Introduction
Carcinosarcomas (CSs), formerly known as malignant
mixed Müllerian tumors (1), are
relatively rare neoplasms of the female genital tract (2–4). These
tumors are generally characterized by aggressive clinical course
and, consequently, demonstrate an unfavorable outcome (3,5). The
incidence of CSs within the female genital tract is low (1–2%),
since they are generally detected at advanced clinical stages in
postmenopausal women. They are distinctly biphasic as they are
composed of malignant epithelial and mesenchymal components
(6,7). Based on the presence or absence of
heterologous mesenchymal components, CSs are divided into two
subtypes: homologous and heterologous, such as rhabdomyosarcomas or
chondrosarcomas (7,8).
Due to diversity of phenotypic manifestation, the
pathogenesis of CSs still remains to be fully elucidated. To date,
three theories, collision, combination and composition theories,
have been proposed to explain this peculiar histogenesis (2,8,9). The
first (collision or ‘multiclonal’) theory suggests that CSs are due
to two intertwining malignant processes producing one final tumor.
The combination or ‘monoclonal’ hypothesis assumes that CSs are
monoclonal in origin, i.e. developing from a single multipotential
stem cell differentiating into epithelial and mesenchymal pathways.
Finally, the composition theory underlines that CS stromal
components are considered to be not truly neoplastic but to
represent a reactive process in response to malignant epithelial
component differentiation. In 2002, McCluggage (6) reported that these neoplasms are
metaplastic carcinomas, where the sarcomatous component is a
manifestation of increased aggressiveness. Recently, this theory
has been questioned due to the fact that anatomopathological data
confirmed that both CS components are truly malignant. However,
some authors reported that a small proportion of female genital
tract CSs represent collision neoplasms, composed of independently
developed carcinomas and sarcomas, although this phenomenon is rare
(10,11).
TP53 pathway alterations have been reported
to be implicated in the development and progression of various
neoplasms originating from the female genital tract organs
(12–19). Several studies concerning the role
of TP53 pathway alterations in female genital CSs have been
conducted (9,10,13,15,1,20–22),
but the presented data are contradictory. Particularly, Blom et
al(20) found that 61% of
uterine tumors overexpressed p53, and that 25% were positive for
mdm-2 immunostaining. Notably, all p53-positive cases showed a
concordant immunostaining within the carcinomatous and sarcomatous
areas. According to another study, 70 and 75% of uterine CSs showed
positive staining for p53 and Ki-67, respectively (21). Wada et al(10) suggested that although uterine CSs
are mostly combination tumors, some of them might develop as
collision neoplasms. Moreover, the evaluation of clonality might
help predict the prognosis of individual cases and improve
subsequent clinical management.
The aim of the present study was to investigate the
immunohistochemical markers, p53 and Ki-67, in 36 CSs derived from
female genital tract organs in order to determine CS histogenesis.
Clinical and pathological variables of the tumors were related to
immunohistochemical data. Finally, based on our data, the role of
combination theory in the development of CS was assessed.
Materials and methods
Patients and tissue samples
The study group was comprised of 36 patients with
CSs originating from the uterus (n=31), cervix (n=3) and ovary
(n=2). These patients were surgically treated during a 12-year
period (2001–2012) in four centers: 2nd Department of Gynecology,
Lublin Medical University, Lublin, Poland; Department of
Gynecology, Otto von Guericke University, Magdeburg, Germany;
Department of Gynecology and Gynecologic Oncology, Medical
University of Białystok, Białystok, Poland; and Oncology Hospital,
Brzozów, Poland. The patients were not administered any additional
treatment prior to surgery. Initially, 42 CSs were collected. Six
cases were excluded from the analysis due to insufficient material.
From the remaining 36 CSs, the mean patient age was 65.2 years
(range, 36–89). The clinical and pathological characteristics of
the included patients are listed in Table I. The study was approved by the
Ethics Committee of the Lublin Medical University.
 | Table IClinicopathological characteristics
of 36 patients with carcinosarcomas (CSs). |
Table I
Clinicopathological characteristics
of 36 patients with carcinosarcomas (CSs).
| Characteristic | No. of patients
(n=36), n (%) |
|---|
| Patient age
(years) |
| <50 | 3 (8) |
| 50–60 | 11 (31) |
| >60 | 22 (61) |
| Clinical stage |
| I | 12 (33) |
| II | 7 (19) |
| III | 13 (36) |
| IV | 4 (12) |
| Histological
type |
| Homologous | 29 (81) |
| Heterologous | 7 (19) |
| Primary tumor
localization |
| Uterine
corpus | 31 (87) |
| Uterine
cervix | 3 (8) |
| Ovary | 2 (5) |
| Myometrial
invasion |
| <50% | 7 (19) |
| >50% | 29 (81) |
| Lymphovascular
space invasion |
| Positive | 24 (67) |
| Negative | 12 (33) |
| Presence of tumor
in the oviduct |
| Yes | 11 (31) |
| No | 25 (69) |
| Ovarian
metastasesa |
| Present | 11 (32) |
| Absent | 23 (68) |
| Presence of tumor
in the uterine cervixb |
| Yes | 13 (39) |
| No | 20 (61) |
| Lymph node
metastases (n=20) |
| Present | 9 (45) |
| Absent | 11 (55) |
| Omental metastases
(n=8) |
| Present | 3 (38) |
| Absent | 5 (62) |
The surgical specimens were immediately fixed in 10%
buffered formalin and representative tissue samples were taken.
Subsequently, the samples were routinely processed, embedded in
paraffin blocks, stained with hematoxylin and eosin (H&E) and
observed under a light microscope. Two experienced pathologists (Dr
Danuta Skomra until 2011, and J.S. thereafter) reviewed and graded
the tumors based on the WHO Staging System (7,23).
Clinical staging was performed according to the modified FIGO
classification (1,24,25).
Regarding ovarian tumors, staging was performed according to FIGO
classification from 1990 (26).
Three metastases of primary uterine tumors, originating from the
ovary, lymph nodes and omentum, were also examined.
Immunohistochemical analysis
Formalin-fixed, paraffin-embedded tissue samples
were used for immunohistochemical analysis. Tissue sections (4-μm)
were gently cut and mounted on adhesive slides (Poly-Prep™; Sigma,
St. Louis, MO, USA). Antigen retrieval technique with microwave
pretreatment was carried out by applying Dako buffer (pH 9.0) for
20 min at 700 W, and then cooled to room temperature. Endogenous
peroxidase activity was blocked by 3% hydrogen peroxidase for 5
min. After washing with TBS buffer, the slides were incubated with
primary antibodies against p53 (clone DO-7; dilution, 1:25;
DakoCytomation, Copenhagen, Denmark) and Ki-67 (clone MIB-1;
dilution, 1:100; DakoCytomation) for 30 min. Dako REAL™ EnVision™
Detection System Peroxidase/DAB+ (DakoCytomation) was then applied,
and visualization was performed using 0.1% 3,3′-diaminobenzidine
tetrahydrochloride (DAB) solution for 5 min. The sections were
finally counterstained with Mayer’s hematoxylin, dehydrated and
coverslipped after being embedded in mounting medium.
Known positive controls (primary human
endometrioid-type endometrial adenocarcinomas overexpressing p53
and Ki-67) were included in each experiment (17,27,28).
Primary antibody was replaced by normal rabbit antibody, diluted
1:100, as a negative control (DakoCytomation).
The representative areas (each of ~500 tumor cells)
were counted. The analysis was performed by 3 independent
researchers (Dr Danuta Skomra, J.S. and A.S.) reaching a full
agreement in 85% of the sections counted. When consensus was not
reached, immunostaining was evaluated cooperatively region by
region. Regarding nuclear p53 reactivity, a semiquantitative
scoring system proposed by Alkushi et al(29) was applied: 0, p53 expressed in
<10% tumor cells; 1, p53 expressed in 10–50% of tumor cells; and
2, p53 expressed in >50% of tumor cells. Nuclear p53 expression
was defined as score 1, while overexpression of p53 was defined as
score 2. Nuclear Ki-67 immunoreactivity was assessed
semi-quantitatively using a two-point score (21): 0, ≤30% of positively stained tumor
cells; and 1, >30% positively stained tumor cells. Positive
nuclear Ki-67 immunoreactivity (overexpression) was defined as
score 1.
Statistical analysis
Statistical analysis was carried out using SPSS
version 14.0 for Windows (SPSS Inc., Chicago, IL, USA).
χ2 or Fisher’s exact test was applied when appropriate.
Spearman’s rank correlation coefficient was used to determine
correlations between the expression of proteins and patient age.
P<0.05 was considered to indicate a statistically significant
difference.
Results
p53 immunostaining
Thirty-six primary human CSs were investigated for
p53 immunoreactivity in the epithelial and stromal components
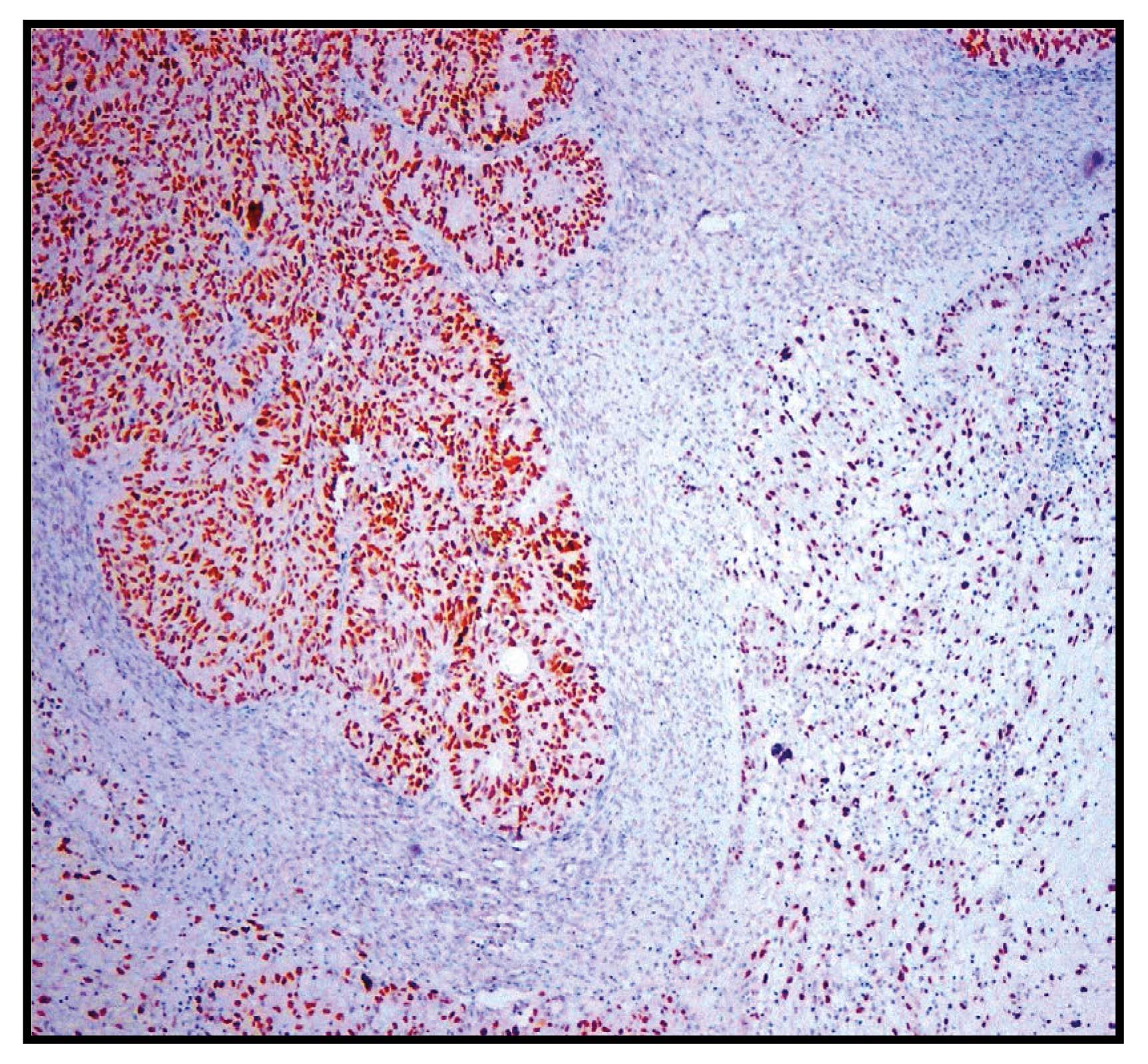
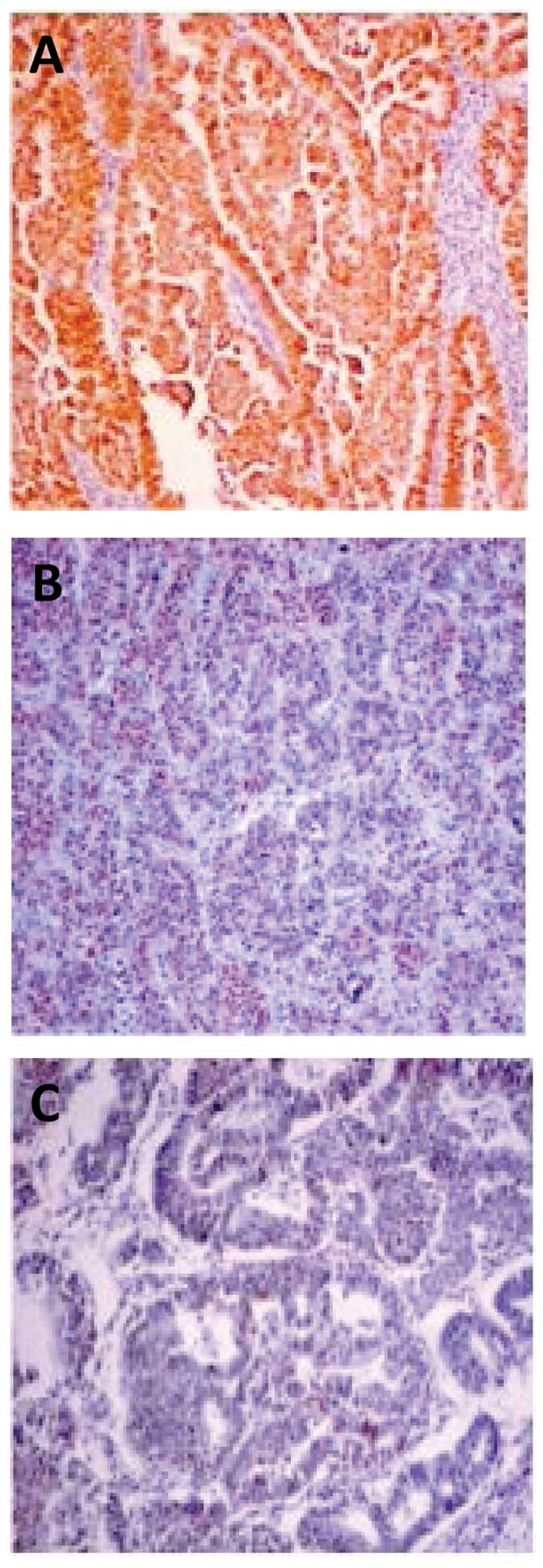
applying immunohistochemical analysis (Fig. 1). p53 was overexpressed in 23 of 36
(64%) tumors at the epithelial component and in 20 of 36 (56%)
tumors at the mesenchymal component (Figs. 2 and 3). Significant difference of p53
immunoreactivity between the two components was established in 3
cases (Table II). p53 protein was
not overexpressed neither by the epithelial nor mesenchymal
component in 5 (14%) cases.
 | Table IIDifferences of p53 and Ki-67
immunoreactivity in epithelial and mesenchymal components of the
carcinosarcomas. |
Table II
Differences of p53 and Ki-67
immunoreactivity in epithelial and mesenchymal components of the
carcinosarcomas.
| | p53 | Ki-67 |
|---|
| |
|
|
|---|
| Patient no. | Primary tumor
localization | Epithelial
component | Mesenchymal
component | Epithelial
component | Mesenchymal
component |
|---|
| 1 | Uterine corpus | 2 | 2 | 86 | 20 |
| 4 | Uterine corpus | 2 | 2 | 60 | 0 |
| 14 | Ovary | 2 | 1 | 20 | 5 |
| 18 | Uterine corpus | 2 | 0 | 20 | 10 |
| 20 | Uterine corpus | 2 | 2 | 30 | 20 |
| 33 | Uterine corpus | 2 | 2 | 15 | 60 |
| 34 | Uterine corpus | 2 | 1 | 30 | 20 |
| 36 | Uterine corpus | 1 | 1 | 15 | 80 |
A significant correlation between p53 overexpression
and patient age was found in both epithelial and mesenchymal
components (P=0.009 and P=0.034, respectively). Moreover, p53
immunostaining was related to cases harboring ovarian metastases
(P=0.025 and P=0.032 for the epithelial and mesenchymal components,
respectively). There was no significant correlation between p53
overexpression and other clinical and pathological characteristics
of cancer, including clinical stage, depth of myometrial
infiltration or lymphovascular space invasion. There was no
correlation between primary tumor localization (uterine corpus,
cervix or ovary) and p53 immunoreactivity.
p53 immunostaining was also evaluated in 3 primary
and paired metastatic tumors (Table
III). Simultaneous p53 overexpression was found in 2
tumor-metastasis pairs, while in the remaining case a marked p53
expression in the primary tumor was accompanied by only weak
immunostaining in lymph node metastasis.
 | Table IIIp53 and Ki-67 immunostaining in
primary uterine carcinosarcomas and the corresponding
metastases. |
Table III
p53 and Ki-67 immunostaining in
primary uterine carcinosarcomas and the corresponding
metastases.
| | p53a | Ki-67a |
|---|
| |
|
|
|---|
| Patient no. | Type of tissue and
metastasis | Epithelial
component | | Mesenchymal
component | Epithelial
component | | Mesenchymal
component |
|---|
| 1 | Primary tumor | 2 | | 2 | 86 | | 20 |
| Ovarian
metastasis | | 2 | | | 90 | |
| 5 | Primary tumor | 2 | | 2 | 20 | | 20 |
| Omental
metastasis | | 2 | | | 20 | |
| 11 | Primary tumor | 1 | | 1 | 30 | | 30 |
| Lymph node
metastasis | | 0 | | | 27 | |
Ki-67 immunoreactivity
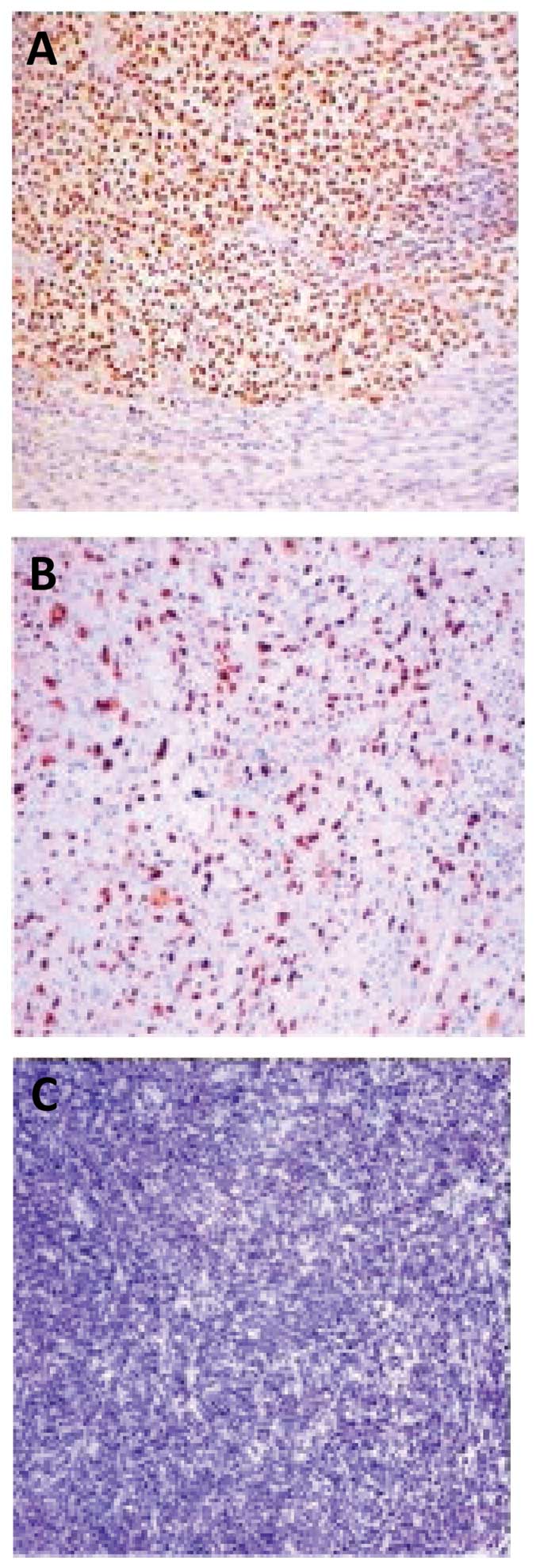
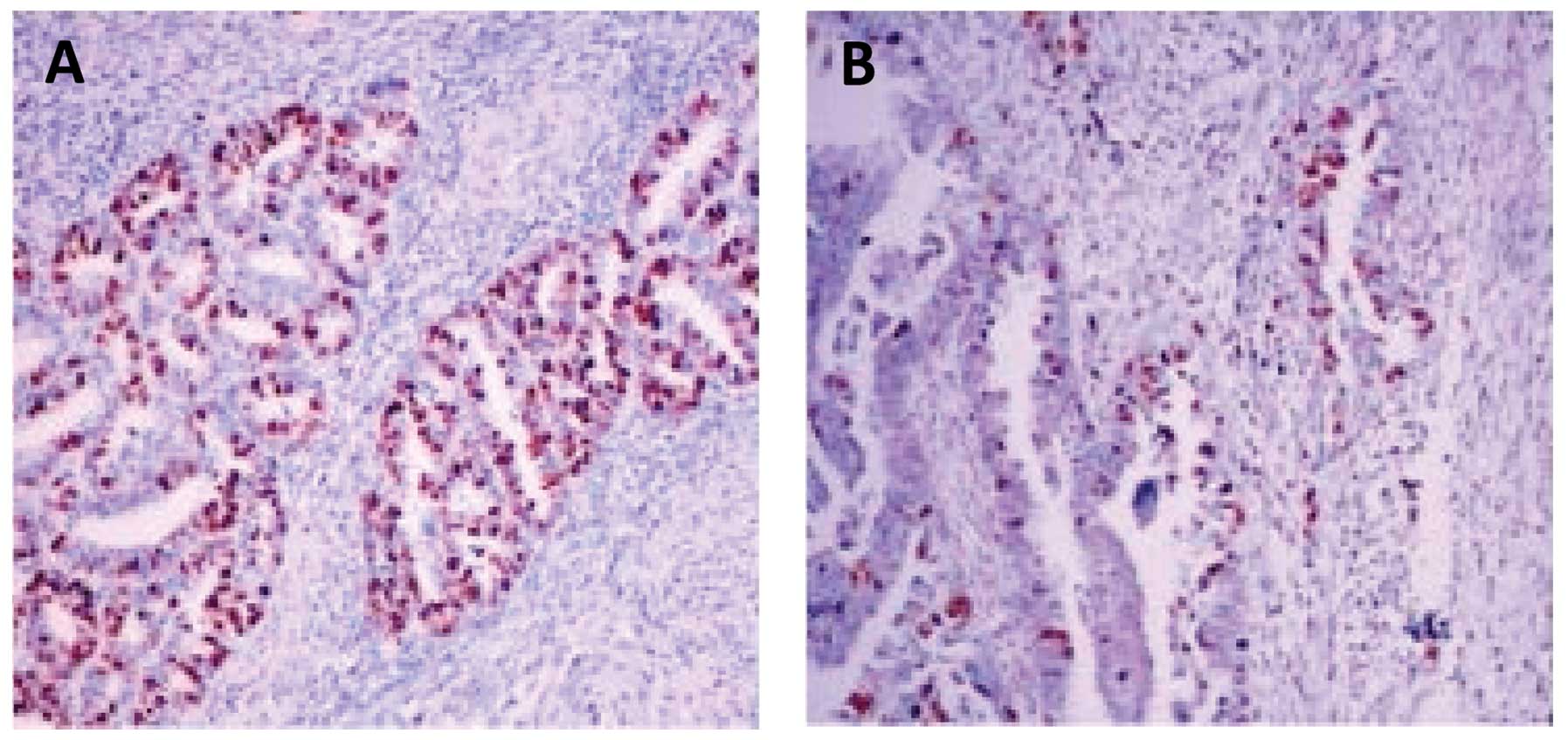
Ki-67 overexpression was observed in 15 of 36 (42%)
tumors in the epithelial component, while 13 of 36 (36%) tumors
displayed a Ki-67 index of ≥30% in the mesenchymal component
(Fig. 4). Only one primary
tumor-metastasis pair showed a significant difference in Ki-67
immunoreactivity (Table II). When
clinicopathological characteristics of Ki-67-immunoreactivity were
related to known clinical and pathological variables of CSs, no
correlation was found between Ki-67 overexpression in the
epithelial or mesenchymal components. Moreover, there was no
correlation between primary tumor localization and Ki-67
immunoreactivity in both tumor components.
Correlation between p53 and Ki-67
expression
There was a significant correlation between p53
overexpression in the epithelial and mesenchymal components
(R=0.884, P<0.001; Table IV). A
significant correlation was also found between the Ki-67
immunoreactivity of the two CS components (R=0.676, P<0.001;
Table IV). However, p53
overexpression was not correlated to Ki-67 immunostaining in both
components. Moreover, neither p53 nor Ki-67 reactivity correlated
with patient age in 36 cases of CSs.
 | Table IVCorrelation between patient age, p53
and Ki-67 immunostaining in epithelial and mesenchymal components
of carcinosarcoma. |
Table IV
Correlation between patient age, p53
and Ki-67 immunostaining in epithelial and mesenchymal components
of carcinosarcoma.
| Patient age | p53 epithelial
component | p53 mesenchymal
component | Ki-67 epithelial
component | Ki-67 mesenchymal
component |
|---|
| Patient age | | R=0.283 | R=0.280 | R=−0.064 | R=−0.028 |
| P=0.095 | P=0.098 | P=0.711 | P=0.873 |
| p53 epithelial
component | R=0.283 | | R=0.884 | R=0.209 | R=−0.068 |
| P=0.095 | |
P<0.001 | P=0.222 | P=0.693 |
| p53 mesenchymal
component | R=0.280 | R=0.884 | | R=0.185 | R=0.008 |
| P=0.098 |
P<0.001 | | P=0.280 | P=0.965 |
| Ki-67 epithelial
component | R=−0.064 | R=0.209 | R=0.185 | | R=0.676 |
| P=0.711 | P=0.222 | P=0.280 | |
P<0.001 |
| Ki-67 mesenchymal
component | R=−0.028 | R=−0.068 | R=0.008 | R=0.676 | |
| P=0.873 | P=0.693 | P=0.965 |
P<0.001 | |
Discussion
Carcinosarcomas are rare female genital tract tumors
composed of two distinct carcinomatous and sarcomatous components
(2,3). These highly aggressive neoplasms are
characterized by a median overall survival of only 21 months, and
for patients with advanced or recurrent disease this survival time
could be shorter (3,5). The survival of women with uterine CSs
was found to be substantially shorter compared with high-risk grade
3 endometrioid and non-endometrioid endometrial carcinomas
(18). Clinical stage, low
myometrial infiltration and late onset of menopause appear to be
independent, prognostic indicators of overall survival (30,31).
CSs are characterized by more aggressive tumor biology and reveal a
wider pattern of spread compared with high-risk endometrioid-type
endometrial carcinomas (32).
The aim of the present study was to independently
investigate the expression of p53 in two coexisting components of
female genital tract CSs. p53 was overexpressed in more than half
of the CSs investigated. A highly significant correlation of p53
overexpression between CS components was established, thus
supporting the combination theory of histogenesis in the majority
of the included patients. The results of this study are in
accordance with previously published studies, where p53
overexpression was observed in 58–78% of female genital tract CSs
(14,20,21,33,34).
However, some data demonstrated a significantly lower rate of
p53-positive CSs. Particularly, Mayall et al(35) found p53-positivity only in 5 of 17
(30%) uterine CSs, a finding leaning towards the monoclonality of
the neoplasm. According to another study, p53 overexpression was
detected only in 30% of uterine CSs, while no p53 overexpression
was detected in uterine adenosarcomas (36). Taken together, the differences in
the frequency of p53 overexpression in female genital tract CSs
could be associated with the application of different antibodies,
detection systems and scoring counting. This variability could be
also related to the relatively small numbers of observations
involved.
p53 overexpression has been associated with
TP53 alterations in various human malignancies, particularly
at ‘hot-spot’ regions of the gene (10,13,16,37,38).
Notably, both point mutations and allelic lost at TP53 occur
in female genital CSs and have been used for clonal tumor analysis
(10,39). As high as 32% (8/25) of uterine CSs
revealed TP53 point mutations, confirming the identical
alterations in both tumor components (10). Based on combined application of
molecular and immunohistochemical markers, Wada et
al(10) suggested that most
cases of CSs represent combination tumors. A high incidence of p53
expression concordance between two CS components was reported by
Szukala et al(40). The same
exon 8 TP53 point mutation (codon 282, CGG→TGG) was detected
in both components of a uterine CS (16). TP53 alterations and protein
overexpression are considered to be early events during CS
tumorigenesis (10,14,20,40,41).
Alterations in the TP53 gene have been reported not only in
primary tumors (14,15), but also in cell lines derived from
female genital CSs (42).
The metastatic process involves several mechanisms
including decreased adhesion between cells, basement membrane
degradation, and invasion into the bloodstream and to locoregional
lymph nodes (43). The presence of
metastases (local or distant) at diagnosis is one of the most
unfavorable prognostic indicators for women with CSs (43,44).
In our laboratory, TP53 alterations have been studied not
only in primary human endometrial carcinomas, but also in
corresponding metastases (45,46).
According to Swisher et al(36), p53 overexpression was detected in 2
of 4 cases with primary tumors and in corresponding metastases,
while immunoreactivity for Ki-67 was comparable. Recently, de Jong
et al(18) demonstrated that
there was similar p53 expression in 18 primary tumors and paired
metastatic tissues. In the present study, 2 out of 3 cases
displayed similar p53 immunoreactivity in primary tumors and
corresponding metastases. Studies evaluating the molecular
mechanisms, particularly the underlying mechanisms of the p53
pathway, involved in the formation of metastases in CS patients
should be conducted.
The established correlation between p53 and Ki-67
overexpression in both tumor components strongly supports the
combination theory in most cases of female genital CSs. Monoclonal
origin of CSs stemming from the cervix, uterus, ovary and oviduct
has been suggested by Fujii et al(39). Several other studies have reported
similar p53 immunoreactivity in both tumor components (10,13,35,40,41,47–50).
Moreover, in a model of uterine CS histogenesis proposed by Taylor
et al(41), more than 71% of
uterine CSs shared similar genetic alterations, while molecular
defects acquired at a later stage were proved to be discordant
between the two components. However, further studies are needeed
for the investigation of the genetic mechanisms that are involved
in the development of CSs into collision tumors. Different patterns
of chromosome X inactivation in tumor cells genotyped from
epithelial and mesenchymal lesions support the collision
histogenesis (11). In the present
study, 3 out of 36 (8%) cases showed a distinct p53 reactivity in
both components, suggesting the ‘biclonal’ (collision)
histogenesis. Future verification of genetic alterations in the
TP53 gene in different p53-stained components of CS is
needed.
In conclusion, based on immunohistochemical data,
p53 is overexpressed in more than half of the female genital tract
CSs included in the present study, either in the epithelial or
mesenchymal component. The correlation between p53 or Ki-67
overexpression in both tumor components supports the combination
theory of histogenesis in the majority of these tumors.
Acknowledgements
This study is dedicated to the unforgettable memory
of Dr Danuta Skomra (7.9.1956–10.3.2011), pathologist, who was a
friend and co-investigator for many years. The authors would like
to express their gratitude to Robert Klepacz for his excellent
technical assistance with the microphotographs. This study was
supported by a grant from Lublin Medical University, Lublin, Poland
(no. 326/12) to A.S.
References
|
1
|
FIGO Committee on Gynecologic Oncology.
FIGO staging for uterine sarcomas. Int J Gynaecol Obstet.
104:1792009. View Article : Google Scholar
|
|
2
|
McCluggage WG: Uterine carcinosarcomas
(malignant mixed Mullerian tumors) are metaplastic carcinomas. Int
J Gynecol Cancer. 12:687–690. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arend R, Doneza JA and Wright J: Uterine
carcinosarcoma. Curr Opin Oncol. 23:531–536. 2011.PubMed/NCBI
|
|
4
|
Serkies K and Jassem J: Uterine
carcinosarcoma. Ginekol Pol. 83:609–612. 2012.(In Polish).
|
|
5
|
D’Angelo E and Prat J: Uterine sarcomas: a
review. Gynecol Oncol. 116:131–139. 2010.
|
|
6
|
McCluggage WG: Malignant biphasic uterine
tumours: carcinosarcomas or metaplastic carcinomas? J Clin Pathol.
55:321–325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zaloudek C and Hendrickson MR: Mesenchymal
tumors of the uterus. Blaustein’s Pathology of the Female Genital
Tract. Kurman RJ: 5th edition. Springer; New York: pp. 561–615.
2002
|
|
8
|
Lopez-Garcia MA and Palacios J: Pathologic
and molecular features of uterine carcinosarcomas. Semin Diagn
Pathol. 27:274–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abeln ECA, Smit VT, Wessels JW, de Leeuw
WJ, Cornelisse CJ and Fleuren GJ: Molecular genetic evidence for
the conversion hypothesis of the origin of malignant mixed
mullerian tumours. J Pathol. 183:424–431. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wada H, Enomoto T, Fujita M, et al:
Molecular evidence that most but not all carcinosarcomas of the
uterus are combination tumors. Cancer Res. 57:5379–5385.
1997.PubMed/NCBI
|
|
11
|
Jin Z, Ogata S, Tamura G, et al:
Carcinosarcomas (malignant mullerian mixed tumors) of the uterus
and ovary: a genetic study with special reference to histogenesis.
Int J Gynecol Pathol. 22:368–373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berchuck A, Kohler MF, Marks JR, Wiseman
R, Boyd J and Bast RC Jr: The p53 tumor suppressor gene frequently
is altered in gynecologic cancers. Am J Obstet Gynecol.
170:246–252. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Costa MJ, Vogelsan J and Young LJ:
p53 gene mutation in female genital tract carcinosarcomas
(malignant mixed mullerian tumors): a clinicopathologic study of 74
cases. Mod Pathol. 7:619–627. 1994.
|
|
14
|
Liu FS, Kohler MF, Marks JR, Bast RC Jr,
Boyd J and Berchuck A: Mutation and overexpression of the p53 tumor
suppressor gene frequently occurs in uterine and ovarian sarcomas.
Obstet Gynecol. 83:118–124. 1994.PubMed/NCBI
|
|
15
|
Soong R, Knowles S, Hammond IG, Michael C
and Iacopetta BJ: p53 protein overexpression and gene mutation in
mixed Mullerian tumors of the uterus. Cancer Detect Prev. 23:8–12.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Watanabe M, Shimizu K, Kato H, et al:
Carcinosarcoma of the uterus: immunohistochemical and genetic
analysis of clonality of one case. Gynecol Oncol. 82:563–567. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Semczuk A, Marzec B, Skomra D, et al:
Allelic loss at TP53 is not related to p53 protein
overexpression in primary human endometrial carcinomas. Oncology.
69:317–325. 2005.
|
|
18
|
de Jong RA, Nijman HW, Wijbrandi TF,
Reyners AK, Boezen HM and Hollema H: Molecular markers and clinical
behavior of uterine carcinosarcomas: focus on the epithelial tumor
component. Mod Pathol. 24:1368–1379. 2011.PubMed/NCBI
|
|
19
|
Yemelyanova A, Vang R, Kshirsagar M, et
al: Immunohistochemical staining pattern of p53 can serve as a
surrogate marker for TP53 mutations in ovarian carcinoma: an
immunohistochemical and nucleotide sequencing analysis. Mod Pathol.
24:1248–1253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blom R, Guerrieri C, Stâl O, Malmström H,
Sullivan S and Simonsen E: Malignant mixed Müllerian tumors of the
uterus: a clinicopathologic, DNA flow cytometric, p53, and mdm-2
analysis of 44 cases. Gynecol Oncol. 68:18–24. 1998.
|
|
21
|
Lee SJ, Kim HS, Kim HS, Chun YK, Hong SR
and Lee JH: Immunohistochemical study of DNA topoisomerase I, p53,
and Ki-67 in uterine carcinosarcomas. Hum Pathol. 38:1226–1231.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Semczuk A, Skomra D, Chyzynska M, Szewczuk
W, Olcha P and Korobowicz E: Immunohistochemical analysis of
carcinomatous and sarcomatous components in the uterine
carcinosarcoma: a case report. Pathol Res Pract. 204:203–207. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scully RE, Bonfiglio TA, Kurman RJ,
Silverberg SG and Wilkinson EJ: Histological Typing of Female
Genital Tract Tumours. Springer-Verlag; Berlin, Heidelberg: 1994,
View Article : Google Scholar
|
|
24
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prat J: FIGO staging for uterine sarcomas.
Int J Gynaecol Obstet. 104:177–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
FIGO. Changes in gynecologic staging by
the International Federation of Gynecology and Obstetrics. Am J
Obstet Gynecol. 162:610–611. 1990.
|
|
27
|
Semczuk A, Skomra D, Cybulski M and
Jakowicki JA: Immunohistochemical analysis of MIB-1 proliferative
activity in human endometrial cancer. Correlation with
clinicopathological parameters, patient outcome, retinoblastoma
immunoreactivity and K-ras codon 12 point mutations.
Histochem J. 33:193–200. 2001. View Article : Google Scholar
|
|
28
|
Olcha P, Cybulski M, Skomra D, et al: The
pattern of p14ARF expression in primary and metastatic
human endometrial carcinomas: correlation with clinicopathological
features and TP53 pathway alterations. Int J Gynecol Cancer.
20:993–999. 2010.
|
|
29
|
Alkushi A, Lim P, Coldman A, Huntsman D,
Miller D and Gilks CB: Interpretation of p53 immunoreactivity in
endometrial carcinoma: establishing a clinically relevant cut-off
level. Int J Gynecol Pathol. 23:129–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Iwasa Y, Haga H, Konishi I, et al:
Prognostic factors in uterine carcinosarcoma: a clinicopathologic
study of 25 patients. Cancer. 82:512–519. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bodner-Adler B, Bodner K, Obermair A, et
al: Prognostic parameters in carcinosarcomas of the uterus: a
clinico-pathologic study. Anticancer Res. 21:3069–3074.
2001.PubMed/NCBI
|
|
32
|
Amant F, Cadron I, Fuso L, et al:
Endometrial carcinosarcomas have a different prognosis and pattern
of spread compared to high-risk epithelial endometrial cancer.
Gynecol Oncol. 98:274–280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kounelis S, Jones MW, Papadaki H, Bakker
A, Swalsky P and Finkelstein SD: Carcinosarcomas (malignant mixed
mullerian tumors) of the female genital tract: comparative
molecular analysis of epithelial and mesenchymal components. Hum
Pathol. 29:82–87. 1998. View Article : Google Scholar
|
|
34
|
Kanthan R, Senger J-LB and Diudea D:
Malignant mixed Mullerian tumors of the uterus: histopathological
evaluation of cell cycle and apoptotic regulatory proteins. World J
Surg Oncol. 8:602010. View Article : Google Scholar
|
|
35
|
Mayall F, Rutty K, Campbell F and Goddard
H: p53 immunostaining suggests that uterine carcinosarcomas are
monoclonal. Histopathology. 24:211–214. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Swisher EM, Gown AM, Skelly M, et al: The
expression of epidermal growth factor receptor, HER-2/Neu,
p53, and Ki-67 antigen in uterine malignant mixed mesodermal tumors
and adenosarcoma. Gynecol Oncol. 60:81–88. 1996.PubMed/NCBI
|
|
37
|
Sherman ME, Bur ME and Kurman RJ: p53 in
endometrial cancer and its putative precursors: evidence for
diverse pathways of tumorigenesis. Hum Pathol. 26:1268–1274. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Petitjean A, Achatz MI, Borresen-Dale AL,
Hainaut P and Olivier M: TP53 mutations in human cancers:
functional selection and impact on cancer prognosis and outcomes.
Oncogene. 26:2157–2165. 2007. View Article : Google Scholar
|
|
39
|
Fujii H, Yoshida M, Gong ZX, et al:
Frequent genetic heterogeneity in the clonal evolution of
gynecological carcinosarcoma and its influence on phenotypic
diversity. Cancer Res. 60:114–120. 2000.PubMed/NCBI
|
|
40
|
Szukala SA, Marks JR, Burchette JL,
Elbendary AA and Krigman HR: Co-expression of p53 by epithelial and
stromal elements in carcinosarcoma of the female genital tract: an
immunohistochemical study of 19 cases. Int J Gynecol Cancer.
9:131–136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Taylor NP, Zighelboim I, Huettner PC, et
al: DNA mismatch repair and TP53 defects are early events in
uterine carcinosarcoma tumorigenesis. Mod Pathol. 19:1333–1338.
2006.PubMed/NCBI
|
|
42
|
Yuan Y, Kim WH, Han HS, et al:
Establishment and characterization of cell lines derived from
uterine malignant mixed Müllerian tumor. Gynecol Oncol. 66:464–474.
1997.PubMed/NCBI
|
|
43
|
Hoon DS, Kitago M, Kim J, et al: Molecular
mechanisms of metastasis. Cancer Metastasis Rev. 25:203–220. 2006.
View Article : Google Scholar
|
|
44
|
Sreenan JJ and Hart WR: Carcinosarcomas of
the female genital tract. A pathologic study of 29 metastatic
tumors: further evidence for the dominant role of the epithelial
component and the conversion theory of histogenesis. Am J Surg
Pathol. 19:666–674. 1995. View Article : Google Scholar
|
|
45
|
Jeczen R, Skomra D, Cybulski M, et al:
P53/MDM2 overexpression in metastatic endometrial cancer:
correlation with clinicopathological features and patient outcome.
Clin Exp Metastasis. 24:503–511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Semczuk A, Schneider-Stock R and Szewczuk
W: Prevalence of allelic loss at TP53 in endometrial
carcinomas. Oncology. 78:220–228. 2010. View Article : Google Scholar
|
|
47
|
Nicòtina PA, Ferlazzo G and Vincelli AM:
Proliferation indices and p53-immunocytochemistry in uterine mixed
mullerian tumors. Histol Histopathol. 12:967–972. 1997.PubMed/NCBI
|
|
48
|
Abargel A, Avinoach I, Kravtsov V, Boaz M,
Glezerman M and Menczer J: Expression of p27 and p53: comparative
analysis of uterine carcinosarcoma and endometrial carcinoma. Int J
Gynecol Cancer. 14:354–359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Buza N and Tavassoli FA: Comparative
analysis of P16 and P53 expression in uterine malignant mixed
mullerian tumors. Int J Gynecol Pathol. 28:514–521. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Koivisto-Korander R, Butzow R, Koivisto AM
and Leminen A: Immunohistochemical studies on uterine
carcinosarcoma, leiomyosarcoma, and endometrial stromal sarcoma:
expression and prognostic importance of ten different markers.
Tumour Biol. 32:451–459. 2011. View Article : Google Scholar
|