor (Fig. 3B). Measured by
Bandscan Software according to grayscale of each band, MPP-14
protein is downregulated to 37 and 31% in A549 and NCI-H1299 cells,
respectively. These results indicated that miR-133a can inhibit
lung cancer cell proliferation, which may be mediated by
downregulation of direct target MMP-14 at mRNA and protein
levels.
miR-133a inhibits lung cancer cell
migration and invasion through targeting MMP-14
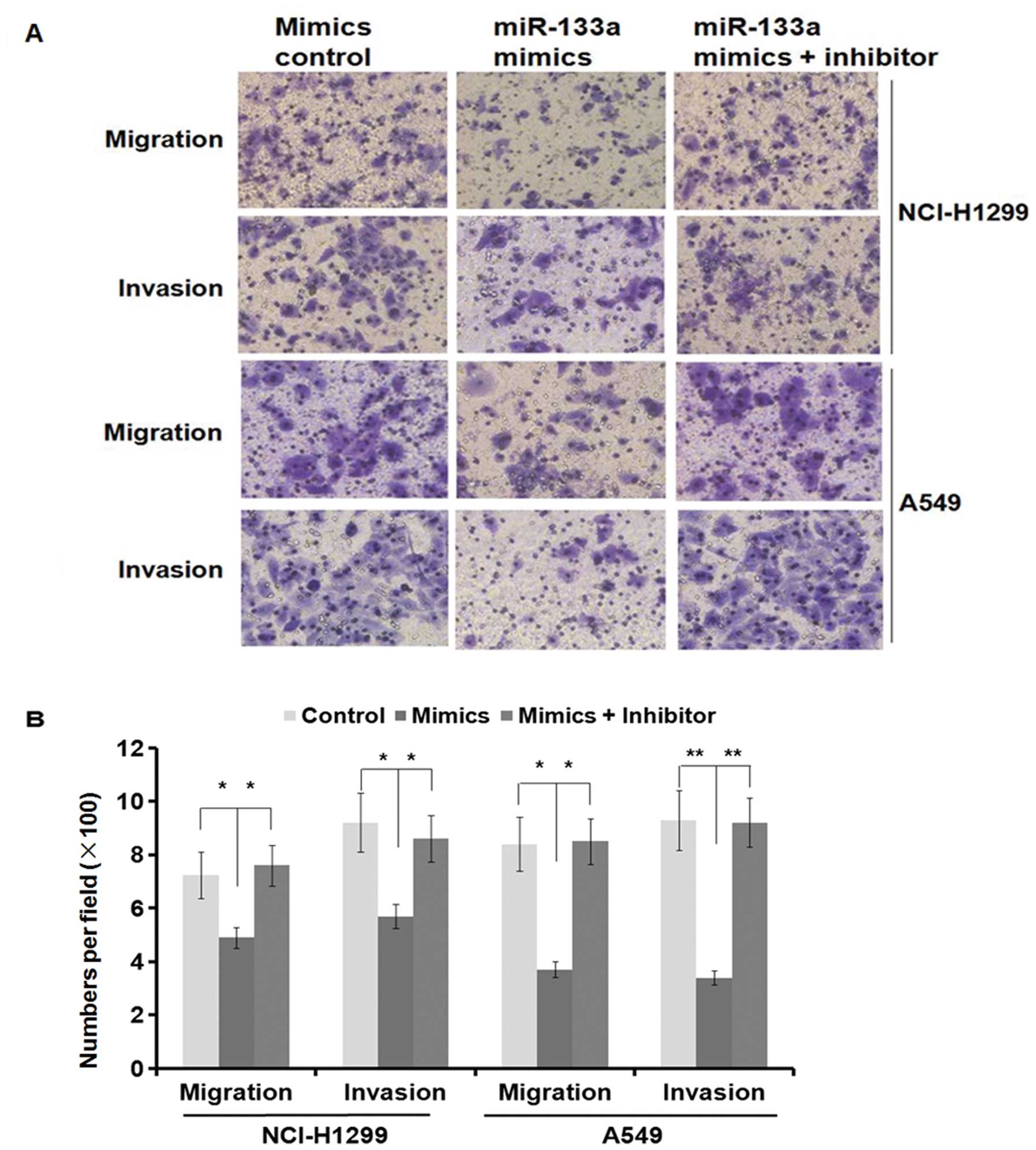
MMP-14 is an MT1-MMP as a primary regulator of
interstitial collagenolysis and there is evidence to suggest that
MMP-14 is enhanced in metastatic PDAC lesions compared with the
primary tumors and myogenic tumors (31,32).
The downregulation of MMP-14 by miR-133a overexpression prompted us
to examine whether miR-133a could modulate migration and invasion
in lung cancer cell lines. We performed the transwell migration and
Matrigel invasion assays in A549 and NCI-H1299 cells transfected
with either miR-133a mimics or control mimics. As shown in Fig. 3A, miR-133a mimics suppressed cell
migration and invasion in both A549 and NCI-H1299 cells (migration
and invasion, P<0.05 in the NCI-H1299 cell line and P<0.01 in
the A549 cell line) (Fig. 4A). The
specific inhibitor of miR-133a almost restored the inhibition
effect in both cell lines (Fig. 4A and
B). Moreover, the MMP-14 protein levels were also restored
(Fig. 3B). Collectively, these
results clearly indicate that miR-133a suppresses cell migration
and invasion in the A549 and NCI-H1299 cells lines by reducing
MMP-14 expression.
Discussion
miRNAs play important roles in a broad range of
biological processes including development, cellular
differentiation, proliferation and apoptosis (18). Increasing evidence shows that
several miRNAs including the miR-200 family, miR-10b and miR-205
are involved in the lung cancer metastasis process (19–21).
In the present study, we identified that miR-133a is another miRNA
which can inhibit cell proliferation, migration and invasion in
lung cancer cell lines by targeting MMP-14. Several lines of
evidence support this idea. First, downregulation of MMP-14 by
miR-133a was confirmed in A549 and NCI-H1299 cell lines at the
protein and mRNA levels. Second, miR-133a repressed luciferase
reporter gene containing the MMP-14 3′UTR through its binding site
and mutation of the putative binding site on MMP-14 3′UTR almost
abolished the suppression effect. Third, overexpression of miR-133a
in A549 and NCI-H1299 cells suppressed cell proliferation,
migration and invasion through reducing MMP-14 expression.
Moreover, the specific antisense miR-133a inhibitor can almost
restore the inhibition effect as well.
A number of recent studies analyzed the genetic
expression of MMP-14 in several types of human cancer, including
tumors of the breast, colon, head, neck and oral cavity (6,8),
indicating the importance of MMP-14 in cancer progression. MMP-14
is an essential molecule whose function is known to include roles
in the activation of other MMP family members, pericellular
proteolysis, modulation of cellular signaling and cell migration
(5). It is not surprising that the
expression of MMP-14 is tightly regulated at multiple levels. For
example, TGF-β1 was reported to promote migration in an MMP-14
dependent manner in pancreatic ductal epithelial (HPDE) cells
(9). In the present study, we
confirmed that MMP-14 is indeed a functional target of miR-133a and
found that the expression of MMP-14 is negatively regulated by
miR-133a. It should be noted that mature miR-133a sequence
(5′-UUUGGUCCCCUUCAACCAGCUA-3′) is highly conserved among mammalian
species. The potential miR-133a binding site (5′-UUUGGUC-3′) is
also presented in the known MMP-14 3′UTR of rat, dog, horse and
cattle, suggesting that miR-133a may regulate MMP-14 expression of
these species in a similar manner. Collectively, it is reasonable
to hypothesize that post-transcriptional regulation of MMP-14 by
miRNAs may be a major determinant of MMP-14 expression in lung
cancer growth and metastasis.
The functions of mammalian miR-133a and its targets
have been reported in several publications. Ectopic expression of
miR-133a significantly inhibited the invasion capacity of various
human cancer cell lines (27,28).
It is reported that miR-133a targets FSCN1 in MCF-7 and MDA-MB-231
breast cancer cell lines. In breast cancer, miR-133a expression was
found reduced by a microarray-based analysis (27), which indicated the tumor suppressive
function of miR-133a. As is known, miRNAs can target hundreds of
targets (16), miRNAs may play
different roles in different cell lines or under different signals.
We cannot exclude the possibility that a single miRNA can exert
multiple functions by targeting multiple targets. In the present
study, ectopic expression of miR-133a was more than 50-fold
compared with endogenous expression, but MMP-14 is only
downregulated to approximately 30%, indicating that the endogenous
expression of miR-133a is extremely low and that MMP-14 may not be
the only target in this condition. The specific inhibitor of
miR-133a can almost restore the suppression of cell migration and
invasion. These results suggest the function of miR-133a in lung
cancer is prominently inhibition of tumor metastasis rather than
tumor growth.
In summary, in the present study we described for
the first time that miR-133a plays an anti-metastatic role in lung
cancer and miR-133a may be a suitable tumor marker for lung cancer
metastasis. We also proved that MMP-14 is a new functional target
of miR-133a. In further studies, we will uncover the complex
functions of miR-133a in modulating lung cancer progression.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81273814).
Abbreviations:
|
MMP
|
matrix metalloproteinase
|
|
MT-MMP
|
membrane-type MMP
|
|
miRNA
|
microRNA
|
|
PVDF
|
polyvinylidene difluoride membrane
|
References
|
1
|
Mehlen P and Puisieux A: Metastasis: a
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steeg PS: Metastasis suppressors alter the
signal transduction of cancer cells. Nat Rev Cancer. 3:55–63. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Segura MF, Hanniford D, Menendez S, et al:
Aberrant miR-182 expression promotes melanoma metastasis by
repressing FOXO3 and microphthalmia-associated transcription
factor. Proc Natl Acad Sci USA. 106:1814–1819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yana I and Seiki M: MT-MMPs play pivotal
roles in cancer dissemination. Clin Exp Metastasis. 19:209–215.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Genís L, Gálvez BG, Gonzalo P and Arroyo
AG: MT1-MMP: universal or particular player in angiogenesis? Cancer
Metastasis Rev. 25:77–86. 2006.PubMed/NCBI
|
|
7
|
Plaisier M, Kapiteijn K, Koolwijk P, et
al: Involvement of membrane-type matrix metalloproteinases
(MT-MMPs) in capillary tube formation by human endometrial
microvascular endothelial cells: role of MT3-MMP. J Clin Endocrinol
Metab. 89:5828–5836. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sounni NE and Noel A: Membrane type-matrix
metalloproteinases and tumor progression. Biochimie. 87:329–342.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ottaviano AJ, Sun L, Ananthanarayanan V
and Munshi HG: Extracellular matrix-mediated membrane-type 1 matrix
metalloproteinase expression in pancreatic ductal cells is
regulated by transforming growth factor-β1. Cancer Res.
66:7032–7040. 2006.PubMed/NCBI
|
|
10
|
Atkinson JM, Pennington CJ, Martin SW, et
al: Membrane type matrix metalloproteinases (MMPs) show
differential expression in non-small cell lung cancer (NSCLC)
compared to normal lung: correlation of MMP-14 mRNA expression and
proteolytic activity. Eur J Cancer. 43:1764–1771. 2007. View Article : Google Scholar
|
|
11
|
Laudański P, Swiatecka J, Kozłowski L, et
al: Increased serum level of membrane type 1-matrix
metalloproteinase (MT1-MMP/MMP-14) in patients with breast cancer.
Folia Histochem Cytobiol. 48:101–103. 2010.PubMed/NCBI
|
|
12
|
Crispi S, Calogero RA, Santini M, et al:
Global gene expression profiling of human pleural mesotheliomas:
identification of matrix metalloproteinase 14 (MMP-14) as potential
tumour target. PLoS One. 4:e70162009. View Article : Google Scholar
|
|
13
|
Zhang H, Liu M, Sun Y and Lu J: MMP-14 can
serve as a prognostic marker in patients with supraglottic cancer.
Eur Arch Otorhinolaryngol. 266:1427–1434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suojanen J, Salo T, Koivunen E, et al: A
novel and selective membrane type-1 matrix metalloproteinase
(MT1-MMP) inhibitor reduces cancer cell motility and tumor growth.
Cancer Biol Ther. 8:2362–2370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Winter J, Jung S, Keller S, et al: Many
roads to maturity: microRNA biogenesis pathways and their
regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eulalio A, Huntzinger E and Izaurralde E:
Getting to the root of miRNA-mediated gene silencing. Cell.
132:9–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gregory PA, Bert AG, Paterson EL, et al:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Renthal NE, Chen CC, Williams KC, et al:
miR-200 family and targets, ZEB1 and ZEB2, modulate uterine
quiescence and contractility during pregnancy and labor. Proc Natl
Acad Sci USA. 107:20828–20833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burk U, Schubert J, Wellner U, et al: A
reciprocal repression between ZEB1 and members of the miR-200
family promotes EMT and invasion in cancer cells. EMBO Rep.
9:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan LX, Huang XF, Shao Q, et al: MicroRNA
miR-21 overexpression in human breast cancer is associated with
advanced clinical stage, lymph node metastasis and patient poor
prognosis. RNA. 14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gee HE, Camps C, Buffa FM, et al:
MicroRNA-10b and breast cancer metastasis. Nature. 455:E8–E9. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu C, Kelnar K, Liu B, et al: The
microRNA miR-34a inhibits prostate cancer stem cells and metastasis
by directly repressing CD44. Nat Med. 17:211–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma L, Young J, Prabhala H, et al: miR-9, a
MYC/MYCN-activated microRNA, regulates E-cadherin and cancer
metastasis. Nat Cell Biol. 12:247–256. 2010.PubMed/NCBI
|
|
27
|
Xiao J, Luo X, Lin H, et al: MicroRNA
miR-133 represses HERG K+ channel expression
contributing to QT prolongation in diabetic hearts. J Biol Chem.
282:12363–12367. 2007.PubMed/NCBI
|
|
28
|
Kato Y, Miyaki S, Yokoyama S, et al:
Real-time functional imaging for monitoring miR-133 during myogenic
differentiation. Int J Biochem Cell Biol. 41:2225–2231. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Hassan MQ, Volinia S, et al: A
microRNA signature for a BMP2-induced osteoblast lineage commitment
program. Proc Natl Acad Sci USA. 105:13906–13911. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshino H, Chiyomaru T, Enokida H, et al:
The tumour-suppressive function of miR-1 and miR-133a targeting
TAGLN2 in bladder cancer. Br J Cancer. 104:808–818. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shields MA, Dangi-Garimella S, Krantz SB,
et al: Pancreatic cancer cells respond to type I collagen by
inducing snail expression to promote membrane type 1 matrix
metalloproteinase-dependent collagen invasion. J Biol Chem.
286:10495–10504. 2011. View Article : Google Scholar
|
|
32
|
Gong YL, Xu GM, Huang WD and Chen LB:
Expression of matrix metalloproteinases and the tissue inhibitors
of metalloproteinases and their local invasiveness and metastasis
in Chinese human pancreatic cancer. J Surg Oncol. 73:95–99. 2000.
View Article : Google Scholar : PubMed/NCBI
|