Introduction
Breast cancer is the most common cancer in women
with more than one million new cases. Breast cancer is one of the
leading causes of cancer-related mortality in women (1). Apart from surgery, adjuvant radiation
therapy and chemotherapy, treatment of breast cancer is based on
the identification of molecular targets, mainly estrogen receptor
(ER) expression, HER2 overexpression and amplification. In
ER-expressing tumors, endocrine therapy consisting of mainly
tamoxifen and anti-aromatase drugs is prescribed. In human
epidermal growth factor receptor 2 (HER2)-positive
(HER2+) tumors, anti-HER2-targeted therapies such as the
anti-HER2 monoclonal antibody trastuzumab and the tyrosine kinase
inhibitor lapatinib have been proposed. Moreover, in
HER2+ tumors also expressing ER, the combination of both
endocrine and anti-HER2 therapies have been proven to improve the
outcome of patients through the blockade of signaling crosstalk
leading to resistance to ER-targeted therapy. In triple-negative
breast cancers (TNBCs) i.e. ER−, PR− and
HER2− tumors, no targeted therapy has been proven
effective to date, and the molecular characteristics of TNBCs have
been extensively studied in order to identify putative molecular
targets for drug development. Since several different growth factor
pathways can stimulate breast cell growth, targeting a unique
pathway may have limited effect on the inhibition of breast cancer
proliferation, and the inhibition of signal transduction at a
deeper point in the cascade has been envisaged.
In this context, mammalian target of rapamycin
(mTOR) has been identified as the point of convergence of many
mitogenic signals, which has led to the recent registration of the
mTOR inhibitor everolimus in association with anti-aromatase
therapy for ER+ breast cancers (2). Another point of convergence of
intracellular downstream growth factor receptor signaling is the
P38 mitogen-activated protein kinase family (P38 MAPK). P38 MAPK is
a member of the MAPK family which includes the extracellular
signal-regulated kinase (ERK), the c-Jun N-terminal kinase (JNK),
and P38 MAPK (3). P38 MAPK is
comprised of four isoforms (α, β, γ and δ) that can be activated by
various growth factors, inflammatory cytokines, and chemical or
physical stress. The α isoform is the most abundant and is subject
to a larger inter-individual variability than the other three
isoforms (12). P38 MAPK plays a
complex role in the regulation of cell growth, differentiation,
apoptosis, and responses to inflammation or stress (4,5). P38
MAPK activity was found to be upregulated in breast, head and neck
carcinomas, lymphomas, gliomas and squamous cell carcinomas
(6).
In breast cancer, a high level of expression of P38
MAPK has been found to correlate with poor prognosis and to be
involved in invasiveness and metastasis in relation with the
urokinase plasminogen activator system (7). Activation of P38 MAPK has been
observed in Scarff-Bloom-Richardson (SBR) grade 2 or 3 ductal
tumors (8). Expression of
phosphorylated-P38 MAPK (p-P38MAPK) has been reported in ~20% of
primary breast carcinomas (9). P38
MAPK overexpression has been correlated with HER2 amplification and
tamoxifen resistance (10) and has
been proposed as a potential prognostic marker in breast cancer
(11). Specifically,
phosphorylation of P38 MAPK was found to be a negative prognostic
indicator in HER2-negative, lymph node-positive breast cancers
(9).
The role of P38 MAPK in the regulation of breast
cancer cell proliferation remains to be elucidated and has been
suggested to have dual activities that include regulation of
survival and proliferation depending on the expression of mutant
TP53 (12) as observed in most
ER− breast tumors therefore justifying the development
of P38 MAPK inhibitors for the treatment of TP53-mutated,
ER− breast cancers or TNBCs. Furthermore, the activation
of P38 MAPK has recently been reported to regulate signaling by
EGFR/c-Src crosstalk in breast cancer (13).
In addition, P38 MAPK has been recently reported to
play a role in the resistance of ER+ breast tumors to
endocrine therapy (14). Although
the cellular mechanisms underlying the development of tamoxifen
resistance in breast cancer cells are not totally understood,
recent research has found that alteration of the signaling pathways
(15–17) can decrease the cell sensitivity to
tamoxifen. More precisely, the development of crosstalk between ER
and growth factor-mediated activation of the MAPK cascade, through
the activation of HER2 has been reported to increase both genomic
and non-genomic ER actions in breast cancer leading to tamoxifen
resistance. This justifies the combination of endocrine therapy
together with aromatase inhibitors and anti-HER2 therapies with
trastuzumab-based or lapatinib-based therapies for breast cancer
(18). Recent studies have noted a
positive correlation between activated P38 MAPK levels and
tamoxifen resistance (19). P38
MAPK has been reported to potentiate ER agonist activity through
increased phosphorylation of ER and enhanced ER signaling through
coactivator regulation (20). P38
MAPK has been shown to play a role in breast cancer progression and
invasion (21) in association with
other signaling proteins such as integrins and urokinase
plasminogen activator (22) as well
as H-RAS (23).
P38 MAPK isoform γ has been recently shown to be
selectively activated by exposure to tamoxifen, consequently
recruiting nonclassical ER signaling and increased estrogen cell
sensitivity (24). Therefore,
increased P38 MAPK activation could define a more malignant,
resistant and metastatic breast cancer phenotype and justify the
evaluation of P38 MAPK inhibitors in the treatment of invasive and
tamoxifen-resistant breast carcinomas (14). A number of P38 MAPK inhibitors are
currently being investigated in clinical trials (25).
In the present study, we investigated the expression
of P38 MAPK and p-P38 MAPK in clinical specimens of invasive breast
carcinomas. We first investigated the correlation of their
expression with ER and HER2 expression, and subsequently evaluated
the correlation with expression levels of MAPK and PI3K signaling
phosphorylated proteins such as p-AKT, p-GSK3β, p-S6 kinase, p-MEK1
and p-ERK1/2 quantitatively determined using multiplex bead
immunoassay as previously described and validated in breast cancer
(26).
Materials and methods
Patients and tumor characteristics
Frozen tumor samples of breast cancer from 45
patients with infiltrative ductal carcinoma were obtained from the
tumor bank of our Institute (agreement with French National Cancer
Institute and Ministry of Health). All patients were informed of
the tumor banking procedure and no opposition was expressed. The
median age at diagnosis was 56.3 years (range, 28–91).
Breast cancer tissues macroscopically selected by
the pathologists were obtained immediately after surgery and were
shock frozen in liquid nitrogen then cryopreserved at −80°C. The
mean tumor (SD) specimen weight was 15.2 (4.2) mg. None of the
patients received any preoperative adjuvant endocrine therapy or
chemotherapy. Thirty-four tumors were SBR grade 3
(Scarff-Bloom-Richardson) and 11 tumors were grade 2.
Immunohistochemistry (BenchMark Ventana) was used to
detect estrogen and progesterone receptor expression and HER-2
overexpression as part of the routine clinical diagnostics using
polyclonal antibody A485 (Dako, Trappes, France) immunostaining of
HER2 and monoclonal antibodies 6F11 and Pgr312 (both from
Novocastra, Leica Microsystèmes, Nanterre, France) for
determination of estrogen and progesterone expression,
respectively.
Protein extraction
The tumor specimens were first disrupted using steel
bead TissueLyser (Qiagen, Courtaboeuf, France) for 15 min, and then
exposed to the lysis solution (Cell Lysis kit, Bio-Rad,
Marnes-la-Coquette, France) containing PMSF anti-protease for 10
sec. After centrifugation (4,500 × g for 20 min at 4°C), the
protein-containing supernatants were collected and stored frozen at
−80°C until analysis. Before being analyzed, the protein
concentration was determined in each extract using 690 nm
colorimetric DC protein assay kit (Bio-Rad) based on Lowry
technique and adjusted to 250 μg/ml.
Multiplex bead immunoassay
The expression of the signaling phosphoproteins was
analyzed using multiplex bead immunoassay as described and
validated previously (26).
Briefly, protein extracts were transferred into 96-well dishes and
diluted with 25 μl buffered solution. Fluorescence capturing beads
coupled to antibodies directed against P38 MAPK, p-P38 MAPK, p-AKT,
p-GSK3β, p-P70S6K, p-MEK1, p-ERK1/2 phosphoproteins were mixed. The
beads were added into each well and incubated overnight at 37°C.
Biotinylated antibodies and then streptavidin-phycoerythrin
solution were then added. The positive control consisting of
standard protein extract from cell lines was added to each series.
The multi-well plates were then analyzed according to the
manufacturer’s instructions (Bio-Plex; Bio-Rad, Hercules, CA, USA).
Frozen protein extracts from an EGFR-overexpressing human breast
cancer cell line exposed to EGF were used as positive controls as
reported previously (26). The
results were recorded as mean fluorescence intensities expressed as
arbitrary units and considered as significant when exceeding a
signal/noise ratio of 3.
Statistics
All analyses were performed as triplicate and are
presented as mean fluorescence intensities (SD). All statistical
analyses were performed using the Wilcoxon test using R software
(v.2.15.1.; the R Foundation for Statistical Computing) and the
level of significance was set at P<0.05.
Results
Immunohistochemistry
The breat cancer tumor characteristics are
summarized in Table I. The ER
status as determined by immunohistochemistry was positive in 29
patients (64%) and negative in 16 patients (36%). Progesterone
receptor (PR) status was positive in 18 patients (40%) and negative
in 27 patients (60%). Fifteen tumors (33%) were HER2+
and 30 tumors (67%) were HER2−. Ten (22%) tumors were
triple-negative breast cancers (TNBCs), i.e. ER−,
PR− and HER2−.
 | Table IPatient demographics and tumor
characteristics. |
Table I
Patient demographics and tumor
characteristics.
| Characteristics | Patients, n (%) |
|---|
| No. of patients | 45 (100.0) |
| Age (years) |
| ≤50 | 13 (28.9) |
| 51–69 | 24 (53.3) |
| ≥70 | 8 (17.8) |
| Tumor size (mm) |
| T1 (10–20) | 16 (35.6) |
| T2 (21–50) | 26 (57.8) |
| T3 (>51) | 3 (6.7) |
| SBR grade |
| SBR 2 | 11 (24.4) |
| SBR 3 | 34 (75.6) |
| Hormone receptor
status |
|
ER+ | 29 (64.4) |
|
ER− | 16 (35.6) |
|
PR+ | 18 (40.0) |
|
PR− | 27 (60.0) |
| HER2 status |
| Positive | 15 (33.3) |
| Negative | 30 (66.7) |
|
Triple-negative | 10 (22.2) |
P38 MAPK and phosphorylated-P38 MAPK
expression
P38 MAPK and p-P38 MAPK were found to be expressed
in nearly all tumor specimens (44/45, 98%) and were significantly
(P=0.0016) overexpressed in ER+ tumors (Fig. 1A). The median expression of p-P38
MAPK was also higher in ER+ when compared with that in
ER− tumors. HER2 status had no influence on P38 MAPK and
p-P38 MAPK expression (Fig. 1B).
P38 MAPK expression was lower in TNBCs (Fig. 1C) when compared with the expression
level in all other tumor types as was p-P38MAPK expression but
without reaching statistical significance. No significant variation
in the phosphorylation rate of P38 MAPK, as calculated from the
phosphorylated/unphosphorylated P38 MAPK expression ratios, was
observed in association with ER, HER2 and TNBC status, or SBR
grade.
Phosphorylated-AKT and
phosphorylated-ERK1/2 expression
ignificant expression of p-AKT and p-ERK1/2 was
detected in 33/45 (73%) and 17/45 (38%) of the tumor extracts,
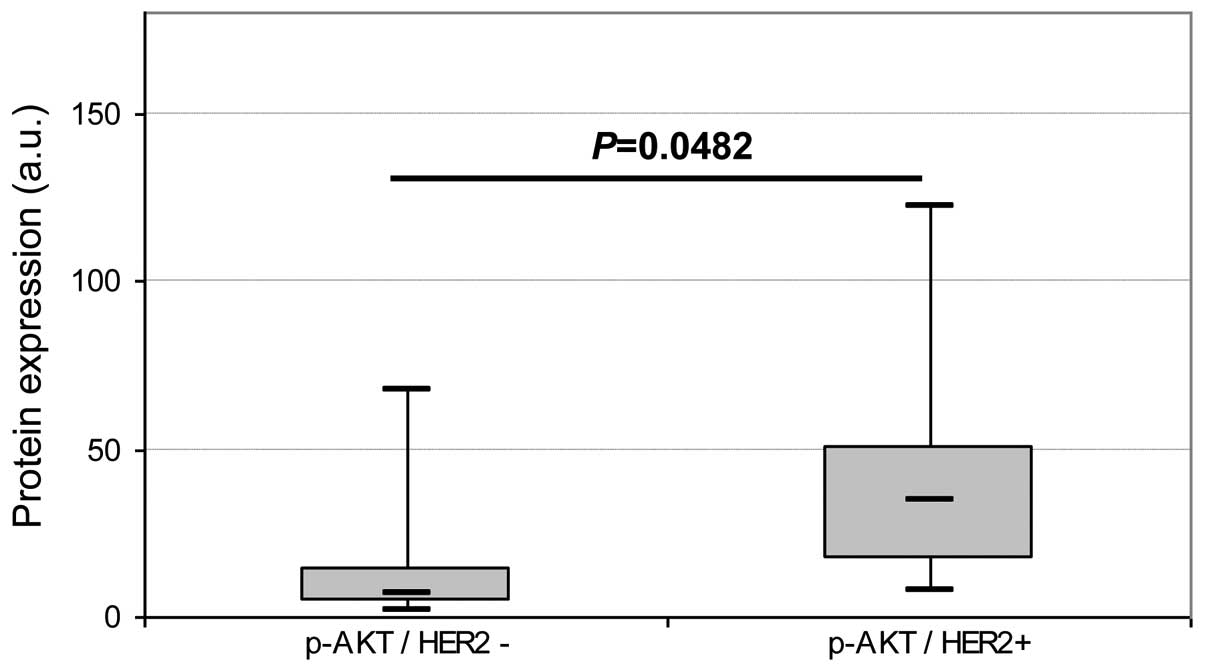
respectively. p-AKT expression was found to be significantly higher
(P=0.0048) in HER2+ tumors (Fig. 2) than in HER2− tumors. No
other significant difference was observed regarding either ER and
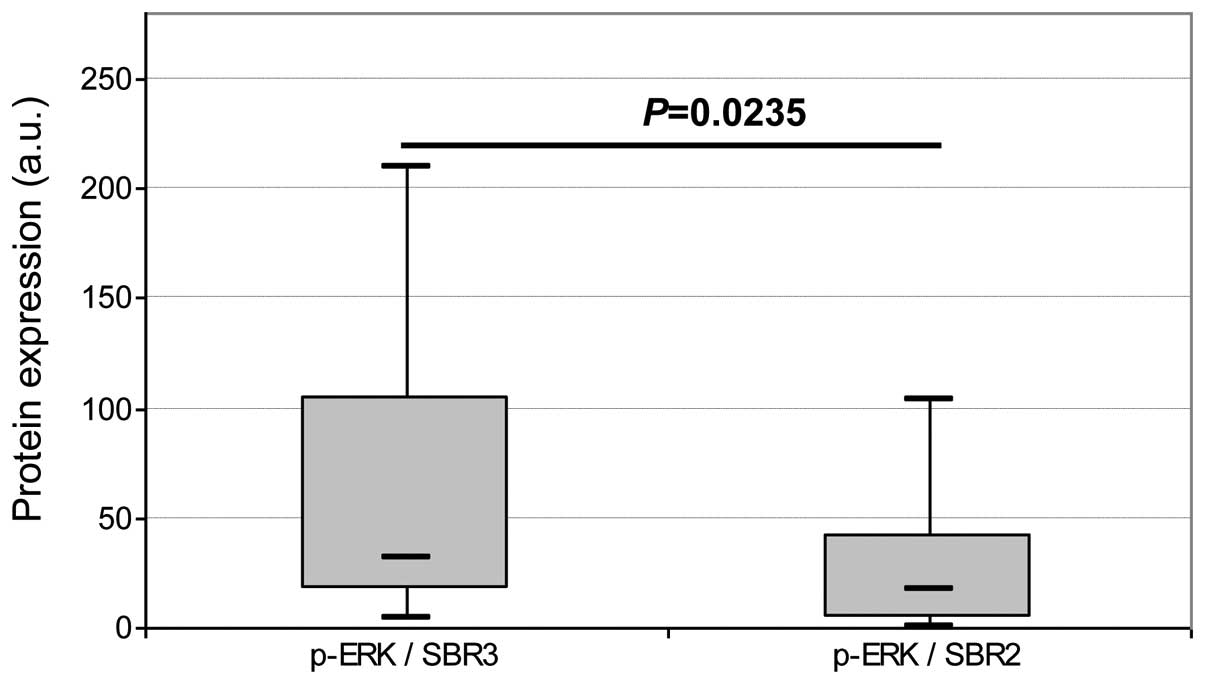
TNBC status or SBR grade. No difference in p-ERK1/2 expression was
observed regarding ER, HER2 and TNBC status. Expression of p-ERK1/2
was found to be significantly higher (P=0.0235) in SBR grade 3 than
in SBR grade 2 tumors (Fig. 3).
Expression of other
phosphorylated-signaling proteins MEK1, GSK3β, S6K
Significant expression of p-MEK1, p-GSK3β and p-S6K
was detected in 39/45 (87%), 31/45 (69%) and 37/45 (82%) of the
protein extracts, respectively. No significant difference in
p-MEK1, p-GSK3β and p-S6K expression was evidenced regarding either
ER and TNBC status or SBR grade (data not shown). No significant
correlation was found between the expression levels of any of the
phosphorylated proteins.
Discussion
In breast cancer, P38 MAPK expression has previously
been correlated with invasiveness and poor prognosis (8–11).
In the present study, we compared the expression of
P38 MAPK and p-P38 MAPK in clinical specimens of invasive breast
carcinomas in association with ER, HER2 and SBR grade and aimed to
ascertain a correlation between P38 MAPK expression or activation
of MEK/ERK and the AKT/mTOR signaling pathways.
In our series, expression of P38 MAPK and p-P38 MAPK
was observed in nearly all tumor specimens. This was consistent
with previously published data (11) reporting P38 MAPK and p-P38 MAPK
expression in 100 and 89% of specimens, respectively, using western
blot analysis, and 70% when IHC was used.
We report here that P38 MAPK was expressed at a
higher level in ER+ when compared with ER−
tumors without post-transductional activation since no variation in
the phosphorylation rate of P38 MAPK was evidenced. This is
consistent with previously published data (10,27)
revealing the great interest in P38 MAPK in ER+ tumors.
P38 MAPK has been reported to be activated by anti-estrogens apart
from ER their main target, resulting in a switch in ER signaling
from its classical pathway, involving the estrogen response element
(ERE) DNA domain, to the AP1-dependent non-classical pathways;
therefore, activation of P38 MAPK can ultimately decrease the
cellular response to endocrine therapy. Based on this concept, P38
MAPK has been proposed as a biomarker for resistance to endocrine
therapy, and quantitative assessment of P38 MAPK expression and the
detection of its activation in breast tumors may represent a new
approach to predict the resistance of breast cancer to endocrine
therapy. Furthermore, inhibition of P38 MAPK in ER−
tumors could restore ER expression and therefore restablish the
sensivity to endocrine therapy (28).
Moreover, evaluation of the molecular pathway may
even be proposed for specimens obtained at recurrence since the
molecular pathways driving tumor growth could be altered along with
tumor progression (10).
An incomplete understanding of the complex
mechanisms exists concerning the relationship between MAPK
activation and expression of hormone receptors and HER2 in breast
carcinoma in vitro. In effusion specimens, p38 activation
was reported to be inversely associated with the intensity of HER2
membrane expression (11).
In this context, although we did not observe any
inverse relationship between HER2 and p-P38 MAPK expression, our
results revealed that expression of P38 MAPK was significantly
lower in TNBCs than in the other tumor subtypes. This may be
reconsidered if a more specific approach of selective inhibition of
P38 MAPK isoforms can be envisaged, as recent preclinical studies
have demonstrated the important role played by the P38 MAPK γ
isoform in TNBCs in relation with its marked induction of cell
cycle arrest in the G(2)/M phase (29) and its effect on the cellular
sensitivity to topoisomerase II inhibitors (30). Stimulation of topo IIα gene
expression by P38 MAPKγ may contribute to increased topo IIα levels
and enhanced antitumor activity of topo II inhibitors (24), therefore opening the field for the
investigation of selective inhibitors of the P38 MAPK γ isoform in
combination with chemotherapy.
p-ERK was detected in 73% of the tumor specimens,
consistent with data reporting significant p-ERK expression in 69
to 96% of breast tumors (11,31,32). A
low expression rate (35%) was only reported in one cohort (33). In our study, the expression of p-ERK
was higher in high grade tumors (SBR3) consistent with data linking
the activation of ERK with breast cancer cell proliferation
(33).
p-AKT was detected in 38% of the tumor specimens and
at a higher level in HER2+ tumors, consistent with data
previously published using IHC which reported p-AKT cytoplasmic and
nuclear expression rates of 36 and 29%, respectively, in invasive
ductal breast tumors and higher activation of AKT in
HER2+ tumors (34). In
this study (34), a correlation was
observed between nuclear p-AKT and nuclear ER and PR expression
while no difference was observed for cytoplasmic p-AKT expression
and cytoplasmic ER and PR expression. This is consistent with our
data showing an absence of a correlation between either ER or PR
and p-AKT expression since when using total protein extract
analysis no difference can be determined between cytoplasmic and
nuclear compartments.
As a whole, we did not find any correlation between
p-AKT, p-ERK expression and P38MAPK or p-P38MAPK expression either
in the total population of this study or in ER, HER2 or TNBC
subgroups. Similar findings have been reported and no significant
correlations were evidenced between ER and levels of p-P38MAPK,
p-AKT, or p-ERK (10,11). Only several correlations have been
reported between p-P38 MAPK and p-AKT and between p-P38 MAPK and
p-ERK in a global population of tumor specimens from untreated
patients analyzed using IHC (10).
Similar to other research (11), we
did not find any relationship between HER2 and p-ERK.
Collectively, our data indicate that the regulation
of P38 MAPK was not directly linked to any of the investigated
signaling pathways and could be considered as an independent
biomarker in breast cancer. This also confirms the complexity of
breast cancer oncogenesis, involving the recruitment of multiple
signaling pathways.
In conclusion, P38 MAPK expression and activation
are frequently observed in breast carcinoma and appear to be
positively associated with the expression of the ER. Our results
confirm the capability of breast cancer cells to activate P38
MAPK-mediated stress mechanisms and that P38 MAPK may represent a
biological target for ER+ breast cancer. The frequent
concomitant activation of P38 MAPK, ERK and AKT indicates that
breast tumor growth involves the activation of multiple signaling
pathways, probably explaining the multiple mechanisms by which
tumor cells develop resistance. Control of tumor growth should
therefore entail the inhibition of all signaling pathways by
combining multiple targeted therapies either in concomitant or in
sequential use.
Acknowledgements
The present study was supported by the French ‘Ligue
contre le Cancer, Comité Inter-régional Grand Est’ and Alexis
Vautrin private research funds.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Baselga J, Campone M, Piccart M, et al:
Everolimus in postmenopausal hormone-receptor-positive advanced
breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemura M, Iino Y, Koibuchi Y, Yokoe T and
Morishita Y: Mitogen-activated protein kinase cascade in breast
cancer. Oncology. 57(Suppl 2): 37–44. 1999. View Article : Google Scholar
|
|
4
|
Ono K and Han J: The p38 signal
transduction pathway: activation and function. Cell Signal.
12:1–13. 2000. View Article : Google Scholar
|
|
5
|
Frigo DE, Basu A, Nierth-Simpson EN, et
al: p38 mitogen-activated protein kinase stimulates
estrogen-mediated transcription and proliferation through the
phosphorylation and potentiation of the p160 coactivator
glucocorticoid receptor-interacting protein 1. Mol Endocrinol.
20:971–983. 2006. View Article : Google Scholar
|
|
6
|
Wagner EF and Nebreda A: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang L and Han X: The urokinase
plasminogen activator system in breast cancer invasion and
metastasis. Biomed Pharmacother. 67:179–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salh B, Marotta A, Wagey R, Sayed M and
Pelech S: Dysregulation of phosphatidylinositol 3-kinase and
downstream effectors in human breast cancer. Int J Cancer.
98:148–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esteva FJ, Sahin AA, Smith TL, et al:
Prognostic significance of phosphorylated P38 mitogen-activated
protein kinase and HER-2 expression in lymph node-positive breast
carcinoma. Cancer. 100:499–506. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gutierrez MC, Detre S, Johnston S, et al:
Molecular changes in tamoxifen-resistant breast cancer:
relationship between estrogen receptor, HER-2, and p38
mitogen-activated protein kinase. J Clin Oncol. 23:2469–2476. 2005.
View Article : Google Scholar
|
|
11
|
Davidson B, Konstantinovsky S, Kleinberg
L, et al: The mitogen-activated protein kinases (MAPK) p38 and JNK
are markers of tumor progression in breast carcinoma. Gynecol
Oncol. 102:453–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Mayer JA, Krisko TI, et al:
Inhibition of the p38 kinase suppresses the proliferation of human
ER-negative breast cancer cells. Cancer Res. 69:8853–8861. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mueller KL, Powell K, Madden JM, Eblen ST
and Boerner JL: EGFR tyrosine 845 phosphorylation-dependent
proliferation and transformation of breast cancer cells require
activation of p38 MAPK. Transl Oncol. 5:327–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Antoon JW, Bratton MR, Guillot LM, et al:
Pharmacology and anti-tumor activity of RWJ67657, a novel inhibitor
of p38 mitogen activated protein kinase. Am J Cancer Res.
2:446–458. 2012.PubMed/NCBI
|
|
15
|
Ghayad SE, Vendrell JA, Ben Larbi S,
Dumontet C, Bieche I and Cohen PA: Endocrine resistance associated
with activated ErbB system in breast cancer cells is reversed by
inhibiting MAPK or PI3K/Akt signaling pathways. Int J Cancer.
126:545–562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Normanno N, Di Maio M, De Maio E, et al:
Mechanisms of endocrine resistance and novel therapeutic strategies
in breast cancer. Endocr Relat Cancer. 12:721–747. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Musgrove EA and Sutherland RL: Biological
determinants of endocrine resistance in breast cancer. Nat Rev
Cancer. 9:631–643. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cortés J, Saura C, Bellet M, et al: HER2
and hormone receptor-positive breast cancer - blocking the right
target. Nat Rev Clin Oncol. 8:307–311. 2011.PubMed/NCBI
|
|
19
|
Massarweh S, Osborne CK, Creighton CJ, et
al: Tamoxifen resistance in breast tumors is driven by growth
factor receptor signaling with repression of classic estrogen
receptor genomic function. Cancer Res. 68:826–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee H and Bai W: Regulation of estrogen
receptor nuclear export by ligand-induced and p38-mediated receptor
phosphorylation. Mol Cell Biol. 22:5835–5845. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Baskerville C, Han Q, Pan ZK and
Huang S: α(v) integrin, p38 mitogen-activated protein kinase, and
urokinase plasminogen activator are functionally linked in invasive
breast cancer cells. J Biol Chem. 276:47901–47905. 2001.
|
|
23
|
Kim MS, Lee EJ, Kim HR and Moon A: p38
kinase is a key signaling molecule for H-Ras-induced cell motility
and invasive phenotype in human breast epithelial cells. Cancer
Res. 63:5454–5461. 2003.PubMed/NCBI
|
|
24
|
Qi X, Zhi H, Lepp A, et al: p38γ
mitogen-activated protein kinase (MAPK) confers breast cancer
hormone sensitivity by switching estrogen receptor (ER) signaling
from classical to nonclassical pathway via stimulating ER
phosphorylation and c-Jun transcription. J Biol Chem.
287:14681–14691. 2012.
|
|
25
|
Banerjee A, Koziol-White C and Panettieri
R Jr: p38 MAPK inhibitors, IKK2 inhibitors, and TNFα inhibitors in
COPD. Curr Opin Pharmacol. 12:287–292. 2012.
|
|
26
|
Chergui F, Chrétien AS, Bouali S, et al:
Validation of a phosphoprotein array assay for characterization of
human tyrosine kinase receptor downstream signaling in breast
cancer. Clin Chem. 55:1327–1336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Svensson S, Jirström K, Rydén L, et al:
ERK phosphorylation is linked to VEGFR2 expression and Ets-2
phosphorylation in breast cancer and is associated with tamoxifen
treatment resistance and small tumours with good prognosis.
Oncogene. 24:4370–4379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhatt S, Xiao Z, Meng Z and
Katzenellenbogen BS: Phosphorylation by p38 mitogen-activated
protein kinase promotes estrogen receptor α turnover and functional
activity via the SCF(Skp2) proteasomal complex. Mol Cell Biol.
32:1928–1943. 2012.
|
|
29
|
Meng F, Zhang H, Liu G, et al: p38γ
mitogen-activated protein kinase contributes to oncogenic
properties maintenance and resistance to poly
(ADP-ribose)-polymerase-1 inhibition in breast cancer. Neoplasia.
13:472–482. 2011.
|
|
30
|
Qi X, Hou S, Lepp A, et al:
Phosphorylation and stabilization of topoisomerase IIα protein by
p38γ mitogen-activated protein kinase sensitize breast cancer cells
to its poison. J Biol Chem. 286:35883–35890. 2011.
|
|
31
|
Milde-Langosch K, Bamberger AM, Rieck G,
et al: Expression and prognostic relevance of activated
extracellular-regulated kinases (ERK1/2) in breast cancer. Br J
Cancer. 92:2206–2215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Linderholm BK, Hellborg H, Johansson U,
Skoog L and Lehtiö J: Vascular endothelial growth factor receptor 2
and downstream p38 mitogen-activated protein kinase are possible
candidate markers of intrinsic resistance to adjuvant endocrine
treatment in steroid receptor-positive breast cancer. Breast Cancer
Res Treat. 125:457–465. 2011. View Article : Google Scholar
|
|
33
|
Hermanto U, Zong CS and Wang LH:
Inhibition of mitogen-activated protein kinase kinase selectively
inhibits cell proliferation in human breast cancer cells displaying
enhanced insulin-like growth factor I-mediated mitogen-activated
protein kinase activation. Cell Growth Differ. 11:655–664.
2000.
|
|
34
|
Park SS and Kim SW: Activated Akt
signaling pathway in invasive ductal carcinoma of the breast:
Correlation with HER2 overexpression. Oncol Rep. 18:139–143.
2007.PubMed/NCBI
|