Introduction
Cisplatin (cis-diamminedichloroplatinum II,
CDDP) is one of the most effective chemotherapeutic agents widely
used for the treatment of solid tumors. However, the side-effects
of cisplatin chemotherapy and resistance during the course of the
treatment limit its clinical use (1–3).
Cisplatin is generally considered as a cytotoxic drug which kills
cancer cells by damaging DNA and inhibiting DNA synthesis.
Cisplatin-induced DNA damage activates various signaling pathways
to prevent or promote cell death predominantly by inducing
apoptosis (4). Based on the
mechanism of action of cisplatin-induced cell death, modifications
to the combination methods used in chemotherapy are required to
reduce the side-effects and increase the therapeutic effects of
cisplatin. Autophagy inhibitors, such as 3-methyladenine and
chloroquine, have been shown to effectively enhance cisplatin
cytotoxity (5–8). Additional agents have been recently
assessed such as bortezomib (PS-341, Velcade), a proteasome
inhibitor (9,10), and histone acetyltransferase
inhibitor, suberoylanilide hydroxamic acid (SAHA) (11,12).
Some of these agents have been shown to confer sensitizing effects
to cisplatin combination therapy.
Ammonium chloride (NH4Cl) is an agent
used for the treatment of metabolic alkalosis in clinical practice
(13). NH4Cl was
recently used as an autophagy inhibitor in in vitro studies,
where it was found to affect the pH of lysosomes and to disturb the
activity of autolysosomes (14–16).
In the present study, we used NH4Cl combined with
cisplatin to investigate the potentially effective use of
NH4Cl as an agent in cisplatin combination
chemotherapy.
In the present study, we tested the hypothesis that
the use of NH4Cl increases the apoptosis induced by
cisplatin treatment in human cervical cancer (HCC) HeLa cells.
Cisplatin was found to inhibit the cell growth, as well as to
induce cell apoptosis and DNA double-strand breaks (DSBs).
Treatment with NH4Cl increased the rate of cell
apoptosis and the activation of caspase-3. NH4Cl
treatment combined with cisplatin was found to increase
cisplatin-induced apoptosis by inducing severe DNA damage.
Materials and methods
Cell culture
The HCC HeLa cell line was cultured at 37°C in an
atmosphere containing 5% CO2 and 95% air, using Iscove’s
modified Dulbecco’s medium (IMDM; Life Technologies, Inc.,
Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco-BRL, Carlsbad, CA, USA), 100 U/ml penicillin and 100
U/ml streptomycin. The cells were divided into 4 groups:
non-treated cells, cells treated with 5 μg/ml cisplatin (Sigma, St.
Louis, MO, USA), cells treated with 2 mM NH4Cl (Sigma)
and cells treated with 5 μg/ml cisplatin combined with 2 mM
NH4Cl.
MTT assay
Cell viability was determined using MTT assay.
Exponentially growing HeLa cells were seeded into 96-well culture
plates in 100 μl medium at a density of 1×104
cells/well. After a 24-h incubation, the indicated dose (as
described in ‘Cell culture’) of cisplatin and/or NH4Cl
was added for a 24-h incubation in 4 parallel wells. MTT assays
(Beyotime, China) were subsequently performed. Briefly, 20 μl of
MTT solution [5 mg/ml in phosphate-buffered saline (PBS)] was added
followed by a 4-h incubation. Then, 150 μl of dimethyl sulfoxide
(Beijing Chemical Industry Co., Ltd., China) was added to each
well. After shaking for 10 min, absorbance was measured at 570 nm
using a microplate reader (Bio-Rad, Hercules, CA, USA). The
survival rate was calculated as follows: Survival rate (%) =
Absorbance of experimental group/Absorbance of control group ×
100%. The mean value of 4 wells per treatment group was calculated
in each experiment.
Western blot analysis
HeLa whole-cell protein extracts were prepared with
cell lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM
Na2EDTA, 1 mM EDTA, 1% Triton X-100, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4, 1 mM NaF, 1 μg/ml leupeptin and 1 mM
PMSF) for western blot analysis. The protein extracts were
quantified using the Bio-Rad kit (Pierce). For Western blot
analysis, lysate proteins (30–50 μg) were resolved on 10%
SDS-polyacrylamide gel electrophoresis and transferred onto
Immobilon-P transfer membranes (Millipore, Boston, MA, USA). The
membranes were blocked with 5% nonfat dry milk in buffer [10 mM
Tris-HCl (pH 7.6), 100 mM NaCl and 0.1% Tween-20] for 1 h at room
temperature. The membranes were then incubated with the appropriate
primary antibody [anti-caspase-3 or anti-β-actin antibody
(dilution, 1:200); Eptomics, Burlingame, CA, USA] at 4°C overnight,
followed by incubation with horseradish peroxidase-conjugated
secondary antibody (HuaAn Biotechnology, Hangzhou, China) at a
dilution of 1:2,000 for 1 h at room temperature. The immunoreactive
bands were visualized using the diaminobenzidine method (Sigma).
Representative bands were measured with a Tanon GIS gel imaging
system and analyzed. The levels of proteins were normalized to
those of β-actin and the ratios of normalized protein to β-actin
were presented as means ± SD from three independent experiments.
Protein levels were quantified by densitometry using Quantity One
software (Bio-Rad).
Immunofluorescence staining and confocal
laser microscopy
Following treatment with the indicated doses of
cisplatin and/or NH4Cl (as described in ‘Cell culture’)
for 24 h, the cells were cultured on coverslips overnight.
Subsequently, the cells were fixed with 4% paraformaldehyde, cell
nuclei were stained with Hoechst 33342 (2 μg/ml; Sigma) for 30 min,
followed by washing with PBS. The cells were then observed using
Olympus FV1000 confocal laser microscope to examine cell chromatin
condensation. The expression levels of active caspase-3 and
γ-H2AX were examined using an indirect
immunofluorescence method. Briefly, the cells were cultured on
coverslips overnight, treated with the indicated doses of cisplatin
and/or NH4Cl (as described in ‘Cell culture’) for 24 h,
and then rinsed thrice with PBS. After incubation, the cells were
fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.1%
Triton X-100 for 5 min, blocked with bovine serum albumin (BSA) and
incubated with the primary antibodies against active caspase-3
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
γ-H2AX (Cell Signaling Technology, Inc., Beverly, MA,
USA) (dilution, 1:100) at 4°C overnight. The cells were then
incubated with FITC/Texas Red conjugated secondary antibodies
(dilution, 1:400) (Invitrogen, Carlsbad, CA, USA) for 1 h, stained
with Hoechst 33342 (2 μg/ml) for 2 min, and washed thrice with PBS.
Following staining, the cells were mounted and examined under a
Olympus FV1000 confocal laser microscope.
Flow cytometric analysis
Propidium iodide (PI, 1 μg/ml; Invitrogen) was used
for the determination of cell death. After exposure to the
different experimental conditions, the cells were trypsinized and
incubated with PI for 30 min at 37°C. The samples were then
analyzed using a FACScan flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA). All the experiments were performed in
triplicate.
Statistical analysis
Data are representative of three independent
experiments each performed in triplicate. Statistical analysis of
the data was performed using one-way ANOVA. Tukey’s post hoc test
was used to determine the significance for all pairwise comparisons
of interest. P-values of <0.05 were considered to indicate a
statistically significant difference.
Results
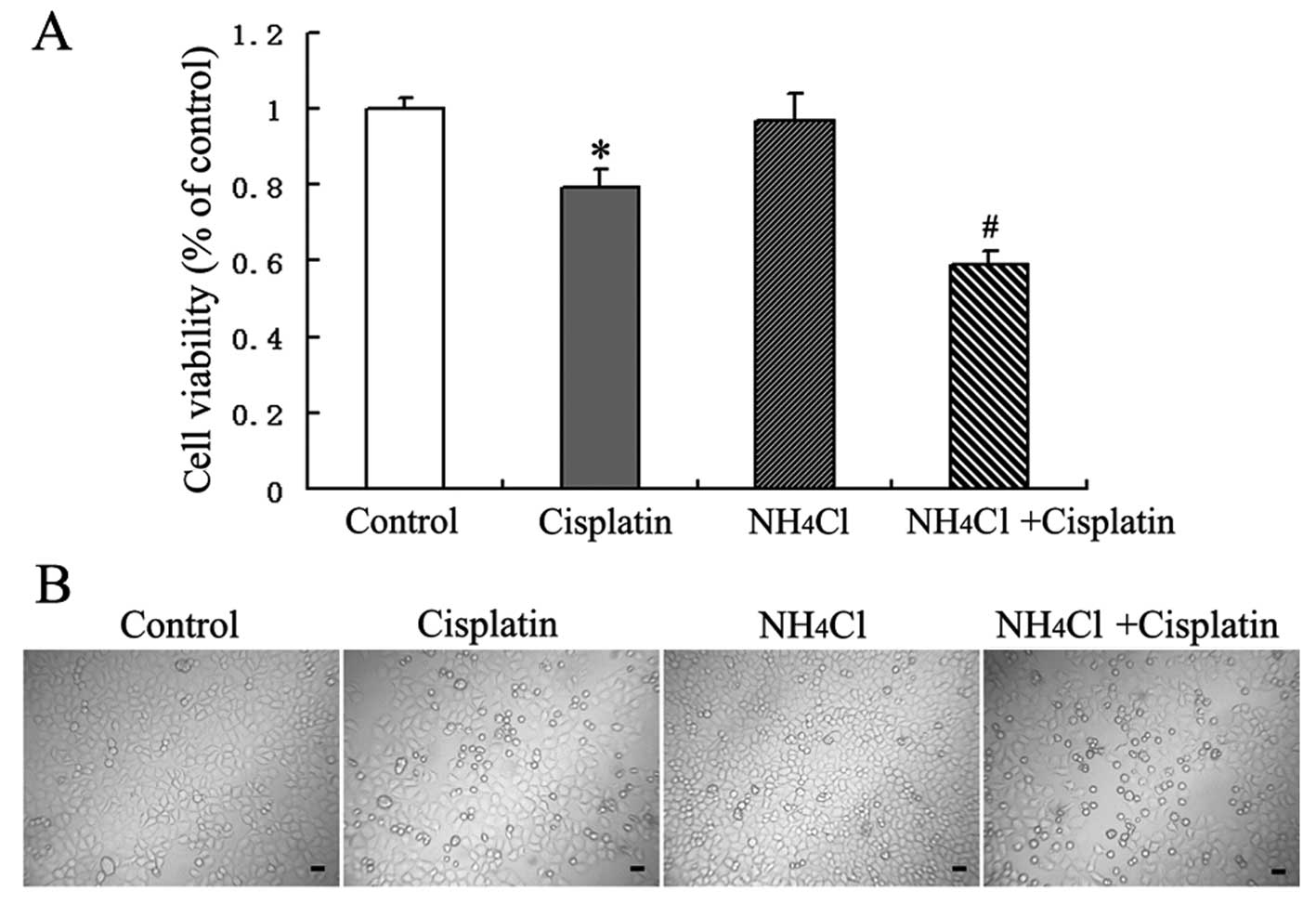
NH4Cl increases cell growth
inhibition induced by cisplatin
Based on the results of our preliminary studies,
HeLa cells were treated with the indicated doses of cisplatin
and/or NH4Cl for 24 h, and cell growth inhibition was
then assessed using MTT assays. Cisplatin was found to inhibit the
growth of HeLa cells. MTT assays indicated that there was no
significant effect of treatment with NH4Cl alone on cell
viability, while NH4Cl enhanced the cytotoxic effect of
cisplatin when NH4Cl was used in combination with
cisplatin (Fig. 1A).
Morphological changes were also examined using an
inverted phase contrast microscope. Cisplatin-treated cells were
observed to be round and fragile in comparison with the control
cells. The number of round and fragile cells following treatment
with cisplatin in combination with NH4Cl was increased
compared to the number of cells exposed to cisplatin alone
(Fig. 1B).
Thus, we hypothesized that NH4Cl
increases the apoptotic rate of HeLa cells induced by cisplatin.
The apoptosis rate was then detected by confocal microscopy and
flow cytometric analysis.
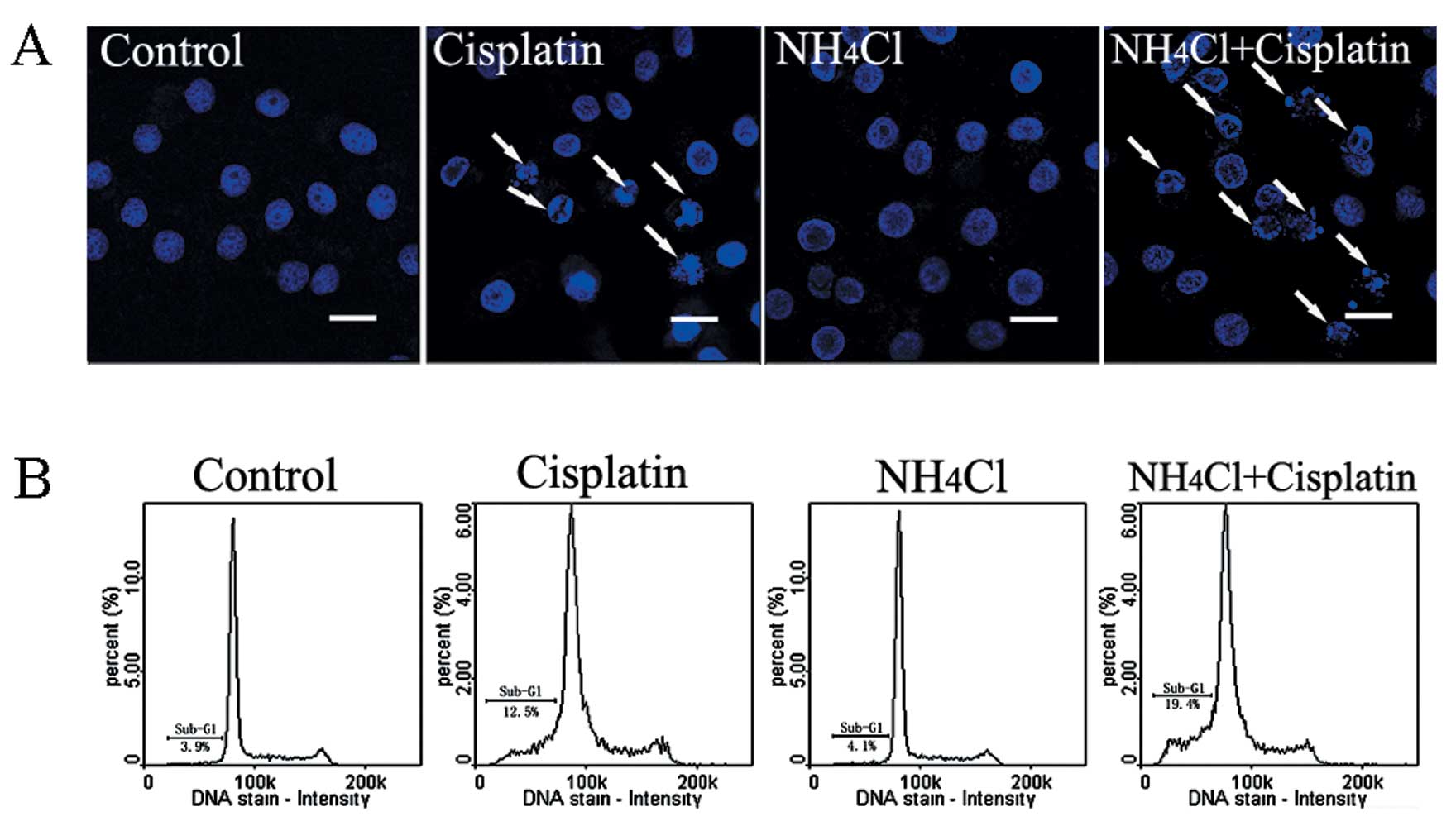
NH4Cl increases
cisplatin-induced cell apoptosis
Apoptotic chromatin condensation was assessed with
Hoechst 33342 staining and confocal microscopy. Cisplatin-induced
apoptotic chromatin condensation was observed in HeLa cells
compared with control cells. The cells treated with cisplatin
combined with NH4Cl exhibited obvious apoptotic
chromatin condensation when compared with cells treated with
cisplatin alone (Fig. 2A).
The effect of NH4Cl on cisplatin-induced
apoptosis in HeLa cells was assessed using flow cytometric
analysis. As shown in Fig. 2B, a
higher apoptotic rate (sub-G1 peak) was observed in cells treated
with cisplatin combined with NH4Cl (19.4%), when
compared with the apoptotic rate of the cells treated with
cisplatin alone (12.5%).
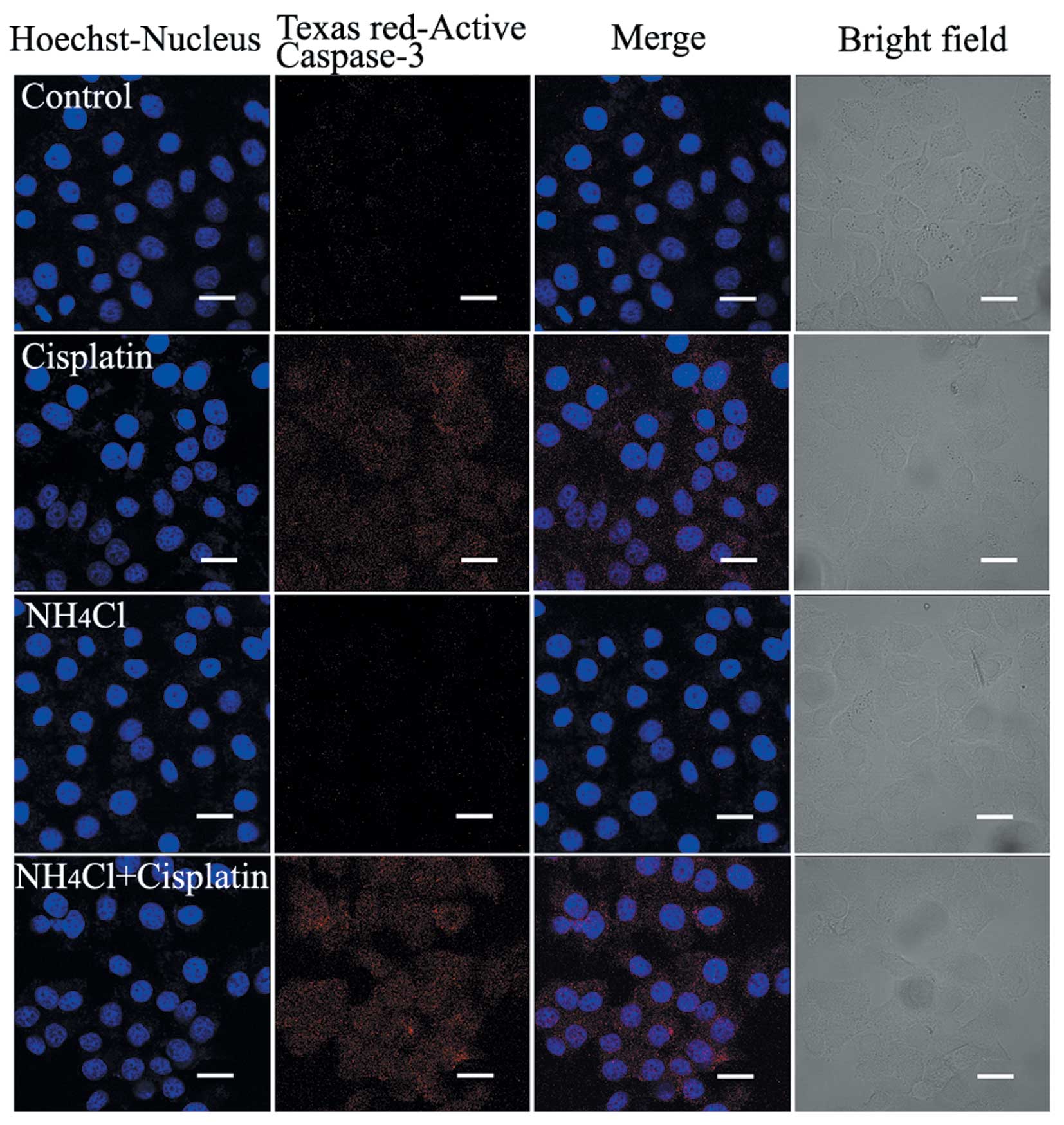
NH4Cl combined with cisplatin
increases the activation of caspase-3
Caspase-3 plays an important role in the execution
of apoptosis, and its activation reflects the process of apoptosis.
The activation of caspase-3 in HeLa cells treated with cisplatin
alone and cells treated with cisplatin combined with
NH4Cl was assessed using confocal microscopy. Red
fluorescence was more intense in cells treated with cisplatin
combined with NH4Cl compared with cells treated with
cisplatin alone, suggesting that treatment with the combination of
cisplatin and NH4Cl increased the activation of
caspase-3 (Fig. 3).
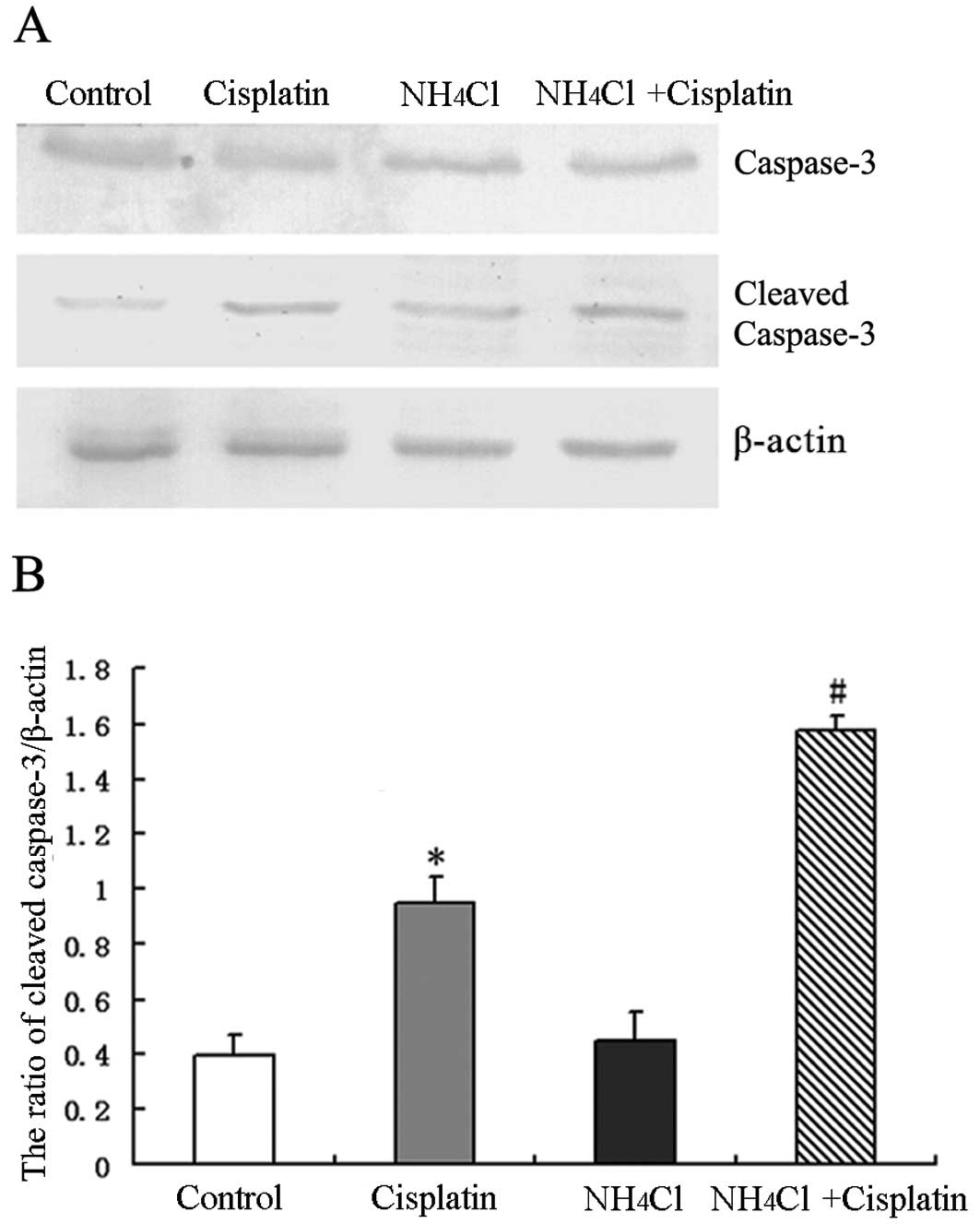
Furthermore, the activation of caspase-3 was
assessed by detecting the expression of cleaved caspase-3 by
western blot analysis. Cisplatin was found to increase the
expression of cleaved caspase-3 in HeLa cells compared to control
cells. However, treatment with cisplatin combined with
NH4Cl increased the expression of cleaved caspase-3
compared to cells treated with cisplatin alone (Fig. 4A and B). These results indicate that
NH4Cl efficiently increased the apoptosis induced by
cisplatin in HeLa cells.
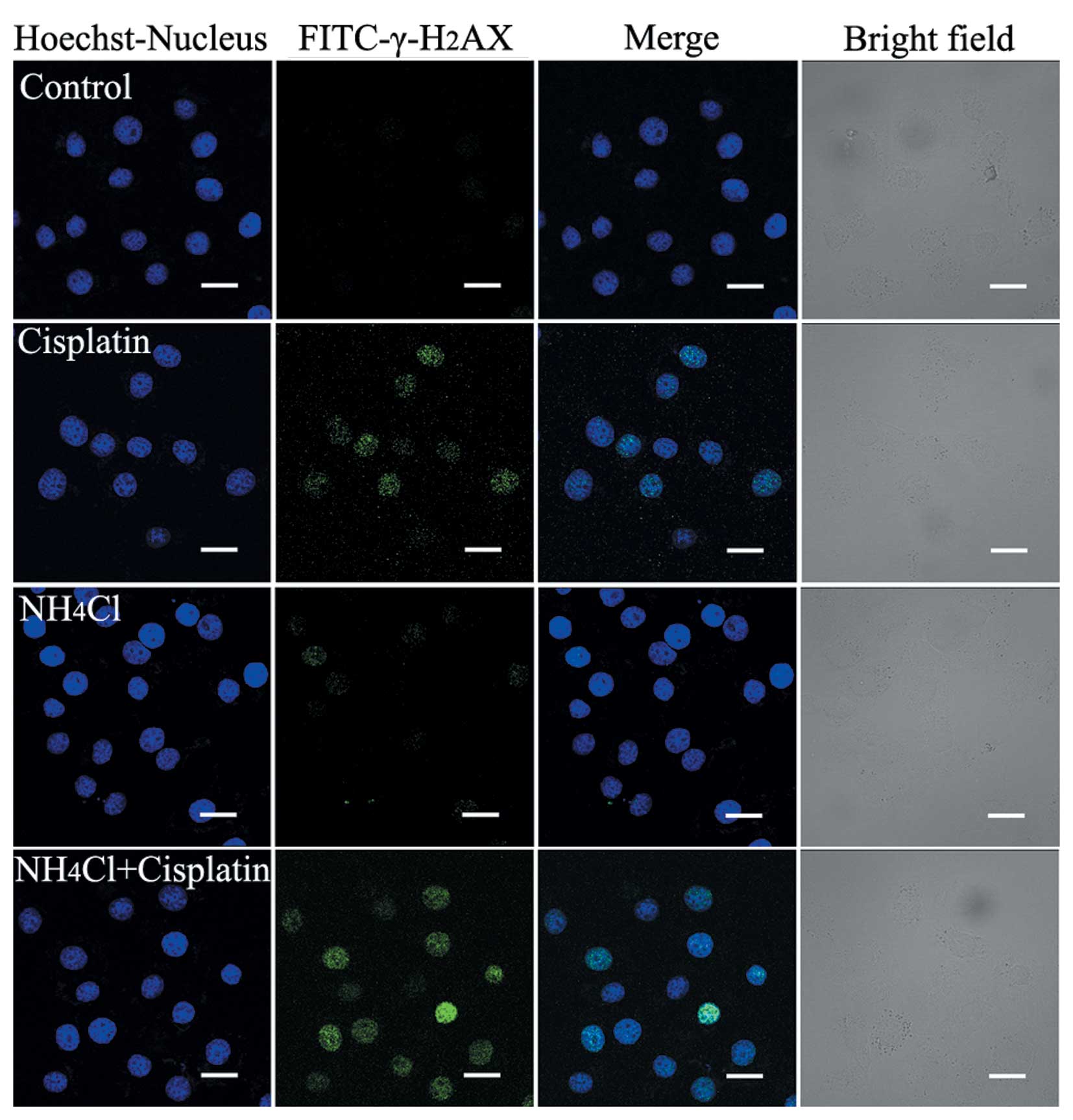
NH4Cl increases
cisplatin-induced DNA DSBs
Cisplatin has been reported to kill cancer cells by
damaging DNA and inhibiting DNA synthesis. Thus, we hypothesized
that NH4Cl increases the DNA damage induced by
cisplatin. DSBs induce the phosphorylation of H2AX at a
conservative C-terminal region of serine 139 leading to the
formation of γ-H2AX. Thus, γ-H2AX is usually
used as a DNA DSB marker.
The expression of γ-H2AX in HeLa cells
treated with cisplatin alone and cells treated with cisplatin
combined with NH4Cl was determined using confocal
microscopy (Fig. 5). After a 24-h
treatment, green fluorescence was observed in cells treated with
cisplatin alone. Moreover, less intense green fluorescence was
observed in cells treated with NH4Cl alone compared with
cisplatin-treated cells. The strongest green fluorescence was
observed in cells treated with cisplatin combined with
NH4Cl (Fig. 5). These
results indicate that NH4Cl efficiently enhances DSBs
induced by cisplatin in HeLa cells.
Discussion
Cisplatin is a widely used chemotherapeutic agent
against several types of solid tumors, including cervical cancer,
and is used either alone or in combination with other anticancer
agents. However, the clinical use of cisplatin is limited due to
its side-effects and drug resistance (17–19).
The antitumor activity of cisplatin is attributed to its ability to
cause DNA damage, leading to the subsequent induction of apoptosis
(20–22). DNA is the primary biological target
of cisplatin (22). The platinum
atom of cisplatin forms covalent bonds with the N7 position of
purine bases to form 1,2- or 1,3-intrastrand cross-links and a
lower percentage of interstrand cross-links. The interstrand and
intrastrand cross-links disrupt the structure of the DNA. Following
DNA damage, cells either repair the damage and start progressing
through the cell cycle or, when the damage cannot be repaired, the
cells proceed to apoptosis (4). The
main death pathway activated by specific cellular damage induced by
cisplatin is a caspase-dependent intrinsic apoptotic pathway that
involves mitochondria and the endoplasmic reticulum (ER) (7,23–27).
Apoptosis is a physiological process of cell self
destruction, which plays important roles in embryo development,
homeostasis and immune defense (28). Caspases are a group of proteases
that are the key components involved in the process of apoptosis.
Active caspase-3 cleaves certain proteins and triggers the
inactivation of cell structure-, cell cycle- and DNA
repair-associated proteins or kinases, leading to cell apoptosis
(29). H2AX
phosphorylation occurs in response to replication fork damage
caused by cisplatin-induced DNA lesions, probably interstrand
cross-links. Although the early kinetics of γ-H2AX
formation is uninformative, retention of γ-H2AX foci 24
h after treatment was shown to be a useful indicator of cell
response to killing by cisplatin (30).
Cisplatin is a reactive drug that interacts not only
with DNA, but also with proteins; damage to cytoplasmic proteins is
an early process that has been suggested to initiate
cisplatin-induced apoptosis (31–33).
The ER was recently reported to be a cytosolic target of
cisplatin-induced apoptosis via the ER stress pathway; sustained
and unabated ER stress induces caspase-mediated apoptosis (7,27,34).
In addition, ER stress and mitochondrial dysfunction have also been
suggested to cooperatively regulate apoptotic-signaling cascades
(35–37).
The results of the present study showed that
cisplatin treatment inhibited cell growth and induced cell
apoptosis. In addition, exposure to cisplatin activated caspase-3
and increased phosphorylation of H2AX. These findings
collectively indicate that cisplatin induces apoptosis through DNA
damage in HCC HeLa cells.
NH4Cl, an expectorant, diuretic and
systemic acidifying agent, is used in the treatment of severe
metabolic alkalosis, to maintain the urine at an acidic pH in the
treatment of certain urinary tract disorders or in forced acid
diuresis (13). The excess ammonia
(NH4Cl) interferes with brain energy metabolism possibly
in part by inhibiting the tricarboxylic acid (TCA) cycle (38). In experimental studies,
NH4Cl can be used as an autophagy inhibitor, which
inhibits the activation of lysosomal enzymes, thus blocking the
degradation of autolysosome components (14–16).
According to a previous study by our group, NH4Cl was
found to prevent autophagy flux by inhibiting the fusion of
autophagosomes with lysosomes and to enhance apoptosis induced by
menadione via the mitochondrial pathway. These results indicated
the generation and accumulation of reactive oxygen species, as well
as increased levels of ubiquitinated proteins and GRP78 in cells
treated with both menadione and NH4Cl (16). In the present study, we found that 2
mM NH4Cl had no toxic effects on HeLa cells. Moreover,
treatment with NH4Cl increased the apoptotic rate and
the activation of caspase-3. Notably, NH4Cl treatment
combined with cisplatin increased H2AX phosphorylation
reflecting severe DNA damage.
In conclusion, cisplatin treatment was found to
induce apoptosis and H2AX phosphorylation in HCC HeLa
cells. NH4Cl treatment combined with cisplatin increased
cell growth inhibition rate, cell apoptosis rate and activation of
caspase-3. Moreover, NH4Cl treatment increased
H2AX phosphorylation induced by cisplatin. These
findings indicate that NH4Cl could be potentially used
as an effective agent for the improvement of cisplatin
chemotherapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81100808), the Natural
Science Foundation of Jilin Province (no. 201015240), the
Department of Education of Jilin Province Project (no. 2013361),
and Scientific Research Foundation of Jilin Medical College for
University Students (201101).
References
|
1
|
Loehrer PJ and Einhorn LH: Drugs five
years later. Cisplatin Ann Intern Med. 100:704–713. 1984.PubMed/NCBI
|
|
2
|
WoŸniak K and Błasiak J: Recognition and
repair of DNA-cisplatin adducts. Acta Biochim Pol. 49:583–596.
2002.
|
|
3
|
Reedijk J: New clues for platinum
antitumor chemistry: kinetically controlled metal binding to DNA.
Proc Natl Acad Sci USA. 100:3611–3616. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Basu A and Krishnamurthy S: Cellular
responses to cisplatin-induced DNA damage. J Nucleic Acids.
2010:2013672010. View Article : Google Scholar
|
|
5
|
Liu D, Yang Y, Liu Q and Wang J:
Inhibition of autophagy by 3-MA potentiates cisplatin-induced
apoptosis in esophageal squamous cell carcinoma cells. Med Oncol.
28:105–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang R, Wang ZH, Wang BQ, et al:
Inhibition of autophagy-potentiated chemosensitivity to cisplatin
in laryngeal cancer Hep-2 cells. Am J Otolaryngol. 33:678–684.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Y, Yu H, Qin H, et al: Inhibition of
autophagy enhances cisplatin cytotoxicity through endoplasmic
reticulum stress in human cervical cancer cells. Cancer Lett.
314:232–243. 2012. View Article : Google Scholar
|
|
8
|
Kimura T, Takabatake Y, Takahashi A and
Isaka Y: Chloroquine in cancer therapy: a double-edged sword of
autophagy. Cancer Res. 73:3–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elstrom RL, Andemariam B, Martin P, et al:
Bortezomib in combination with rituximab, dexamethasone,
ifosfamide, cisplatin and etoposide chemoimmunotherapy in patients
with relapsed and primary refractory diffuse large B-cell lymphoma.
Leuk Lymphoma. 53:1469–1473. 2012. View Article : Google Scholar
|
|
10
|
Kubicek GJ, Axelrod RS, Machtay M, et al:
Phase I trial using the proteasome inhibitor bortezomib and
concurrent chemoradiotherapy for head-and-neck malignancies. Int J
Radiat Oncol Biol Phys. 83:1192–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ong PS, Wang XQ, Lin HS, Chan SY and Ho
PC: Synergistic effects of suberoylanilide hydroxamic acid combined
with cisplatin causing cell cycle arrest independent apoptosis in
platinum-resistant ovarian cancer cells. Int J Oncol. 40:1705–1713.
2012.
|
|
12
|
Jin KL, Park JY, Noh EJ, Hoe KL, Lee JH,
Kim JH and Nam JH: The effect of combined treatment with cisplatin
and histone deacetylase inhibitors on HeLa cells. J Gynecol Oncol.
21:262–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mathew JT and Bio LL: Injectable ammonium
chloride used enterally for the treatment of persistent metabolic
alkalosis in three pediatric patients. J Pediatr Pharmacol Ther.
17:98–103. 2012.PubMed/NCBI
|
|
14
|
Kawai A, Uchiyama H, Takano S, Nakamura N
and Ohkuma S: Autophagosome-lysosome fusion depends on the pH in
acidic compartments in CHO cells. Autophagy. 3:154–157. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang YP, Liang ZQ, Gao B, Jia YL and Qin
ZH: Dynamic effects of autophagy on arsenic trioxide-induced death
of human leukemia cell line HL60 cells. Acta Pharmacol Sin.
29:123–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu C, Huang X, Xu Y, et al: Lysosome
dysfunction enhances oxidative stress-induced apoptosis through
ubiquitinated protein accumulation in Hela cells. Anat Rec
(Hoboken). 296:31–39. 2013. View
Article : Google Scholar
|
|
17
|
Safirstein R, Winston J, Goldstein M, Moel
D, Dikman S and Guttenplan J: Cisplatin nephrotoxicity. Am J Kidney
Dis. 8:356–367. 1986. View Article : Google Scholar
|
|
18
|
Choudhury D and Ahmed Z: Drug-associated
renal dysfunction and injury. Nat Clin Pract Nephrol. 2:80–91.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alexander S, Swatson WS and Alexander H:
Pharmacogenetics of resistance to cisplatin and other anticancer
drugs and the role of sphingolipid metabolism. Methods Mol Biol.
983:185–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sherman SE, Gibson D, Wang AH and Lippard
SJ: X-ray structure of the major adduct of the anticancer drug
cisplatin with DNA: cis-[Pt(NH3)2{d(pGpG)}].
Science. 230:412–417. 1985.
|
|
21
|
Chaney SG, Campbell SL, Bassett E and Wu
Y: Recognition and processing of cisplatin- and oxaliplatin-DNA
adducts. Crit Rev Oncol Hematol. 53:3–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rebillard A, Lagadic-Gossmann D and
Dimanche-Boitrel MT: Cisplatin cytotoxicity: DNA and plasma
membrane targets. Curr Med Chem. 15:2656–2663. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang M, Wang CY, Huang S, Yang T and Dong
Z: Cisplatin-induced apoptosis in p53-deficient renal cells via the
intrinsic mitochondrial pathway. Am J Physiol Renal Physiol.
296:F983–F993. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu J, Yang Y and Wu J: Bcl-2 cleavages at
two adjacent sites by different caspases promote cisplatin-induced
apoptosis. Cell Res. 17:441–448. 2007.PubMed/NCBI
|
|
25
|
Sharma H, Sen S and Singh N: Molecular
pathways in the chemosensitization of cisplatin by quercetin in
human head and neck cancer. Cancer Biol Ther. 4:949–955. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei Q, Dong G, Franklin J and Dong Z: The
pathological role of Bax in cisplatin nephrotoxicity. Kidney Int.
72:53–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peyrou M, Hanna PE and Cribb AE:
Cisplatin, gentamicin, and p-aminophenol induce markers of
endoplasmic reticulum stress in the rat kidneys. Toxicol Sci.
99:346–353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hotchkiss RS, Strasser A, McDunn JE and
Swanson PE: Cell death. N Engl J Med. 361:1570–1583. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Slee EA, Adrain C and Martin SJ:
Executioner caspase-3, -6, and -7 perform distinct, non-redundant
roles during the demolition phase of apoptosis. J Biol Chem.
276:7320–7326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olive PL and Banáth JP: Kinetics of
H2AX phosphorylation after exposure to cisplatin.
Cytometry B Clin Cytom. 76:79–90. 2009.
|
|
31
|
Fuertesa MA, Castillab J, Alonsoa C and
Perez JM: Cisplatin biochemical mechanism of action: from
cytotoxicity to induction of cell death through interconnections
between apoptotic and necrotic pathways. Curr Med Chem. 10:257–266.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gonzalez VM, Fuertes MA, Alonso C and
Perez JM: Is cisplatin-induced cell death always produced by
apoptosis? Mol Pharmacol. 59:657–663. 2001.PubMed/NCBI
|
|
33
|
Perez RP: Cellular and molecular
determinants of cisplatin resistance. Eur J Cancer. 34:1535–1542.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mandic A, Hansson J, Linder S and Shoshan
MC: Cisplatin induces endoplasmic reticulum stress and
nucleus-independent apoptotic signaling. J Biol Chem.
278:9100–9106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ruiz A, Matute C and Alberdi E:
Intracellular Ca2+ release through ryanodine receptors
contributes to AMPA receptor-mediated mitochondrial dysfunction and
ER stress in oligodendrocytes. Cell Death Dis. 1:e542010.
|
|
36
|
Lee JW, Kim WH, Yeo J and Jung MH: ER
stress is implicated in mitochondrial dysfunction-induced apoptosis
of pancreatic beta cells. Mol Cells. 30:545–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takemoto K, Miyata S, Takamura H, Katayama
T and Tohyama M: Mitochondrial TRAP1 regulates the unfolded protein
response in the endoplasmic reticulum. Neurochem Int. 58:880–887.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haghighat N, McCandless DW and Geraminegad
P: The effect of ammonium chloride on metabolism of primary neurons
and neuroblastoma cells in vitro. Metab Brain Dis. 15:151–162.
2000. View Article : Google Scholar : PubMed/NCBI
|