Introduction
Hepatocellular carcinoma (HCC) is a significant
health issue and is associated with poor prognosis and high
mortality, accounting for more than 700,000 new cases and more than
600,000 cancer-related deaths each year worldwide. Half of these
new cases and deaths are estimated to occur in China due to the
epidemic prevalence of chronic hepatitis B virus infection. Other
risk factors include hepatitis C virus infection, food contaminated
with aflatoxin B1, alcohol-related cirrhosis, and nonalcoholic
fatty liver disease (1). To date,
there has been no significant breakthrough in the management of
HCC; treatment options include surgery, liver transplantation,
chemotherapy and targeted therapy. Most HCC patients are diagnosed
at the late stage of the disease or have concomitant medical
conditions, which account for the extremely poor survival rates
(1). Thus, novel approaches or
strategies to effectively control progression, detect the disease
early, and predict prognosis are international priorities in HCC.
Towards this end, our research focused on biomarker discovery and
identification, and verification of novel biological markers for
early detection or prediction of survival and treatment
outcome.
p57 is a member of the Cip/Kip (CDK-interacting
protein/kinase inhibition protein) family, a putative
tumor-suppressor gene implicated in different types of human
cancers, including HCC (2,3). The gene p57 is maternally expressed
and paternally imprinted, and encodes a 316-amino acid protein. It
is located on chromosome 11p15.5, a region that has been associated
with chromosomal abnormalities in multiple types of sporadic
cancers and in familial Beckwith-Wiedemann syndrome (4,5). Loss
of p57 expression frequently occurs in various types of human
cancers, such as cancers of the thyroid, mammary glands,
gastrointestinal tract, pancreas, prostate and bladder (6–9).
Functionally, p57 has a key role in the timing of the cell cycle
exit before cell differentiation. Previous studies have shown that
p57 protein is a regulator of the G1/S transition of the cell cycle
via cyclin-dependent kinases (CDKs) and ultimately affects
proliferation and apoptosis of tumor cells (10,11).
In contrast, the knockout of p57 in mice resulted in much larger
creatures, since p57 is a negative regulator of the cell cycle
(12,13).
p57 protein is also involved in the regulation of
cell migration (14,15). The metastasis of primary tumors is
facilitated by the migration and invasion capabilities of tumor
cells. This aggressive phenotype is regulated through cytoskeletal
dynamics, and in particular involves the Rho family of GTPases
(16). RhoA is a member of this
family, and participates in regulating the actin cytoskeleton
during cell locomotion and adhesion (17). During cell movement, RhoA
contributes to the formation of actin stress fibers and the focal
adhesion assembly, a key regulator of cell adhesion and motility in
cancer cells (18,19). Overexpression of RhoA has been
observed in many types of malignancies, including non-inflammatory
breast, lung, pancreatic, colorectal and gastric cancers, and
melanomas (20,21).
Previous studies have shown that both p57 and RhoA
proteins have inverse effects on the malignant behavior of cancers.
What is more, the Cip/Kip family has been reported to control
cytoskeletal organization and cell migration by regulating the
Rho-ROCK-LIMK-cofilin signaling pathways (14,22).
It now appears that there may be direct crosstalk between
cell-cycle proteins and the cytoskeletal regulatory proteins. Thus,
in the present study, we performed immunohistochemistry and qRT-PCR
to analyze the expression levels of p57 and RhoA in HCC tissue
specimens and evaluate their association with clinicopathological
parameters and survival of HCC patients.
Materials and methods
Patients and tissue samples
This study was approved by the Ethics Committee of
Clinical Research of the First Affiliated Hospital, Medical
College, Xi’an Jiaotong University. All patients provided written
informed consent.
We obtained tissue specimens from 80 HCC patients
who underwent surgical resection of HCC lesions at the First
Affiliated Hospital, College of Medicine, Xi’an Jiaotong University
between December 2007 and December 2009. None of the patients had
received prior radiotherapy or chemotherapy treatment. All patients
were histologically confirmed with HCC by 3 independent and
experienced pathologists. There were 56 men and 24 women patients,
aged from 23 to 79 years (median age 45.62±10.89 years) (Table I).
 | Table IClinicopathological features of HCC
patients by p57 and RhoA expression. |
Table I
Clinicopathological features of HCC
patients by p57 and RhoA expression.
| | p57 | | RhoA | |
|---|
| |
| |
| |
|---|
| Characteristic | n (%) | Positive, n | Negative, n | P-value | Positive, n | Negative, n | P-value |
|---|
| Age (years) |
| <45 | 30 (37.5) | 11 | 19 | 0.809 | 19 | 11 | 0.637 |
| ≥45 | 50 (63.5) | 17 | 33 | | 29 | 21 | |
| Gender |
| Male | 56 (70.0) | 20 | 36 | 0.838 | 36 | 20 | 0.232 |
| Female | 24 (30.0) | 8 | 16 | | 12 | 12 | |
| Histopathology |
| Massive | 29 (36.3) | 9 | 20 | 0.575 | 15 | 14 | 0.255 |
| Nodular | 51 (63.7) | 19 | 32 | | 33 | 18 | |
| HBsAg |
| Positive | 72 (90.0) | 23 | 49 | 0.086 | 45 | 27 | 0.171 |
| Negative | 8 (10.0) | 5 | 3 | | 3 | 5 | |
| AFP (ng/ml) |
| <400 | 32 (40.0) | 7 | 25 | 0.044a | 21 | 11 | 0.402 |
| ≥400 | 48 (60.0) | 21 | 27 | | 27 | 21 | |
| Tumor size
(cm) |
| <5 | 28 (35.0) | 4 | 24 | 0.004b | 20 | 8 | 0.126 |
| ≥5 | 52 (65.0) | 24 | 28 | | 28 | 24 | |
| Histological
grade |
| Well/moderate | 34 (42.5) | 7 | 27 | 0.020a | 16 | 18 | 0.042a |
| Poor | 46 (57.5) | 21 | 25 | | 32 | 14 | |
| TNM stage |
| I + II | 27 (33.8) | 5 | 22 | 0.027a | 20 | 7 | 0.067 |
| III + IV | 53 (66.2) | 23 | 30 | | 28 | 25 | |
| Capsule
invasion |
| Positive | 52 (65.0) | 23 | 29 | 0.018a | 36 | 16 | 0.022a |
| Negative | 28 (35.0) | 5 | 23 | | 12 | 16 | |
| Tumor
thrombosis |
| Positive | 44 (55.0) | 21 | 23 | 0.008b | 33 | 11 | 0.002b |
| Negative | 36 (45.0) | 7 | 29 | | 15 | 21 | |
TNM stages were assigned to each patient according
to the criteria of the 2002 Union for International Cancer Control.
Tumor differentiation was assessed using Edmondson’s
classification. The 3-year follow-up for all of the patients was
completed in December 2012. Cases lost to follow-up and those
ending in death from causes other than HCC were regarded as
censored data during the survival analysis.
Paraffin tissue blocks were retrospectively
retrieved at the Pathology Department, which contained both
cancerous and paired distant normal tissues (5 cm away from tumor
lesions) for each patient. The tissue samples had been obtained in
the operating room during surgery and were immediately snap-frozen
in liquid nitrogen and stored at −80°C for RNA isolation.
Real-time reverse
transcriptase-polymerase chain reaction (qRT-PCR)
Total mRNA was isolated from tissue samples using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
and reverse-transcribed into cDNA using an RT-PCR kit (Takara,
Dalian, China) in accordance with the manufacturer’s instructions.
Amplification of these cDNA samples was performed using SYBR Premix
Ex Taq™ II (Takara) in accordance with the manufacturer’s
instructions in an iQ5 Multicolor real-time PCR detection system
(Bio-Rad, Hercules, CA, USA).
The primers for p57, RhoA, and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; internal control)
were designed and synthesized by Takara. The primer sequences were:
p57, 5′-GCGGCGATCAAGAAGCTGT-3′ and 5′-ATCGCCCGAC GACTTCTCA-3′;
RhoA, 5′-GACTCGGATTCGTTGCC TGA-3′ and 5′-TGGGAACTGGTCCTTGCTGA-3;
GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCC
TGTTGCTGTA-3′.
Each experiment was performed in duplicate and
repeated 3 times. A dissociation curve analysis was conducted for
each qPCR. Expression levels of the target gene were evaluated
using a relative quantification approach (2−ΔΔCt method)
against GAPDH levels.
Immunohistochemistry
To detect p57 and RhoA expression, we performed
immunohistochemical staining. Briefly, after deparaffinization and
re-hydration of tissue sections, the sections were first subjected
to antigen retrieval in a pressure cooker in citric buffer for 10
min. The sections were then incubated with 3%
H2O2 for 10 min at room temperature to block
potential endogenous peroxidase activity and then incubated with
20% normal serum and further with a primary antibody against p57
(sc-56341, 1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) or RhoA (10749-1-AP, 1:100; Proteintech Group, Chicago, IL,
USA) at 4°C overnight. The negative control sections were incubated
with phosphate buffered saline (PBS) only to replace the primary
antibody. The next day the sections were washed thrice with PBS and
then incubated with a biotinylated secondary antibody for 30 min at
room temperature. Diaminobenzidine substrate was used to reveal
immunoreactive products in the sections. After counterstaining with
hematoxylin to reveal nuclei, the sections were mounted on slides
and coverslipped.
To assess immunopositive cells, 3 pathologists
reviewed the immunostained sections under a light microscope and
scored the sections in 10 random ×20 power fields. The staining
intensity was graded as: 0, no staining; 1, weak; 2, moderate; 3,
strong. The percentage of positive cells was scored as: 1, <25%;
2, 26–50%; 3, 51–75%; 4, >76%. These 2 scores were added
together. Based on the sum of the scores, each tissue sample was
categorized into 4 groups: 0, ≤5% cells were stained; 1–3, weak
expression; 4–5, moderate expression; and 6–7, strong expression.
Finally, we compared statistically the numbers of cells with
low-to-weak expression with those with moderate-to-strong
expression.
Statistical analysis
Data were analyzed using the Student’s t-test and
the Chi-square (χ2) test. Spearman r test was used to
analyze the correlation between p57 and RhoA expression. Survival
curves were plotted using the Kaplan-Meier method. The log-rank
test for trend was used for the ordinal datum of univariate
analysis, and a Cox proportional hazards regression model was used
for the multivariate analysis of survival duration. Statistical
Package for Social Science (SPSS) version 16.0 (Chicago, IL, USA)
was used to generate the P-value for each test, and all reported
P-values were two-sided. A P-value <0.05 was considered to
indicate a statistically significant result.
Results
Expression of p57 mRNA and protein
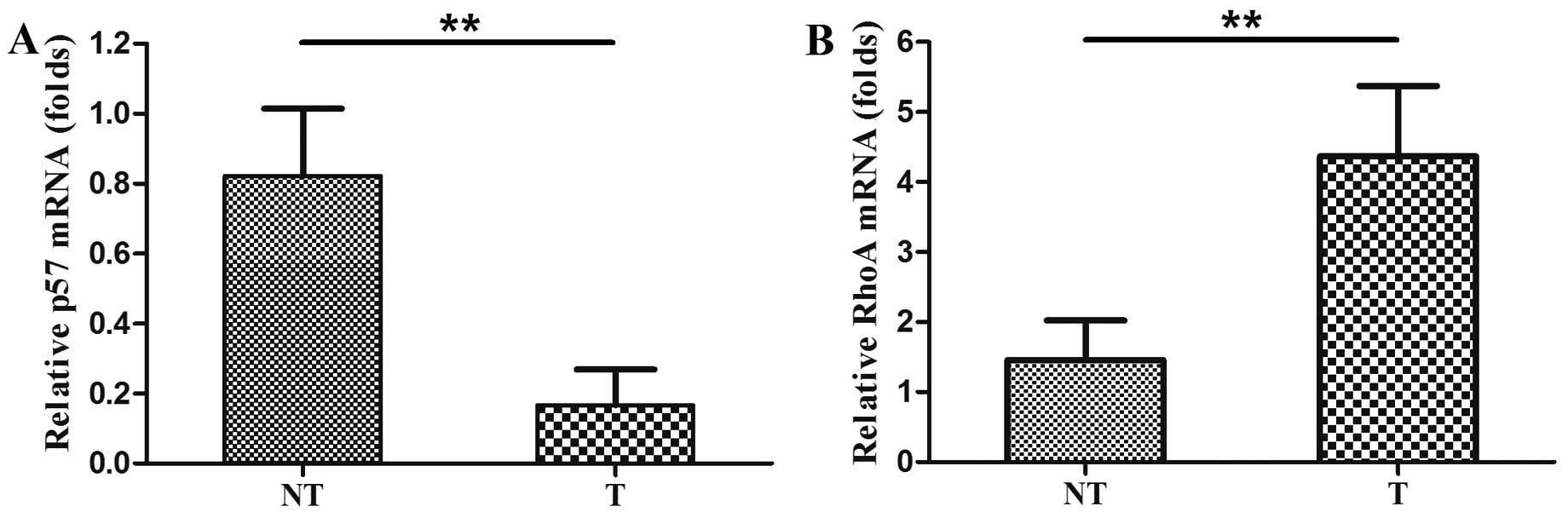
We assessed levels of p57 mRNA using qRT-PCR in the
80 pairs of HCC and adjacent non-cancerous tissues. The level of
p57 mRNA was significantly lower in the HCC tissues than the level
in the distant non-cancerous tissues (0.1670±0.1014 compared with
0.8214±0.1926, P<0.01, Fig. 1A).
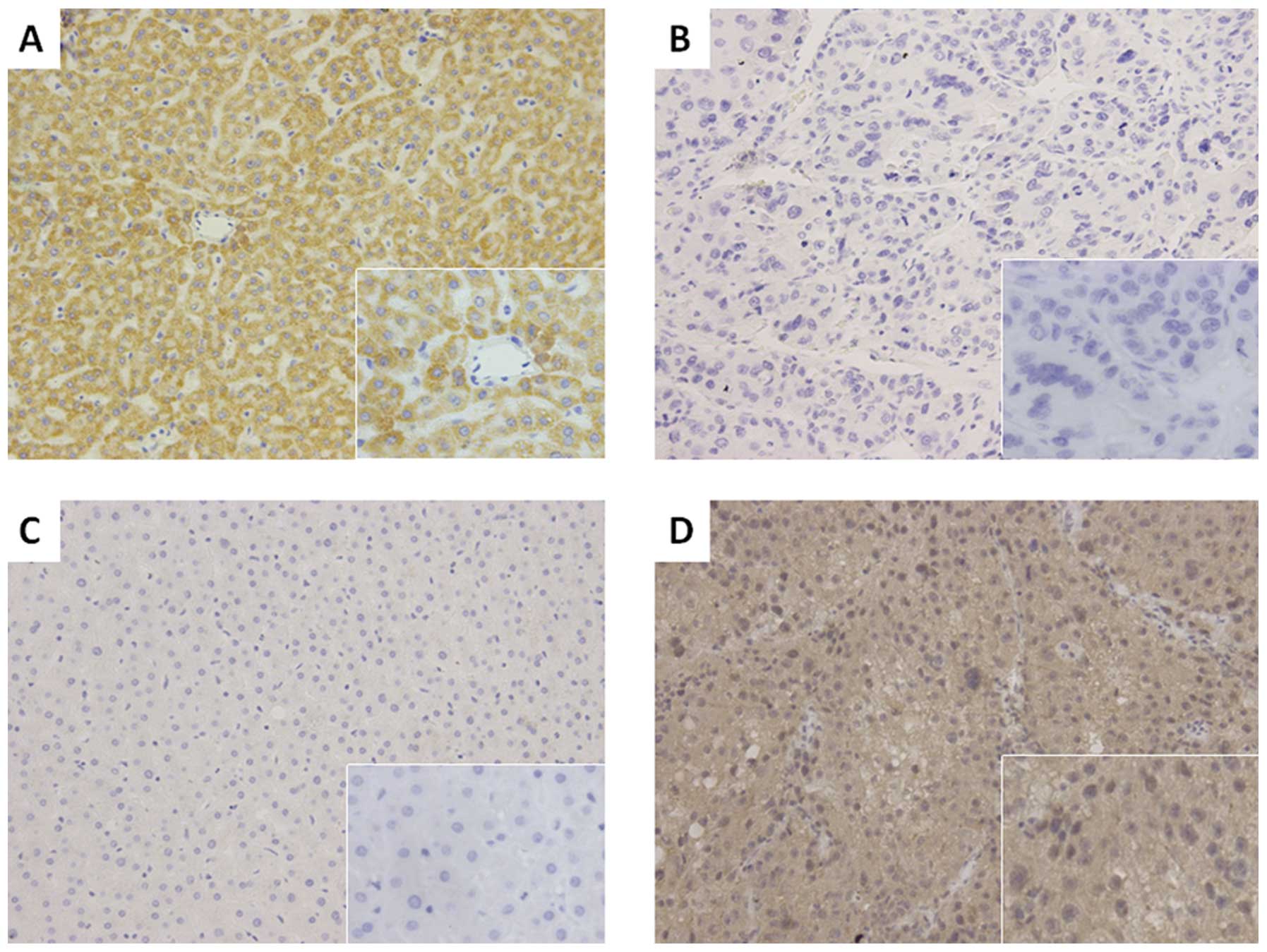
Moreover, we analyzed the expression of p57 protein using
immunohistochemistry and found that compared to the p57 expression
in paired normal tissues (50/80, 62.5%), expression of p57 was
absent in 52 of the 80 HCC tissues (65.0%). The immunohistochemical
data confirmed the qRT-PCR data and indicated that p57 was
significantly reduced in HCC tissues when compared with that of the
distant non-cancerous tissues (χ2=12.108, P=0.001,
Fig. 2).
We also investigated the association between p57
expression and clinicopathological data of the HCC patients. Our
data showed that loss of expression of p57 was associated with HCC
with higher α-fetoprotein (AFP) levels (>400 ng/ml; P=0.044),
larger tumor size (>5 cm, P=0.004), poor tumor differentiation
(P=0.020), advanced TNM stage (P=0.027), presence of capsule
invasion (P=0.018) and tumor thrombosis (P=0.008; Table I).
Expression of RhoA mRNA and protein
We analyzed RhoA expression in the 80 HCC tissues
and the corresponding non-cancerous tissues. We found that the
average level of RhoA mRNA expression in HCC tissues was
significantly higher than that of the distant non-cancerous tissues
(4.3659±1.0056 compared with 1.4571±0.5641, P<0.01, Fig. 1B). Similarly, expression of RhoA
protein was also significantly higher in HCC tissues (60.0%, 48/80)
when compared with the paired normal tissues (42.5%, 34/80;
χ2=4.903, P=0.027, Fig.
2).
Expression of RhoA protein was found to be
significantly associated with poor tumor differentiation (P=0.042),
the presence of capsule invasion (P=0.022), and tumor thrombosis
(P=0.002; Table I).
Association between p57 and RhoA
expression in HCC tissue specimens
As discussed in the Introduction, expression of p57
protein inhibits tumor cell migration and invasion, and the latter
may be associated with RhoA activation; thus, we investigated the
association between p57 and RhoA levels in the HCC and matched
normal tissues. For analysis, we divided the 80 HCC specimens into
groups according to expression: p57+/RhoA−,
p57+/RhoA+, p57−/RhoA−
and p57−/RhoA+. We found that there was a
strong inverse relationship between p57 and RhoA expression in the
HCC tissues. Notably, co-expression (p57-lower and RhoA-higher) was
detected in 38 of the 80 tumors (47.5%), which was statistically
significant (r=−0.364, P=0.001; Table
II). These results indicate that loss of p57 expression may
contribute to RhoA overexpression in HCC tissues. In addition, we
found that there also was an inverse relationship between p57 and
RhoA expression in distant normal tissues (r=−0.270, P=0.016;
Table II).
 | Table IIAssociation between p57 and RhoA
expression in normal and HCC tissue specimens. |
Table II
Association between p57 and RhoA
expression in normal and HCC tissue specimens.
| p57 | | |
|---|
|
| | |
|---|
| RhoA | Positive n (%) | Negative n (%) | r | P-value |
|---|
| Tumor tissue | | | | |
| Positive | 10 (12.5) | 38 (47.5) | −0.364 | 0.001 |
| Negative | 18 (22.5) | 14 (17.5) | | |
| Normal tissue | | | | |
| Positive | 17 (21.2) | 17 (21.2) | −0.270 | 0.016 |
| Negative | 35 (43.8) | 11 (14.8) | | |
Association between p57 and RhoA
expression and overall survival of HCC patients
All of the 80 patients were followed up for survival
until December 2012, and their survival data were stratified
according to p57 and RhoA expression. Of the 80 patients, 77 died
during the follow-up period and the 3-year survival rate was 3.75%.
Survival time ranged from <4 months to >33 months, with a
median survival time of 11.0 months.
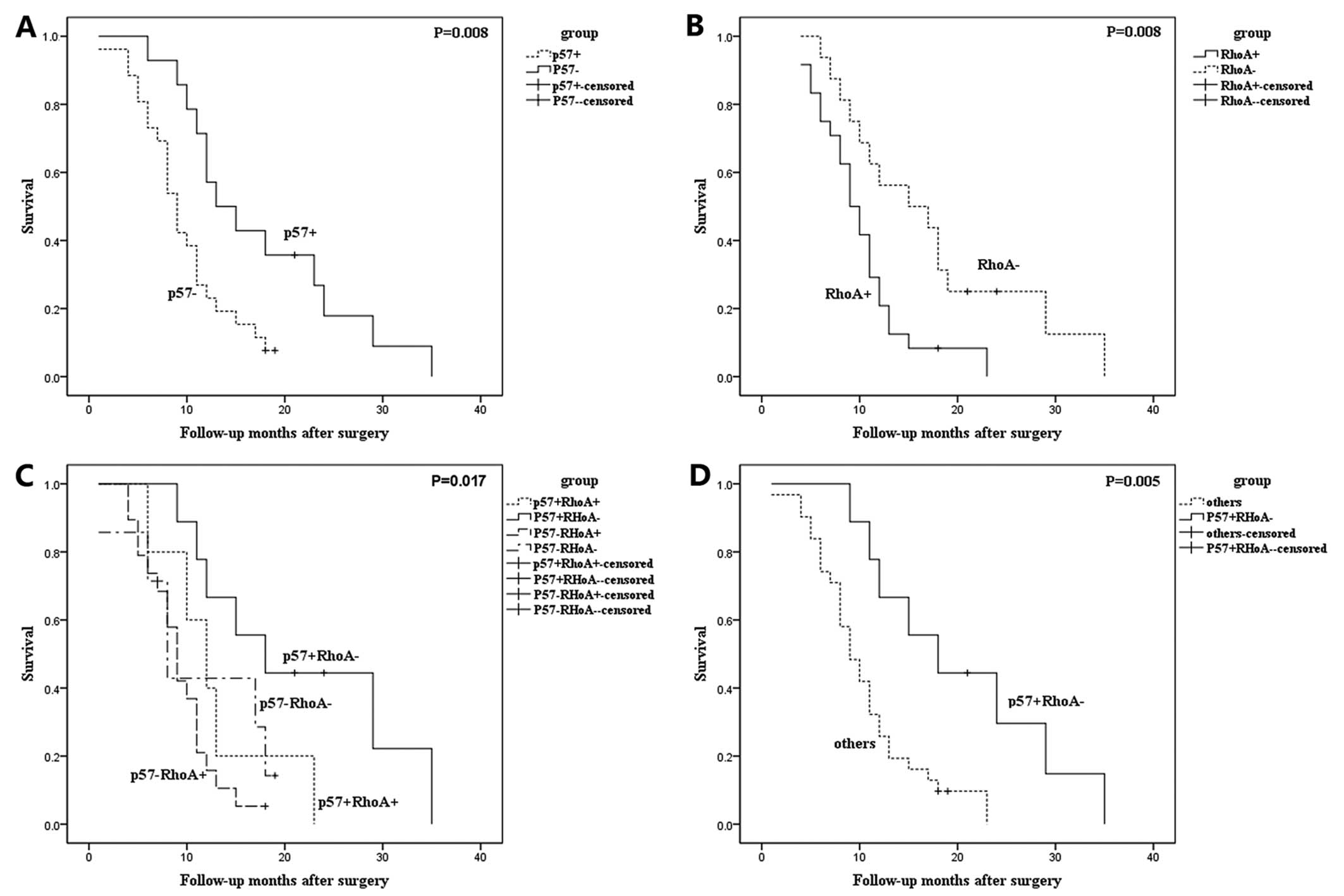
The median survival time of HCC patients with
p57+ and p57− was 13.0 and 9.0 months,
respectively, whereas the median survival time of HCC patients with
RhoA+ and RhoA− was 9.0 and 15.0 months,
respectively. This supports the usefulness of p57 and RhoA proteins
as prognostic markers for HCC patients (P<0.05, Fig. 3A and B).
We then analyzed the association between the
expression groups (p57+/RhoA−,
p57+/RhoA+, p57−/RhoA−
and p57−/RhoA+) with the survival of the HCC
patients. The median survival times were 18.0 months
(p57+/RhoA− patients), 12.0 months
(p57+/RhoA+), 9.0 months
(p57−/RhoA−) and 8.0 months
(p57−/RhoA+). These differences were
statistically significant (P=0.017, Fig. 3C). Moreover, HCC patients with RhoA
expression but with loss of p57 expression
(p57−/RhoA+) tended to have poorer outcomes
than those with other expression combinations (P=0.007, Fig. 3D).
Relatedness of survival with
clinicopathological factors and molecular markers
We grouped significant factors for the analysis of
prognostic factors (Table III).
The log-rank test data revealed that the levels of AFP, tumor size,
TNM stage, histological grade, capsule invasion, tumor thrombosis,
p57, RhoA, and co-expression of p57 and RhoA were all significant
prognostic indicators for overall survival of HCC patients
(P<0.05, Table III). According
to the results of the multivariate analysis of these factors, the
predictive ability of tumor size, TNM stage, p57, RhoA, and
p57−/RhoA+ expression was confirmed
(P<0.05, Table IV). However,
there were no significant associations between prognosis and the
other clinicopathological features.
 | Table IIIUnivariate analysis of survival
data. |
Table III
Univariate analysis of survival
data.
| Clinicopathological
characteristics | P-value |
|---|
| Age, <45 vs. ≥45
years | 0.382 |
| Gender, male vs.
female | 0.331 |
| Histopathology,
massive vs. nodular | 0.446 |
| AFP, <400 vs.
≥400 ng/ml | 0.034a |
| Hepatitis B virus
infection, positive vs. negative | 0.660 |
| Tumor size, <5
vs. ≥5 cm | 0.010a |
| Histological grade,
well/moderate vs. poor | 0.031a |
| TNM stage, I + II
vs. III + IV | 0.020a |
| Capsule invasion,
positive vs. negative | 0.025a |
| Tumor thrombosis,
positive vs. negative | 0.005b |
| p57, positive vs.
negative | 0.008b |
| RhoA, positive vs.
negative | 0.008b |
| Co-expression of
p57 and RhoA, p57−/RhoA+ vs. others | 0.007b |
 | Table IVMultivariate analysis of overall
survival in the 80 HCC cases. |
Table IV
Multivariate analysis of overall
survival in the 80 HCC cases.
| Clinicopathological
characteristics | P-value | Exp(B) | 95% CI |
|---|
| AFP, <400 vs.
≥400 ng/ml | 0.492 | 3.335 | 0.107–103.590 |
| Tumor size, ≥5 vs.
<5 cm | 0.011a | 2.251 | 1.102–4.216 |
| Histological grade,
well/moderate vs. poor | 0.447 | 3.617 | 0.132–99.431 |
| TNM stage, I + II
vs. III + IV | 0.033a | 4.274 | 1.127–16.212 |
| Capsule invasion,
positive vs. negative | 0.780 | 0.671 | 0.041–11.024 |
| Tumor thrombosis,
positive vs. negative | 0.101 | 0.105 | 0.007–1.551 |
| p57, high vs. low
expression | 0.032a | 0.369 | 0.101–0.853 |
| RhoA, high vs. low
expression | 0.020a | 1.694 | 1.086–2.640 |
| Co-expression of
p57 and RhoA, p57−/RhoA+ vs. others | 0.010a | 6.162 | 1.551–24.485 |
Discussion
In the present study, we analyzed the expression of
p57 and RhoA mRNA and p57 and RhoA protein for associations with
clinicopathological data and survival. We found that the
combination of the loss of expression of p57 mRNA and protein and
overexpression of RhoA mRNA and protein in HCC tissue specimens was
significantly more apparent than in distant normal tissues. The
loss of expression of p57 associated with HCC was also correlated
with higher AFP levels, larger tumor size, and poor differentiation
of the tumor, advanced TNM stages, tumor capsule invasion and tumor
thrombosis. However, among the clinicopathological features we
analyzed, expression of RhoA protein was positively associated only
with poor tumor differentiation, tumor capsule invasion and tumor
thrombosis. Furthermore, loss of p57 expression was associated with
RhoA overexpression in HCC tissues, and the
p57−/RhoA+ protein combination contributed to
the poor survival of HCC patients. Multivariate analysis verified
that tumor size, TNM stage, p57, RhoA, and loss of p57 with RhoA
all were risk factors for predicting the poor survival of HCC
patients. This study indicates that detection of p57 and RhoA,
taken together, will predict HCC overall survival.
Tumor cell proliferation, invasion, and metastasis
are defining characteristics of malignant phenotypes in human
cancers. Although much is known regarding the molecular alterations
associated with cancer invasion and metastasis, more research is
needed to fully understand the underlying molecular mechanisms.
Recent studies have shown that p57 protein has many functions in
cancer cells, including regulation of cell cycle distribution,
apoptosis, cell migration and cytoskeletal dynamics. The latter
contributes not only to tumor cell proliferation but also invasion
and metastasis (14,15). We found in the present study that
loss of expression of p57 mRNA and protein was associated with
advanced HCC stage and poor prognosis. These results imply that p57
is a tumor-suppressor gene.
Although the process by which p57 protein functions
to suppress HCC development or progression is still unknown, we did
find that loss of expression of p57 protein was associated with
upregulation of RhoA protein, i.e., RhoA protein was elevated in 48
cases of HCC tissue samples, while p57 was downregulated in 52 of
80 cases. This suggests that the combination of loss of p57
expression and elevated RhoA has a synergistic effect on HCC
tumorigenesis and progression.
RhoA family proteins are known for their roles in
the regulation of actin-stress fiber formation, focal-adhesion
assembly, as well as actin-myosin contractility (19). Thus, activation of the
Rho-ROCK-LIMK-cofilin signaling pathway promotes cell mobility and
migration. The latter are characterized by less or poor cell
differentiation, and our current data showed that overexpression of
RhoA protein was associated with poor tumor differentiation, tumor
capsule invasion, and tumor thrombosis in HCC lesions, all of which
are phenotypes of tumor invasion and metastasis.
Previous studies have shown that expression and
activation of RhoA protein are correlated with tumor progression
(23) and a poor prognosis in human
breast cancer (24). However,
different mechanisms may be involved in regulating the
overexpression and function of RhoA protein. For example, it has
been proven that p27 and p21 proteins may have a role in modulating
cytoskeletal dynamics by binding to RhoA or ROCK (25–27).
Notably, RhoA can also negatively regulate the levels of p27 and
p21 proteins (28–33). Thus, there may be a
negative-feedback loop between cyclin-dependent kinase inhibitors
(CKIs) and Rho family proteins. Our previous data showed that p57
protein inhibited the proliferation and invasion of HCC cells
through inhibition of LIM domain kinase 1 (LIMK1)/phospho-cofilin
signaling (3). LIMK1 principally
acts downstream of Rho GTPases. However, the precise mechanism by
which p57 inhibits RhoA signaling in HCC cells warrants further
investigation.
In previous clinical studies, altered expression of
p57 or RhoA protein was associated with biologically aggressive
tumor phenotypes and with poor prognosis of cancer patients
(34–36). The present study supports these
published data. However, we further showed that tumor size, TNM
stage, p57, RhoA, and loss of p57 combined with RhoA expression all
were independent factors for survival of HCC patients. These data
suggest that altered expression of p57 and RhoA may be associated
with advanced aggressive tumor phenotypes, and both p57 and RhoA
may be independent predictors of the survival of HCC patients.
Acknowledgements
This study was supported in part by a grant from the
National Natural Science Foundation of China (nos. 81172361 and
81201923). The authors thank Professor Chen Huang of Xi’an Jiaotong
University (Xi’an, China) for providing the experimental platform
and expert opinions, and Mr. Song Ren for his technical
assistance.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Pateras IS, Apostolopoulou K, Niforou K,
Kotsinas A and Gorgoulis VG: p57KIP2: ‘Kip’ing the cell
under control. Mol Cancer Res. 7:1902–1919. 2009.
|
|
3
|
Guo H, Lv Y, Tian T, Hu TH, Wang WJ, Sui
X, Jiang L, Ruan ZP and Nan KJ: Downregulation of p57 accelerates
the growth and invasion of hepatocellular carcinoma.
Carcinogenesis. 32:1897–1904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee MH, Reynisdóttir I and Massagué J:
Cloning of p57KIP2, a cyclin-dependent kinase inhibitor
with unique domain structure and tissue distribution. Genes Dev.
9:639–649. 1995.
|
|
5
|
Malik K and Brown KW: Epigenetic gene
deregulation in cancer. Br J Cancer. 83:1583–1588. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee MH and Yang HY: Negative regulators of
cyclin-dependent kinases and their roles in cancers. Cell Mol Life
Sci. 58:1907–1922. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee SH, Lee JK, Jin SM, Lee KC, Sohn JH,
Chae SW and Kim DH: Expression of cell-cycle regulators (cyclin D1,
cyclin E, p27kip1, p57kip2) in papillary
thyroid carcinoma. Otolaryngol Head Neck Surg. 142:332–337. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Larson PS, Schlechter BL, King CL, Yang Q,
Glass CN, Mack C, Pistey R, de Las Morenas A and Rosenberg CL:
CDKN1C/p57kip2 is a candidate tumor suppressor
gene in human breast cancer. BMC Cancer. 8:682008. View Article : Google Scholar
|
|
9
|
Li JQ, Wu F, Usuki H, Kubo A, Masaki T,
Fujita J, Bandoh S, Saoo K, Takeuchi H, Kuriyama S, Ishida T and
Imaida K: Loss of p57KIP2 is associated with colorectal
carcinogenesis. Int J Oncol. 23:1537–1543. 2003.
|
|
10
|
Jin RJ, Lho Y, Wang Y, Ao M, Revelo MP,
Hayward SW, Wills ML, Logan SK, Zhang P and Matusik RJ:
Down-regulation of p57Kip2 induces prostate cancer in
the mouse. Cancer Res. 68:3601–3608. 2008.PubMed/NCBI
|
|
11
|
Schwarze SR, Shi Y, Fu VX, Watson PA and
Jarrard DF: Role of cyclin-dependent kinase inhibitors in the
growth arrest at senescence in human prostate epithelial and
uroepithelial cells. Oncogene. 20:8184–8192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan Y, Frisén J, Lee MH, Massagué J and
Barbacid M: Ablation of the CDK inhibitor p57Kip2 results in
increased apoptosis and delayed differentiation during mouse
development. Genes Dev. 11:973–983. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang P, Liégeois NJ, Wong C, Finegold M,
Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA and Elledge
SJ: Altered cell differentiation and proliferation in mice lacking
p57KIP2 indicates a role in Beckwith-Wiedemann syndrome.
Nature. 387:151–158. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Besson A, Assoian RK and Roberts JM:
Regulation of the cytoskeleton: an oncogenic function for CDK
inhibitors? Nat Rev Cancer. 4:948–955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo H, Tian T, Nan K and Wang W: p57: A
multifunctional protein in cancer (Review). Int J Oncol.
36:1321–1329. 2010.PubMed/NCBI
|
|
16
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aspenström P, Fransson A and Saras J: Rho
GTPases have diverse effects on the organization of the actin
filament system. Biochem J. 377:327–337. 2004.PubMed/NCBI
|
|
18
|
Ridley AJ and Hall A: The small
GTP-binding protein rho regulates the assembly of focal adhesions
and actin stress fibers in response to growth factors. Cell.
70:389–399. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar
|
|
20
|
Gómez del Pulgar T, Benitah SA, Valerón
PF, Espina C and Lacal JC: Rho GTPase expression in tumourigenesis:
evidence for a significant link. Bioessays. 27:602–613.
2005.PubMed/NCBI
|
|
21
|
Pan Y, Bi F, Liu N, Xue Y, Yao X, Zheng Y
and Fan D: Expression of seven main Rho family members in gastric
carcinoma. Biochem Biophys Res Commun. 315:686–691. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong CC, Wong CM, Au SL and Ng IO:
RhoGTPases and Rho-effectors in hepatocellular carcinoma
metastasis: ROCK N’Rho move it. Liver Int. 30:642–656.
2010.PubMed/NCBI
|
|
23
|
Pillé JY, Denoyelle C, Varet J, Bertrand
JR, Soria J, Opolon P, Lu H, Pritchard LL, Vannier JP, Malvy C,
Soria C and Li H: Anti-RhoA and anti-RhoC siRNAs inhibit the
proliferation and invasiveness of MDA-MB-231 breast cancer cells in
vitro and in vivo. Mol Ther. 11:267–274. 2005.PubMed/NCBI
|
|
24
|
Fritz G, Just I and Kaina B: Rho GTPases
are over-expressed in human tumors. Int J Cancer. 81:682–687. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee S and Helfman DM: Cytoplasmic
p21Cip1 is involved in Ras-induced inhibition of the
ROCK/LIMK/cofilin pathway. J Biol Chem. 279:1885–1891. 2004.
|
|
26
|
Wang XQ, Lui EL, Cai Q, Ching WY, Liu KS,
Poon RT and Fan ST: p27Kip1 promotes migration of
metastatic hepatocellular carcinoma cells. Tumour Biol. 29:217–223.
2008.
|
|
27
|
Larrea MD, Hong F, Wander SA, da Silva TG,
Helfman D, Lannigan D, Smith JA and Slingerland JM: RSK1 drives
p27Kip1 phosphorylation at T198 to promote RhoA
inhibition and increase cell motility. Proc Natl Acad Sci USA.
106:9268–9273. 2009.PubMed/NCBI
|
|
28
|
Croft DR and Olson MF: The Rho GTPase
effector ROCK regulates cyclin A, cyclin D1, and p27Kip1
levels by distinct mechanisms. Mol Cell Biol. 26:4612–4627. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lai JM, Wu S, Huang DY and Chang ZF:
Cytosolic retention of phosphorylated extracellular
signal-regulated kinase and a Rho-associated kinase-mediated signal
impair expression of p21Cip1/Waf1 in phorbol
12-myristate-13-acetate-induced apoptotic cells. Mol Cell Biol.
22:7581–7592. 2002. View Article : Google Scholar
|
|
30
|
Mammoto A, Huang S, Moore K, Oh P and
Ingber DE: Role of RhoA, mDia, and ROCK in cell shape-dependent
control of the Skp2-p27kip1 pathway and the
G1/S transition. J Biol Chem. 279:26323–26330. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sahai E, Ishizaki T, Narumiya S and
Treisman R: Transformation mediated by RhoA requires activity of
ROCK kinases. Curr Biol. 9:136–145. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sahai E, Olson MF and Marshall CJ:
Cross-talk between Ras and Rho signalling pathways in
transformation favours proliferation and increased motility. EMBO
J. 20:755–766. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng
Y, Liu M, Chen J, Liu S, Qiu M, Liao Z, Li Z, Luo D, Shi F, Zheng Y
and Bi F: RhoA regulates G1-S progression of gastric
cancer cells by modulation of multiple INK4 family tumor
suppressors. Mol Cancer Res. 7:570–580. 2009.PubMed/NCBI
|
|
34
|
Fan GK, Xu F, Yang B and Fujieda S:
p57kip2 expression is related to carcinogenesis and
tumor progression in laryngeal tissues. Acta Otolaryngol.
126:301–305. 2006.
|
|
35
|
Biaoxue R, Xiguang C, Hua L, Hui M,
Shuanying Y, Wei Z, Wenli S and Jie D: Decreased expression of
decorin and p57(KIP2) correlates with poor survival and lymphatic
metastasis in lung cancer patients. Int J Biol Markers. 26:9–21.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma L, Liu YP, Geng CZ, Wang XL, Wang YJ
and Zhang XH: Overexpression of RhoA is associated with progression
in invasive breast duct carcinoma. Breast J. 16:105–107. 2010.
View Article : Google Scholar : PubMed/NCBI
|