Introduction
Cervical carcinoma is the third most common
malignancy affecting women worldwide (1). With the development of surgery and the
utilization of radiation and chemotherapy, the death rate has been
reduced; however, some tumors are more refractory to treatment, and
certain patients are still less sensitive to recently developed
treatments (2). Many
clinicopathological parameters, including the clinical stage of the
disease, tumor volume and metastatic status, have been reported to
be associated with prognosis, while they are not directly
correlated with tumor aggressiveness. Further research is needed to
identify more effective prognostic markers for the improvement of
the clinical management of cervical carcinoma patients. This would
also support the development of novel individually tailored
strategies for use in the treatment of high-risk patients due to
molecular risk factors (3–6).
Astrocyte-elevated gene-1 (AEG-1), also known as
metadherin (MTDH), was initially identified as a human
immunodeficiency virus (HIV)-1-inducible gene in human fetal
astrocytes (7,8). AEG-1 has recently received
considerable attention in an increasing spectrum of tumor
indications due to its multiple roles in regulating cancer
progression and metastasis (9).
Aberrant elevation of AEG-1 expression frequently occurs in human
cancers, including salivary gland, glioma, hepatocellular,
esophageal squamous cell and renal cell carcinomas, as well as
breast, prostate, melanoma, gastric and non-small cell lung cancers
(10–18), which correlates with poor clinical
outcomes. Loss- and gain-of-function studies revealed the
importance of AEG-1 in regulating multiple signal transduction
pathways and various pathologically relevant processes (19–26).
AEG-1 has been reported to function as a downstream mediator of the
transforming activity of oncogenic Ha-Ras and c-Myc 27, to promote
proliferation via suppression of FOXO1, as well as to suppress
apoptosis and increase anchorage-independent growth of
non-tumorigenic astrocytes by activating PI3K-Akt and NF-κB
pathways (28–30). These studies suggest that AEG-1
overexpression plays a dominant positive role in the progression of
numerous types of cancer. However, the biological significance of
AEG-1 in cervical carcinoma has not been fully elucidated yet.
In the present study, we demonstrated that the
expression of AEG-1 was upregulated in 3 cervical carcinoma cell
lines as well as in cervical carcinoma tissue (CCT) specimens, and
that overexpression of AEG-1 was correlated with the clinical
characteristics of the disease. Moreover, we also showed that AEG-1
overexpression promoted angiogenesis through the increased
expression of the angiogenesis-related genes HIF-1α,
Tie2, VEGF and TEM1/CD248. Our results suggest
that AEG-1 constitutes a novel and valuable predictive factor for
the prognostic evaluation of cervical carcinoma patients.
Materials and methods
CCT specimens and cell culture
The 3 human cervical carcinoma cell lines HeLa,
CaSki and SiHa were purchased from the American Type Culture
Collection (ATCC) and cultured in complete RPMI-1640 medium (Gibco,
Grand Island, NY, USA). Normal cervical epithelial cells (NCECs)
and human umbilical vein endothelial cells (HUVECs) were
established and maintained in our laboratory (31,32).
Eight paired primary CCT specimens and matched normal tissues
obtained from the same patients were collected at the Department of
Gynecology and Obstetrics of Tangdu Hospital (Fourth Military
Medical University, Xi’an, China). Tissue arrays containing 200
paraffin-embedded archival CCT specimens and 8 normal cervical
tissues (nos. CR208-1 and CR208-3) were purchased from Chaoying
Biotech Company (Xian, China). Patient informed consent and
approval from the Institutional Research Ethics Committee of Tangdu
Hospital were obtained prior to use of the clinical material for
research purposes. Patient clinicopathological characteristics are
provided in Table I.
 | Table IPatient clinicopathological
characteristics. |
Table I
Patient clinicopathological
characteristics.
| Characteristic | No. of cases (%) |
|---|
| Age (years) |
| ≤50 | 115 (57.5) |
| >50 | 85 (42.5) |
| Clinical stage |
| I | 35 (17.5) |
| II | 130 (65) |
| III | 25 (12.5) |
| IV | 10 (5) |
| Tumor
differentiation |
| G1 | 36 (18) |
| G2 | 148 (74) |
| G3 | 16 (8) |
| T
classification |
| T1 | 124 (62) |
| T2 | 65 (32.5) |
| T3 | 6 (3) |
| T4 | 5 (2.5) |
| N
classification |
| N0 | 177 (88.5) |
| N1 | 23 (11.5) |
| M classification
(distant metastasis) |
| M0 | 191 (95.5) |
| M1 | 9 (4.5) |
Immunohistochemical analysis (IHC)
IHC was performed as previously described (31) in 200 human CCT and 8 normal cervical
tissue specimens. Rabbit anti-human AEG-1 polyclonal antibody
(Proteintech Group, Chicago, IL, USA) was used as a primary
antibody and goat anti-rabbit IgG coupled to horseradish peroxidase
(HRP) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was
used as a secondary antibody. Immunostaining was observed in 5
areas of each slide and scored independently by two observers,
based on both the proportion of positively stained tumor cells and
the intensity of staining. The proportion of positive cells was
scored as follows: 0, no positive cells; 1, <10% positive cells;
2, 10–50% positive cells; and 3, >50% positive cells. The
intensity of staining was stratified into 4 categories: 0, no
staining; 1, weak staining (light yellow); 2, moderate staining
(yellow brown); and 3, strong staining (brown). The staining index
(SI) was calculated as follows: Staining intensity score ×
proportion of positive tumor cells. Using this method of
assessment, we evaluated the expression of AEG-1 in 200 archival
CCT specimens by determining SI which was scored as 0, 1, 2, 3, 4,
6 and 9. An SI score of 0 was marked as ‘−’, 1 and 2 as ‘+’, 3 and
4 as ‘++’, 6 and 9 as ‘+++’.
Vector construction and cell
transfection
shRNA expression vectors were generated by annealing
single-stranded oligonucleotides and inserting them into the
BamHI and HindIII enzyme sites of pSilencer4.1-CMVneo
vector (Ambion, Austin, TX, USA). The target sequences were as
follows: shA1 (AEG-1; GenBank, AF411226.1; 1825–1843 bp): 5′-GTGCCG
CCAATACTACAAG-3′ (recommended by P.B. Fisher, Departments of
Pathology and Urology, Columbia University, USA); shA2 (AEG-1;
GenBank, AF411226.1; 666–686 bp): AACAGAAGAAGAAGAACCGGA (27); and a scrambled sequence was used as
a negative control (NC): 5′-TTCTCC GAACGTGTCACGT-3′ (provided by
Ambion). The recombinant shRNA vectors were named pshA1, pshA2 and
pshNC. The full-length open reading frame cDNA of AEG-1 was
amplified using RT-PCR from total mRNA of HeLa cells, and then
inserted into the pcDNA3.1 expression vector; the recombinant
vector was named pAEG1. All recombinant vectors were confirmed by
enzyme digestion and DNA sequencing analysis. Cell transfection was
performed using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA)
following the manufacturer’s instructions and selected with 600
μg/ml of G418 (Invitrogen, San Diego, CA, USA) after
transfection.
RT-PCR and quantitative real-time
RT-PCR
RT-PCR was conducted as previously described
(33). Briefly, total RNA was
extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA),
and 1 μg RNA was used to synthesize cDNA using the M-MLV reverse
transcriptase kit (Takara Biotechnology, Dalian, China) according
to the manufacturer’s protocol. The cDNA was then used to amplify
the mRNA fragment. β-actin was also amplified as an internal
standard. The corresponding primer sequences for RT-PCR
amplification are provided in Table
II. The RT-PCR products were then electrophoresed through a
1.5% agarose gel, and signals were quantified by densitometric
analysis using Multilmage™ Light Cabinet (Alpha Innotech
Corporation, San Leandro, CA, USA).
 | Table IIPrimer pairs used in RT-PCR and
real-time RT-PCR. |
Table II
Primer pairs used in RT-PCR and
real-time RT-PCR.
| Gene | GenBank ID | Primer pairs
(5′→3′) |
|---|
| RT-PCR | AEG-1 | AF411226 | F:
GTGAAGCTGTTCGAACACCTCAAAG
R: GACAGTGAGGTTTTCATTCAATCCTG |
| TEM1 | NM_020404 | F:
TCGAGTGTTATTGTAGCGAGGGACATG
R: AGGTGGGCTCCGGGTAGGGTAT |
| HIF-1α | NM_181054 | F:
GCAAGACTTTCCTCAGTCGACACA
R: GCATCCTGTACTGTCCTGTGGTGA |
| Tie2 | NM_000459 | F:
GCTGTCATCAACATCAGCTCTG
R: GAGGAGGGAGTCCGATAGAAGC |
| VEGF | NM_001025366 | F:
ATGGCAGAAGGAGGAGGG
R: TTGGACTCCTCAGTGGGC |
| β-actin | NM_001101.3 | F:
GACTTAGTTGCGTTACACCCTTTC
R: TGCTGTCACCTTCACCGTTC |
| Real-time
RT-PCR | AEG-1 | AF411226 | F:
GGGGAAGGAGTTGGAGTGAC
R: GTAGACTGAGAAACTGGCTCAGCAG |
| GAPDH | NG 007073.2 | F:
CCACATCGCTCAGACACCAT
R: GGCAACAATATCCACTTTACCAGAGT |
Real-time RT-PCR was performed using ABI 7500
Real-Time RT-PCR system and SYBR Premix Ex Taq II for individual
mRNAs according to the manufacturer’s protocol (Takara
Biotechnology). Primer pairs are shown in Table II. Expression data were normalized
to the geometric mean of the GAPDH gene to control the
variability in expression levels.
Western blot analysis
Protein levels were determined by western blot
analysis as previously described (33). Target proteins were detected using
specific antibodies against AEG-1 (Proteintech, Chicago, IL, USA);
TEM1 (Abcam, Cambridge, UK); Tie2, HIF-1α and VEGF (Santa Cruz
Biotechnology, Inc.). All secondary antibodies of IgG coupled to
HRP were purchased from Santa Cruz Biotechnology, Inc.
Densitometric analysis was performed by photoimage analysis using
the Multilmage™ Light Cabinet. β-actin was used as a loading
control and the results are expressed as the protein/β-actin
absorbance ratio.
Flow cytometric analysis
For cell cycle analysis, the cells were fixed with
70% ethanol and stained with propidium iodide (PI). Cell cycle
distribution was then analyzed on a FACSCalibur system (BD
Biosciences, Bedford, MA, USA) using ModFit software (Verity
Software House, Topsham, ME, USA). For apoptosis detection, the
cells were pelleted, suspended in Annexin V-fluorescein
isothiocyanate (FITC) (0.5 mg/ml) and PI (0.6 mg/ml), and then
analyzed with WinMDI software (The Scripps Research Institute, La
Jolla, CA, USA) using FACSCalibur system.
Scratch wound migration assay
The cells were seeded onto 6-well plates and grown
until they reached 90% confluence. A linear wound was then created
in the confluent monolayer using a pipette tip. The wounded
monolayer was washed to remove cell debris, and wounds were then
observed and photographed at various indicated times. The migrated
cells were analyzed under a light microscope. Each experiment was
repeated three times.
Tube formation assay
Matrigel (BD Biosciences) was dissolved at 4°C
overnight and 96-well plates were prepared with 55 μl Matrigel in
each well and incubated at 37°C for 30 min. HUVECs were seeded on
coated plates at a density of 2×104 cells/well in
Dulbecco’s modified Eagle’s medium (DMEM) containing 2% fetal
bovine serum (FBS). The culture media of HUVECs were replaced with
the media from HeLa cells, pshAEG1-transfected HeLa cells, NCECs
and pAEG1-transfected NCECs, and then all treated HUVECs were
incubated at 37°C overnight. Five different fields were randomly
chosen in each well and images were captured. The length of the
tubes was measured using Olympus DP2-BSW software (Soft Imaging
System GmbH, Münster, Germany) and was expressed as total length
(mm) per microscopic field for each well.
Statistical analysis
Results are expressed as means ± standard deviation
(SD). Statistical analyses were performed using SPSS version 17.0
(IBM Company, Chicago, IL, USA). The Mann-Whitney U test and the
Kruskal-Wallis H test were used to analyze the correlation between
AEG-1 expression and clinicopathologic characteristics. P<0.05
was considered to indicate a statistically significant difference
which is indicated by letters or asterisks, respectively, in the
tables and figures.
Results
AEG-1 expression is upregulated in
cervical carcinoma cells and primary CCT specimens
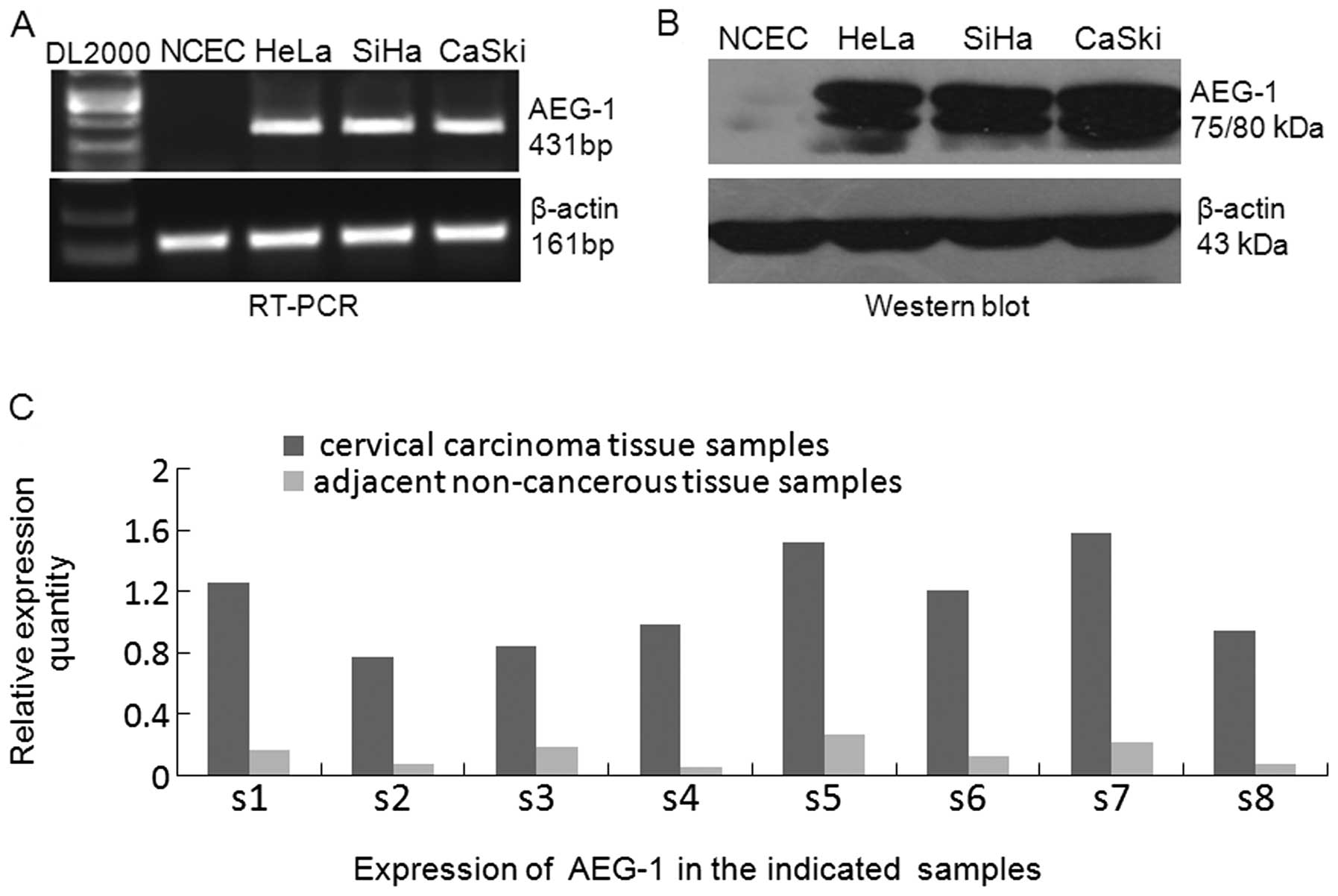
To determine AEG-1 protein and mRNA expression
levels in cervical carcinomas, we first performed semi-quantitative
RT-PCR and western blot analysis in the following cell lines: SiHa,
HeLa and CaSki. RT-PCR results revealed that AEG-1 mRNA was
overexpressed in all the 3 cervical carcinoma cell lines, while it
was weakly detected in NCECs (Fig.
1A). In parallel with the upregulated AEG-1 mRNA expression,
western blot analysis showed that all cervical carcinoma cell lines
exhibited significantly higher levels of AEG-1 protein compared
with that of the NCECs (Fig. 1B,
P<0.05). Furthermore, real-time RT-PCR analysis of 8 cases of
paired primary CCT and adjacent non-cancerous tissue specimens
demonstrated that the levels of AEG-1 expression in all 8 cervical
CCT specimens were found to be obviously upregulated compared with
their matched adjacent non-cancerous tissues (Fig. 1C). These results demonstrate that
AEG-1 is overexpressed in CCTs which is suggested to be primarily
caused by transcriptional upregulation.
Overexpression of AEG-1 in archived CCT
specimens is associated with clinical staging of cervical carcinoma
patients
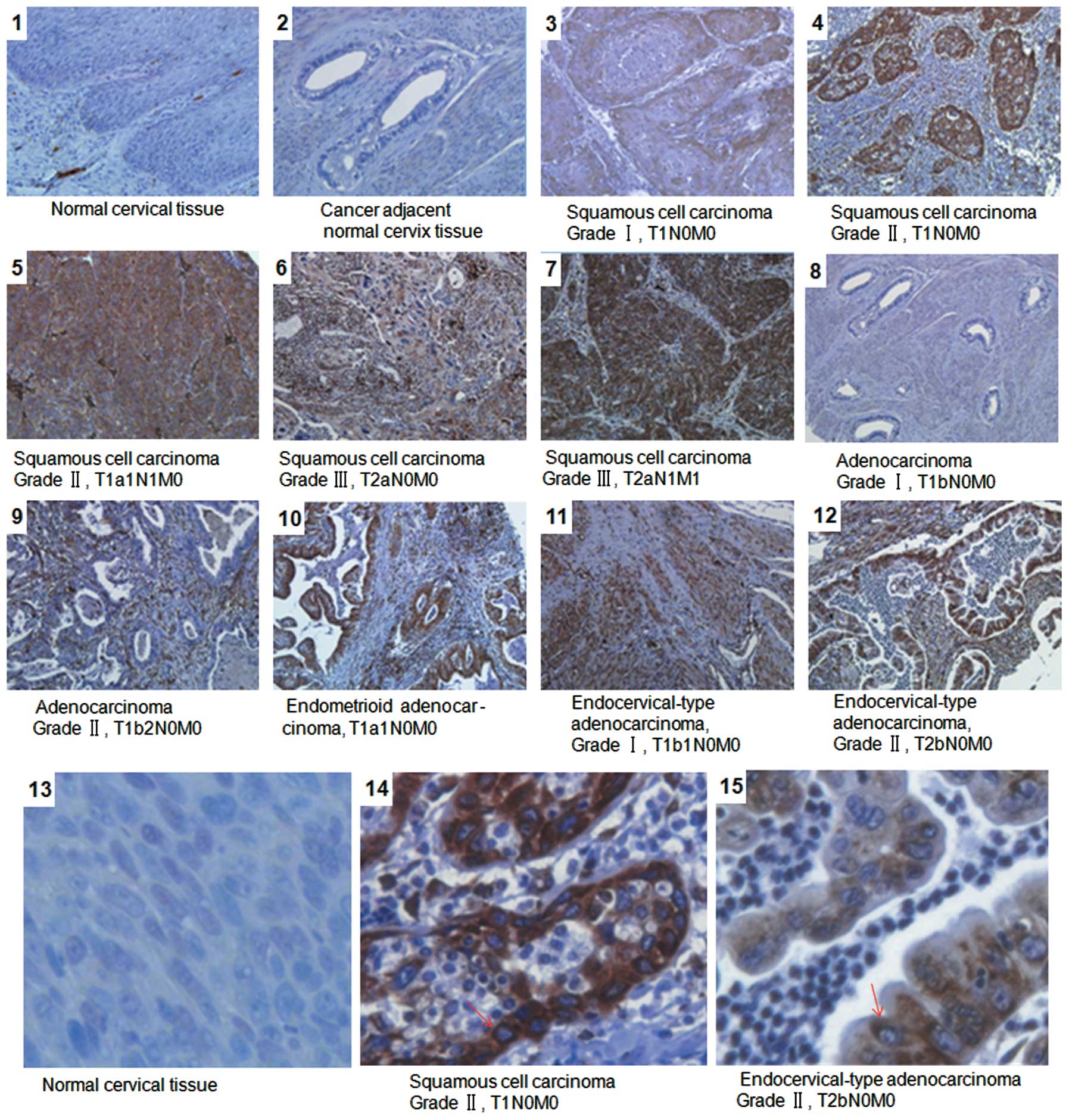
To further elucidate whether AEG-1 overexpression is
associated with clinicopathological characteristics of cervical
carcinoma, 200 archived CCT and 8 normal cervical tissue specimens
were examined by IHC staining with anti-AEG-1 antibody. As shown in
Fig. 2, AEG-1 protein was detected
in 180/200 (90%) cases. By contrast, weak or negative signals were
observed in normal cervical tissues. Statistical analysis revealed
that the expression of AEG-1 was strongly correlated with the
clinical staging of cervical carcinoma patients (P=0.034) and tumor
differentiation (P=0.043). Furthermore, significant differences
were also found in AEG-1 expression in patients categorized
according to T (P=0.019), N (P=0.038) and M classification
(P=0.018), indicating that advanced clinical stages and T
classification were correlated with higher AEG-1 expression
(Table III). However, there were
no obvious correlations between the levels of AEG-1 protein
expression and patient age. These results showed that higher
clinical stage and T classification or metastasis were correlated
with a higher level of AEG-1 protein expression.
 | Table IIICorrelation of AEG-1 expression with
the clinicopathological characteristics of 200 cervical carcinoma
specimens. |
Table III
Correlation of AEG-1 expression with
the clinicopathological characteristics of 200 cervical carcinoma
specimens.
| Expression of AEG-1
protein |
|---|
|
|
|---|
| Characteristic | Negative (−)
(n=20), n (%) | Positive (+)
(n=30), n (%) | Positive (++)
(n=68), n (%) | Positive (+++)
(n=82), n (%) | P-value |
|---|
| Age (years) |
| ≤50 | 15 (75) | 17 (56.7) | 37 (54.4) | 46 (56.1) | 0.248 |
| >50 | 5 (25) | 13 (43.3) | 31 (45.6) | 36 (43.9) | |
| Clinical stage |
| I | 10 (50) | 5 (16.7) | 15 (22.1) | 5 (6.1) | 0.034a |
| II | 10 (50) | 25 (83.3) | 50 (73.5) | 45 (54.9) | |
| III | 0 (0) | 0 (0) | 3 (4.4) | 22 (26.8) | |
| IV | 0 (0) | 0 (0) | 0 (0) | 10 (12.2) | |
| Tumor
differentiation |
| G1 | 0 (0) | 5 (16.7) | 10 (14.7) | 21 (25.6) | 0.043a |
| G2 | 11 (55) | 20 (66.7) | 56 (82.4) | 61 (74.4) | |
| G3 | 9 (45) | 5 (16.7) | 2 (2.9) | 0 (0) | |
| T
classification |
| T1 | 17 (85) | 27 (90) | 47 (69.1) | 33 (40.2) | 0.019a |
| T2 | 3 (15) | 3 (10) | 21 (30.9) | 38 (46.3) | |
| T3 | 0 (0) | 0 (0) | 0 (0) | 6 (7.3) | |
| T4 | 0 (0) | 0 (0) | 0 (0) | 5 (6.1) | |
| N
classification |
| N0 | 20 (100) | 30 (100) | 68 (100) | 59 (71.9) | 0.038a |
| N1 | 0 (0) | 0 (0) | 0 (0) | 23 (28.1) | |
| M classification
(distant metastasis) |
| M0 | 20 (100) | 30 (100) | 68 (100) | 73 (89) | 0.018a |
| M1 | 0 (0) | 0 (0) | 0 (0) | 9 (11) | |
Knockdown of AEG-1 inhibits proliferation
and promotes the apoptosis of cervical carcinoma cells
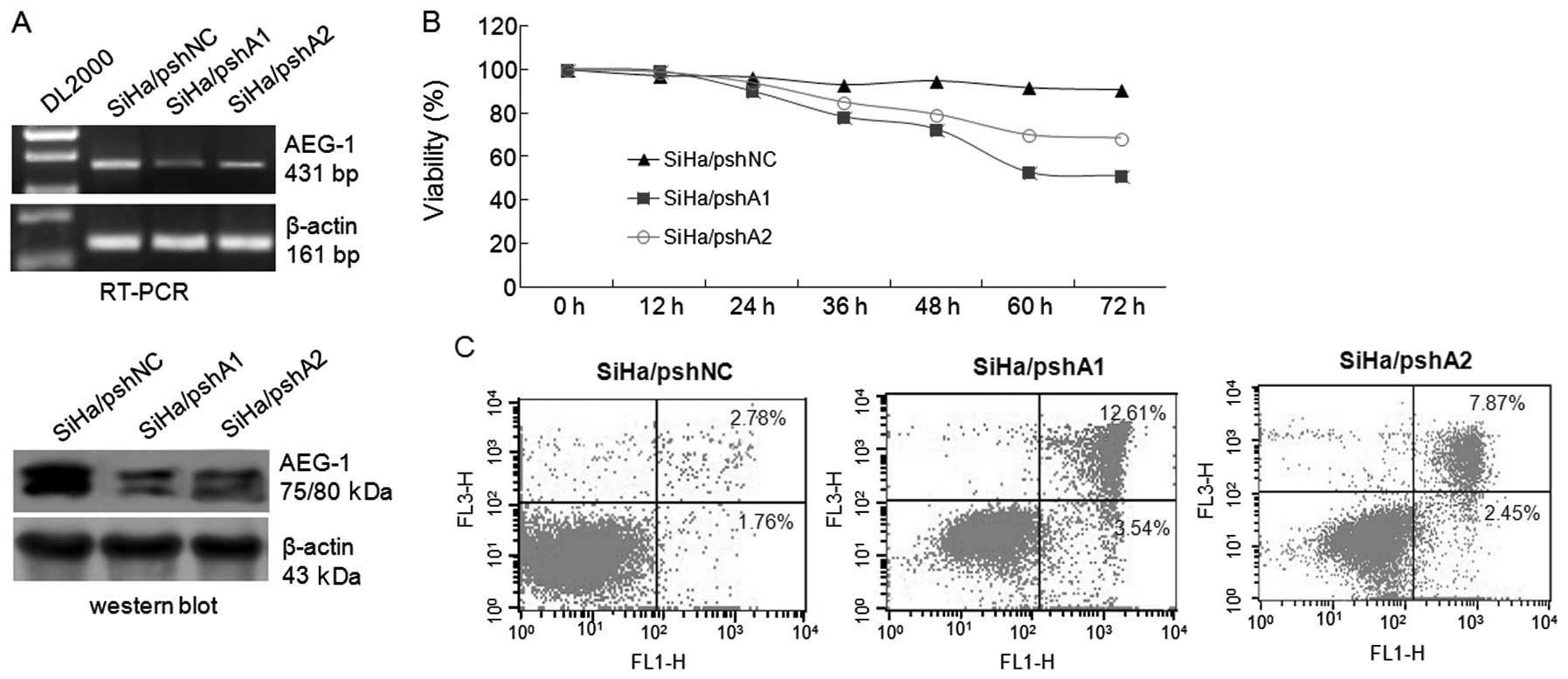
To determine the biological role of AEG-1
overexpression in cervical carcinoma, we transfected the AEG-1
shRNA expression vectors pshA1 and pshA2 into SiHa cells, and
examined the cell growth and apoptosis by flow cytometry. As shown
in Fig. 3A, both pshA1 and pshA2
vectors effectively knocked down AEG-1 expression in SiHa cells,
which was followed by a subsequent decrease in cell proliferation
by 39.95 and 22.39%, respectively, at 72 h compared with the
control cells (Fig. 3B).
Furthermore, analysis of apoptosis showed that the apoptotic rate
of pshA1-transfected SiHa cells was significantly increased to
16.15±4.6 and 10.32±3.4% when compared with the pshNC-transfected
control cells (Fig. 3C, P<0.05).
These results demonstrate that AEG-1 overexpression in cervical
carcinoma cells plays an important role in cell proliferation and
apoptosis, and that downregulation of AEG-1 expression not only
leads to inhibition of cervical carcinoma cell proliferation, but
also promotes tumor cell apoptosis.
Knockdown of AEG-1 reduces the migration
ability of cervical carcinoma cells
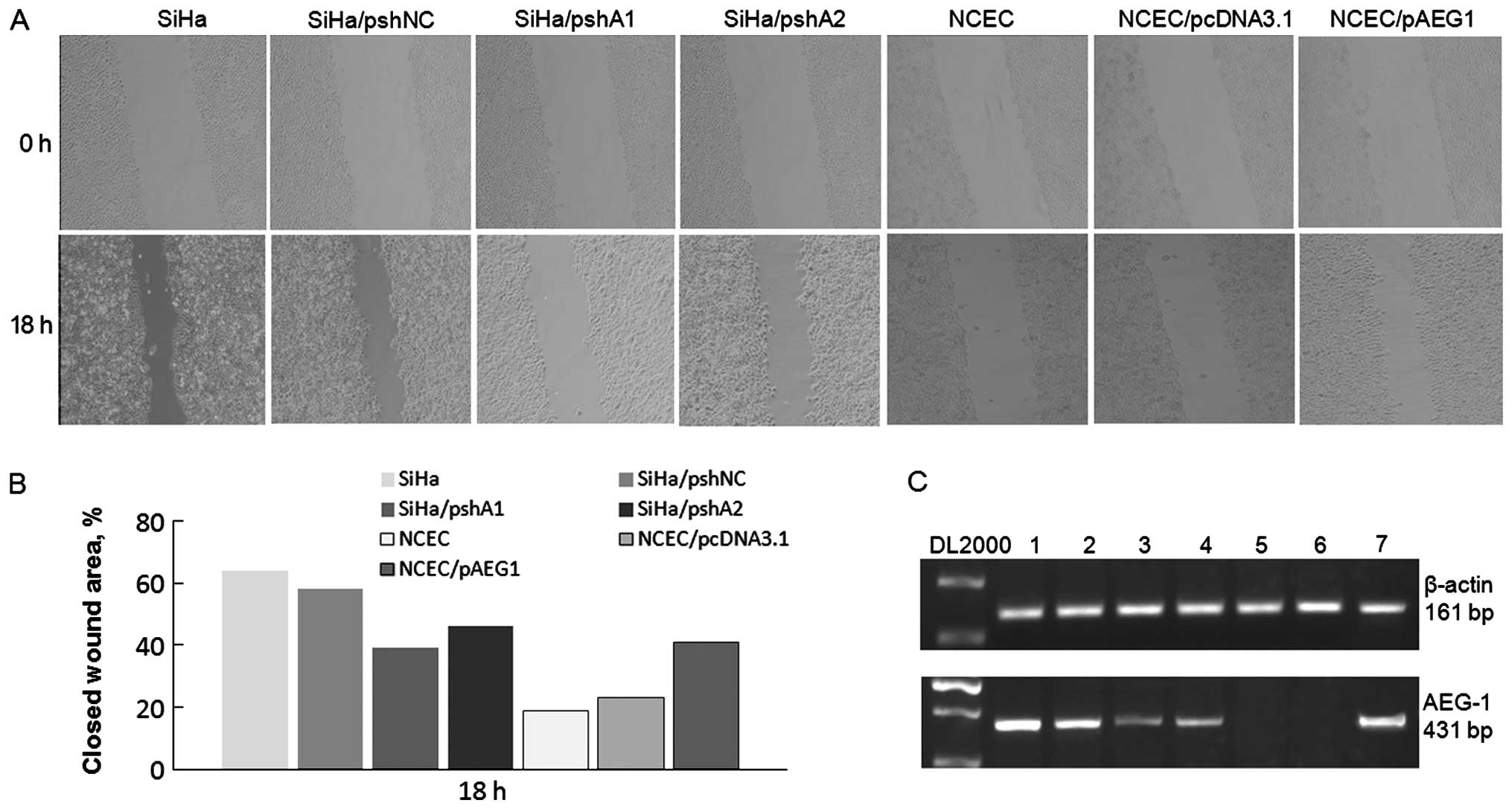
It was previously shown that AEG-1 promotes the
invasive ability of various types of cancer cells (11,18).
To further investigate the role of AEG-1 overexpression in the
migratory ability of cervical carcinoma cells, a scratch wound
migration assay was conducted. As shown in Fig. 4A, the overexpression of AEG-1 in
NCECs led to a significantly increased invasive ability compared
with the ability of the control vector-transfected NCECs.
Conversely, silencing of AEG-1 expression in SiHa cells inhibited
the migratory speed and reduced the invasive ability of the cells.
Meanwhile, the expression of AEG-1 in the cells that were used
during the wound healing assay was detected using RT-PCR as shown
in Fig. 4C. These results confirm
the role of AEG-1 in regulating the invasive ability of cervical
carcinoma cells.
AEG-1 expression influences the migration
and tube formation ability of HUVECs through several
angiogenesis-associated molecular markers
The role of AEG-1 deregulation both in the
angiogenesis of tumor cells and HUVECs was examined in
vitro. We first assessed whether downregulation of AEG-1 in
SiHa cells influences the tube formation ability of HUVECs by
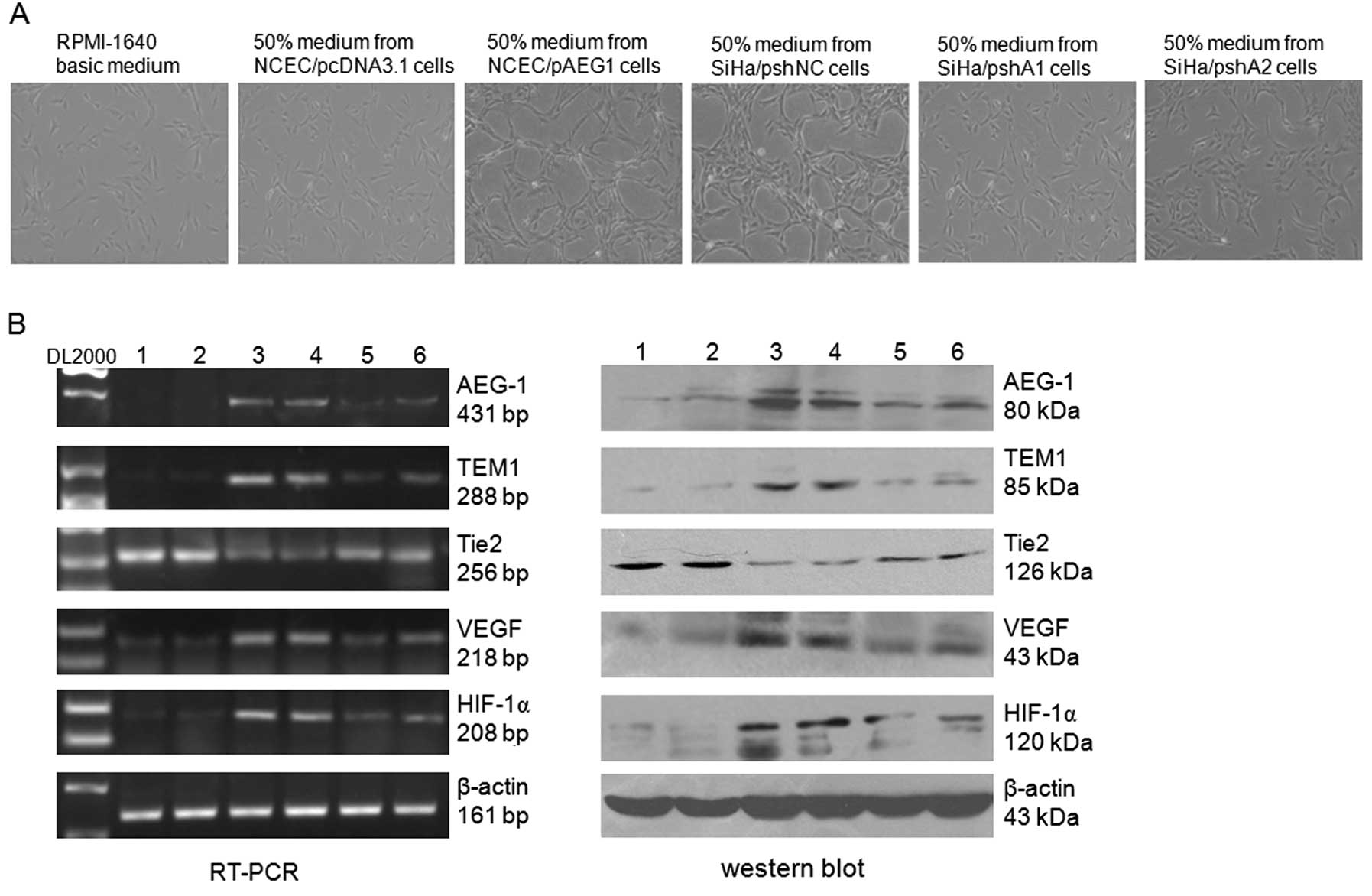
co-culture in conditioned medium. As shown in Fig. 5A, the medium from SiHa and SiHa
cells transfected with the control vector (SiHa/pshNC)
significantly promoted the tube formation ability of HUVECs, while
the medium from AEG-1-depleted SiHa cells (SiHa/pshA1 and
SiHa/pshA2) showed no obvious effect on the tube formation ability
of HUVECs when compared with the medium from control cells. To
further investigate the role of the upregulation of AEG-1 in
angiogenesis, the full-length AEG-1 expression vector pAEG1 was
transfected into NCECs, and the results from co-culture in
conditioned medium showed that ectopic expression of AEG-1 in NCECs
significantly increased the tube formation ability of HUVECs when
compared with the control cells (Fig.
5A). RT-PCR results revealed that co-culture of HUVECs with the
medium from SiHa/pshNC and NCEC/pAEG1 cells significantly decreased
the expression level of Tie2 and increased the expression levels of
HIF-1α, VEGF and TEM1/CD248 (Fig.
5B). These results demonstrate that overexpression of AEG-1 in
HeLa cells plays a dominant positive role in regulating oncogenic
angiogenesis. To the best of our knowledge, this is the first
report that AEG-1 promotes the expression of TEM1/CD248.
Discussion
Cervical carcinoma is the third most common type of
cancer among women worldwide, and the majority of new cases present
with advanced stages (34)
potentially due to the limited access to screening programs. Many
diagnostic markers of cervical carcinoma and markers for disease
follow-up have been identified and investigated; however, a useful
screening marker of cervical carcinoma has not yet been clearly
established. Thus, the identification of novel molecular prognostic
and predictive markers of cervical carcinoma is suggested to
support the accurate evaluation of patient prognosis and the proper
stratification of patients into different risk groups. This would
subsequently lead to the administration of specific adjuvant
therapies and would, finally, increase patient survival time.
Recently, numerous studies have demonstrated that
AEG-1 is upregulated in various types of cancers, and that it is
correlated with cancer progression and patient prognosis (10,13,14,16,18).
The present study was performed in order to investigate whether
AEG-1 plays a role in cervical carcinoma. We showed that AEG-1 is
upregulated at both the mRNA and protein levels in cervical
carcinoma cells when compared with NCECs. Paired CCT and adjacent
non-cancerous tissues were also found to differentially express
AEG-1, with the cancer tissues displaying a significantly higher
expression of AEG-1 (Fig. 1).
Considering the oncogenic properties of AEG-1 protein and its
ability to promote the proliferation of various cell types in
vitro, the high expression of AEG-1 protein observed in
cervical carcinoma cells and tumor tissues suggests that AEG-1 is
involved in the progression of human cervical carcinoma in
vivo. Thus, we analyzed the correlation between AEG-1
expression and the clinical characteristics of cervical carcinoma
patients. IHC staining (Fig. 2)
showed that the expression level of AEG-1 protein in histological
sections was significantly correlated with the clinical stage of
the disease of patients with cervical carcinoma. These results
demonstrated that AEG-1 plays an important role in the pathogenesis
of cervical carcinoma and that it represents a novel prognostic
indicator for cervical carcinoma.
Based on the above-mentioned findings, we further
investigated the biological roles of AEG-1 by loss- and
gain-of-function analyses to determine the potential use of AEG-1
as a therapeutic target for the treatment of cervical carcinoma. As
shown in Fig. 3B, knockdown of
AEG-1 led to a significant inhibition of cell proliferation in
vitro. Moreover, significantly increased rates of cell
apoptosis were detected in the stably pshA1-transfected cervical
carcinoma cells. Moreover, silencing of AEG-1 expression obviously
inhibited the migratory ability of cervical carcinoma cells.
Conversely, ectopic expression of AEG-1 in NCECs led to a
significantly increased invasive ability compared with the control
vector-transfected cells (Fig. 4).
These results confirmed that AEG-1 plays an important role in the
pathogenesis of cervical carcinoma through modulation of the cell
cycle, apoptosis and invasive ability of the cells. Thus, AEG-1 is
suggested to constitute a therapeutic target for the treatment of
cervical carcinoma.
Increasing evidence has shown that AEG-1-expressing
tumors have increased microvessel density (MVD) and that AEG-1
overexpression promotes angiogenesis both in vitro and in
vivo, suggesting that AEG-1 plays an important role in tumor
angiogenesis (23,27). In the present study, co-culture with
conditioned medium was performed to assess the influence of the
downregulation or upregulation of AEG-1 expression in SiHa cells or
NCECs on the tube formation ability of HUVECs. The results showed
that the medium from SiHa or SiHa/pshNC cells significantly
promoted the tube formation ability of HUVECs, while the medium
from AEG-1-depleted SiHa cells showed no obvious effect on the tube
formation ability of HUVECs when compared with that from the
control cells (Fig. 5A). These
results demonstrated that aberrant expression of AEG-1 caused
cervical carcinoma cells to secrete angiogenesis-associated
cytokines which promoted the tube formation ability of HUVECs.
Angiogenesis occurs during the early stages of cancer development
and is essential for the advancement of solid tumors (35). Meanwhile, medium from NCECs
transfected with full-length AEG-1 cDNA of the pAEG1 vector led to
a significant increase in the tube formation ability of HUVECs when
compared with that of the control cells. In addition, ectopic
expression of AEG-1 in HUVECs induced the expression of several
angiogenesis-associated molecular markers, including HIF-1α, Tie2,
VEGF and TEM1/CD248 (Fig. 5B). To
the best of our knowledge, this is the first report that TEM1/CD248
is an AEG-1-induced angiogenesis marker; however, the effects on
angiogenesis and the role of TEM1/CD28 as a predictive factor for
the prognostic evaluation of cervical carcinoma need to be further
investigated. These results confirmed that overexpression of AEG-1
in SiHa cells plays an important role in the regulation of
oncogenic angiogenesis.
In conclusion, our data suggest that AEG-1 is an
important mediator of angiogenesis and a valuable prognostic factor
in patients with cervical carcinoma. AEG-1 protein is also
suggested to provide a therapeutic target for the treatment of
cervical carcinoma.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81201525). We
thank all staff members of the Department of Medical Laboratory and
the Research Center in Tangdu Hospital of the Fourth Military
Medical University (Xi’an, China) for their sincere help and
technical support. We also thank all staff members of the
Department of Gynecology and Obstetrics of the Tangdu Hospital of
the Fourth Military Medical University for kindly providing patient
samples.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Green JA, Kirwan JM, Tierney JF, et al:
Survival and recurrence after concomitant chemotherapy and
radiotherapy for cancer of the uterine cervix: a systematic review
and meta-analysis. Lancet. 358:781–786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin-Loeches M, Ortí RM, Cazorla E,
Asins E and Lixiona J: Multivariate analysis of the morphometric
characteristics of tumours as prognostic factors in the survival of
patients with uterine cervix cancer treated with radical surgery.
Eur J Obstet Gynecol Reprod Biol. 105:170–176. 2002. View Article : Google Scholar
|
|
4
|
Gasinska A, Urbanski K, Adamczyk A,
Pudelek J, Lind BK and Brahme A: Prognostic significance of
intratumour microvessel density and haemoglobin level in carcinoma
of the uterine cervix. Acta Oncol. 41:437–443. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goudy G, Stoeckle E, Thomas L, et al:
Prognostic impact of tumour volume and lymph node involvement in
intermediate stage T1b1 to T2b cancer of the uterine cervix. Bull
Cancer. 96:685–694. 2009.(In French).
|
|
6
|
Grigiene R, Valuckas KP, Aleknavicius E,
Kurtinaitis J and Letautiene SR: The value of prognostic factors
for uterine cervical cancer patients treated with irradiation
alone. BMC Cancer. 7:2342007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su ZZ, Kang DC, Chen Y, et al:
Identification and cloning of human astrocyte genes displaying
elevated expression after infection with HIV-1 or exposure to HIV-1
envelope glycoprotein by rapid subtraction hybridization, RaSH.
Oncogene. 21:3592–3602. 2002. View Article : Google Scholar
|
|
8
|
Brown DM and Ruoslahti E: Metadherin, a
cell surface protein in breast tumors that mediates lung
metastasis. Cancer Cell. 5:365–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarkar D, Emdad L, Lee SG, Yoo BK, Su ZZ
and Fisher PB: Astrocyte elevated gene-1: far more than just a gene
regulated in astrocytes. Cancer Res. 69:8529–8535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao WT, Guo L, Zhong Y, Wu YH, Li J and
Song B: Astrocyte elevated gene-1 (AEG-1) is a marker for
aggressive salivary gland carcinoma. J Transl Med. 9:2052011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Wu J, Ying Z, et al: Astrocyte
elevated gene-1 upregulates matrix metalloproteinase-9 and induces
human glioma invasion. Cancer Res. 70:3750–3759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoo BK, Emdad L, Su ZZ, et al: Astrocyte
elevated gene-1 regulates hepatocellular carcinoma development and
progression. J Clin Invest. 119:465–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu C, Chen K, Zheng H, et al:
Overexpression of astrocyte elevated gene-1 (AEG-1) is associated
with esophageal squamous cell carcinoma (ESCC) progression and
pathogenesis. Carcinogenesis. 30:894–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Zhang N, Song LB, et al: Astrocyte
elevated gene-1 is a novel prognostic marker for breast cancer
progression and overall patient survival. Clin Cancer Res.
14:3319–3326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kikuno N, Shiina H, Urakami S, et al:
Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer
progression through upregulation of FOXO3a activity. Oncogene.
26:7647–7655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu J-B, Wu H, He Y-L, Zhang C-H, Zhang
L-J, Cai S-R and Zhan W-H: Astrocyte-elevated gene-1 overexpression
is associated with poor prognosis in gastric cancer. Med Oncol.
28:455–462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen W, Ke Z, Shi H, Yang S and Wang L:
Overexpression of AEG-1 in renal cell carcinoma and its correlation
with tumor nuclear grade and progression. Neoplasma. 57:522–529.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song LB, Li W, Zhang HZ, et al:
Over-expression of AEG-1 significantly associates with tumour
aggressiveness and poor prognosis in human non-small cell lung
cancer. J Pathol. 219:317–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ash SC, Yang DQ and Britt DE: LYRIC/AEG-1
overexpression modulates BCCIPa protein levels in prostate tumor
cells. Biochem Biophys Res Commun. 371:333–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thirkettle HJ, Mills IG, Whitaker HC and
Neal DE: Nuclear LYRIC/AEG-1 interacts with PLZF and relieves
PLZF-mediated repression. Oncogene. 28:3663–3670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Yang L, Song L, et al: Astrocyte
elevated gene-1 is a proliferation promoter in breast cancer via
suppressing transcriptional factor FOXO1. Oncogene. 28:3188–3196.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santarpia M, Magri I, Sanchez-Ronco M, et
al: mRNA expression levels and genetic status of genes involved in
the EGFR and NF-κB pathways in metastatic non-small-cell lung
cancer patients. J Transl Med. 9:1632011.PubMed/NCBI
|
|
23
|
Li C, Li R, Song H, et al: Significance of
AEG-1 expression in correlation with VEGF, microvessel density and
clinicopathological characteristics in triple-negative breast
cancer. J Surg Oncol. 103:184–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu GH, Chong RA, Yang QF, et al: MTDH
activation by 8q22 genomic gain promotes chemoresistance and
metastasis of poor-prognosis breast cancer. Cancer Cell. 15:9–20.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu H, Song X, Liu C, Li X, Wei L and Sun
R: Knockdown of astrocyte elevated gene-1 inhibits proliferation
and enhancing chemo-sensitivity to cisplatin or doxorubicin in
neuroblastoma cells. J Exp Clin Cancer Res. 28:192009. View Article : Google Scholar
|
|
26
|
Yoo BK, Gredler R, Vozhilla N, et al:
Identification of genes conferring resistance to 5-fluorouracil.
Proc Natl Acad Sci USA. 106:12938–12943. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SG, Su ZZ, Emdad L, Sarkar D and
Fisher PB: Astrocyte elevated gene-1 (AEG-1) is a target gene of
oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc.
Proc Natl Acad Sci USA. 103:17390–17395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SG, Su ZZ, Emdad L, Sarkar D, Franke
TF and Fisher PB: Astrocyte elevated gene-1 activates cell survival
pathways through PI3K-Akt signaling. Oncogene. 27:1114–1121. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ
and Fisher PB: Molecular basis of nuclear factor-kappaB activation
by astrocyte elevated gene-1. Cancer Res. 68:1478–1484. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Emdad L, Sarkar D, Su ZZ, et al:
Activation of the nuclear factor-kappaB pathway by astrocyte
elevated gene-1: implications for tumor progression and metastasis.
Cancer Res. 66:1509–1516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xi W, Rui W, Fang L, Ke D, Ping G and
Zhang H-Z: Expression of stathmin/op18 as a significant prognostic
factor for cervical carcinoma patients. J Cancer Res Clin Oncol.
135:837–846. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li B, Zhang LJ, Zhang ZL, et al:
Synergistic tumor growth inhibition effect of prostate-specific
antigen-activated fusion peptide BSD352 for prostate cancer
therapy. Anticancer Drugs. 22:213–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Long M, Yin G, Liu L, et al:
Adenovirus-mediated Aurora A shRNA driven by stathmin promoter
suppressed tumor growth and enhanced paclitaxel chemotherapy
sensitivity in human breast carcinoma cells. Cancer Gene Ther.
19:271–281. 2012. View Article : Google Scholar
|
|
34
|
Moore MA, Attasara P, Khuhaprema T, et al:
Cancer epidemiology in mainland South-East Asia - past, present and
future. Asian Pac J Cancer Prev. 11(Suppl 2): 67–80.
2010.PubMed/NCBI
|
|
35
|
Folkman J: Tumor angiogenesis. Adv Cancer
Res. 43:175–203. 1985. View Article : Google Scholar
|