Introduction
Cholangiocarcinoma (CCA) is a rare and devastating
malignancy arising from the epithelium of the bile ducts (1). Patient overall survival at 5 years
from diagnosis is <5% (2). CCA
epidemiology is shifting towards a younger population, i.e. <65
years of age, with an increasing incidence of intrahepatic CCA and
an evidently decreasing rate of extrahepatic CCA (3). Currently, surgery is the only curative
therapy available for CCA patients, and palliation is a valuable
aid in relieving symptoms of obstructive jaundice and recurrent
cholangitis (4,5).
Tumor progression has been recognized as the
consequence of a dynamic ‘crosstalk’ between different cell types
of the tumor parenchyma and the surrounding tissue, the tumor
stroma (6,7). Dissimilarities between a ‘normal’ and
a ‘reactive’ stroma are currently the subject of detailed studies
(5,8–10). The
normal stroma in many organs contains a minimal amount of
fibroblasts relative to the extracellular matrix. The reactive
stroma, however, is associated with an increase in fibroblasts,
capillary density and deposition of fibrin and collagen type-1
(7).
Cancer cells may alter the adjacent stroma to create
a microenvironment that permits and supports tumor growth.
Morphological evidence describes it as a desmoplastic reaction
involving many cell types, including endothelial cells and their
precursors, pericytes, smooth muscle cells, cancer-associated
fibroblasts (CAFs), myofibroblasts, neutrophils, eosinophils,
basophils, mast cells, T and B lymphocytes, macrophages and
dendritic cells (11). Endothelial
cells, macrophages and CAFs play a key role in promoting growth and
tumor progression (12).
Compared with fibroblasts present in a quiescent
state in connective tissues or activated in response to tissue
damage or organ fibrosis, CAFs are not susceptible to apoptosis and
as such, are perpetually activated and do not return to a normal
phenotype. Morphologically, CAFs are elongated mesenchymal cells
positive for α-smooth muscle actin (α-SMA), fibroblast activation
protein (FAP), Thy-1, desmin and the S100A4 protein (13). Results of several in vivo and
in vitro studies indicate that CAFs promote tumor
progression both in a proliferative and an invasive manner through
the secretion of various growth factors and the activation of
numerous intracellular signaling pathways (14).
The desmoplastic stroma of intrahepatic
cholangiocarcinoma (ICC) is rich in α-SMA-positive fibroblasts that
surround ducts, glandular structures and aggregates of neoplastic
cholangiocytes (15) (Fig. 2). In fact, patients who have a
desmoplastic reaction rich in CAFs have a significantly lower
overall survival and a worse disease-free survival than patients
with ICC with lower levels of α-SMA positivity (16).
Cholangiocarcinoma 2-D culture models have been
described in the literature (17,18)
but they use long-standing CCA cell lines of various biliary tumor
cell origins with stromal cells derived from non-cholangiocarcinoma
tissues. In 2012, Campbell et al(19) presented their novel 3-D organotypic
co-culture model of rat cholangiocarcinoma. The aim of our study
was to devise a novel in vitro model by which to study CCA
tumor-stroma crosstalk to better investigate the interactions
between neoplastic cholangiocytes and stromal fibroblasts. From
human CCA surgical samples, human biliary epithelial cells (hBECs)
and stromal cells (SCs) were isolated and cultured in selective
media. Cultures were maintained and passaged several times, and
their purity was assessed by fluorescent immunocytochemistry.
Materials and methods
Cholangiocarcinoma specimens
Liver tissues from patients undergoing CCA resection
(Department of Surgery, Regional Center for HPB Surgery, Treviso
Regional Hospital, Treviso, Italy) were collected between 2010 and
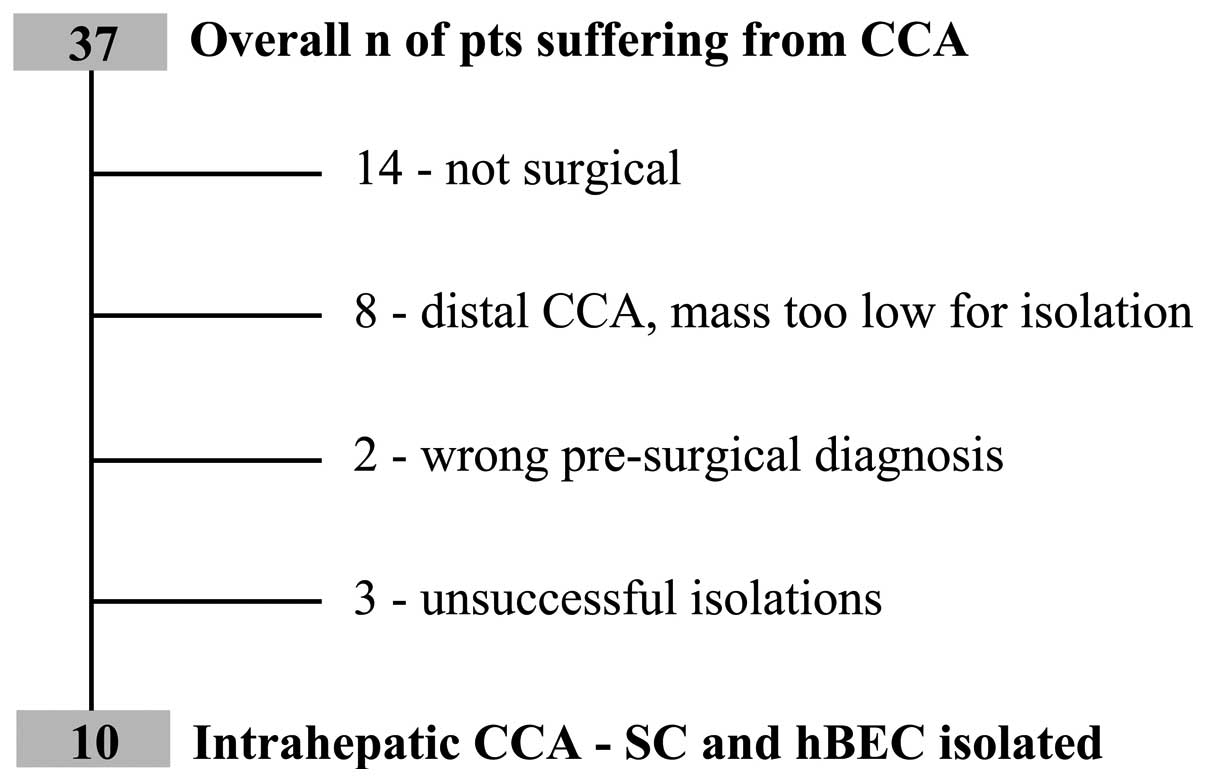
2012; a total of 37 consecutive patients with a diagnosis of
primary CCA were managed. Of the total number of patients, 14 were
not eligible for surgical resection, 8 were extrahepatic CCA with a
mass too low for cell isolation, 2 were not CCA and 3 isolation
processes were not successful due to culture infection (Fig. 1). Cultures of biliary and stromal
cells were, therefore, obtained from 10 patients and their clinical
and biological features are outlined in Table I. According to the classification
proposed by the Liver Cancer Study Group of Japan (20), the macroscopic growth patterns were:
6 mass-forming, 2 mass-forming/periductal-infiltrating and 2 not
detectable since they were isolated from metastatic samplings; all
were adenocarcinomas (Table I). The
study was approved by the local ethical committee of our
Institution.
 | Table IClinical and biochemical findings of
the 10 patients with intrahepatic cholangiocarcinoma enrolled in
the study. |
Table I
Clinical and biochemical findings of
the 10 patients with intrahepatic cholangiocarcinoma enrolled in
the study.
| Findings | Values |
|---|
| Gender |
| Male/female | 5/5 |
| Age (years; average ±
SD) | 67.54±7.07 |
| CCA |
|
Intrahepatic/extrahepatic | 10/0 |
| Dimension (cm) |
|
Average/max/min |
6.36/10.00/3.50 |
| Growth pattern |
|
MF/MF-PI/PI/IG/nd | 6/2/0/0/2 |
| Grading |
| G1/G2/G3/nd | 0/4/4/2 |
| Area
α-SMA+ (%; average ± SD) | 13.59±7.35 |
| CA 19-9 |
| Average/max/min
(U/ml) (nr 0.0–34.0) |
3,537/10,000/44.00 |
| CEA |
| Average/max/min
(ng/ml) (nr 0.0–4.3) |
71.42/267.40/0.80 |
Cell isolation and cultures
CCA cell lines were isolated as described by Fabris
et al(21). Briefly, a
minimum of 30 g of liver tissue was finely diced and digested with
collagenase type 1A (1 mg/ml; Sigma Chemical Co.). After a variable
incubation time, according to the extent of tissue fibrosis, the
liver was sieved through fine mesh (Sigma Screen cup; Sigma
Chemical Co.). The homogenate was semi-purified by centrifugation
on a 33%/77% Percoll (Sigma) gradient to reveal three separate
layers: hepatocytes, hBECs and a mixture of blood cells and CAFs.
The hBEC layer was extracted while the CAF layer was plated and
cultured.
hBECs were immunopurified using an
immunomagnetic technique
First, cells were incubated with the monoclonal
HEA125 antibody (Progen Biotechnik GmBH) and then with IgG1
paramagnetic beads (Dynabeads, Invitrogen). HEA125-positive cells
were plated and cultured in selective medium. By modifying the
Holt’s method (10) the remaining
cells were plated on flasks, and the medium was replaced after 24 h
to eliminate floating cells in order to leave behind the SC
fraction.
Growth media
CCA cell lines were grown using a selective and
complex medium for hBECs and for SCs as described by Fabris et
al(21) and Auth et
al(22).
Cell lines
In these experiments we used 10 hBEC and 7 SC cell
lines obtained from the CCA surgical specimens from the patients
with a primary CCA diagnosis managed at Treviso Regional
Hospital.
Immunohistochemical analysis of
paraffin-embedded tissue sections of CCA
To evaluate α-SMA positivity, slides of
paraffin-embedded CCA tissue sections (5-μm) were used. After
deparaffinization, sections were hydrated in alcohol, and
endogenous peroxidase activity was quenched for 30 min in methanol
and 10% hydrogen peroxide (10%). Antigen retrieval was performed by
heating slides for 20 min in 10 mM citrate buffer pH 6.0, in a
steamer. Sections were incubated overnight at 4°C with the primary
antibodies listed in Table II. The
CCA sections were rinsed with 0.05% Tween-20 in phosphate-buffered
saline (1 M) (PBS) and incubated for 30 min at room temperature
with EnVision anti-mouse antibodies (Dako, Milan, Italy) (Table III). Specimens were developed
using 3,3-diaminobenzidine tetrahydrochloride (0.04 mg/ml) (DAB;
Sigma, Milan, Italy) and H2O2 (0.01%) and
counterstained with Gill’s hematoxylin no. 2 (Sigma). All the
antibodies were diluted in PBS (Sigma) supplemented with 5% normal
human serum type 0 and 0.05% Tween-20 (Sigma).
 | Table IICharacteristics of the primary
antibodies used in the immunohistochemistry and immunofluorescence
experiments. |
Table II
Characteristics of the primary
antibodies used in the immunohistochemistry and immunofluorescence
experiments.
| Antibody | Antigenic
unmasking | Dilution | Isotype | Supplier |
|---|
| CK7 | 20 min in citrate,
10 mmol/l at pH 6.0 | 1:50 | Mouse
monoclonal | Dako |
| CK19 | 20 min in citrate,
10 mmol/l at pH 6.0 | 1:50 | Mouse
monoclonal | Dako |
| CD68 | 20 min in citrate,
10 mmol/l at pH 6.0 | 1:500 | Mouse
monoclonal | Dako |
| Vimentin | 20 min in citrate,
10 mmol/l at pH 6.0 | 1:100 | Mouse
monoclonal | Dako |
| α-SMA | 20 min in citrate,
10 mmol/l at pH 6.0 | 1:100 | Mouse
monoclonal | Dako |
 | Table IIICharacteristics of the secondary
antibodies used in the immunohistochemistry and immunofluorescence
experiments. |
Table III
Characteristics of the secondary
antibodies used in the immunohistochemistry and immunofluorescence
experiments.
| Antibody | Unmasking | Dilution | Isotype | Supplier |
|---|
| Alexa Fluor 488
anti-rabbit IgG | Fluorescence | 1:500 | Donkey
polyclonal | Invitrogen |
| Alexa Fluor 488
anti-mouse IgG | Fluorescence | 1:500 | Donkey
polyclonal | Invitrogen |
| Alexa Fluor 594
anti-mouse IgG | Fluorescence | 1:500 | Goat
polyclonal | Invitrogen |
| EnVision
anti-mouse | HRP | Net | Goat
polyclonal | Dako (K4001) |
| EnVision
anti-rabbit | HRP | Net | Goat
polyclonal | Dako (K4003) |
Immunofluorescence analysis of
paraffin-embedded tissue sections of CCA
Double immunofluorescence was performed in selected
samples to study the co-localization of the phenotypic markers of
hBECs and CAFs. The same methodology from the section above was
used up to and including the incubation with the primary
antibodies. After 3 washes with PBS plus 0.05% Tween-20 (Sigma),
the samples were incubated with the appropriate fluorescent
secondary antibody (Table III)
for 30 min. The sections were mounted with Vectashield supplemented
with 4.6-diaminidino-2-phenylindole (DAPI; Vector Laboratories) or
glycerol supplemented with 5% 1,4-diazabicyclo(2.2.2)octane
(Sigma-Aldrich).
Immunofluorescence of the cell
cultures
In the cultured cells, immunoreactivity was detected
by immunofluorescence. Briefly, cells were fixed with 4%
paraformaldehyde (Carlo Erba, Milan, Italy) and incubated overnight
at 4°C with rabbit anti-mouse antibodies (Table II). After washing with PBS, cells
were supplemented with 0.05% Tween-20, and then incubated at room
temperature with goat or donkey anti-mouse or anti-rabbit antibody
(Invitrogen, Milan, Italy) (Table
II) for 30 min and mounted with Vectastain + DAPI (Vector
Laboratories).
Morphometric analysis
Samples were analyzed with a Nikon Eclipse E800
microscope. Images were captured with a Nikon DS-U1 cooled and
analyzed using LuciaG 5.0 (Nikon, Milan, Italy). In the
histological samples from resected patients, stromal α-SMA
positivity was calculated as the positive percentage over the total
neoplastic area (calculated in 10 random fields captured at a
magnification of ×200 for each histological section).
Statistical analysis
Results are reported as averages ± standard
deviation, and data were statistically compared using the Student’s
t-test. Statistical analysis was performed using the SPSS
statistical package (SPSS Inc., Bologna, Italy); the value of
significance was set at P<0.05.
Results
Desmoplastic reaction is increased in
cholangiocarcinoma samples
In normal liver parenchyma, fibroblasts are absent
with the exception of a few perisinusoidal cells, also known as Ito
cells or hepatic stellate cells. CCA, however, presents an
increased desmoplastic area characterized by diffuse α-SMA
positivity.
In samples obtained from the CCA patients, the
proportion of desmoplastic reaction was determined using
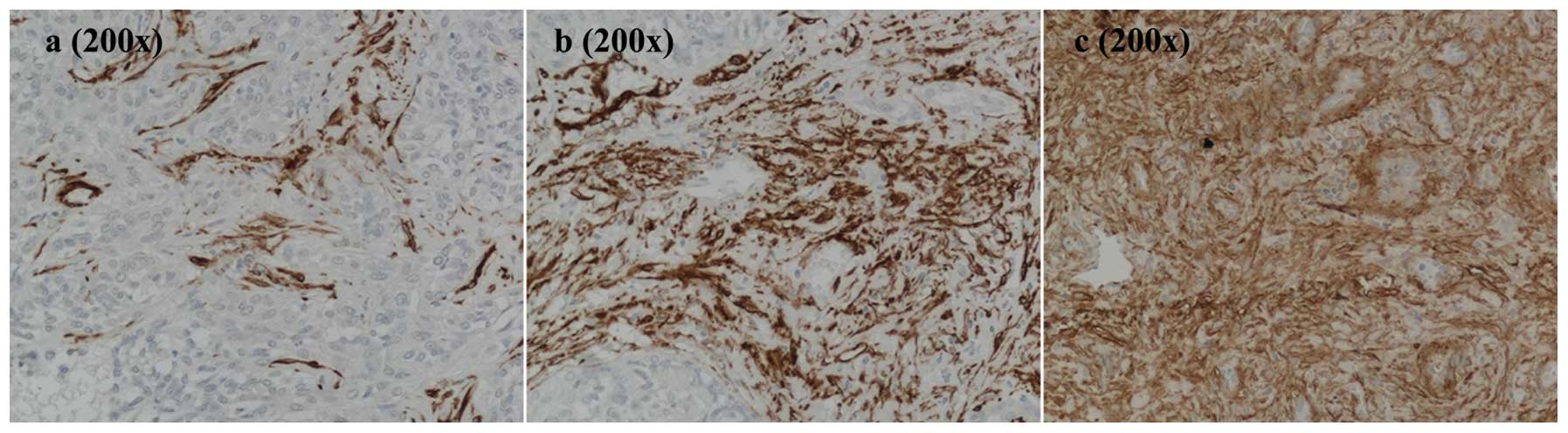
immunohistochemical staining for α-SMA (Fig. 2). Samples were characterized by the
consistent presence of α-SMA positivity at variable degrees. The
α-SMA-positive area was evaluated by morphometric analysis, and the
average distribution of α-SMA positivity was 13.59±7.35% with a
maximum value of 30.08±0.23% and a minimum of 5.45±0.03% (Table IV). In areas with the lowest
percentage (Fig. 2a), nests of
hBECs surrounded by SCs were recognized. As the stromal component
increased, the desmoplastic reaction typical of CCA became more
apparent (Fig. 2b and c).
 | Table IVValues and average distribution of
α-SMA positivity in the CCA samples (TV#) collected during the
study. |
Table IV
Values and average distribution of
α-SMA positivity in the CCA samples (TV#) collected during the
study.
| Patient sample | α-SMA
positivity |
|---|
| TV1 | 10.28±0.05 |
| TV2 | 13.04±0.07 |
| TV3 | 10.66±0.07 |
| TV4 | 5.45±0.03 |
| TV6 | 15.43±0.06 |
| TV7 | 10.18±0.08 |
| TV9 | 30.08±0.23 |
| TV10 | 14.37±0.07 |
| Average | 13.59±7.35 |
Generation of primary biliary and stromal
cell cultures from specimens of human intrahepatic CCA
In order to generate an in vitro model by
which to study tumor-stroma interactions, intra-hepatic CCA hBECs
and SCs were purified by modifying the method described by Fabris
et al(21).
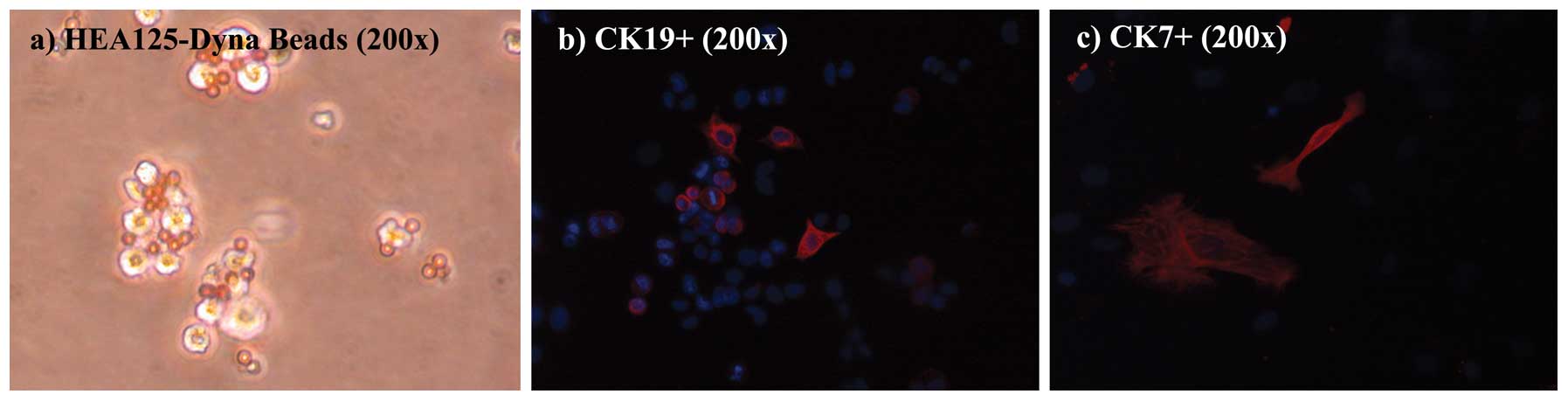
As shown in Fig. 3,
paramagnetic beads conjugated with HEA125 adhered to the cell
surface via epithelial glycoprotein 34, in the purification
process. Once placed, the addition of human hepatocyte growth
factor to the medium, a strong trophic agent for cholangiocytes,
ensured that the cells reached confluence and their classic
epithelial palisade shape was recognized.
As shown in Fig. 4,
hBECs were positive for HEA125, and the immunomagnetic Dynabeads
are shown adhering to the cell surface after plating. In the cell
cultures, immunophenotypic staining of hBECs revealed positivity at
variable degrees for CK7 and CK19, while hBECs were negative for
VMN and α-SMA, a profile consistent with their lineage (24).
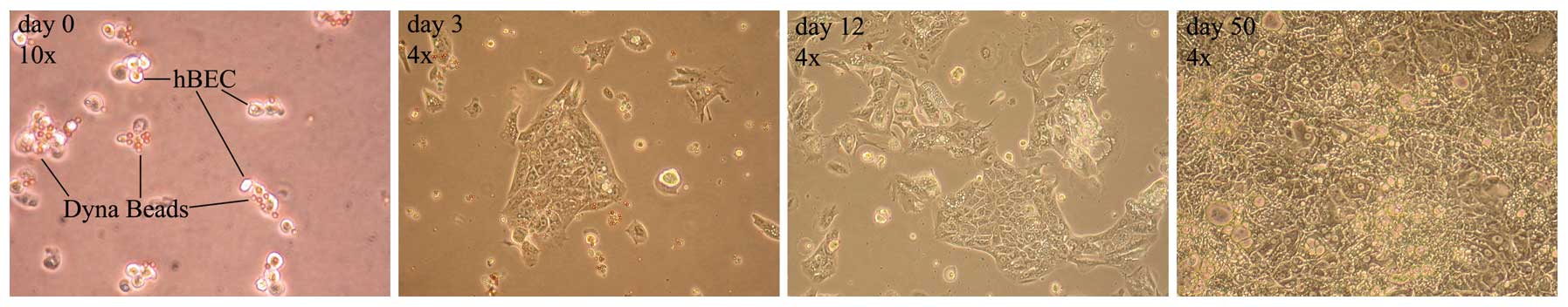
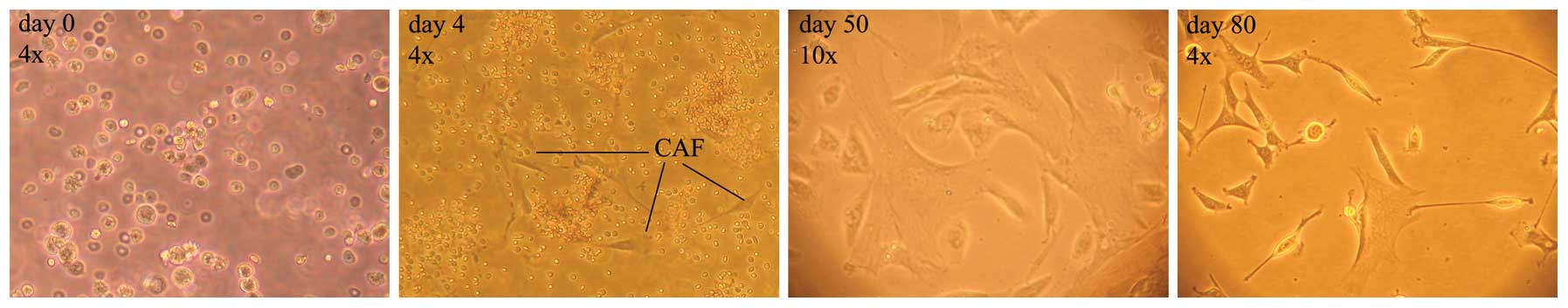
From the early days of cell culture, isolated SCs
were easily detectable by their typical spindle shape, however,
they were clouded by a mixture of varying other cell types and
debris. The cell culture was progressively purified by the
selective growth medium and its replacement after 24 h, and in the
following days allowed only for the growth of cells attached to the
flask, as illustrated in the timeline of Fig. 5.
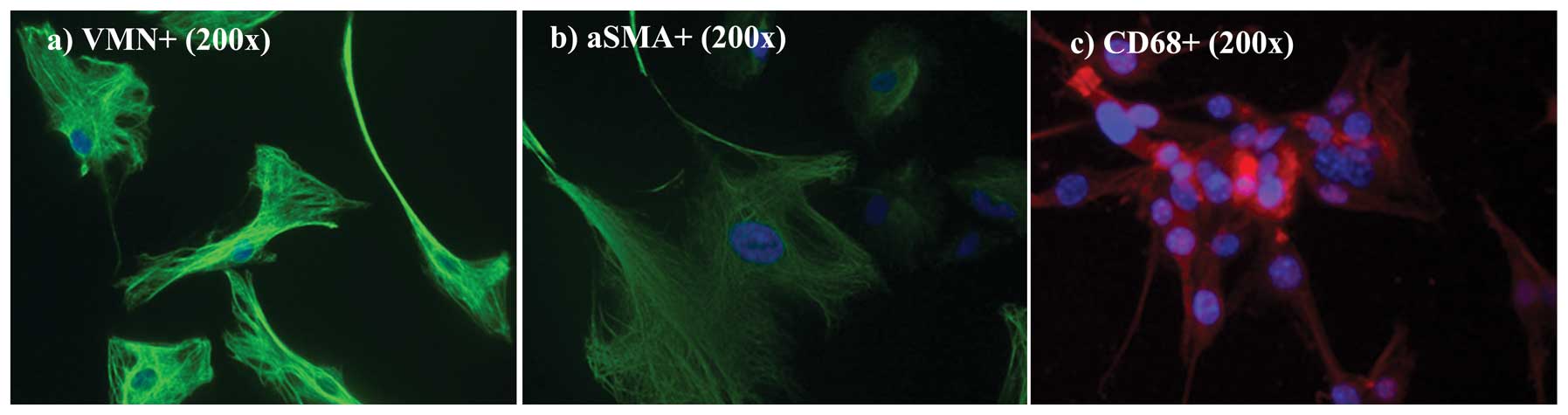
To verify the purity of the cultures,
immunofluorescence for VMN, a specific fibroblast marker, and
α-SMA, an activated fibroblast marker, were performed. In Fig. 6a and b, cultured cells were positive
for both VMN and for α-SMA (both green). As previously reported by
Kalluri and Zeisberg (7) the lack
of specific molecular fibroblast markers is a limiting factor in
studying fibroblasts. Even though none are either exclusive to
fibroblasts or present in all fibroblasts, VMN and α-SMA,
intermediate-filament-associated proteins, are specific fibroblast
markers (25,26). Nuclei were counterstained with DAPI
(blue). As shown in Fig. 6c, only a
limited fraction, <15%, was CD68-positive (CD68-negative fields
are not shown here).
Discussion
CCA is a rare cancer of the bile ducts characterized
by an insidious onset and a poor prognosis. The tumor
microenvironment has emerged as a key player in CCA invasiveness
(7,11). In particular, CAFs are involved in
tumorigenesis by promoting the growth and invasion of cancer cells
through paracrine communications (14). In addition, CAFs are responsible for
the generation of a dense desmoplastic reaction in CCA that
frequently prevents surgical resection (7,12).
In tumor samples collected during this study, CAFs
were found to be present with a mean percent value of 13.59±7.35%.
In some samples, fibroblasts were detected around neoplastic
lobules while, in others, the physiological parenchyma structure
was subverted and a widespread fibroblastic parenchymal
infiltration was present (Fig.
2).
Between 2010 and 2012, a total of 37 patients with
primary CCA were managed at Treviso Hospital (Fig. 1). Of these, 10 intrahepatic CCA
specimens were collected. According to the classification proposed
by the Liver Cancer Study Group of Japan, mass forming was the
predominant macroscopic growth pattern (Table I).
In order to generate an in vitro model by
which to study the interactions between tumoral epithelial and
mesenchymal cells in CCA, neoplastic cholangiocytes were isolated
by modification of the method described by Fabris et
al(21), and stromal
fibroblasts by the modification of Holt’s method (10). Ten CCA primary biliary epithelial
and 7 stromal cell lines were obtained. Both cell types were
maintained and passaged several times in culture. Cells were
semipurified by centrifugation on Percoll gradient and then hBECs
were further immunopurified using anti-HEA125 magnetic beads, and
plated and cultured in selective medium. In contrast, SCs were
plated and the medium was replaced after 24 h to eliminate the
unattached cells and debris. hBECs and SCs were characterized by
immunofluorescence using epithelial (CK7 and CK19) and mesenchymal
(VMN, α-SMA and CD68) cell markers. hBECs were positive for HEA125,
and at variable degrees for CK7 and CK19, while they were negative
for VMN and α-SMA. SCs were consistently positive for VMN and
α-SMA, and negative for CK7 and CK19. Only a limited fraction
(<15%) was positive for CD68.
CCA cells alter the adjacent stroma and create a
microenvironment that permits and supports its growth. Previous
studies describe long-standing CCA cell lines of various biliary
tumor cell origins with stromal cells derived from
non-cholangiocarcinoma tissues. To our knowledge this is the first
report of an in vitro model of primary human CCA cell lines
freshly isolated from resected patients. These cell cultures may
provide a useful tool to further study the tumor-stroma
interactions in human CCA. Future studies will evaluate the
migration of stromal cells to epithelium through a Boyden chamber
using inhibitors of migration. Furthermore, our epithelial/stroma
model will both serve in vitro to investigate the activated
pathways and, consequently, to treat these cells with compounds or
drugs in order to assess growth and survival under different
conditions.
The phenotype and the characterization of the
different stromal cells surrounding the CCA stroma have been widely
investigated by many authors (6,7,11,12,22),
however, this was beyond the specific aim of our study.
Abbreviations:
|
α-SMA
|
α-smooth muscle actin
|
|
CAF
|
cancer-associated fibroblast
|
|
CCA
|
cholangiocarcinoma
|
|
CK7
|
cytokeratin-7
|
|
CK19
|
cytokeratin-19
|
|
ICC
|
intrahepatic cholangiocarcinoma
|
|
hBEC
|
human biliary epithelial cell
|
|
SC
|
stromal cell
|
|
VMN
|
vimentin
|
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aljiffry M, Walsh MJ and Molinari M:
Advances in diagnosis, treatment and palliation of
cholangiocarcinoma: 1990–2009. World J Gastroenterol. 15:4240–4262.
2009.PubMed/NCBI
|
|
3
|
Deoliveira ML, Schulick RD, Nimura Y,
Rosen C, Gores G, Neuhaus P and Clavien PA: New staging system and
a registry for perihilar cholangiocarcinoma. Hepatology.
53:1363–1371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan SA, Thomas HC, Davidson BR and
Taylor-Robinson SD: Cholangiocarcinoma. Lancet. 366:1303–1314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sempoux C, Jibara G, Ward SC, Fan C, Qin
L, Roayaie S, Fiel MI, Schwartz M and Thung SN: Intrahepatic
cholangiocarcinoma: new insights in pathology. Semin Liver Dis.
31:49–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mueller MM and Fusenig NE: Friends or foes
- bipolar effects of the tumour stroma in cancer. Nat Rev Cancer.
4:839–849. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
8
|
Sirica AE: The role of cancer-associated
myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev
Gastroenterol Hepatol. 9:44–54. 2011. View Article : Google Scholar
|
|
9
|
Fabris L, Cadamuro M, Moserle L, Dziura J,
Cong X, Sambado L, Nardo G, Sonzogni A, Colledan M, Furlanetto A,
Bassi N, Massani M, Cillo U, Mescoli C, Indraccolo S, Rugge M,
Okolicsanyi L and Strazzabosco M: Nuclear expression of S100A4
calcium-binding protein increases cholangiocarcinoma invasiveness
and metastasisation. Hepatology. 54:890–899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holt AP, Haughton EL, Lalor PF, Filer A,
Buckley CD and Adams DH: Liver myofibroblasts regulate infiltration
and positioning of lymphocytes in human liver. Gastroenterology.
136:705–714. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Folkman J: Fundamental concepts of the
angiogenic process. Curr Mol Med. 3:643–651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Albini A and Sporn MB: The tumour
microenvironment as a target for chemoprevention. Nat Rev Cancer.
7:139–147. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garin-Chesa P, Old LJ and Rettig WJ: Cell
surface glycoprotein of reactive stromal fibroblasts as a potential
antibody target in human epithelial cancers. Proc Natl Acad Sci
USA. 87:7235–7239. 1990. View Article : Google Scholar
|
|
14
|
Xing F, Saidou J and Watabe K: Cancer
associated fibroblasts (CAFs) in tumor microenvironment. Front
Biosci. 15:166–179. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sirica AE, Campbell DJ and Dumur CI:
Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma.
Curr Opin Gastroenterol. 27:276–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishihara Y, Aishima S, Hayashi A, Iguchi
T, Fujita N, Taketomi A, Honda H and Tsuneyoshi M: CD10+
fibroblasts are more involved in the progression of
hilar/extrahepatic cholangiocarcinoma than of peripheral
intrahepatic cholangiocarcinoma. Histopathology. 55:423–431.
2009.
|
|
17
|
Chuaysri C, Thuwajit P and Paupairoj A:
Alpha-smooth muscle actin-positive fibroblasts promote biliary cell
proliferation and correlate with poor survival in
cholangiocarcinoma. Oncol Rep. 21:957–969. 2009.PubMed/NCBI
|
|
18
|
Utispan K, Thuwajit P and Abiko Y: Gene
expression profiling of cholangiocarcinoma-derived fibroblasts
reveals alterations related to tumor progression and indicates
periostin as a poor prognostic marker. Mol Cancer. 9:132010.
View Article : Google Scholar
|
|
19
|
Campbell DJW, Dumur CI, Lamour NF, DeWitt
JL and Sirica AE: Novel organotypic culture model of
cholangiocarcinoma progression. Hepatol Res. 42:1119–1130. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim JH: Cholangiocarcinoma: morphologic
classification according to growth pattern and imaging findings.
AJR Am J Roentgenol. 181:819–827. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fabris L, Strazzabosco M, Crosby HA,
Ballardini G, Hubscher SG, Kelly DA, Neuberger JM, Strain AJ and
Joplin R: Characterization and isolation of ductular cells
coexpressing neural cell adhesion molecule and Bcl-2 from primary
cholangiopathies and ductal plate malformations. Am J Pathol.
156:1599–1612. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Auth MK, Woitaschek D, Beste M, Schreiter
T, Kim HS, Oppermann E, Joplin RE, Baumann U, Hilgard P, Nadalin S,
Markus BH and Blaheta RA: Preservation of the synthetic and
metabolic capacity of isolated human hepatocytes by coculture with
human biliary epithelial cells. Liver Transpl. 11:410–419. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cannito S, Novo E, Compagnone A, Valfrè di
Bonzo L, Busletta C, Zamara E, Paternostro C, Povero D, Bandino A,
Bozzo F, Cravanzola C, Bravoco V, Colombatto S and Parola M: Redox
mechanisms switch on hypoxia-dependent epithelial-mesenchymal
transition in cancer cells. Carcinogenesis. 29:2267–2278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bateman AC and Hübscher SG: Cytokeratin
expression as an aid to diagnosis in medical liver biopsies.
Histopathology. 56:415–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mork C, van Deurs B and Petersen OW:
Regulation of vimentin expression in cultured human mammary
epithelial cells. Differentiation. 43:146–156. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechanoregulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|