Introduction
Breast cancer is the most common cancer in females
worldwide with an age-standardized rate (ASR) of newly-diagnosed
cases at 39/100,000 people and deaths at 12.5/100,000 people
annually, in 2008 (1). The
incidence rate was reported as increasing, particularly in low- and
middle-income countries (2). While
the management of breast cancer has advanced from molecular studies
to clinical outcome, the standard treatments for breast cancer are
still based on surgery with improvement of tissue conserved,
radiation and addition of chemotherapy both cytotoxic and targeted
types, in the advance stages (3,4).
Breast cancer has a shown variety of cellular marker protein
expressions which could determine responses to chemical treatment,
for example, the estrogen receptor (ER), progesterone receptor (PR)
and human epidermal growth factor receptor 2 (HER2), all of which
may be considered as targets for effective therapies (5). ER indicated the response to estrogenic
agents for regulation of cancer cell proliferation and this could
be inhibited using anti-estrogenic drugs such as tamoxifen and
fulvestrant which have been used in clinical practice (6). Moreover, in the past decades, there
have been numerous reports of patients that demonstrated that
expression of ER showed less sensitivity to cytotoxic agents in
breast cancer cell (7–9). Recently, an in vitro experiment
demonstrated the cellular response to co-treatment with estrogen
and doxorubicin, a first-line cytotoxic chemotherapeutic agent
commonly used in treatment of advanced stage breast cancer
(10). It was shown that
doxorubicin could impair estrogen-induced proliferation in human ER
positive breast cancer cell lines T47D and MCF-7 (10). On the other hand, the role of
estrogen in reducing the cytotoxic effect of doxorubicin cannot be
undermined. The study of the mechanism of doxorubicin resistance in
estrogen-related ER positive breast cancer cells is also
noteworthy.
Estrogen is a group of sex steroid hormones which
functions mainly in the female reproductive system (11,12).
Traditionally, estrogen binds to intracellular ER and then the
hormone-receptor complex should bind directly to the regulatory
elements on DNA sequences, such as the estrogen responsive element
(ERE), and affect gene expressions (11). These estrogen-regulated proteins are
involved in several cellular processes including apoptosis
(12). While the mechanism of
almost all cytotoxic agents including doxorubicin is the induction
of cancer cells to the apoptotic process resulting in cell death,
the proteins that affect apoptosis should be considered as involved
in drug resistance. In fact, estrogen could regulate breast cancer
cells in opposite directions, apoptosis induction and
anti-apoptosis, depending on various factors including estrogen
concentration and cell types (12).
Formerly, the treatment of ER positive breast cancer patients
included the use of high-dose estrogen (13) until the use of anti-estrogen
tamoxifen was implicated (14).
With physiological concentrations of estrogen, the estrogen could
stimulate apoptosis in MCF-7 cells with prolonged estrogen
deprivation conditions (15).
However, in physiological concentrations, estrogen prefers to
inhibit apoptotic processes induced by serum deprivation in ER
positive breast cancer cell lines MCF-7, T47D and ZR-75-1 (16). In addition, estrogen was also able
to inhibit the apoptotic process induced by either hydrogen
peroxide (17) or doxorubicin
(18). One of the significant genes
which contains ERE on the gene 5′ region (19), a site that could affect cell
apoptosis is a the pS2 gene, also known as trefoil factor
family-1 (TFF1) (20,21).
TFF1 is a secreted protein that was first found in
the MCF-7 breast cancer cell line (22). It is the first member of a trefoil
factor family which includes TFF2 and TFF3 in mammals (23). Normally, TFF1 co-expresses with
MUC5AC mucin and forms a stable cross-linked polymer for epithelial
protection in various tissues, mainly in gastric mucosa, and is
also expressed in tracheal mucosa and conjunctival goblet cells
(24,25). TFF1 can also stimulate epithelial
cell migration that promotes a healing process but it is considered
to promote metastasis in carcinoma (23,26).
An in vitro study showed that TFF1 can promote invasion of
human cholangiocarcinoma cell lines and breast cancer cell lines
(26,27). By contrast, TFF1 was reported as a
tumor suppressor protein in gastric cancer (28). TFF1 is upregulated in various
pathological conditions including inflammation and cancer such as
breast, ovarian, lung, prostate, pancreas, colon and
cholangiocarcinoma (23,26). A previous study reported high
expression of TFF1 detected in 74% of breast cancer tissues, with
correlation to the hormonal receptor status (29), while normal breast tissue showed
minimal expression of TFF1 (25,30).
TFF1 was also detected in breast cancer patient sera which
correlated with the tumor proliferative rate (31). In laboratory experiments a
controversy has developed over whether TFF1 enhances (32) or suppresses (33) oncogenicity of breast cancer. The
discussion revolves around the possible differences in cell lines
and methods that might produce the different outcomes. TFF1,
however, could be used as marker for responding to hormonal therapy
such as aromatase inhibitors and ER antagonist tamoxifen in
clinical therapy (34,35).
Previous in vitro experiments showed that
TFF1 could suppress apoptosis stimulated by chemically-induced Bad
expression or anchorage-dependent apoptosis in gastrointestinal rat
IEC18 diploid intestinal cells, human HCT116 colon cancer cells and
AGS gastric cancer cells (20). In
addition, TFF1 could protect Chang conjunctival cells from chemical
or ultraviolet radiation-induced apoptosis (21). TFF1 is also a downstream product of
estrogenic stimulant and estrogen itself could inhibit
induced-apoptosis in breast cancer cells; TFF1 also showed high
expression in breast cancer tissue, particularly in ER positive
tissues, which naturally resist cytotoxic chemotherapeutic agents.
The hypothesis that estrogen could inhibit chemical-induced
apoptosis in breast cancer cells via a TFF1-dependent mechanism
merits further investigation. In the present study, it was
demonstrated that TFF1 stable knockdown of MCF-7 cells as a
tool for studying doxorubicin-induced apoptosis in the presence of
17β-estradiol (E2) could show apoptosis protection or not. The
phenomenon was also confirmed by reversing the experiments using
anti-TFF1 antibody and recombinant TFF1 (rTFF1) for treating the
mock control and knockdown conditions. The apoptotic process was
analyzed using flow cytometry, viable cell counting and an
apoptosis protein array and the proteins of interest were then
chosen for final functional assay. The present study further
clarifies the role of TFF1 in estrogen-induced breast cancer
resistance to chemotherapy.
Materials and methods
Cell line and chemical agents
ER positive human breast adenocarcinoma MCF-7 cells
were grown as a monolayer in Dulbecco's modified Eagle's medium
(DMEM) (Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (FBS), antibiotics and an antimycotic including
0.1 U/ml penicillin G sodium, 0.1 mg/ml streptomycin and 5 mg/ml of
amphotericin B. Cells were cultured in adhesive sterile culture
flasks at 37°C in a 5% CO2 humidified incubator. During
the experiment, cells were passaged to appropriate containers
allowing for adherence for 24 h. Then the media was replaced by
phenol red-free DMEM (Gibco-Invitrogen) supplemented with 10%
charcoal stripped FBS (Gibco-Invitrogen) to diminish the estrogenic
effect of phenol red. In the conditions with estrogen treatment,
media was added with 1 nM E2 (Sigma-Aldrich, St. Louis, MO, USA).
This pre-treatment process was performed for 48 h and the media was
changed to phenol red-free media to proceed with treatment
conditions. Cells were treated with 1 nM E2, 1 μM doxorubicin
(Pfizer, Midtown Manhattan, NY, USA), 10 μM fulvestrant
(Sigma-Aldrich), 100 μg/ml anti-TFF1 antibody (Sigma-Aldrich) or 10
μg/ml rTFF1 protein (Abcam, Cambridge, MA, USA), alone or in
combinations.
TFF1 stable knockdown
MCF-7 breast cancer cells were transfected with
MISSION® shRNA TFF1 pLKO.1-puro plasmid DNA
vectors (Sigma-Aldrich) and selected by puromycin. Empty
pLKO.1-puro plasmid (Sigma-Aldrich) was used as mock control. The
transfection process was performed using Lipofectamine®
2000 (Invitrogen) following the manufacturer's protocol. Stable
transfected cells were selected using an appropriate dose of
puromycin (Sigma-Aldrich) and gradually increased up to 10 mg/ml
with a 7-day duration. The knockdown of TFF1 was analyzed by
immunoblotting analysis. Cells with least expression of TFF1 were
used as TFF1-knockdown (TFF1-KD) cells.
Immunoblotting analysis
Proteins from the whole cell lysate were separated
by 15–20% SDS-polyacrylamide gel electrophoresis. Subsequently,
proteins for western blots were transferred to PVDF membranes for
immunodetection. For TFF1, the membranes were probed with
polyclonal anti-human TFF1 (Sigma-Aldrich; 1:2,000). Then,
detection of antibody binding was performed using horseradish
peroxide-labeled goat anti-rabbit secondary antibody (Abcam;
1:2,000). The signal was developed using SuperSignal West Pico®
Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL,
USA) and detected with autoradiography. β-actin was used as a
loading control with the same procedure.
Apoptosis measurement
An apoptosis assay of mock and TFF1-KD MCF-7
cells treated with doxorubicin or E2 alone or in combination was
performed using flow cytometry after fluorescein isothiocyanate
(FITC)-conjugated Annexin V/propidium iodide (PI) staining by BD
Pharmingen™ FITC Annexin V Apoptosis Detection kit I
(Becton-Dickinson, Franklin Lakes, NJ, USA). Cells were seeded in a
6-well plate. After the pre-treatment process, the media was
changed to similar media and doxorubicin was added in the
doxorubicin-treated conditions. At 18 h after treatment, both
detached and adherent cells were harvested. The cells were
centrifuged to remove the media followed by an ice cold
phosphate-buffer saline (PBS) wash. The cells were gently
resuspended in Annexin V binding buffer (provided by manufacturer)
and ~1×105 cells were transferred to a 5-ml tube, with
FITC-Annexin V and PI added and incubated in the dark at 20–25°C
for 15 min and fixed by freshly prepared 4% paraformaldehyde for 15
min. All processes were performed on ice. The fluorescence
intensity was determined by BD FACsFlow™ FACS analysis
(Becton-Dickinson).
Assessment of cell death determined after
neutralizing with anti-TFF1 antibody or antagonizing estrogen with
fulvestrant or reconstitution of TFF1 to the TFF1-KD cells
Mock or TFF1-KD MCF-7 cells were seeded in a
96-well plate and pre-treated with phenol red-free media with or
without E2 as explained. The respective wells were then treated
with doxorubicin, E2, anti-human TFF1 antibody, full length rTFF1
or fulvestrant alone or in combination prepared in 200 μl of media.
Untreated cells were used as background controls. Eighteen hours
after treatment, total cells were harvested then stained by trypan
blue for viable cell counting under the microscope. The experiments
were performed multiple times independently with duplication each
time.
Protein array
Mock or TFF1-KD MCF-7 cells were seeded in
75-cm2 cell culture flasks. After the pre-treatment
process, the medium was replaced with medium containing doxorubicin
alone or in combination with E2. Eighteen hours after treatment,
the cells were harvested for analysis of apoptosis protein
expression. The apoptosis protein assay used in the present study
was Proteome Profiler™ Human Apoptosis array (R&D Systems,
Minneapolis, MN, USA). According to the manufacturer's
instructions, the remaining PBS was removed and ~1×107
cells were solubilized in lysis buffer provided by the
manufacturer. A total protein assay was performed on the
supernatant using Coomassie Plus™ (Bradford) protein assay (Thermo
Fisher Scientific). Appropriate dilutions of protein in the lysates
were prepared as per the maximum allowable volume per array
recommended by the manufacturer. The recommended quantity of
lysates was diluted and pipetted onto the membranes and incubated
overnight at 2–8°C on a rocking platform shaker. Biotinylated
secondary antibody cocktail provided by the manufacturer was
pipetted onto membranes and incubated for 1 h. After the washing
process, the membranes were incubated with streptavidin-HRP
provided by the manufacturer for 30 min. The signals were developed
using chemiluminescent reagents and then exposed to X-ray films.
The positive signals were analyzed using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Determination of catalase activity
Dichromate in the presence of acetic acid and
hydrogen peroxide (H2O2) with heat was
reduced to chromic acetate that can be measured colorimetrically
and when compared to H2O2 concentration. The
catalase enzyme eliminated H2O2 and the
reaction was stopped by adding the dichromate/acetic acid mixture.
Then the remaining H2O2 was determined by
spectrophotometry at 570–610 nm. A catalase activity assay was
modified from a previous report (36). Briefly, cells were seeded into the
25-cm2 culture flask and pre-treated with phenol
red-free media with or without E2 as explained. The cells were then
treated with doxorubicin with or without E2 with non-treated
controls. After 18 h, the cells were harvested and 3×105
cells were washed with PBS and lysed in 100 μl of chilled 0.1%
Triton X-100 in 10 mM phosphate buffer at pH 7.4. An aliquot was
taken for the Bradford assay and 90 μl of cell lysates were added
with bovine serum albumin and H2O2 to a final
concentration of 0.5% (w/v) and 20 mM. The reaction mixtures were
incubated at 37°C for 10 min and the reactions were stopped by
adding 30 μl of freshly prepared 1.25% (w/v)
K2Cr2O7 in 75% (v/v) acetic acid
and boiled for 10 min. The reaction mixtures were then cooled on
ice for 1 min and centrifuged at 12,000 rpm, 4°C for 5 min.
Supernatants were obtained for measuring optical density at 570 nm
by spectrophotometry. Specific activity of catalase (U/mg protein)
was calculated and compared between conditions.
Statistical analysis
The comparison of the anti-apoptotic effect of TFF1
between the mock MCF-7 cell and the TFF1-KD MCF-7 cells
under different treatment conditions were computed by the
independent t-test using SigmaStat software version 3.5.1.2 (Systat
Software, Richmond, CA, USA). A P-value of <0.05 was considered
to indicate a statistically significant difference. For the protein
array experiment, individual signals from gene expression with
background subtracted were normalized with the average signal from
of positive controls. Upregulation of protein was considered when
the signal was increased more than 1.5-fold and downregulation was
the reduction <0.66-fold.
Results
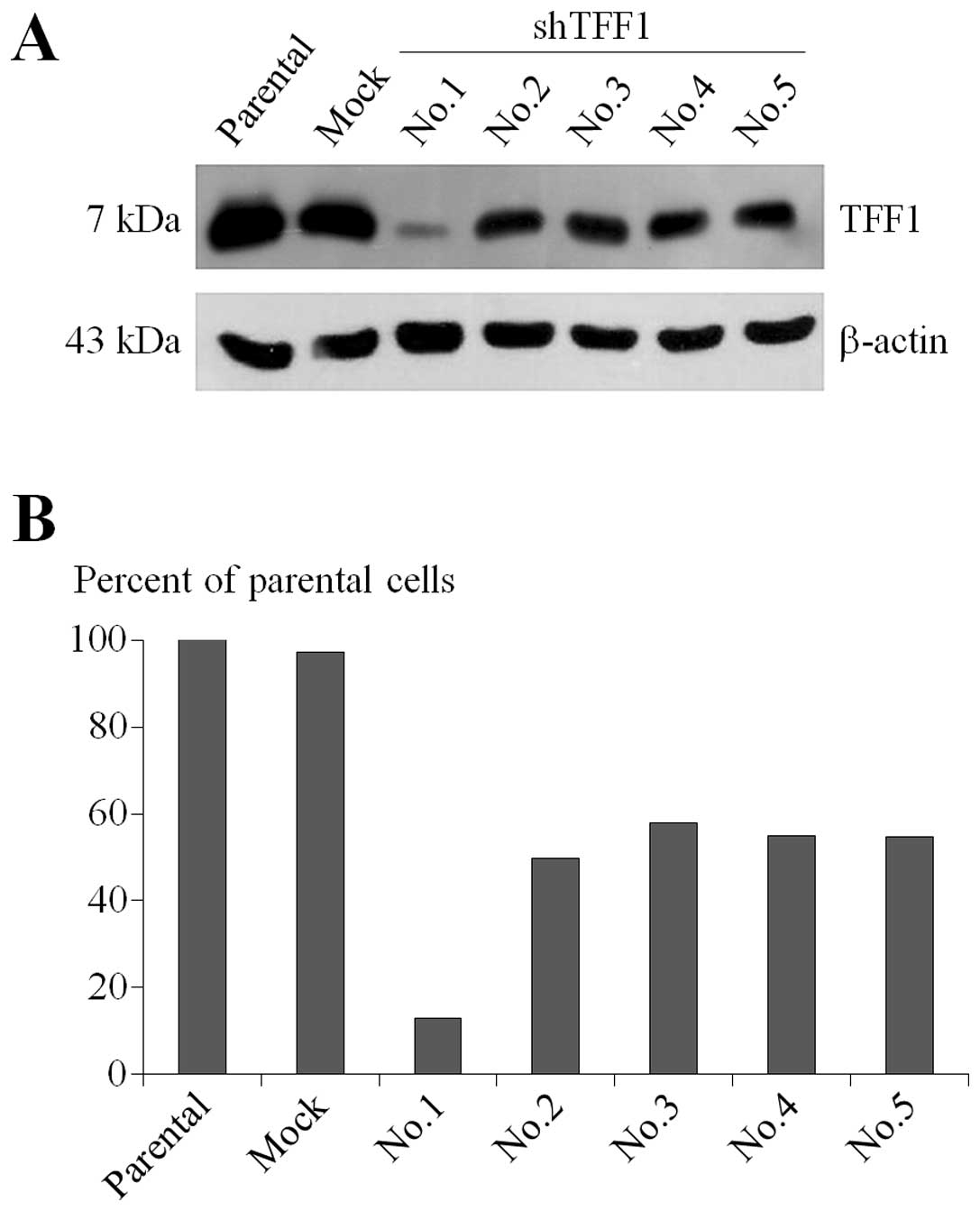
Generation of TFF1-KD MCF7 breast cancer
cells
Stable TFF1-KD MCF-7 cells were created by
knocking down TFF1 using shRNA strategy. Five potential
complementary sequence inserts for TFF1 gene were used to
stably modify MCF-7 cells and an empty vector was used to generate
the mock controls for the experiment. The transfected cells were
then selected by gradually increasing up to 10 μg/ml of puromycin
in complete media. The positively transfected cells, resistant to
puromycin, were collected and propagated until adequate confluency
was achieved. There were 5 sets of plasmids provided and labeled as
Nos.1–5 as referred to in the RNAi Consortium (TRC) numbers
TRCN_00000033614 to TRCN_00000033618. The immunoblotting experiment
showed that only No.1 could generate successful gene knockdown
(Fig. 1A). No.1 showed the
knockdown efficiency of ~87% which had been achieved in comparison
to the parental MCF-7 cells while the others showed <50% and
mock MCF-7 cells retained their intrinsic expression (Fig. 1B). No.1 was chosen as TFF1-KD
cells used in further experiments.
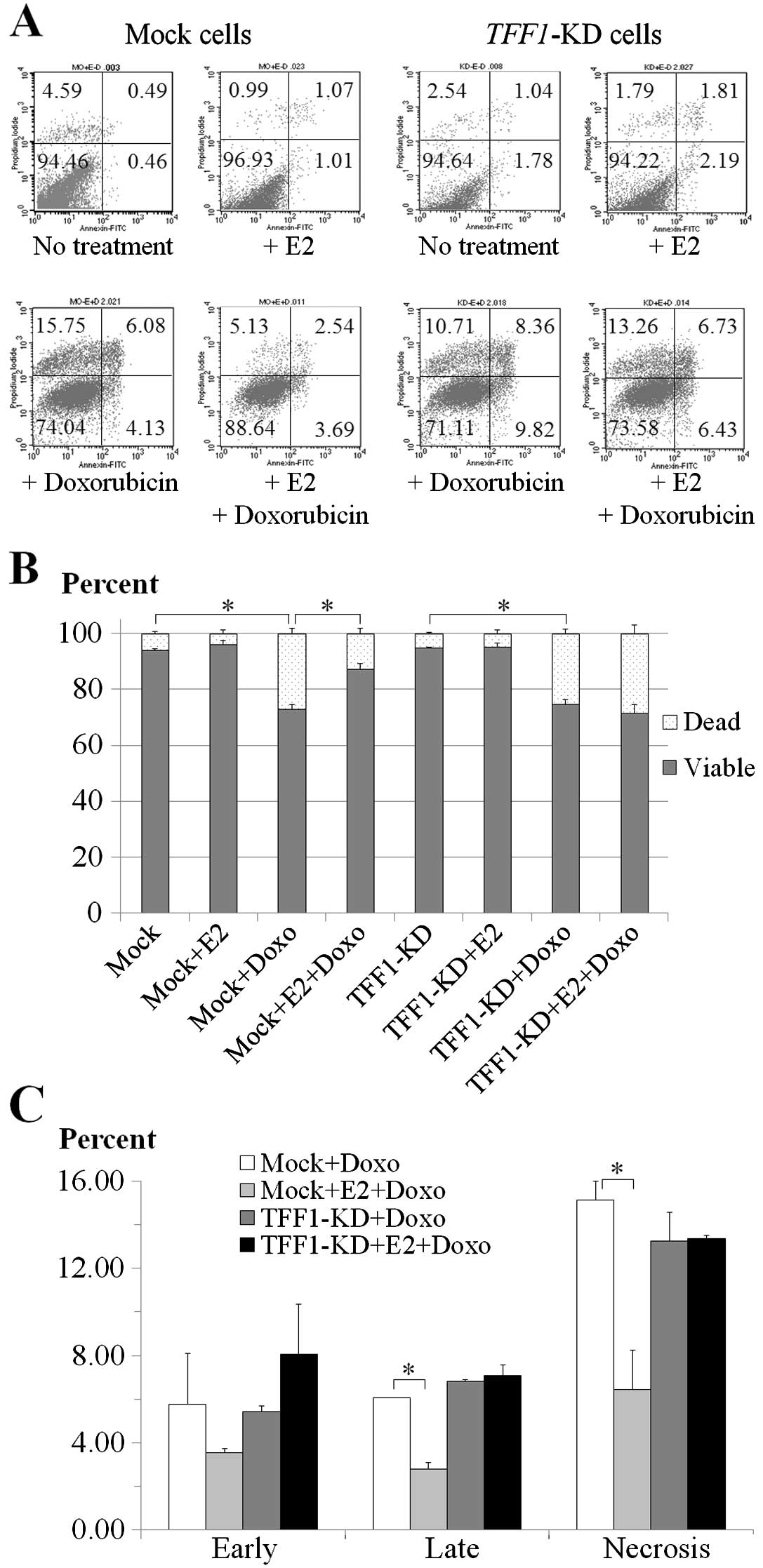
Apoptosis assay
The mock and TFF1-KD cells were incubated
with 1 μM doxorubicin and 1 nM E2 in combination or separately for
18 h and were subjected to FITC-Annexin V and PI staining and
analyzed by flow cytometry as shown by the dot plots in Fig. 2A. Treatment with 1 μM doxorubicin
showed a significant decrease in viability of both mock and
TFF1-KD cells with or without 1 nM E2 (Fig. 2B). In mock cells without E2
treatment, the percentage of viable cells was reduced 21.29%
(P=0.004) but in the presence of E2 the reduction was minimized to
only 8.84% (P=0.032) (Fig. 2B) with
a statistical significance of P=0.016 (Fig. 2B). In TFF1-KD cells the
percentage reductions of viable cells were not significantly
different (P=0.335) (Fig. 2B) as
20.28% (P=0.003) and 23.62% (P=0.009) for the conditions of absence
and presence of E2 (Fig. 2B).
Only mock cells with E2-treatment showed reduction
of percentage in all dead cell components; early apoptosis (2.23%,
P=0.308), late apoptosis (3.31%, P=0.005) and necrosis (8.71%,
P=0.026) after 18-h exposure to doxorubicin when compared to cells
without E2 treatment (Fig. 2C). By
contrast, E2 treatment did not show the significant difference of
dead cell percentages in TFF1-KD cells after treatment with
doxorubicin. The percentages were increased in early apoptosis
(2.64%, P=0.249), late apoptosis (0.27%, P=0.53) and necrosis
(0.10%, P=0.925) (Fig. 2C) in
E2-treated TFF1-KD cells. Collectively, doxorubicin
treatment caused significant apoptosis in both the mock and
TFF1-KD cells but the addition of E2 protected only the mock
cells.
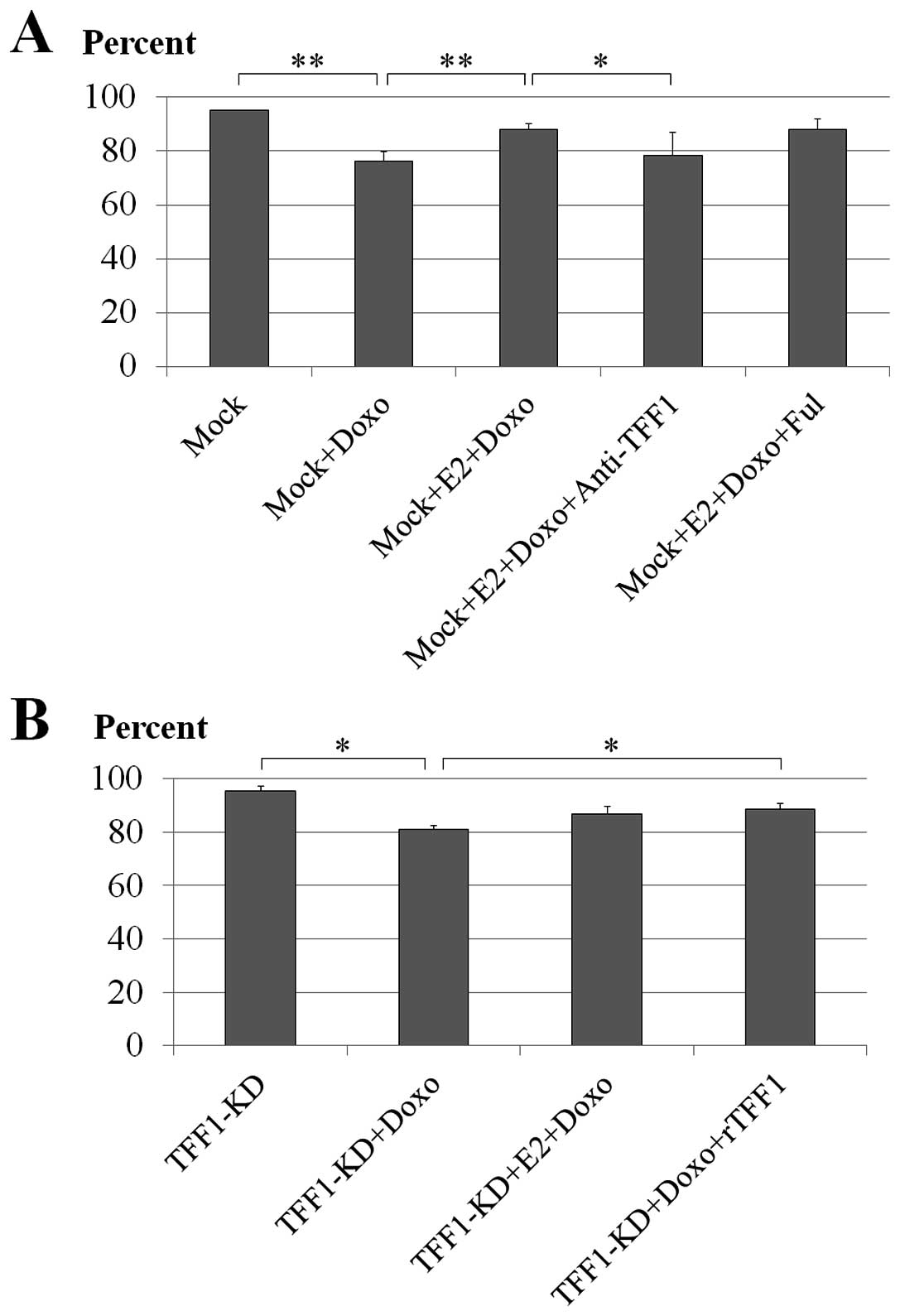
Effect of neutralization of the secreted
TFF1 with anti-TFF1 antibody
To evaluate if the neutralization of the secreted
TFF1 had any effect on the doxorubicin-induced cell death,
anti-TFF1 antibody was applied to mock MCF-7 cells treated with E2
and doxorubicin. Anti-TFF1 antibody used in this experiment was the
same one that was used as primary antibody to detect TFF1 trefoil
protein expression in the immunoblotting experiment and with this
experimental concentration (100 μg/ml) it showed a minimal effect
on cell viability after 18 h (data not shown). Doxorubicin caused
significant cell death in the mock cells (76% viable cells;
P<0.001) compared to untreated cells (95% viable cells) while E2
significantly rescued the cells (88% viable cells; P<0.001). The
addition of anti-TFF1 antibody showed obvious neutralization of
TFF1 by increasing the number of cell deaths significantly (79%
viable cells; P=0.029) compared to the reverse effect exhibited by
the E2 treatment (Fig. 3A).
Effect of ER antagonist fulvestrant
Estrogen has been shown to have pro-survival effects
on the MCF-7 cells. In this experiment, the fulvestrant was
expected to block the action of the added estrogen by
downregulating the effective ERs in the MCF-7 cells, to further
elucidate the pro-survival role of estrogen-induced TFF1 following
chemotherapeutic drug treatment. The result showed, however, that
the fulvestrant failed to diminish the pro-survival effect of E2 on
doxorubicin-treated mock MCF-7 cells (88% viable cells; P=0.953)
compared to E2 and doxorubicin-treated mock cells (88% viable
cells) (Fig. 3A).
Treatment of the TFF1-KD cells with rTFF1
and E2
TFF1 trefoil protein was reconstituted in the
TFF1-KD MCF-7 cells by treating with rTFF1 during incubation
with E2 and doxorubicin. The ability of the rTFF1 to rescue the
TFF1-KD MCF-7 cells was examined in cells treated with E2
and doxorubicin. By comparing to untreated cells (95% viable
cells), doxorubicin treatment showed induction of cell death in the
TFF1-KD cells (81% viable cells; P=0.013) and E2 did not
significantly recover this effect in these cells (87% viable cells;
P=0.124). The pro-survival effect by rTFF1 was evident at 100 μg/ml
with statistical significance (89% viable cells; P=0.037) (Fig. 3B).
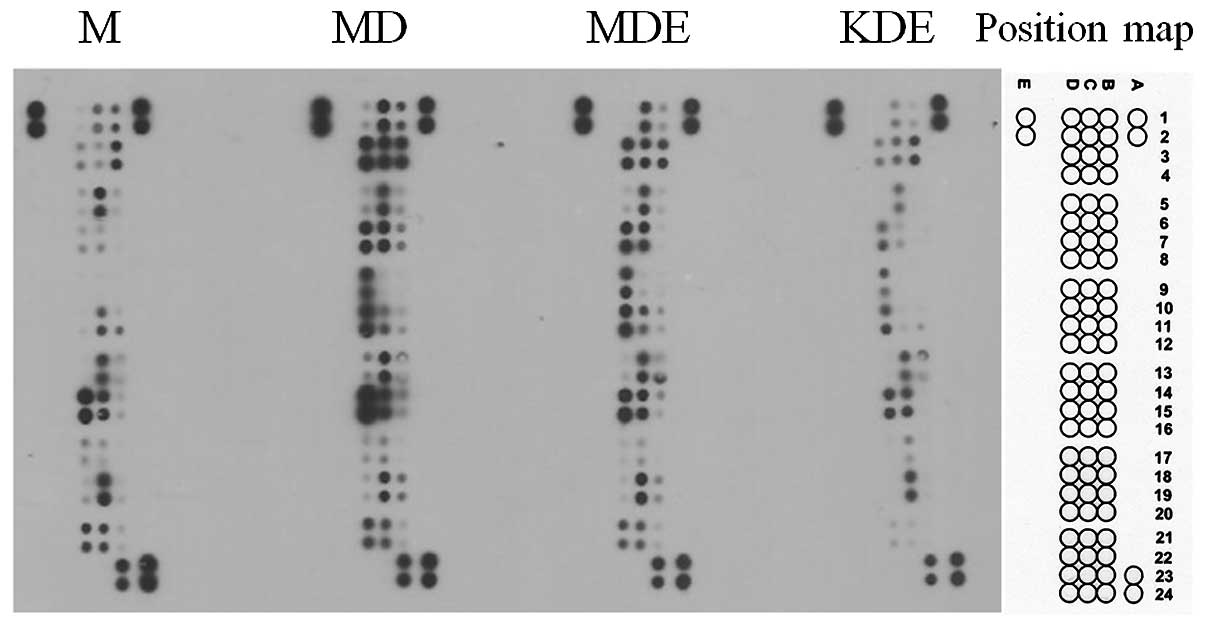
Apoptosis protein array
To investigate changes in the apoptosis protein
expression under different conditions, four different treatment
conditions were set up including non-treated controls.
Doxorubicin-mediated protein expressions were compared to those of
the untreated mock MCF-7 cells. The doxorubicin and E2 co-treated
condition was compared to the doxorubicin-treated mock cells to
examine the E2 effect. The TFF1 effect was compared between the
mock and TFF1-KD cells co-treated with doxorubicin and E2.
Fig. 4 represents the ECL developed
protein array results with the aforementioned conditions.
Of the 35 proteins on the array, doxorubicin-treated
mock MCF-7 cells showed upregulated expression of 24 proteins, 12
pro-apoptosis proteins and 12 anti-apoptosis proteins, compared to
non-treated mock control cells while no significant reduction was
observed between these conditions (Table I). When mock MCF-7 cells, which were
co-treated with doxorubicin and E2, were compared to
doxorubicin-treated cells, 4 upregulated proteins (1 pro-apoptosis
protein and 3 anti-apoptosis proteins) and 5 downregulated proteins
(3 pro-apoptosis proteins 2 anti-apoptosis proteins) were observed
(Table I). The comparison between
TFF1-KD cells and mock cells co-treated with doxorubicin and E2
demonstrated only downregulated expression on 26 proteins (15
pro-apoptosis proteins and 11 anti-apoptosis proteins) but not
upregulated proteins (Table I).
Among these three comparisons, proteins which could be regulated by
E2 treatment and TFF1 expression and correlated with the apoptotic
status of the cell were catalase and clusterin. Since catalase is
the intracellular enzyme, it was chosen to analyze its
activity.
 | Table ICalculated signal from apoptosis
protein arrays. |
Table I
Calculated signal from apoptosis
protein arrays.
| | | Calculated
signalb | Ratioc |
|---|
| | |
|
|
|---|
| Position | Protein | Pro/Antia | M | MD | MDE | KDE | MD/M | MDE/MD | KDE/MDE |
|---|
| B1,2 | Bad | P | 0.144 | 0.230 | 0.113 | 0.056 | 1.6d | 0.5d | 0.5 |
| B3,4 | Bax | P | 0.359 | 0.655 | 0.442 | 0.431 | 1.8d | 0.7d | 1.0 |
| B5,6 | Bcl-2 | A | 0.014 | 0.050 | 0.041 | 0.017 | 3.6d | 0.8 | 0.4e |
| B7,8 | Bcl-x | A | 0.004 | 0.099 | 0.022 | 0.008 | 26.9d | 0.2d | 0.4e |
| B9,10 | Pro-caspase-3 | P | 0.001 | 0.013 | 0.016 | 0.003 | 15.6d | 1.2 | 0.2d |
| B11,12 | Cleaved
caspase-3 | P | 0.055 | 0.039 | 0.073 | 0.044 | 0.7 | 1.9d | 0.6 |
| B13,14 | Catalase | A | 0.035 | 0.063 | 0.323 | 0.192 | 1.8d | 5.1 | 0.6 |
| B15,16 | cIAP-1 | A | 0.021 | 0.092 | 0.107 | 0.023 | 4.5d | 1.2 | 0.2 |
| B17,18 | cIAP-2 | A | 0.003 | 0.010 | 0.009 | 0.007 | 2.9d | 0.9 | 0.8 |
| B19,20 | Claspin | A | 0.017 | 0.105 | 0.116 | 0.026 | 6.2e | 1.1 | 0.2d |
| B21,22 | Clusterin | A | 0.010 | 0.021 | 0.036 | 0.008 | 2.0d | 1.7d | 0.2d |
| B23,24 | Cytochrome
c | P | 0.532 | 0.638 | 0.643 | 0.531 | 1.2d | 1.0 | 0.8 |
| C1,2 | TRAIL R1/DR4 | P | 0.121 | 0.646 | 0.565 | 0.176 | 5.4d | 0.9 | 0.3d |
| C3,4 | TRAIL R2/DR5 | P | 0.030 | 0.849 | 0.520 | 0.296 | 28.0e | 0.6d | 0.6d |
| C5,6 | FADD | P | 0.433 | 0.593 | 0.542 | 0.334 | 1.4d | 0.9 | 0.6e |
| C7,8 | Fas/TNFRSF6 | P | 0.026 | 0.636 | 0.597 | 0.084 | 25.0e | 0.9 | 0.1d |
| C9,10 | HIF-1a | P | 0.003 | 0.044 | 0.045 | 0.009 | 17.7d | 1.0 | 0.2d |
| C11,12 |
HO-1/HMOX/HSP32 | A | 0.210 | 0.282 | 0.381 | 0.032 | 1.3 | 1.4d | 0.1e |
| C13,14 | HO-2/HMOX2 | A | 0.394 | 0.574 | 0.642 | 0.506 | 1.5d | 1.1 | 0.8d |
| C15,16 | HSP27 | A | 0.468 | 0.866 | 0.620 | 0.557 | 1.9d | 0.7d | 0.9 |
| C17,18 | HSP60 | A | 0.021 | 0.061 | 0.095 | 0.125 | 2.9d | 1.6d | 1.3 |
| C19,20 | HSP70 | A | 0.602 | 0.436 | 0.604 | 0.582 | 0.7d | 1.4d | 1.0 |
| C21,22 | HTRA2/Omi | P | 0.227 | 0.261 | 0.337 | 0.025 | 1.2 | 1.3d | 0.1d |
| C23,24 | Livin | A | 0.006 | 0.006 | 0.008 | 0.007 | 1.0 | 1.4 | 0.8 |
| D1,2 | PON2 | A | 0.008 | 0.041 | 0.011 | 0.010 | 4.8d | 0.3d | 0.9 |
| D3,4 |
p21/CIP1/CDNK1A | A | 0.051 | 0.952 | 0.696 | 0.186 | 18.7d | 0.7d | 0.3d |
| D5,6 | p27/Kip1 | A | 0.015 | 0.061 | 0.047 | 0.008 | 4.2d | 0.8d | 0.2d |
| D7,8 | Phospho-p53
(S15) | P | 0.022 | 0.631 | 0.753 | 0.410 | 28.2e | 1.2 | 0.5d |
| D9,10 | Phospho-p53
(S46) | P | 0.002 | 0.711 | 0.636 | 0.305 | 356.4e | 0.9 | 0.5d |
| D11,12 | Phospho-p53
(S392) | P | 0.007 | 0.896 | 0.794 | 0.464 | 127.4d | 0.9 | 0.6d |
| D13,14 | Phospho-Rad17
(S635) | P | 0.012 | 0.076 | 0.047 | 0.019 | 6.4d | 0.6d | 0.4d |
| D15,16 | SMAC/Diablo | P | 0.791 | 1.206 | 0.872 | 0.544 | 1.5d | 0.7d | 0.6d |
| D17,18 | Survivin | A | 0.020 | 0.026 | 0.029 | 0.008 | 1.3 | 1.1 | 0.3d |
| D19,20 | TNF
RI/TNFRSF1A | P | 0.019 | 0.019 | 0.026 | 0.006 | 1.0 | 1.4d | 0.2d |
| D21,22 | XIAP | A | 0.274 | 0.350 | 0.337 | 0.039 | 1.3d | 1.0 | 0.1e |
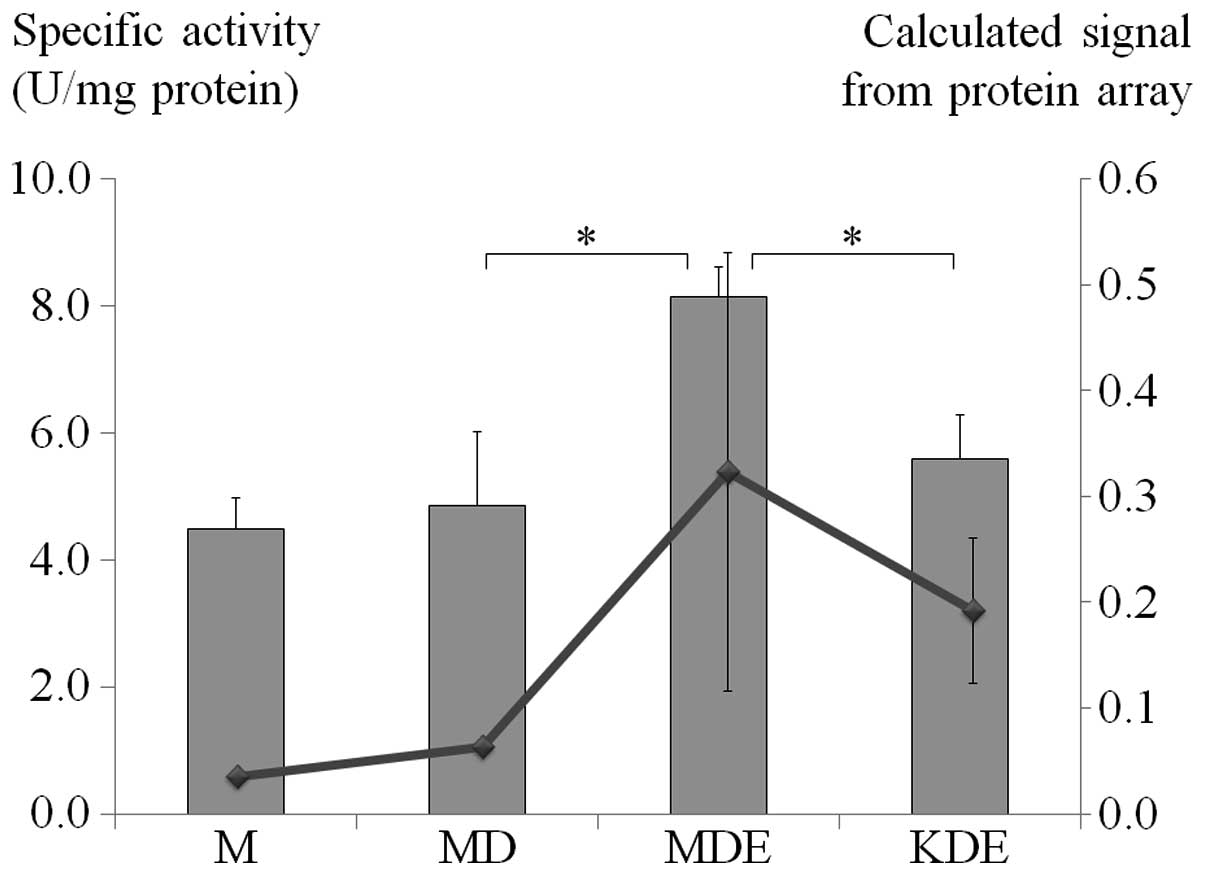
Catalase assay
To correlate catalase expression in the protein
array, the cells were treated under similar conditions and their
specific catalase activity was determined. Doxorubicin-treated mock
MCF-7 cells showed minimal increase of catalase-specific activity
compared to non-treated control cells. However, when mock MCF-7
cells which co-treated with doxorubicin and E2, were compared to
doxorubicin-treated cells, a 1.7-fold increase of catalase specific
activity (P=0.018) was observed and it showed a reduction to
0.69-fold change in TFF1-KD MCF-7 cells co-treated with
doxorubicin and E2 (P=0.006) (Fig.
5).
Discussion
Breast cancer is one of the most common types of
cancer worldwide (1). In advanced
stages, chemotherapy is important for treatment (4). The use of cytotoxic drugs has been
shown to be unsuccessful in ER positive patients (7–9). While
almost all chemotherapeutic agents can induce apoptosis, estrogen
is able to inhibit this action. It has been reported that estrogen
inhibits doxorubicin-induced apoptosis in MFC-7 breast cancer cells
(18). In addition, estrogen could
stimulate expression of TFF1 trefoil protein, a small protein which
has been reported for its function to protect various cell types in
induced-apoptosis in vitro(20,21).
Thus, it is of note that estrogen could stimulate apoptosis
resistance in breast cancer cells via a TFF1 dependent mechanism.
This can be lead to the understanding of some mechanisms in the
drug resistance of breast cancer.
In the present study, the role of TFF1 in
estrogen-promoted resistance to doxorubicin in ER positive MCF-7
breast cancer cells was introduced. Stable knockdown of TFF1
gene in MCF-7 cells was generated (Fig.
1) and used to test the sensitivity to doxorubicin treatment
compared to empty plasmid transfected mock control cells in the
presence or absence of E2. The apoptosis cells were measured by
fluorescence staining by flow cytometry. The results showed that
with the stimulation of apoptosis by doxorubicin, E2 suppressed
this process in mock cells but not in TFF1-KD cells
(Fig. 2). This confirmed that E2
could diminish the cytotoxic effect of doxorubicin by reduced
apoptosis. In addition, with TFF1 gene knockdown, the
anti-apoptosis effect of E2 was decreased. It may be that TFF1 is
involved in this mechanism. In addition, the experiment of mock
cells treated with anti-TFF1 antibody showed that E2 could not
inhibit doxorubicin-induced cell death similar to TFF1-KD
cells (Fig. 3A). Moreover, the
experiment of TFF1-KD cells treated with rTFF1 demonstrated
resistance to doxorubicin cytotoxicity as shown in mock cells
treated with E2 (Fig. 3B). These
results confirmed the anti-apoptotic function of TFF1 trefoil
protein.
The apoptosis protein array experiment indicated
that mock MCF-7 cells treated with doxorubicin showed upregulation
of expression of almost all apoptosis-related proteins, including
both pro- and anti-apoptosis proteins, but no significantly
downregulated proteins (Fig. 4).
These results showed either a similarity or difference to previous
studies of doxorubicin treated MCF-7 cells by an oligonucleotide
microarray (37–39). The differences in either cell
treatment protocols or gene expression detecting methods might lead
to different interpretation, which should be confirmed in protein
expression levels and functional analysis. In the estrogen
treatment condition, the results demonstrated that E2 could help
mock cells from doxorubicin-induced cell death and showed that some
apoptosis proteins changed as compared to mock cells treated with
only doxorubicin (Fig. 4 and
Table I). Catalase, clusterin and
Hsp60, which are anti-apoptosis proteins, were upregulated while
Bad, Trail R2 and phospho-Rad17 (S635), which are pro-apoptosis
proteins, were downregulated. This may suggest that changes of
these proteins were necessary for E2 protection of cell apoptosis
induced by doxorubicin. By contrast, pro-apoptosis protein cleaved
caspase-3 was detected at a higher level in E2 and
doxorubicin-treated mock cells than in only doxorubicin-treated
mock cells but pro-caspase-3 protein was not significantly altered.
In addition, anti-apoptosis proteins Bcl-x and Pon2 were
downregulated. These results did not correspond to cell death, so
it could not be concluded that gene expression of either caspase-3,
Bcl-x or Pon2 had a role in this situation.
The comparison between TFF1-KD cells and mock
cells co-treated with E2 and doxorubicin showed downregulated
expression of 15 pro-apoptosis proteins and 11 anti-apoptosis
proteins (Table I). Among these
protein expressions and modifications, only catalase and clusterin
showed to correspond to cell death, which might be regulated by E2
and TFF1. Functional analyses of catalase in the similar conditions
with apoptosis protein array studies were performed and the results
confirmed the expression of catalase proteins (Fig. 5). By contrast, previous studies
showed that estrogen could inhibit catalase expression in MCF-7
cells (40,41). This could be explained by the fact
that the conditions used in previous studies did not stimulate
apoptosis by a cytotoxic agent, so catalase expression might be the
compensation for induced-apoptosis stimulated by E2 and TFF1. To
the best of our knowledge, no previous report demonstrates the
relationship between catalase expression and TFF1. Clusterin is an
intracellular protein which showed protective activity to apoptosis
and was expressed in various types of cancer (42). Clusterin expression could be induced
by estrogen and may lead to resistance to the chemotherapeutic
agent paclitaxel (43). Thus,
clusterin is another attractive protein for further study into the
mechanism of estrogen and TFF1-related drug resistance in breast
cancer.
Fulvestrant, formerly known as ICI 182,780, is an
anti-estrogenic agent which is used in clinical practice (44). It has been shown that fulvestrant
enhances doxorubicin cytotoxicity in MCF-7 cells in both in
vitro and in vivo studies (44,45).
Therefore, in the present study fulvestrant was expected to show an
antagonistic effect to estrogen in E2-doxorubicin co-treated MCF-7
cells; however, the results showed that fulvestrant could not
express this effect. This may be explained by the fact that in the
presence of estrogen and with a short time of 18-h exposure to
fulvestrant this effect might not be as clear as with 72-h
treatment or more as in previous reports (44,45).
Thus, fulvestrant may be considered for study in clinical trials in
combination with chemotherapeutic agents using another system
(44).
In conclusion, estrogen showed protection of MCF-7
cells from doxorubicin-induced apoptosis but not in TFF1-KD
cells. This indicated a possible TFF1 role in estrogen-induced
apoptosis resistance. In this experiment, fulvestrant was not able
to inhibit estrogen-induced apoptosis resistance. Catalase was
shown as an effective mediator in TFF1-mediated estrogen-induced
apoptosis resistance. Therefore, the TFF1 gene may be a
target for enhancing sensitivity to chemotherapy in breast cancer
treatment.
Acknowledgements
The present study was supported by the Faculty of
Medicine, Siriraj Hospital, Mahidol University. The authors thank
Professor Pornchai O-chareonrat (Department of Surgery, Faculty of
Medicine Siriraj Hospital, Mahidol University) for providing the
MCF-7 breast cancer cell line. The authors would also like to thank
Professor James A. Will (University of Wisconsin-Madison) for
editorial comments on the manuscript.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Porter P: ‘Westernizing’ women's risks?
Breast cancer in lower-income countries. N Engl J Med. 358:213–216.
2008.
|
|
3
|
Tuttle TM, Rueth NM, Abbott A and Virnig
BA: United States trends in the surgical treatment of primary
breast cancer. World J Surg. 36:1475–1479. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arslan C, Altundag K and Dizdar O:
Emerging drugs in metastatic breast cancer: an update. Expert Opin
Emerg Drugs. 16:647–667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stopeck AT, Brown-Glaberman U, Wong HY,
Park BH, Barnato SE, Gradishar WJ, Hudis CA and Rugo HS: The role
of targeted therapy and biomarkers in breast cancer treatment. Clin
Exp Metastasis. 29:807–819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jordan VC and O'Malley BW: Selective
estrogen-receptor modulators and antihormonal resistance in breast
cancer. J Clin Oncol. 25:5815–5824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allegra JC, Lippman ME, Thompson EB and
Simon R: An association between steroid hormone receptors and
response to cytotoxic chemotherapy in patients with metastatic
breast cancer. Cancer Res. 38:4299–4304. 1978.
|
|
8
|
Kuerer HM, Newman LA, Smith TL, Ames FC,
Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N,
Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN and
Singletary SE: Clinical course of breast cancer patients with
complete pathologic primary tumor and axillary lymph node response
to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol.
17:460–469. 1999.
|
|
9
|
Conforti R, Boulet T, Tomasic G, Taranchon
E, Arriagada R, Spielmann M, Ducourtieux M, Soria JC, Tursz T,
Delaloge S, Michiels S and Andre F: Breast cancer molecular
subclassification and estrogen receptor expression to predict
efficacy of adjuvant anthracyclines-based chemotherapy: a biomarker
study from two randomized trials. Ann Oncol. 18:1477–1483. 2007.
View Article : Google Scholar
|
|
10
|
Pritchard JE, Dillon PM, Conaway MR, Silva
CM and Parsons SJ: A mechanistic study of the effect of
doxorubicin/adriamycin on the estrogen response in a breast cancer
model. Oncology. 83:305–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dickson RB and Stancel GM: Estrogen
receptor-mediated processes in normal and cancer cells. J Natl
Cancer Inst Monogr. 2000:135–145. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lewis-Wambi JS and Jordan VC: Estrogen
regulation of apoptosis: how can one hormone stimulate and inhibit?
Breast Cancer Res. 11:2062009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haddow A, Watkinson JM, Paterson E and
Koller PC: Influence of synthetic oestrogens on advanced malignant
disease. Br Med J. 2:393–398. 1944. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ward HW: Anti-oestrogen therapy for breast
cancer: a trial of tamoxifen at two dose levels. Br Med J. 1:13–14.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lewis JS, Meeke K, Osipo C, Ross EA,
Kidawi N, Li T, Bell E, Chandel NS and Jordan VC: Intrinsic
mechanism of estradiol-induced apoptosis in breast cancer cells
resistant to estrogen deprivation. J Natl Cancer Inst.
97:1746–1759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gompel A, Somaï S, Chaouat M, Kazem A,
Kloosterboer HJ, Beusman I, Forgez P, Mimoun M and Rostène W:
Hormonal regulation of apoptosis in breast cells and tissues.
Steroids. 65:593–598. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teixeira C, Reed JC and Pratt MA: Estrogen
promotes chemotherapeutic drug resistance by a mechanism involving
Bcl-2 proto-oncogene expression in human breast cancer cells.
Cancer Res. 55:3902–3907. 1995.PubMed/NCBI
|
|
18
|
Perillo B, Sasso A, Abbondanza C and
Palumbo G: 17β-estradiol inhibits apoptosis in MCF-7 cells,
inducing bcl-2 expression via two estrogen-responsive
elements present in the coding sequence. Mol Cell Biol.
20:2890–2901. 2000.
|
|
19
|
Rio MC and Chambon P: The pS2 gene, mRNA,
and protein: a potential marker for human breast cancer. Cancer
Cells. 2:269–274. 1990.PubMed/NCBI
|
|
20
|
Bossenmeyer-Pourié C, Kannan R, Ribieras
S, Wendling C, Stoll I, Thim L, Tomasetto C and Rio MC: The trefoil
factor 1 participates in gastrointestinal cell differentiation by
delaying G1-S phase transition and reducing apoptosis. J Cell Biol.
157:761–770. 2002.PubMed/NCBI
|
|
21
|
Buron N, Guery L, Creuzot-Garcher C,
Lafontaine PO, Bron A, Rio MC and Solary E: Trefoil factor
TFF1-induced protection of conjunctival cells from apoptosis at
premitochondrial and postmitochondrial levels. Invest Ophthalmol
Vis Sci. 49:3790–3798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nunez AM, Jakowlev S, Briand JP, Gaire M,
Krust A, Rio MC and Chambon P: Characterization of the
estrogen-induced pS2 protein secreted by the human breast cancer
cell line MCF-7. Endocrinology. 121:1759–1765. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomasetto C and Rio MC: Pleiotropic
effects of Trefoil Factor 1 deficiency. Cell Mol Life Sci.
62:2916–2920. 2005.PubMed/NCBI
|
|
24
|
Ruchaud-Sparagano MH, Westley BR and May
FE: The trefoil protein TFF1 is bound to MUC5AC in human gastric
mucosa. Cell Mol Life Sci. 61:1946–1954. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Madsen J, Nielsen O, Tornøe I, Thim L and
Holmskov U: Tissue localization of human trefoil factors 1, 2, and
3. J Histochem Cytochem. 55:505–513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thuwajit P, Chawengrattanachot W, Thuwajit
C, Sripa B, May FE, Westley BR, Tepsiri NN, Paupairoj A and Chau-In
S: Increased TFF1 trefoil protein expression in Opisthorchis
viverrini-associated cholangiocarcinoma is important for
invasive promotion. Hepatol Res. 37:295–304. 2007.PubMed/NCBI
|
|
27
|
Prest SJ, May FE and Westley BR: The
estrogen-regulated protein, TFF1, stimulates migration of human
breast cancer cells. FASEB J. 16:592–594. 2002.PubMed/NCBI
|
|
28
|
Soutto M, Belkhiri A, Piazuelo MB,
Schneider BG, Peng D, Jiang A, Washington MK, Kokoye Y, Crowe SE,
Zaika A, Correa P, Peek RM Jr and El-Rifai W: Loss of TFF1 is
associated with activation of NF-κB-mediated inflammation and
gastric neoplasia in mice and humans. J Clin Invest. 121:1753–1767.
2011.
|
|
29
|
Gillesby BE and Zacharewski TR: pS2 (TFF1)
levels in human breast cancer tumor samples: correlation with
clinical and histological prognostic markers. Breast Cancer Res
Treat. 56:253–265. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Poulsom R, Hanby AM, Lalani EN, Hauser F,
Hoffmann W and Stamp GW: Intestinal trefoil factor (TFF 3) and pS2
(TFF 1), but not spasmolytic polypeptide (TFF 2) mRNAs are
co-expressed in normal, hyperplastic, and neoplastic human breast
epithelium. J Pathol. 183:30–38. 1997. View Article : Google Scholar
|
|
31
|
Reshkin SJ, Tedone T, Correale M, Mangia
A, Casavola V and Paradiso A: Association of pS2 (TFF1) release
with breast tumour proliferative rate: in vitro and in vivo
studies. Cell Prolif. 32:107–118. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amiry N, Kong X, Muniraj N, Kannan N,
Grandison PM, Lin J, Yang Y, Vouyovitch CM, Borges S, Perry JK,
Mertani HC, Zhu T, Liu D and Lobie PE: Trefoil factor-1 (TFF1)
enhances oncogenicity of mammary carcinoma cells. Endocrinology.
150:4473–4483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buache E, Etique N, Alpy F, Stoll I,
Muckensturm M, Reina-San-Martin B, Chenard MP, Tomasetto C and Rio
MC: Deficiency in trefoil factor 1 (TFF1) increases tumorigenicity
of human breast cancer cells and mammary tumor development in
TFF1-knockout mice. Oncogene. 30:3261–3273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miller WR: Clinical, pathological,
proliferative and molecular responses associated with neoadjuvant
aromatase inhibitor treatment in breast cancer. J Steroid Biochem
Mol Biol. 118:273–276. 2010. View Article : Google Scholar
|
|
35
|
Zhou L, Yan T, Jiang Y, Di G, Shen Z, Shao
Z and Lu J: Prognostic and predictive value of TFF1 for adjuvant
endocrine therapy in Chinese women with early ER positive breast
cancer: comparing aromatase inhibitors with tamoxifen. Breast.
20:15–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sinha AK: Colorimetric assay of catalase.
Anal Biochem. 47:389–394. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kudoh K, Ramanna M, Ravatn R, Elkahloun
AG, Bittner ML, Meltzer PS, Trent JM, Dalton WS and Chin KV:
Monitoring the expression profiles of doxorubicin-induced and
doxorubicin-resistant cancer cells by cDNA microarray. Cancer Res.
60:4161–4166. 2000.PubMed/NCBI
|
|
38
|
Troester MA, Hoadley KA, Sorlie T, Herbert
BS, Borresen-Dale AL, Lonning PE, Shay JW, Kaufmann WK and Perou
CM: Cell-type-specific responses to chemotherapeutics in breast
cancer. Cancer Res. 64:4218–4226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Elmore LW, Di X, Dumur C, Holt SE and
Gewirtz DA: Evasion of a single-step, chemotherapy-induced
senescence in breast cancer cells: implications for treatment
response. Clin Cancer Res. 11:2637–2643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mobley JA and Brueggemeier RW: Estrogen
receptor-mediated regulation of oxidative stress and DNA damage in
breast cancer. Carcinogenesis. 25:3–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sastre-Serra J, Valle A, Company MM, Garau
I, Oliver J and Roca P: Estrogen down-regulates uncoupling proteins
and increases oxidative stress in breast cancer. Free Radic Biol
Med. 48:506–512. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
O'Sullivan J, Whyte L, Drake J and
Tenniswood M: Alterations in the post-translational modification
and intracellular trafficking of clusterin in MCF-7 cells during
apoptosis. Cell Death Differ. 10:914–927. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Won YS, Lee SJ, Yeo SG and Park DC:
Effects of female sex hormones on clusterin expression and
paclitaxel resistance in endometrial cancer cell lines. Int J Med
Sci. 9:86–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ikeda H, Taira N, Nogami T, Shien K, Okada
M, Shien T, Doihara H and Miyoshi S: Combination treatment with
fulvestrant and various cytotoxic agents (doxorubicin, paclitaxel,
docetaxel, vinorelbine, and 5-fluorouracil) has a synergistic
effect in estrogen receptor-positive breast cancer. Cancer Sci.
102:2038–2042. 2011. View Article : Google Scholar
|
|
45
|
de Vincenzo R, Scambia G, Benedetti Panici
P, Bonanno G, Ercoli A, Fattorossi A, Pernisco S, Isola G and
Mancuso S: Chemosensitizing effect of tamoxifen and ICI 182,780 on
parental and adriamycin-resistant MCF-7 human breast cancer cells.
Ann NY Acad Sci. 784:517–520. 1996.
|