Introduction
Medulloblastoma (MB) is the most common pediatric
malignant brain tumor, and satisfactory treatment results are
notoriously difficult to achieve. MB has a high propensity to
metastasize, as ~30% of patients have evidence of leptomeningeal
dissemination at initial diagnosis (1). Despite recent advances in surgery,
radiotherapy and chemotherapy, only 60 to 75% of affected children
are cured, and the majority of them suffer from considerable
long-term morbidity after aggressive multimodal therapy (2). Attempts to further improve outcomes
and decrease the morbidity associated with MB have been restricted
by the conventional cytotoxic approaches used and the infiltrative
nature of the disease (2–4). Therefore, increased understanding of
the mechanisms underlying MB is necessary to develop novel
therapeutic approaches.
The oncogenic transcription factor FOXM1 is known to
stimulate proliferation by promoting cell cycle transition and is
involved in the proper execution of mitosis (5,6).
Elevated expression of FOXM1 has been detected in a wide range of
human tumors and has been implicated in cellular transformation,
tumor initiation and progression (7). Accumulating evidence demonstrates that
increased expression of FOXM1 is associated with poor prognosis in
various types of cancers (8–11),
including MB (12). Furthermore,
previous studies have shown that FOXM1 mediates resistance to a
diverse spectrum of anticancer drugs in breast cancer (13–15). A
recent study confirmed that suppression of FOXM1 enhanced the
chemosensitivity of various types of cancer cells to the
DNA-damaging reagent doxorubicin (16). Therefore, the inhibition of FOXM1
activity has emerged as an attractive goal for cancer therapy.
However, since FOXM1 displays a proliferation-specific expression
pattern and is essential for embryonic development (5), the impact of inhibiting FOXM1 activity
in non-malignant cells must be considered.
Thiostrepton, a natural product originally isolated
from Streptomyces azureus, has captured a great deal of
attention because of its potent anticancer activity as a FOXM1
inhibitor (17–19). The mechanism by which thiostrepton
affects FOXM1 remains unknown. Hegde et al(18) reported that thiostrepton interacts
directly with FOXM1 protein to inhibit the transcriptional activity
of FOXM1, whereas Bhat et al(19) suggested that thiostrepton functions
as a proteasomal inhibitor. However, in a wide variety of tumor
cell types, thiostrepton-induced apoptosis is dependent on the
expression of FOXM1 (10,17,20).
Importantly, thiostrepton appears to exert minimal toxicity against
non-malignant cells (17,21). Thiostrepton has shown anticancer
activity in rodent xenograft models without observable toxicity
(10,22,23).
Collectively, these data suggest that thiostrepton is an ideal
treatment for MB, particularly in children. In this study, we
examined the antitumor effects of thiostrepton in Daoy MB cells.
More importantly, we assessed the ability of thiostrepton to
sensitize MB cell lines to cisplatin, which is commonly used for
the treatment of MB.
Materials and methods
Chemicals and reagents
Thiostrepton purchased from Tocris Cookson Inc.
(Ellisville, MO, USA) was freshly dissolved in dimethyl sulfoxide
(DMSO) to make a 10 mmol/l stock solution. Cisplatin (Qilu
Pharmaceutical Co., Ltd., Shandong, China) was dissolved at a stock
concentration of 2 mmol/l and divided into aliquots. An antibody
specific for FOXM1 was obtained from Santa Cruz Biotechnology Inc.
(Santa Cruz, CA, USA) Antibodies specific for Bcl-2, Bax,
caspase-3, PARA and β-actin were obtained from Cell Signaling
Technology, Inc.
Cell culture
The Daoy human MB cell line was obtained from ATCC
(Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (both
from Gibco-BRL) and incubated at 37°C in a humidified incubator in
the presence of 5% CO2.
siRNA transfection
RNA interference was performed by transfecting Daoy
cells with 21-nucleotide RNA duplexes. FOXM1 siRNA and mock siRNA
were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China). The siRNA-NC did not target any known mammalian gene and
was synthesized by Shanghai GenePharma Co., Ltd. siRNA transfection
was carried out with Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA), according to the procedure
recommended by the manufacturer. Six hours after transfection with
siRNA NC at various concentrations, the cells were analyzed on a
FACSCalibur flow cytometer equipped with Cell Quest software
(Becton-Dickinson, San Jose, CA, USA).
Western blot analysis
The cells were collected and lysed in lysis buffer
on ice. The cell lysates were centrifuged at 10,000 × g for 10 min
at 4°C, and the protein content in the supernatants was determined
using a BCA protein assay kit (Pierce Biotechnology, Inc., USA).
Equal amounts of protein lysate were electrophoretically separated
on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred
to polyvinylidene difluoride (PVDF) membranes (Millipore, USA). The
membranes were blocked with 1% bovine serum albumin (BSA) for 2 h
at room temperature and then incubated with anti-FOXM1, anti-Bcl-2,
anti-Bax, anti-caspase 3, anti-PARA or anti-β-actin primary
antibody overnight at 4°C. Horseradish peroxidase (HRP)-conjugated
secondary antibody was added for 2 h at room temperature. Detection
was performed by enhanced chemiluminescence (ECL; Pierce
Biotechnology, Inc.).
Cell proliferation analysis
Cell proliferation assays were performed using the
CCK-8 kit (Cell Counting Kit-8; Dojindo Laboratories), according to
the manufacturer’s instructions. In brief, Daoy cells were seeded
in a 96-well plate at a density of 5×104 cells/ml. The
following day, the medium was replaced with DMEM containing 10% FBS
with or without agents. The cells were incubated for 24, 48 or 72
h, and CCK-8 was used according to the manufacturer’s instructions.
Extinction was measured at 450 nM, and the reference extinction was
subtracted. Each experiment was performed in triplicate and
repeated 3 times.
Cell cycle analysis
Daoy cells were seeded in 6-well plates in DMEM
containing 10% FBS. The following day, the medium was replaced with
DMEM containing 10% FBS with or without agents. The cells were
detached after 24 h and fixed with 500 μl of 70% ethanol at −20°C
for 2 h. Subsequently, the cells were washed twice with PBS and
then stained with propidium iodide (PI) (50 μg/ml propidium iodide
and 100 μg/ml RNase A in PBS) at 37°C for 30 min. Cell cycle
analysis was performed on a FACScan flow cytometer
(Becton-Dickinson).
Apoptosis analysis
Daoy cells were seeded in 6-well plates in DMEM
containing 10% FBS. The following day, the medium was replaced with
DMEM containing 10% FBS without or with agents. The cells were
collected after 24 h, and cell apoptosis was detected by Annexin
V-FITC/PI staining. The experiments were performed in triplicate
for each sample, and analyses were performed using a FACScan flow
cytometer in accordance with the manufacturer’s guidelines.
Colony formation assays
Daoy cells growing in log phase were seeded at a
density of 1,000 cells/well in a 6-well plate in complete growth
medium containing 10% FBS. The cells were allowed to adhere for 24
h, and the medium was replaced with fresh complete growth medium
containing the indicated concentrations of thiostrepton. The cells
were cultured at 37°C for 10 days with medium changes every third
or fourth day. Colony formation was detected by crystal violet
staining.
Cell invasion and migration assay
Daoy cells were treated with the indicated
concentrations of thiostrepton for 48 h, and equal numbers of cells
were suspended in serum-free medium and seeded into either uncoated
Transwell inserts (for migration assays) or growth factor-reduced
Matrigel-coated Transwell inserts (for invasion assays) (BD
Biosciences, Bedford, MA, USA). The bottom wells were filled with
complete medium, and after 12 h, the cells were stained with
crystal violet and photographed under a fluorescence microscope.
The number of cells that penetrated the membrane was determined by
counting the mean cell number of 5 randomly selected high-power
fields.
Statistical analysis
All data were analyzed using GraphPad Prism version
5 (GraphPad Software Inc., La Jolla, CA, USA). t-tests were used
for pairwise comparisons. The synergistic or antagonistic effects
of 2 drugs were evaluated according to the formula [Q = Ea + b/Ea +
Eb − Ea × Eb]. In this equation, Ea + b represents the inhibition
rate of the combined drug therapy on tumor cell proliferation, and
Ea and Eb represent the inhibition rates of drug A and drug B
individually. Q-values ranging from 0.85 to 1.15 indicate that the
effects of the 2 drugs are simply additive, Q-values >1.15
indicate a synergistic effect, and Q-values <0.85 indicate an
antagonistic effect for the combined drug therapy.
Results
Thiostrepton inhibits cell proliferation
and decreases the colony formation capacity of Daoy cells
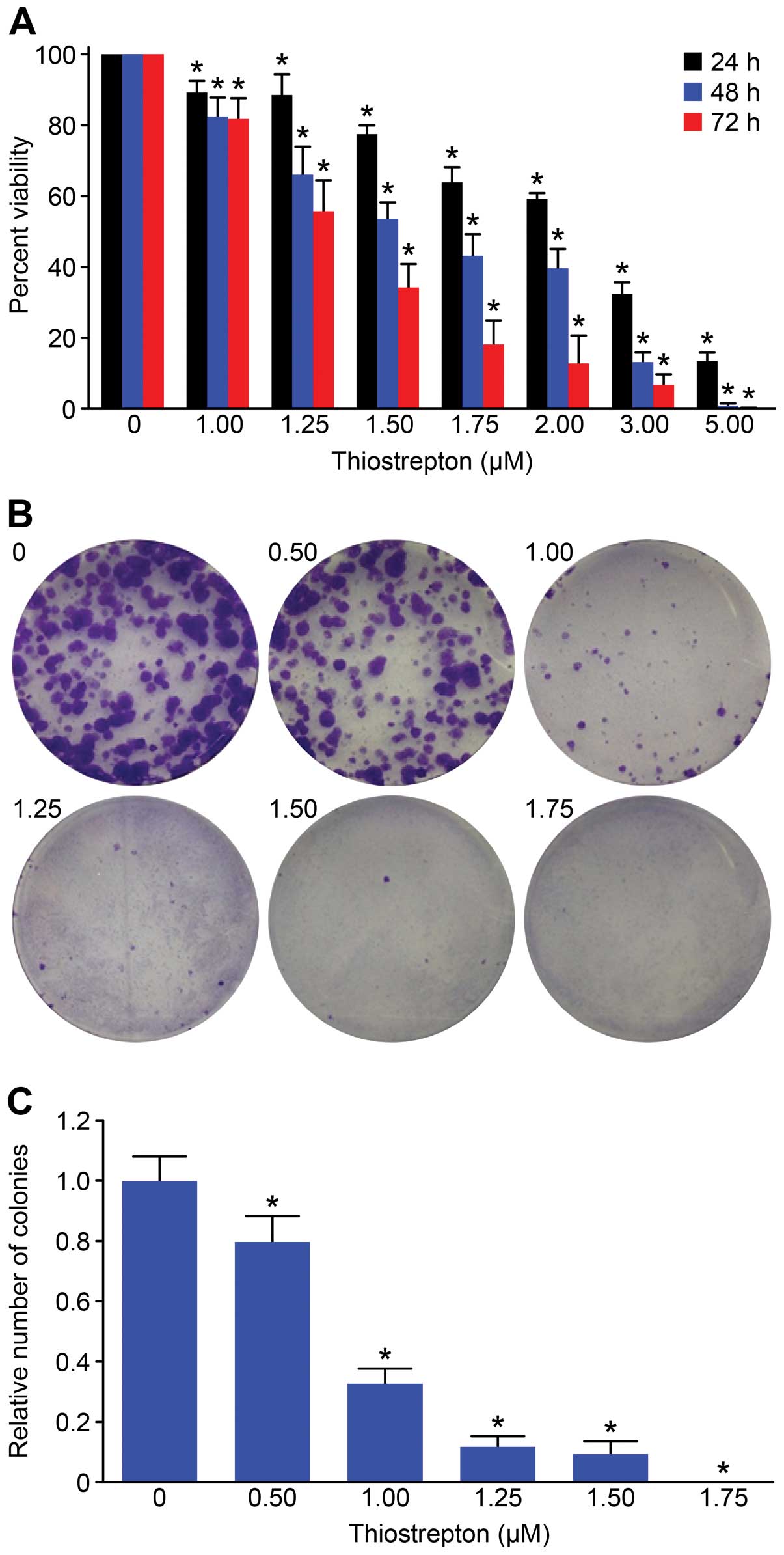
We first investigated the effect of thiostrepton on
Daoy cell proliferation. Daoy cells were treated with various
concentrations of thiostrepton (1–5 μM) for 24, 48 or 72 h, and
cell proliferation was measured by performing CCK-8 assays. Our
results demonstrated that Daoy cells were sensitive to
thiostrepton, and thiostrepton inhibited cell growth in a time- and
dose-dependent manner (P<0.05) (Fig.
1A). The IC50 values of Daoy cells to thiostrepton
after 24, 48 or 72 h of treatment are shown in Table I. To evaluate the long-term impact
of thiostrepton on MB cells, we performed colony formation assays.
We found that Daoy cells had a robust ability to form colonies, and
colony formation was significantly inhibited by thiostrepton.
Treatment with 1.75 μM thiostrepton completely abolished the colony
formation ability of Daoy cells (Fig.
1B and C).
 | Table IIC50 values for
thiostrepton in Daoy cells were calculated at 24, 48 or 72 h. |
Table I
IC50 values for
thiostrepton in Daoy cells were calculated at 24, 48 or 72 h.
| Treatment time |
|---|
|
|
|---|
| 24 h | 48 h | 72 h |
|---|
| IC50 (μM)
for thiostrepton | 2.14 | 1.69 | 1.39 |
Thiostrepton-mediated inhibition of FOXM1
induces cell cycle arrest and leads to apoptosis of Daoy cells
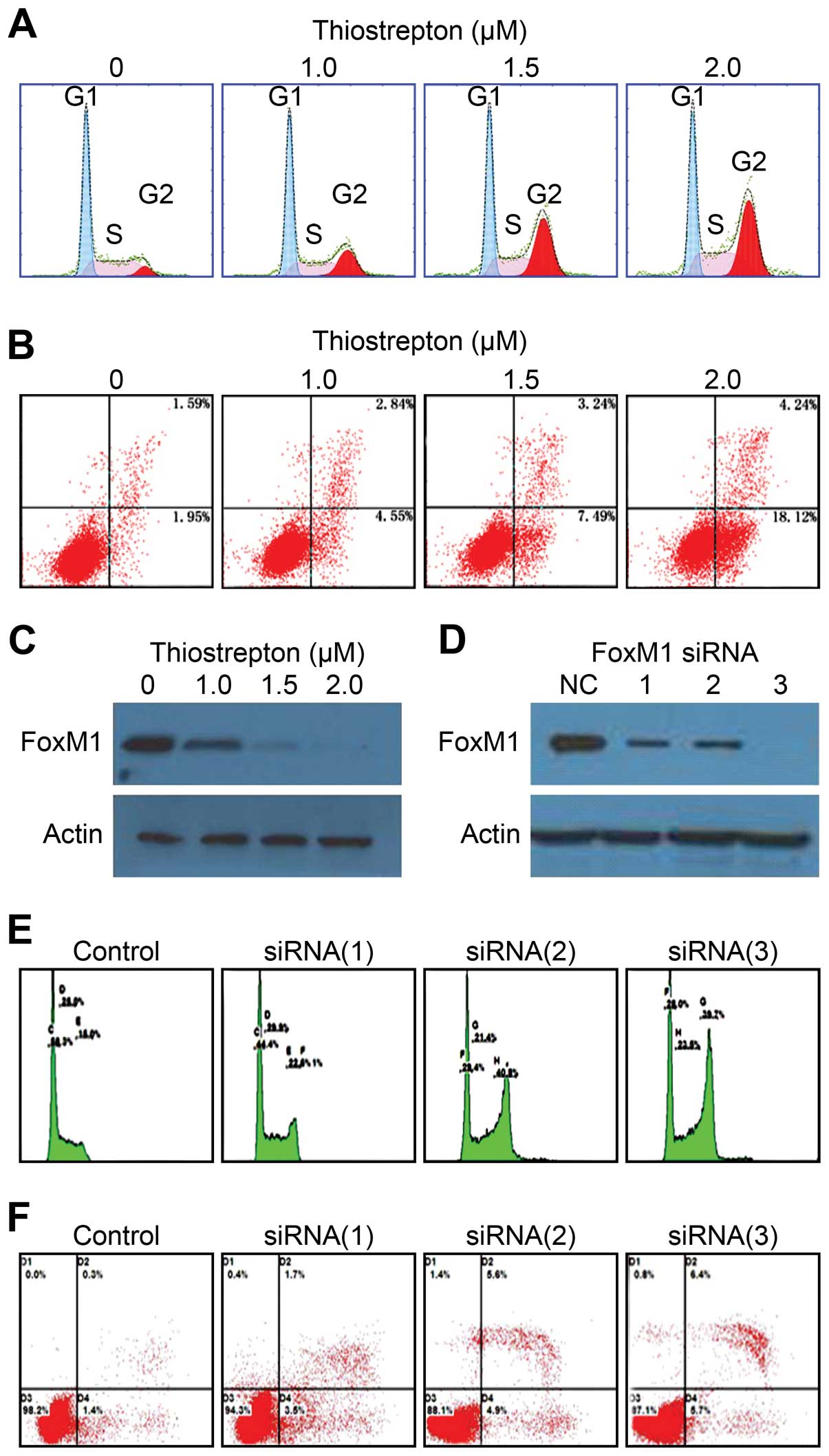
To further characterize the mechanism underlying the
antitumor activity of thiostrepton on Daoy cells, we investigated
the expression of FOXM1 following thiostrepton treatment and
examined whether thiostrepton affects cell cycle distribution and
apoptosis. The results of these experiments showed that
thiostrepton inhibited FOXM1 expression in a dose-dependent manner
and induced a gradual dose-dependent G2/M arrest and apoptosis in
Daoy cells (Fig. 2A–C). To validate
the target specificity of thiostrepton, we downregulated FOXM1
expression using RNAi. Three different FOXM1 siRNA
oligonucleotides, but not the negative control (NC)
oligonucleotides, efficiently knocked down FOXM1 protein expression
(Fig. 2D) and similarly resulted in
a significant G2/M cell cycle phases arrest and apoptosis in Daoy
cells (Fig. 2E and F). These
results indicate that thiostrepton induces G2/M cell cycle phase
arrest and leads to apoptosis in Daoy cells by targeting FOXM1.
Thiostrepton enhances the
anti-proliferative effects of cisplatin on Daoy cells
The upregulation of FOXM1 has recently been reported
to be closely related to chemotherapy resistance, and the
inhibition of FOXM1 has been shown to increase the sensitivity of
tumor cells to chemotherapeutic agents (13,15,24–26).
We next aimed to ascertain whether combining FOXM1 inhibition with
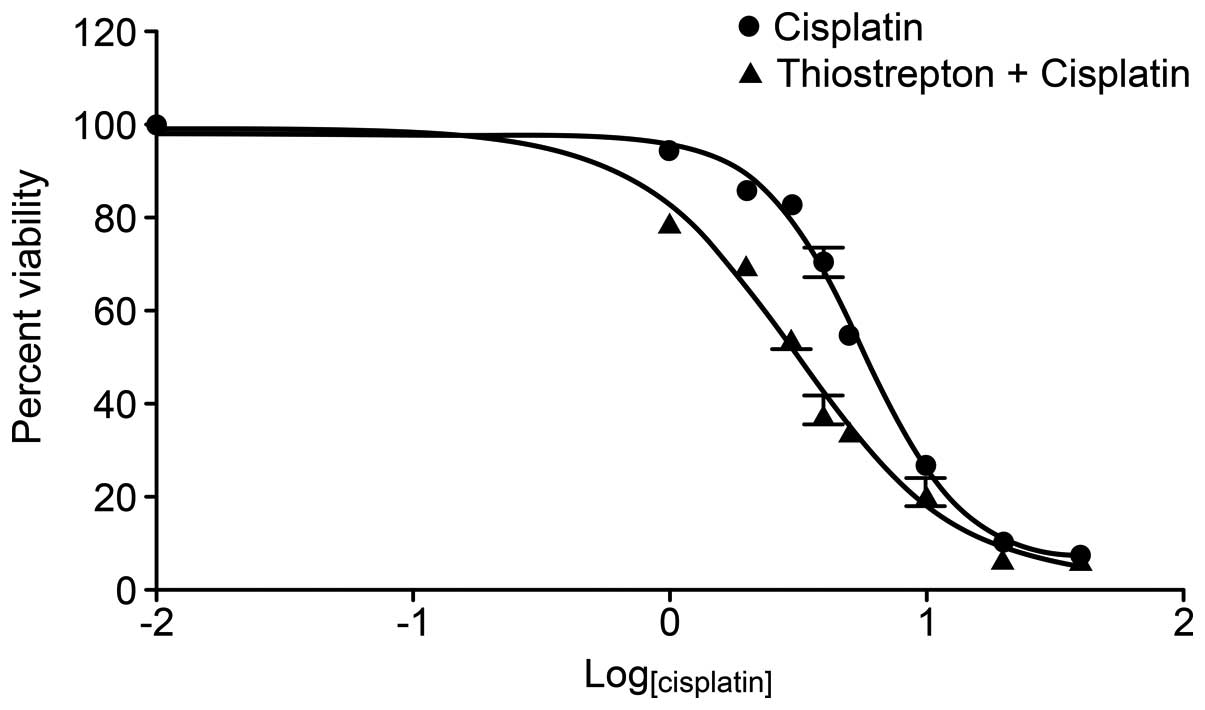
standard chemotherapeutic agents would enhance the effect. Daoy
cells were cultured in the presence of cisplatin (0–40 μm/l) with
or without thiostrepton (1 μm/l) for 24 h. Cytotoxicity was
assessed using CCK-8 proliferation assays. The results demonstrated
that combined treatment reduced the IC50 value of
cisplatin in Daoy cells from 5.622 to 3.116 μmol/l (Fig. 3). Thiostrepton and cisplatin were
shown to synergistically inhibit cell growth (Q >1.15) when the
cells were treated with suboptimal concentrations of cisplatin
(Table II).
 | Table IIAnalysis was employed to characterize
the interactions between the drugs. |
Table II
Analysis was employed to characterize
the interactions between the drugs.
| Cisplatin (μM) + Thio
(1 μM)a |
|---|
|
|
|---|
| 1 | 2 | 3 | 4 | 5 | 10 | 20 | 40 |
|---|
| Q-value | 1.35 | 1.31 | 1.78 | 1.66 | 1.29 | 1.04 | 1.03 | 1 |
Thiostrepton enhances cisplatin-induced
caspase-mediated apoptosis in Daoy cells
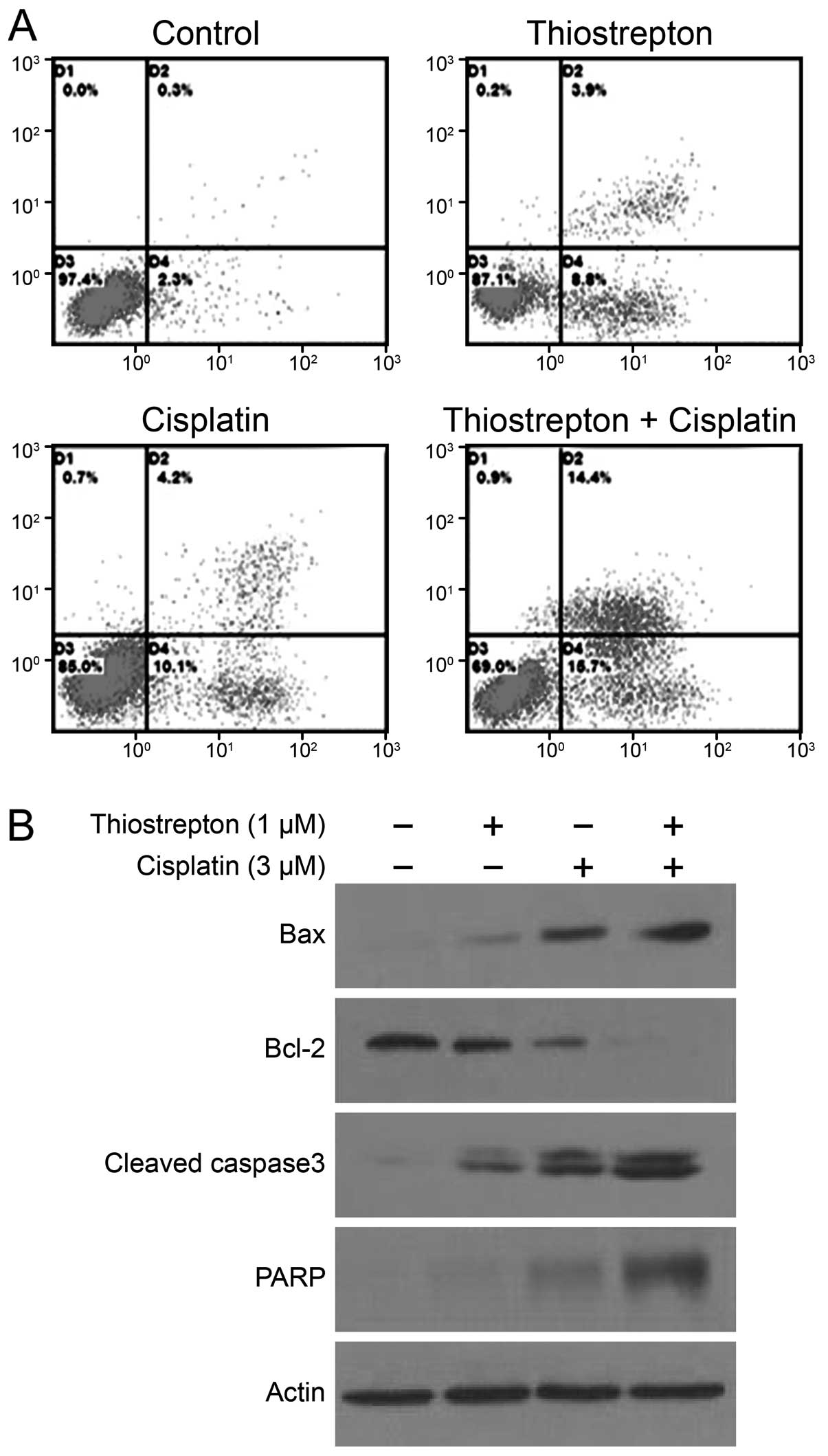
To characterize thiostrepton-mediated apoptotic
enhancement, Daoy cells were treated with a low concentration of
either thiostrepton (1 μm/l) or cisplatin (3 μm/l) or treated with
both drugs for 24 h. Flow cytometric analysis showed that
thiostrepton increased cisplatin-induced apoptosis from 14.3±2.46
to 30.2±3.16% (P<0.01) (Fig.
4A). To gain a better understanding of the mechanism leading to
cell death, we measured the combined effect of thiostrepton and
cisplatin on the expression of Bcl-2, Bax, caspase-3 and PARP
proteins. As shown in Fig. 4B, the
decreases in Bcl-2 expression were significantly greater in samples
treated with both thiostrepton (1 μM) and cisplatin (3 μM) than
those in the single treatment group. Moreover, combination
treatment resulted in greater increases in Bax, caspase-3 and PARP
protein expression than either drug alone (Fig. 4B).
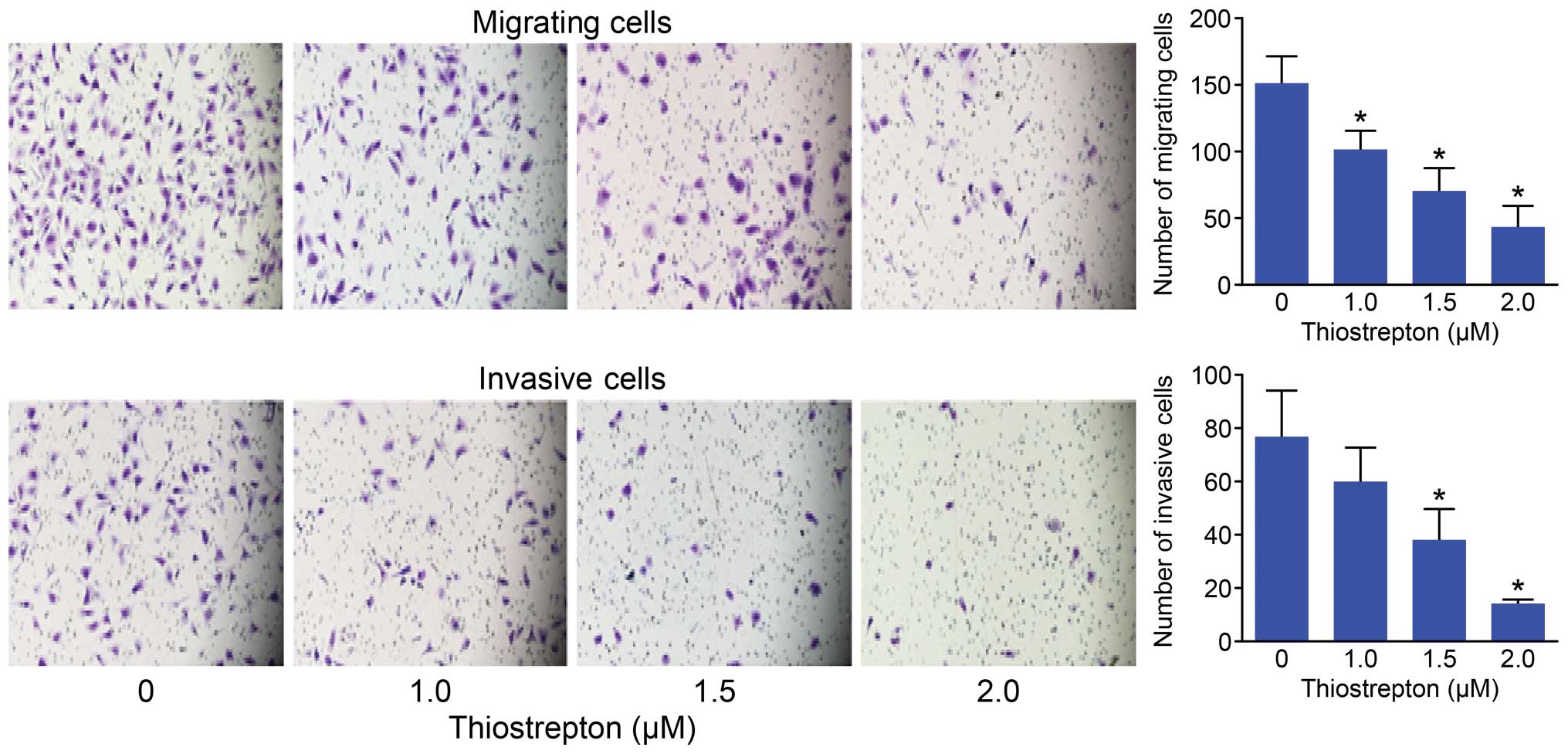
Thiostrepton impairs the migration and
invasion of Daoy cells
FOXM1 play a critical role in tumor cell metastasis
(27–29). Since tumor cell migration and
invasion are essential steps in tumor metastasis, we further
examined the effects of thiostrepton on cell migration and invasion
in Daoy cells. Our data clearly showed that thiostrepton inhibited
the migration and invasion capacity of Daoy cells in a
dose-dependent manner with minimal involvement of cell inhibition
(Fig. 5).
Discussion
As the most common malignant brain tumor in
children, medulloblastoma is characterized by aggressive invasion
and early metastasis. Current standard therapies have
unsatisfactory effects on survival, and therapy-associated
side-effects have led to a concentrated search for novel
therapeutic approaches for MB. Here, we report for the first time
that thiostrepton inhibits FOXM1 in Daoy MB cells. We found that
thiostrepton inhibited Daoy cell proliferation and triggered
apoptosis in a dose-dependent manner. We further demonstrated that
thiostrepton chemosensitized Daoy cells to cisplatin by enhancing
cisplatin-induced apoptosis. Thiostrepton also showed important
antiprogressive effects such as inhibition of migration and
invasion in Daoy cells.
We found that thiostrepton significantly inhibited
the proliferation of Daoy cells. The IC50 value of
thiostrepton in Daoy cells after 48 h of treatment was 1.69 μmol/l,
suggesting the sensitivity of Daoy cells to thiostrepton is
comparable to that of other cancer cell types (20,22).
We next performed colony formation assays, which are an excellent
indication of the long-term survival of tumor cells. These assays
showed that thiostrepton strongly suppressed the ability of Daoy
cells to form colonies. Notably, 1.75 μM thiostrepton treatment had
a more marked effect on colony formation than on cell proliferation
(100 vs. 35.81% inhibition, respectively). These data suggest that
thiostrepon may impact tumor cell self-renewal in addition to
mitosis.
Furthermore, our studies demonstrated that
thiostrepton treatment induced cell cycle arrest at the G2/M phase
transition and triggered apoptosis. Similar results were obtained
with RNAi-mediated inhibition of FOXM1 in Daoy cells. These results
support the idea proposed by Priller et al(12) that FOXM1 inhibition results in
failure of mitosis and leads to mitotic catastrophe in MB.
Furthermore, our results demonstrated the efficient antitumor
activity of thiostrepton in Daoy cells and suggest that the effects
of thiostrepton may depend on the downregulation of FOXM1 target
genes, which have been shown to be involved in the regulation of
cell growth, apoptosis, cell cycle and progression (10).
Previous studies suggest that silencing of FOXM1
enhances sensitivity to chemotherapeutic agents in a wide range of
human cancers (15,16,25).
This prompted us to investigate whether thiostrepton-mediated FOXM1
inhibition sensitizes Daoy cells to chemotherapy. In this study,
the IC50 value of cisplatin in Daoy cells decreased from
5.622 to 3.116 μmol/l when it was combined with thiostrepton (1
μmol/l). Notably, all of the synergistic effects identified in this
study occurred when thiostrepton was combined with suboptimal
concentrations of chemotherapeutic drugs. Cisplatin is a standard
chemotherapy agent used in MB treatment; however, its use results
in drug-related morbidities including hearing loss and renal
dysfunction. Therefore, a combination therapy approach using
cisplatin and thiostrepton would not only improve the effect of
cisplatin but also reduce the adverse side-effects of cisplatin by
allowing it to be used at lower doses.
The ability to trigger tumor cell death is critical
for successful treatment with chemotherapeutic agents. In the
present study, flow cytometric analysis revealed a more marked
increase in apoptosis in cells treated with a combination of
thiostrepton and cisplatin than in those treated with either drug
alone. To further explore the mechanism underlying the increased
efficacy of combination treatment, we examined proteins known to
regulate apoptosis. Bcl-2 is best known for its role in preventing
apoptosis and conferring resistance to chemotherapeutic agents in
various cell lines (30), whereas
Bax accelerates cell death in response to certain apoptotic
stimuli. Cells treated with both thiostrepton and cisplatin
displayed a greater decrease in Bcl-2 protein expression than cells
treated only with cisplatin, and thiostrepton also enhanced
cisplatin-induced increases in Bax, PARP and caspase-3 protein
expression. These results suggest that thiostrepton sensitizes Daoy
cells to cisplatin partly through enhanced apoptosis, possibly via
a caspase-3-dependent pathway.
FOXM1 has been reported to play a central role in
tumor metastasis (27–29), and few studies have explored the
antitumor activity of thiostrepton. In our study, thiostrepton
significantly decreased the migration and invasion capacity of Daoy
cells. As these processes are both necessary steps for metastasis,
we believe that thiostrepton may be a promising agent with which to
antagonize the highly metastatic behavior of MB.
Collectively, our findings showed that thiostrepton
induces apoptosis and significantly decreases the proliferation and
metastatic potential of Daoy cells. Thiostrepton also acts
synergistically with cisplatin by enhancing cisplatin-induced
apoptosis. These results demonstrate that thiostrepton may have
wide therapeutic and/or adjuvant applications for MB chemotherapy.
Thiostrepton may be particularly useful in very young children with
metastatic disease who currently must receive more aggressive
chemotherapeutics.
Acknowledgements
This study was supported by The National Natural
Science Foundation of China (81001119), The Grant Awarded to New
Teachers from the Chinese Ministry of Education (20110171120112)
and The Fundamental Research Funds for the Central Universities
(11ykzd06).
References
|
1
|
von Hoff K, Hinkes B, Gerber NU, Deinlein
F, Mittler U, Urban C, Benesch M, Warmuth-Metz M, Soerensen N,
Zwiener I, Goette H, Schlegel PG, Pietsch T, Kortmann RD, Kuehl J
and Rutkowski S: Long-term outcome and clinical prognostic factors
in children with medulloblastoma treated in the prospective
randomised multicentre trial HIT’91. Eur J Cancer. 45:1209–1217.
2009.PubMed/NCBI
|
|
2
|
Massimino M, Giangaspero F, Garrè ML,
Gandola L, Poggi G, Biassoni V, Gatta G and Rutkowski S: Childhood
medulloblastoma. Crit Rev Oncol Hematol. 79:65–83. 2011. View Article : Google Scholar
|
|
3
|
Gilbertson RJ: Medulloblastoma: signalling
a change in treatment. Lancet Oncol. 5:209–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leary SE and Olson JM: The molecular
classification of medulloblastoma: driving the next generation
clinical trials. Curr Opin Pediatr. 24:33–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wierstra I and Alves J: FOXM1, a typical
proliferation-associated transcription factor. Biol Chem.
388:1257–1274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laoukili J, Kooistra MR, Brás A, Kauw J,
Kerkhoven RM, Morrison A, Clevers H and Medema RH: FoxM1 is
required for execution of the mitotic programme and chromosome
stability. Nat Cell Biol. 7:126–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koo CY, Muir KW and Lam EW: FOXM1: from
cancer initiation to progression and treatment. Biochim Biophys
Acta. 1819:28–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu M, Dai B, Kang SH, Ban K, Huang FJ,
Lang FF, Aldape KD, Xie TX, Pelloski CE, Xie K, Sawaya R and Huang
S: FoxM1B is overexpressed in human glioblastomas and critically
regulates the tumorigenicity of glioma cells. Cancer Res.
66:3593–3602. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bektas N, Haaf At, Veeck J, Wild PJ,
Lüscher-Firzlaff J, Hartmann A, Knüchel R and Dahl E: Tight
correlation between expression of the Forkhead transcription factor
FOXM1 and HER2 in human breast cancer. BMC Cancer. 8:422008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmed M, Uddin S, Hussain AR, Alyan A,
Jehan Z, Al-Dayel F, Al-Nuaim A, Al-Sobhi S, Amin T, Bavi P and
Al-Kuraya KS: FoxM1 and its association with matrix
metalloproteinase (MMP) signaling pathway in papillary thyroid
carcinoma. J Clin Endocrinol Metab. 97:E1–E13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia JT, Wang H, Liang LJ, Peng BG, Wu ZF,
Chen LZ, Xue L, Li Z and Li W: Overexpression of FOXM1 is
associated with poor prognosis and clinicopathologic stage of
pancreatic ductal adenocarcinoma. Pancreas. 41:629–635. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Priller M, Pöschl J, Abrão L, von Bueren
AO, Cho YJ, Rutkowski S, Kretzschmar HA and Schüller U: Expression
of FoxM1 is required for the proliferation of medulloblastoma cells
and indicates worse survival of patients. Clin Cancer Res.
17:6791–6801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carr JR, Park HJ, Wang Z, Kiefer MM and
Raychaudhuri P: FoxM1 mediates resistance to herceptin and
paclitaxel. Cancer Res. 70:5054–5063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Millour J, Constantinidou D, Stavropoulou
AV, Wilson MS, Myatt SS, Kwok JM, Sivanandan K, Coombes RC, Medema
RH, Hartman J, Lykkesfeldt AE and Lam EW: FOXM1 is a
transcriptional target of ERalpha and has a critical role in breast
cancer endocrine sensitivity and resistance. Oncogene.
29:2983–2995. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kwok JM, Peck B, Monteiro LJ, Schwenen HD,
Millour J, Coombes RC, Myatt SS and Lam EW: FOXM1 confers acquired
cisplatin resistance in breast cancer cells. Mol Cancer Res.
8:24–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Halasi M and Gartel AL: Suppression of
FOXM1 sensitizes human cancer cells to cell death induced by
DNA-damage. PLoS One. 7:e317612010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwok JM, Myatt SS, Marson CM, Coombes RC,
Constantinidou D and Lam EW: Thiostrepton selectively targets
breast cancer cells through inhibition of forkhead box M1
expression. Mol Cancer Ther. 7:2022–2032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hegde NS, Sanders DA, Rodriguez R and
Balasubramanian S: The transcription factor FOXM1 is a cellular
target of the natural product thiostrepton. Nat Chem. 3:725–731.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhat UG, Halasi M and Gartel AL: FoxM1 is
a general target for proteasome inhibitors. PLoS One. 4:e65932009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhat UG, Halasi M and Gartel AL: Thiazole
antibiotics target FoxM1 and induce apoptosis in human cancer
cells. PLoS One. 4:e55922009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halasi M, Schraufnagel DP and Gartel AL:
Wild-type p53 protects normal cells against apoptosis induced by
thiostrepton. Cell Cycle. 8:2850–2851. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Halasi M, Zhao H, Dahari H, Bhat UG,
Gonzalez EB, Lyubimo AV, Tonetti DA and Gartel AL: Thiazole
antibiotics against breast cancer. Cell Cycle. 9:1214–1217. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang M and Gartel AL: Micelle-encapsulated
thiostrepton as an effective nanomedicine for inhibiting tumor
growth and for suppressing FOXM1 in human xenografts. Mol Cancer
Ther. 10:2287–2297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Wen L, Zhao SH, Ai ZH, Guo JZ and
Liu WC: FoxM1 expression is significantly associated with
cisplatin-based chemotherapy resistance and poor prognosis in
advanced non-small cell lung cancer patients. Lung Cancer.
79:173–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang N, Wu X, Yang L, Xiao F, Zhang H,
Zhou A, Huang Z and Huang S: FoxM1 inhibition sensitizes resistant
glioblastoma cells to temozolomide by downregulating the expression
of DNA-repair gene Rad51. Clin Cancer Res. 18:5961–5971.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu N, Zhang X, Wang X, Ge HY, Wang XY,
Garfield D, Yang P, Song YL and Bai CX: FoxM1 mediated resistance
to gefitinib in non-small-cell lung cancer cells. Acta Pharmacol
Sin. 33:675–681. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park HJ, Gusarova G, Wang Z, Carr JR, Li
J, Kim KH, Qiu J, Park YD, Williamson PR, Hay N, Tyner AL, Lau LF,
Costa RH and Raychaudhuri P: Deregulation of FoxM1b leads to tumour
metastasis. EMBO Mol Med. 3:21–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lok GT, Chan DW, Liu VW, Hui WW, Leung TH,
Yao KM and Ngan HY: Aberrant activation of ERK/FOXM1 signaling
cascade triggers the cell migration/invasion in ovarian cancer
cells. PLoS One. 6:e237902011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang C, Qiu Z, Wang L, Peng Z, Jia Z,
Logsdon CD, Le X, Wei D, Huang S and Xie K: A novel FoxM1-caveolin
signaling pathway promotes pancreatic cancer invasion and
metastasis. Cancer Res. 72:655–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim R, Emi M, Tanabe K and Toge T:
Therapeutic potential of antisense Bcl-2 as a chemosensitizer for
cancer therapy. Cancer. 101:2491–2502. 2004. View Article : Google Scholar : PubMed/NCBI
|