Introduction
Paclitaxel (Taxol®), a potent drug of
natural origin isolated from the bark of the Pacific yew, is an
important antitumor drug with significant activity against ovarian,
lung and breast cancer (1–3). As a drug of cancer chemotherapy,
paclitaxel has an unusual chemical structure that is a complex
diterpene having a taxane ring with a four-membered oxetane ring
and an ester side chain at position C-13 endowed with a unique
mechanism of action: it inhibits microtubules from disassembly
(4). For example, paclitaxel
enhances the polymerization of tubulin to stable microtubules and
also interacts directly with microtubules (4) and β-tubulin gene mutations are strong
predictors of resistance response to the antitubulin drug
paclitaxel (5–7).
Since the unique action of paclitaxel against
microtubules was discovered in the 1970s (8), considerable work has been carried out
to characterize the mechanisms by which paclitaxel disrupts the
normal function of microtubules and arrests the cell cycle at the
G2/M phase. Little attention has been paid to other
possible cellular actions of this antineoplastic agent. In 1993,
Bhalla et al(9) demonstrated
for the first time that the exposure of human myeloid leukemia
HL-60 and KG-1 cells to clinically achievable concentrations of
paclitaxel produced internucleosomal DNA fragmentation of ~200
base-pair multiples, and also showed the morphologic changes
characteristic of cells undergoing programmed cell death (PCD) or
apoptosis. In recent years, apoptotic cell death induced by
paclitaxel has been characterized by several laboratories in
epithelial ovarian cancer (10),
human gastric carcinoma cell lines (11), prostate tumors (12), adrenocortical carcinoma cell line
(13) and human glioma cells
(14).
These results clearly indicate that paclitaxel, in
addition to its classical effect on microtubules and the arrest of
cell cycle, may also possess significant cell-killing activity by
induction of apoptosis. In the clinic, paclitaxel has been widely
used in several malignant solid tumors, such as ovarian cancer,
breast cancer and lung cancer (15). However, a poor level of in
vivo induction of apoptosis was achieved during a phase I
clinical study with paclitaxel therapy in 26 leukemia patients
(16). In addition, paclitaxel has
a significantly weaker cytotoxic effect on CD34 positive AML cells
than CD34 negative leukemia cell lines, such as HL-60 and U937
(16). Considering the fact that
leukemia is a heterogeneous disease with different cell types and
immune phenotypes, it is necessary to investigate the effect of
paclitaxel on different types of leukemia cell lines.
Materials and methods
Cell culture
Two types of established leukemic cell lines (MEL
and K562) used in the present study were preserved in our
laboratory, and were generous gifts from the Chinese Academy of
Medical Sciences and Peking Union Medical College. The cell lines
K562 and MEL were cultured in DMEM medium (Gibco-BRL Life
Technologies) supplemented with 10% heat-inactivated fetal bovine
serum (Sijiqing Biotech, Beijing, China) at 37°C in a humidified
atmosphere of 5% CO2.
Cell proliferation assay
MTT assay was used to determine the effect of
proliferative inhibition on the two types of leukemia cell lines
induced by incubation with paclitaxel (Huadong Medicine Co., Ltd.,
Hanzhou, China). Cells at the exponential growth phase were seeded
at 3×103 cells/well in 96-well microtiter plates in a
volume of 180 μl medium/well in the presence of various paclitaxel
concentrations at a volume of 20 μl or in the presence of isochoric
PBS as the control. Then, 10 μl of 5 mg/ml MTT (Sigma) was added to
each well and incubated for 4 h at 24, 48, 72 and 96 h,
respectively. DMSO (150 μl) was added to dissolve the formazan
crystals after centrifugation of the microtiter plates (3,000 × g
for 15 min) and the supernatant was gently removed. The absorbance
was determined using microplate reader (Varioscan Flash; Thermo
Scientific) at 570 nm.
Inverted fluorescent microscope
MEL and K562 cells were incubated in the presence of
paclitaxel or buffer alone for 24, 48, 72 and 96 h. The final
concentrations of paclitaxel for MEL and K562 cells were 100, 30,
10, 3, 1 and 0.3 ng/ml; Hoechst 33342 (10 μg/ml, 50 μl) was added
and the suspension was further incubated for 5 min at room
temperature in the dark. Microscopic analysis was carried out with
an inverted fluorescent microscope (TE2000-U, Nikon, Tokyo,
Japan).
Flow cytometric analysis of apoptosis and
necrosis
Cells were first harvested at the exponential growth
phase and mixed with paclitaxel at a concentration of 50 ng/ml for
MEL cells, and 21 ng/ml for K562 cells, at 37°C in a humidified
atmosphere of 5% CO2 for 48 h. For cell apoptosis and
necrosis analysis, cells were resuspended at 2×106
cells/ml, and stained by Annexin V-FITC (5 μl for 15 min) and PI
(10 μl for 5 min) according to the instructions of the Cell
Apoptosis kit (Kaiji Bio Co., Nanjing, China) and samples were
analyzed with flow cytometry (FACSAria; BD Biosciences, Mountain
View, CA, USA).
Flow cytometric analysis of cell
cycle
Cells were first harvested at the exponential growth
phase and mixed with paclitaxel at a concentration of 50 ng/ml for
MEL cells and 21 ng/ml for K562 cells, at 37°C in a humidified
atmosphere of 5% CO2 for 24 h. For cell cycle analysis,
cells were resuspended at 2×106 cells/ml and fixed in
ice-cold 70% ethanol. According to the instructions of the Cell
Cycle kit (Kaiji Bio Co.), each sample was resuspended in propidium
iodide (PI) stain buffer (0.1% Triton X-100, 10 μg/ml DNase-free
RNase A, 50 μg/ml PI) for 30 min and samples were analyzed with
flow cytometry (FACSAria; BD Biosciences).
Statistical analysis
All results were confirmed by at least three
independent experiments. The data are expressed as means ± SEM.
Comparisons of mean values were performed using the independent
samples t-test in SPSS for Windows 11.5 software. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Proliferation of leukemia cells is
inhibited by paclitaxel
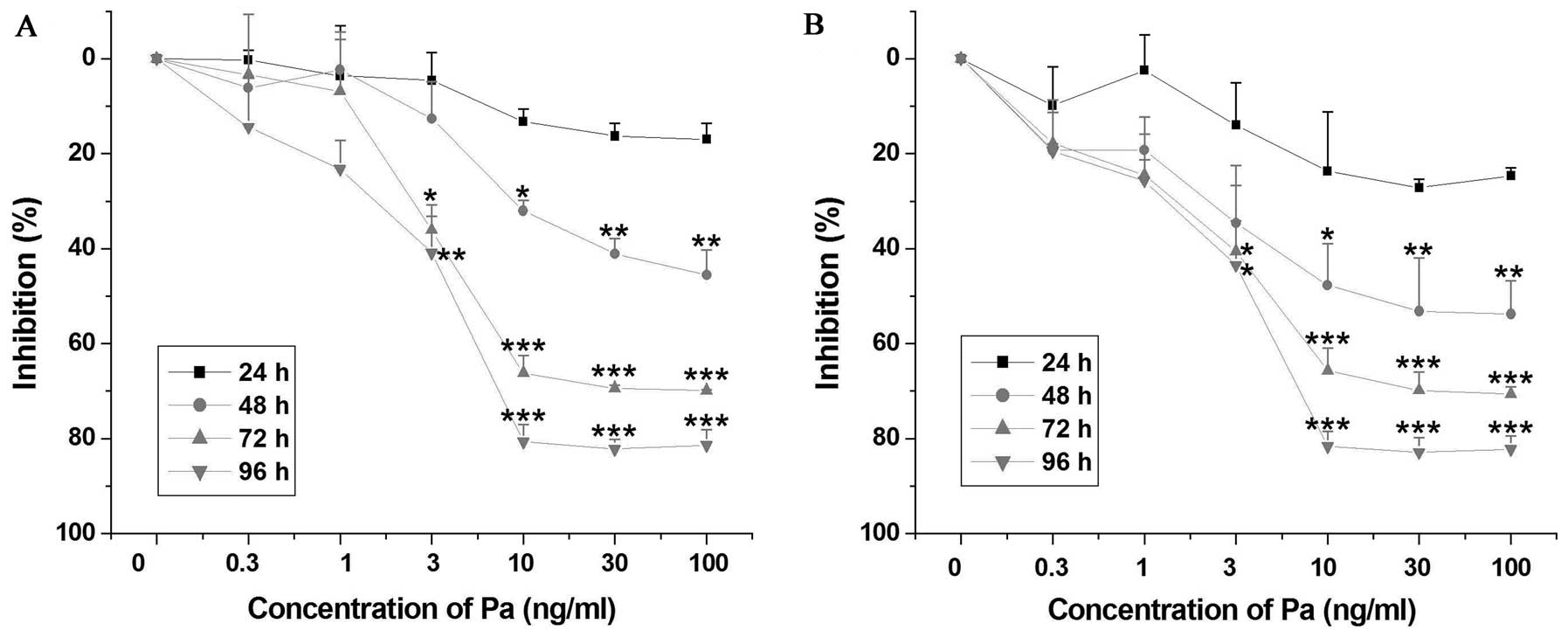
Using the MTT assay, our results showed that
paclitaxel inhibited the proliferation of MEL and K562 cells in a
dose- and time-dependent manner (Fig.
1). The IC50 values of paclitaxel in MEL and K562
cells at 48 h were 99.5 and 42.7 ng/ml, respectively. Furthermore,
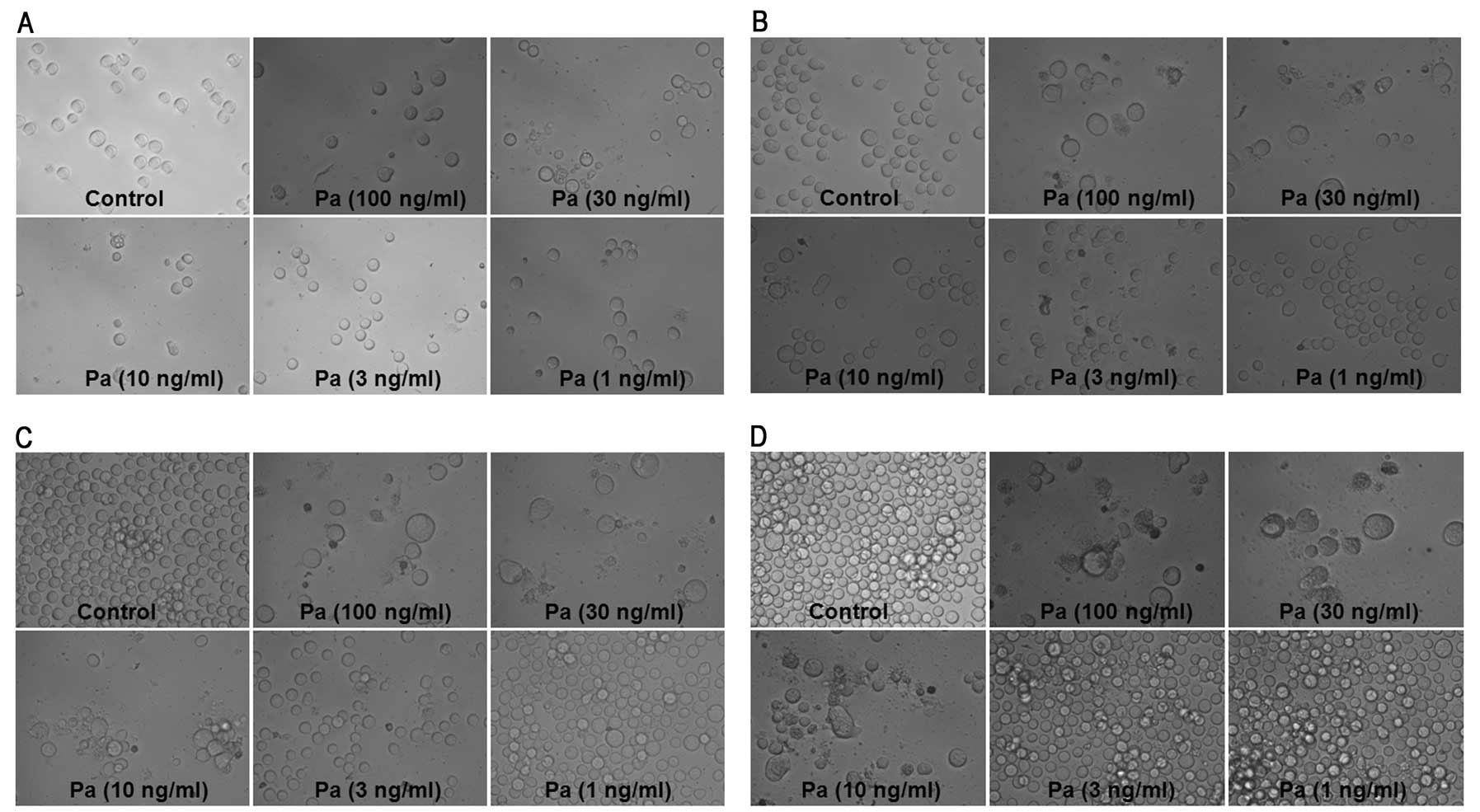
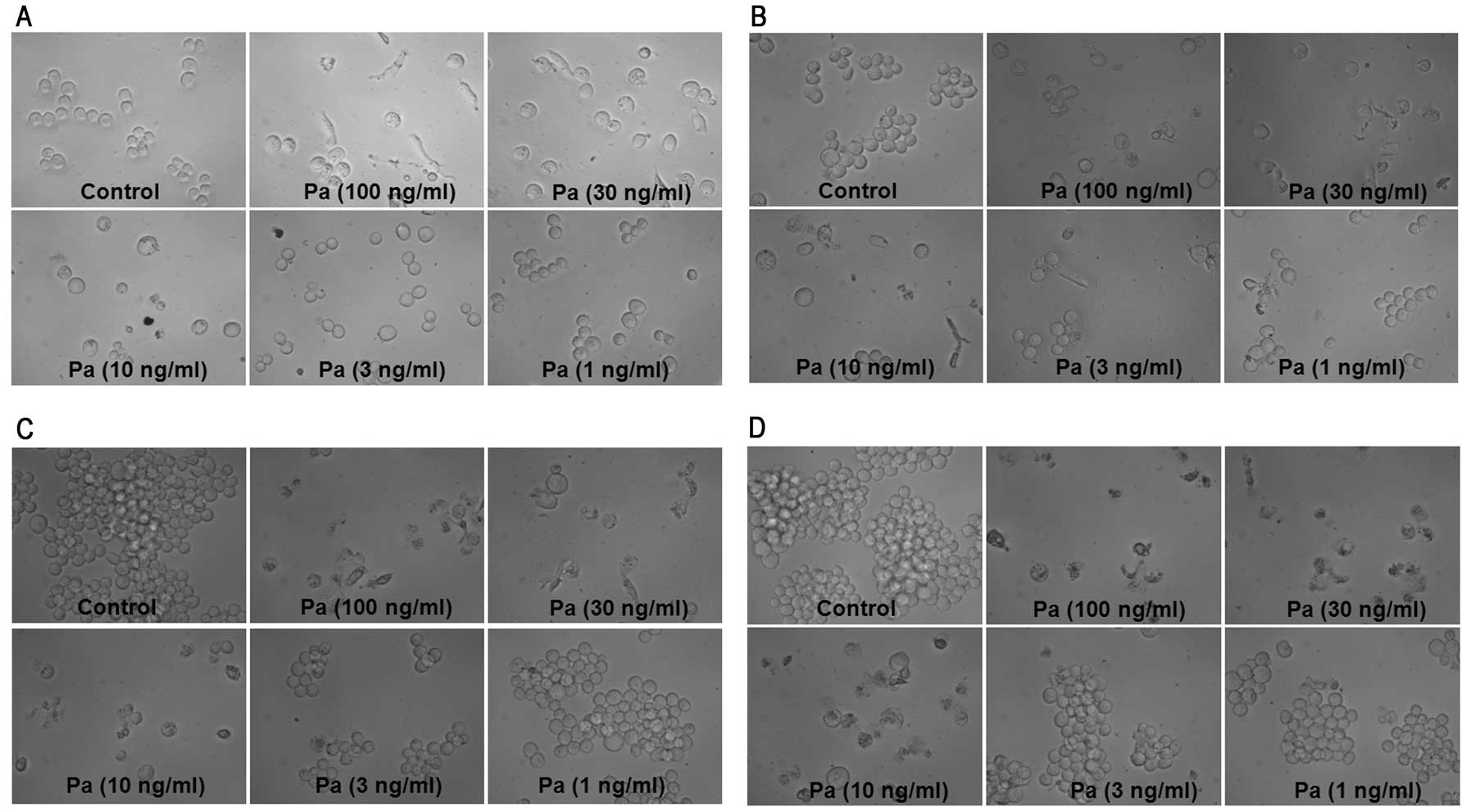
morphological assessment of MEL cultures revealed that
paclitaxel-induced marked cellular swelling should be called
oncosis which leads to necrosis (Fig.
2), while a small part of the K562 cells incubated with
paclitaxel showed marked cellular shrinking (Fig. 3).
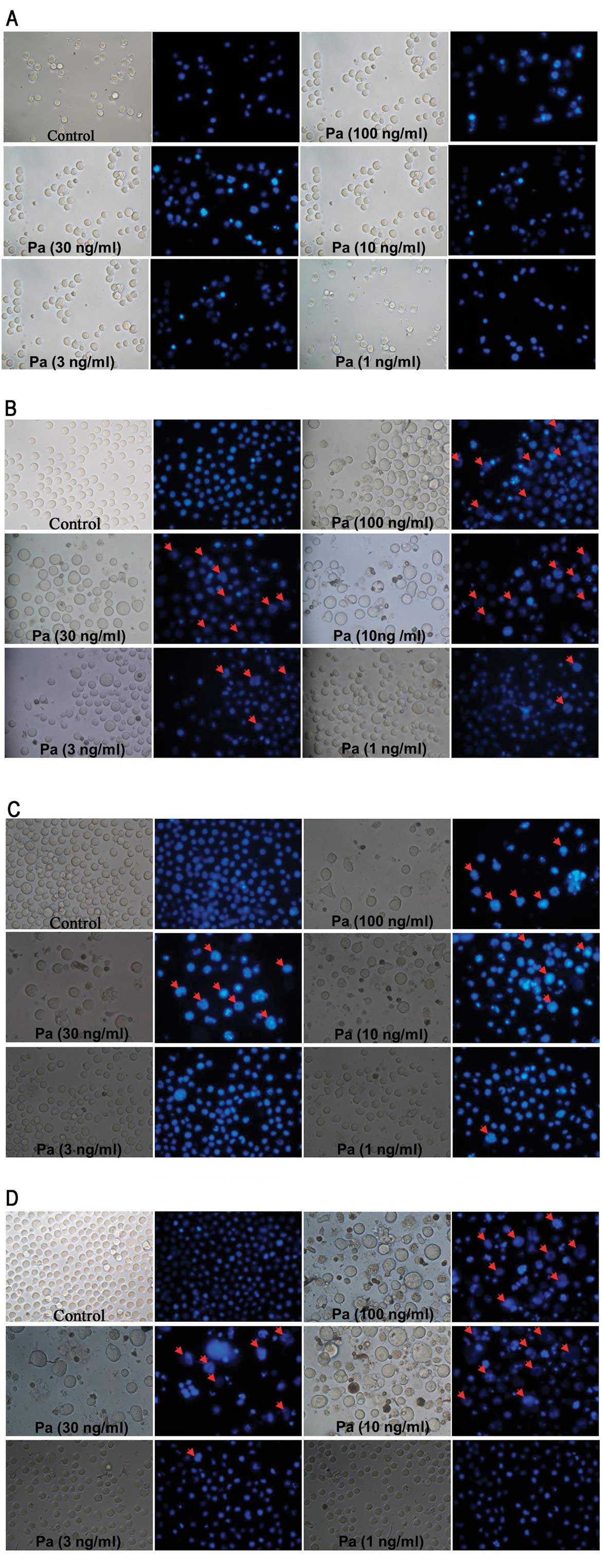
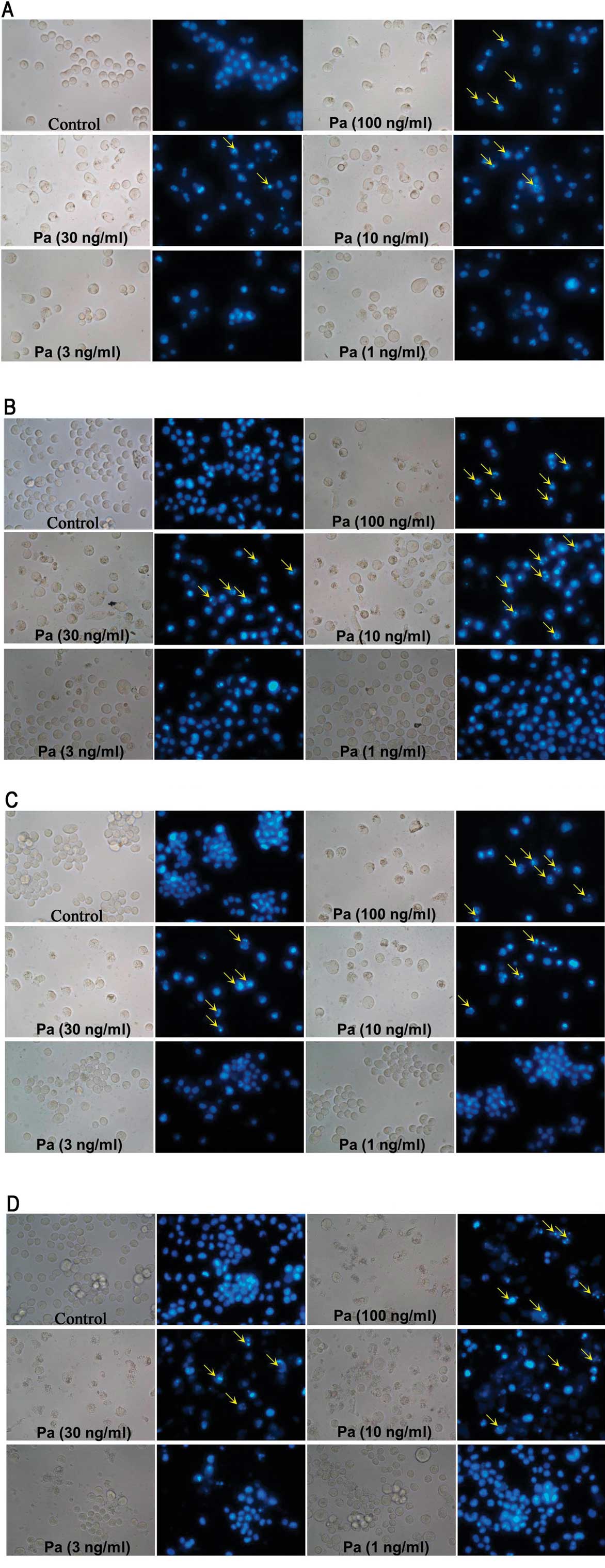
Morphological observation using Hoechst
33342 stain assay
Hoechst 33342 is a non-toxic specific vital stain
for DNA (17). Using Hoechst 33342
stain assay, our results showed that paclitaxel clearly induced
necrosis in MEL cells (Fig. 4).
However, there were evident apoptotic instead of necrotic cells in
K562 cells treated by paclitaxel (Fig.
5).
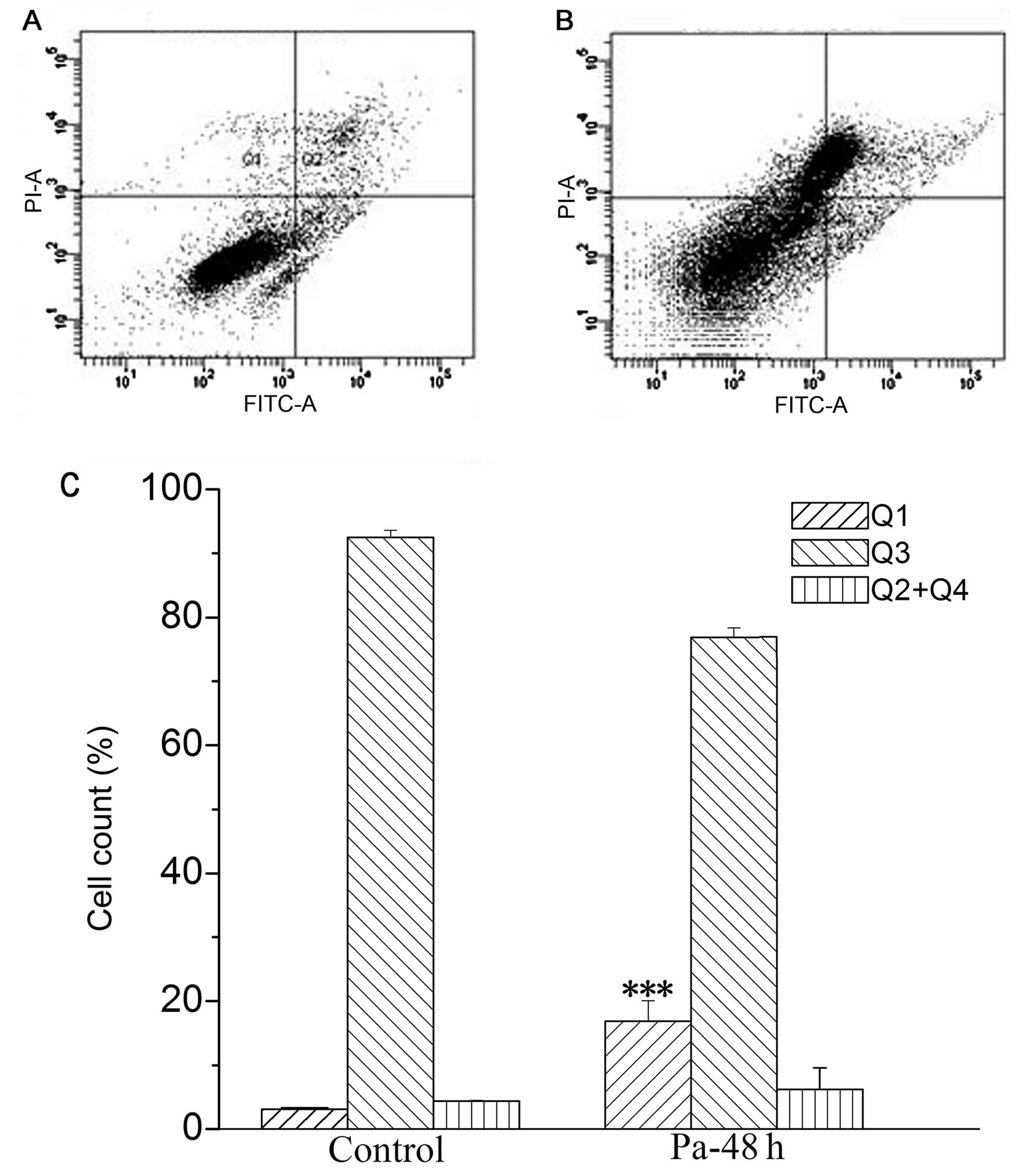
Flow cytometry for the detection of
apoptosis and necrosis
Cell apoptosis and necrosis were detected by flow
cytometry with Annexin V-FITC/PI dual staining. The amount of
normal cells, early apoptosis, late apoptosis and necrosis was
determined as the percentage of Annexin
V−/PI− (Q3), Annexin
V+/PI− (Q4), Annexin
V+/PI+ (Q2) and Annexin
V−/PI+ (Q1) cells, respectively (18). Our results showed that necrotic
cells were significantly increased (from 3.10±0.21 to 14.68±1.76),
while apoptotic cells were not significantly increased following
paclitaxel treatment (50 ng/ml) in MEL cells for 48 h, when
compared to those in the control group that was treated with
vehicle only (Fig. 6). However, in
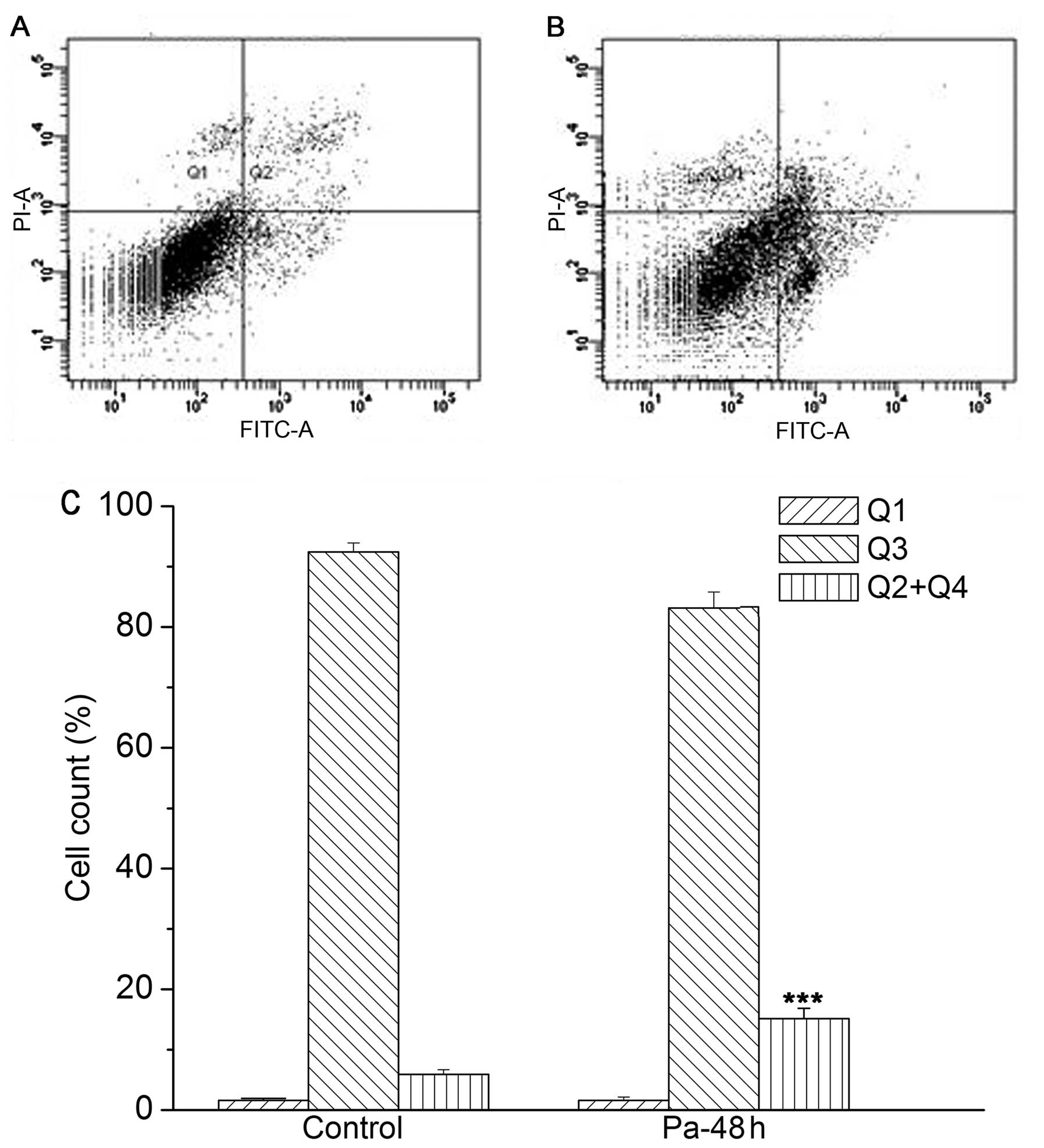
K562 cells, there was a marked increase of apoptotic cells from
5.87±0.77 to 14.53±2.78 following paclitaxel treatment (21 ng/ml)
for 48 h compared with the control group, while there was no
significant difference of necrotic cells between the paclitaxel
treated group and the control group (from 2.16±0.53 to 3.93±1.46)
(Fig. 7). These results are in
accordance with the changes observed in cell morphology (Figs. 4 and 5).
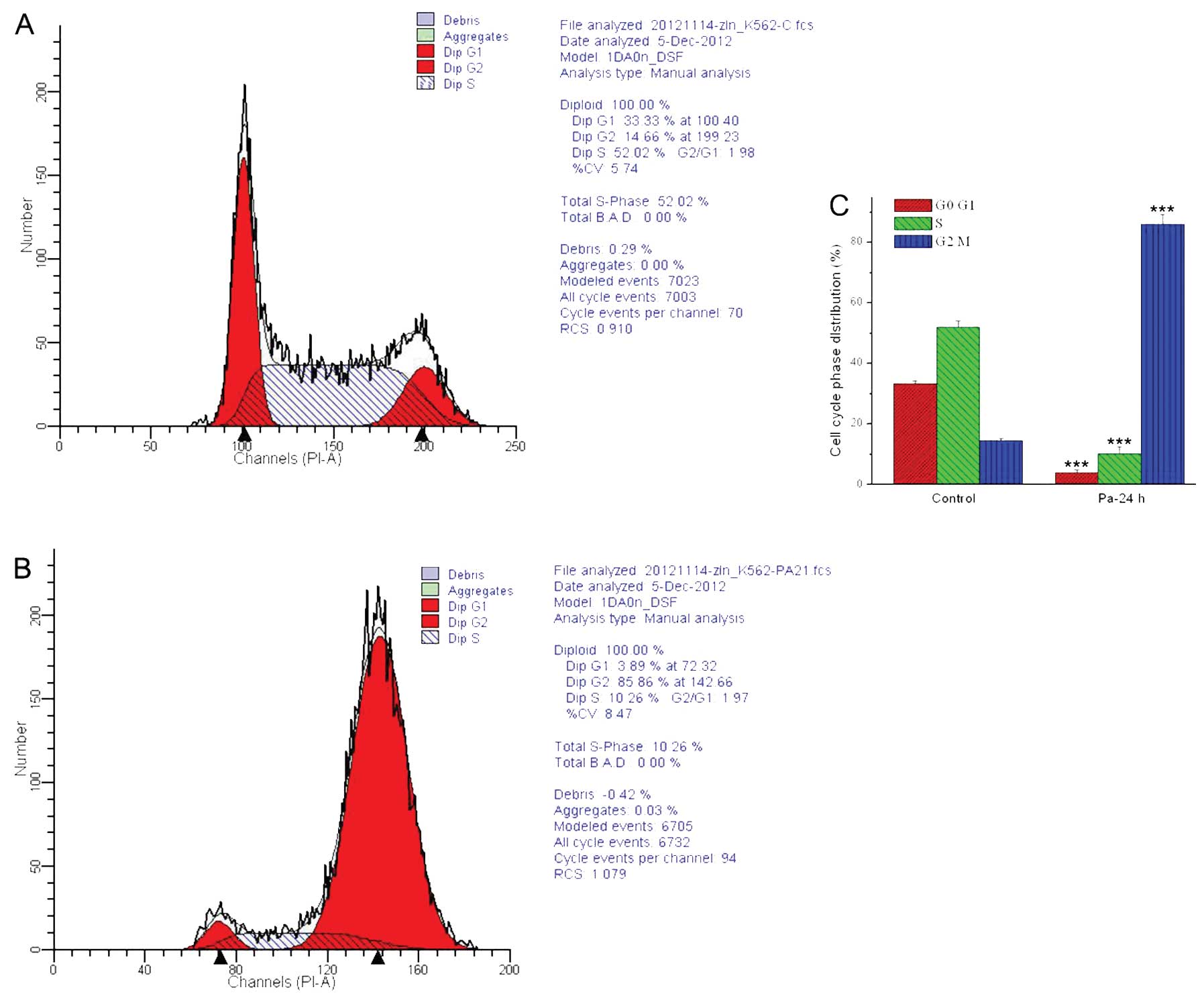
Paclitaxel induces significant
G2/M phase arrest of the cell cycle in K562 cells
Flow cytometry analysis (PI stain) was used to
determine the effect of paclitaxel on cell cycle distribution of
K562 cells. As shown in Fig. 8, the
percentage of K562 cells in the G2/M phase was increased
significantly (P<0.01) from 14.66±0.50% (control group) to
85.96±3.24% after the treatment with paclitaxel at the
concentration of 21 ng/ml for 24 h, while the percentage of
G0/G1 phase and S phase was decreased
markedly, indicating that paclitaxel inhibited the proliferation of
K562 cells by causing G2/M phase arrest of the cell
cycle. However, it could not detect the normal cell cycle
distribution in MEL cells treated by paclitaxel due to the necrosis
(data not shown).
Discussion
Previous studies showed the efficacy of taxanes on
human leukemic cell lines (16,19–21),
as well as their effectiveness in inducing apoptosis in
vivo(22) and in fresh leukemia
cells in primary cultures (23).
However, to the best of our knowledge, there is no report on the
effect of paclitaxel on leukemia induced by virus. Friend virus is
an acutely oncogenic retrovirus that causes erythroblastosis and
polycythemia in mice (24,25). In the present study, the MEL cell
lines induced by friend virus were used to investigate the effect
of paclitaxel on leukemia induced by virus, as well as to compare
with that of human erythroleukemic cell line (K562 cells). Our
present results showed the dose- and time-dependency of the
antitumor effects of paclitaxel on these two types of leukemia
cells, and the potency of paclitaxel in K562 cells was stronger
than in MEL cells. Paclitaxel clearly induced apoptosis in K562
cells, which is in accordance with previous results (21). Also, there was a significant arrest
of cell cycle to G2/M phase induced by paclitaxel in
K562 cells, similar to the findings of a previous report (26). These findings suggest that
paclitaxel induced K562 cell death involving the cell cycle and
apoptosis (27).
By contrast, in MEL cells, paclitaxel could not
induce significant apoptosis, which is different from that in K562
cells. Cell death is the process which culminates with cessation of
biological activity. It is generally accepted that apoptosis and
necrosis are two distinct, mutually exclusive, modes of cell death
(28). One of the early events in
apoptosis is cell dehydration. Loss of intracellular water leads to
condensation of the cytoplasm followed by a change in cell shape
and size: the originally round cells may become elongated and are
generally smaller. Another change, perhaps the hallmark of
apoptosis, is condensation of nuclear chromatin. In the present
study, the phenomenon of apoptosis was evident in K562 cells
treated by paclitaxel, while it was barely observed in MEL cells
treated by paclitaxel. Necrosis is a passive, catabolic, and
degenerative process with karyorrhexis and cell swelling prior to
rupture of the plasma membrane, which is in contrast to apoptosis
(29). Our present results showed
that the MEL cells treated by paclitaxel exhibited significant
characteristics of necrosis. Additionally, the normal cell cycle
distribution in MEL cells treated by paclitaxel could not be
detected due to the necrosis. It is therefore evident that the mode
of cell death induced by paclitaxel in the two types of leukemia
cells used in the present study is different.
In summary, paclitaxel is the prototype of a group
of promising chemotherapeutic agents, taxanes, which specifically
interact with microtubules. However, the present study showed that
paclitaxel clearly induced necrosis in leukemia cells induced by
virus, which is different from that of human erythroleukemic cells.
Advances in the research of cell cycle, apoptosis and necrosis will
extend our understanding of the mechanisms involved in
paclitaxel-induced cell death, particularly in leukemia cells.
Further elucidation of the necrotic mechanism in MEL cells may
expedite the development of enhanced paclitaxel-based regimens for
cancer therapy.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 31000496), the
521 talent of Zhejiang Sci-Tech University and the Project
supported by the Zhejiang Open Foundation of the Most Important
Subjects (no. SWYX0903).
References
|
1
|
Burris HA III: Docetaxel (Taxotere) plus
trastuzumab (Herceptin) in breast cancer. Semin Oncol. 28:38–44.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Belani C and Lynch T: Docetaxel (Taxotere)
in combination with platinums in patients with non-small cell lung
cancer: trial data and implications for clinical management. Semin
Oncol. 28:10–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaye S, Piccart M, Aapro M, Francis P and
Kavanagh J: Phase II trials of docetaxel (taxotere®) in
advanced ovarian cancer - an updated overview. Eur J Cancer.
33:2167–2170. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horwitz S: Taxol (paclitaxel): mechanisms
of action. Ann Oncol. 5:S3–S6. 1994.
|
|
5
|
Monzó M, Rosell R, Sánchez JJ, Lee JS,
O’Brate A, González-Larriba JL, Alberola V, Lorenzo JC, Núñez L, Ro
JY and Martín C: Paclitaxel resistance in non-small-cell lung
cancer associated with beta-tubulin gene mutations. J Clin Oncol.
17:1786–1793. 1999.PubMed/NCBI
|
|
6
|
Giannakakou P, Sackett DL, Kang YK, Zhan
Z, Buters JT, Fojo T and Poruchynsky MS: Paclitaxel-resistant human
ovarian cancer cells have mutant β-tubulins that exhibit impaired
paclitaxel-driven polymerization. J Biol Chem. 272:17118–17125.
1997.
|
|
7
|
Ranganathan S, Benetatos C, Colarusso P,
Dexter D and Hudes G: Altered beta-tubulin isotype expression in
paclitaxel-resistant human prostate carcinoma cells. Br J Cancer.
77:562–566. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilson L, Bamburg J, Mizel S, Grisham L
and Creswell K: Interaction of drugs with microtubule proteins. Fed
Proc. 33:158–166. 1974.PubMed/NCBI
|
|
9
|
Bhalla K, Ibrado A, Tourkina E, Tang C,
Mahoney M and Huang Y: Taxol induces internucleosomal DNA
fragmentation associated with programmed cell death in human
myeloid leukemia cells. Leukemia. 7:563–568. 1993.PubMed/NCBI
|
|
10
|
Havrilesky LJ, Elbendary A, Hurteau JA,
Whitaker RS, Rodriguez GC and Berchuck AW: Chemotherapy-induced
apoptosis in epithelial ovarian cancers. Obstet Gynecol.
85:1007–1010. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang YF, Li LL, Wu CW, Liu TY, Lui WY,
P’eng FK and Chi CW: Paclitaxel-induced apoptosis in human gastric
carcinoma cell lines. Cancer. 77:14–18. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yen WC, Wientjes MG and Au JLS:
Differential effect of taxol in rat primary and metastatic prostate
tumors: site-dependent pharmacodynamics. Pharm Res. 13:1305–1312.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fallo F, Pilon C, Barzon L, Pistorello M,
Pagotto U, Altavilla G, Boscaro M and Sonino N: Effects of taxol on
the human NCI-H295 adrenocortical carcinoma cell line. Endocr Res.
22:709–715. 1996.PubMed/NCBI
|
|
14
|
Terzis AJ, Thorsen F, Heese O, Visted T,
Bjerkvig R, Dahl O, Arnold H and Gundersen G: Proliferation,
migration and invasion of human glioma cells exposed to paclitaxel
(Taxol) in vitro. Br J Cancer. 75:1744–1752. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rowinsky EK: The development and clinical
utility of the taxane class of antimicrotubule chemotherapy agents.
Annu Rev Med. 48:353–374. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Alami O, Sammons J, Martin J and Hassan
H: Divergent effect of taxol on proliferation, apoptosis and nitric
oxide production in MHH225 CD34 positive and U937 CD34 negative
human leukaemia cells. Leuk Res. 22:939–945. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richards W, Song MK, Krutzsch H, Evarts R,
Marsden E and Thorgeirsson S: Measurement of cell proliferation in
microculture using Hoechst 33342 for the rapid semiautomated
microfluorimetric determination of chromatin DNA. Exp Cell Res.
159:235–246. 1985. View Article : Google Scholar
|
|
18
|
Shi Y, Wei Y, Qu S, Wang Y, Li Y and Li R:
Arsenic induces apoptosis of human umbilical vein endothelial cells
through mitochondrial pathways. Cardiovasc Toxicol. 10:153–160.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gangemi RM, Tiso M, Marchetti C, Severi AB
and Fabbi M: Taxol cytotoxicity on human leukemia cell lines is a
function of their susceptibility to programmed cell death. Cancer
Chemother Pharmacol. 36:385–392. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haldar S, Basu A and Croce CM: Bcl2 is the
guardian of microtubule integrity. Cancer Res. 57:229–233.
1997.PubMed/NCBI
|
|
21
|
Gangemi RM, Santamaria B, Bargellesi A,
Cosulich E and Fabbi M: Late apoptotic effects of taxanes on K562
erythroleukemia cells: apoptosis is delayed upstream of caspase-3
activation. Int J Cancer. 85:527–533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seiter K, Feldman EJ, Traganos F, Li X,
Halicka HD, Darzynkiewicz Z, Lederman CA, Romero MB and Ahmed T:
Evaluation of in vivo induction of apoptosis in patients with acute
leukemia treated on a phase I study of paclitaxel. Leukemia.
9:1961–1966. 1995.PubMed/NCBI
|
|
23
|
Consolini R, Pui C-H, Behm FG, Raimondi SC
and Campana D: In vitro cytotoxicity of docetaxel in childhood
acute leukemias. J Clin Oncol. 16:907–913. 1998.PubMed/NCBI
|
|
24
|
Ney PA and D’Andrea AD: Friend
erythroleukemia revisited. Blood. 96:3675–3680. 2000.PubMed/NCBI
|
|
25
|
Friend C: Cell-free transmission in adult
Swiss mice of a disease having the character of a leukemia. J Exp
Med. 105:307–318. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yusuf R, Duan Z, Lamendola D, Penson R and
Seiden M: Paclitaxel resistance: molecular mechanisms and
pharmacologic manipulation. Curr Cancer Drug Targets. 3:1–19. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang TH, Wang HS and Soong YK:
Paclitaxel-induced cell death: where the cell cycle and apoptosis
come together. Cancer. 88:2619–2628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Darzynkiewicz Z, Juan G, Li X, Gorczyca W,
Murakami T and Traganos F: Cytometry in cell necrobiology: analysis
of apoptosis and accidental cell death (necrosis). Cytometry.
27:1–20. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Majno G and Joris I: Apoptosis, oncosis,
and necrosis. An overview of cell death. Am J Pathol. 146:3–15.
1995.PubMed/NCBI
|