Introduction
Gastric cancer is the fourth most common cancer
worldwide and is the second most common causes of cancer-related
mortality; it also has a poor prognosis (1). Although the 5-year survival rate in
patients with early-stage disease is ~90%, since the vast majority
present with distant metastasis, the overall 5-year survival rate
is typically <20% (2). Patients
5-year survival rate has also been significantly correlated with
the degree of tumor invasion, the presence of lymph node and/or
distant metastases and the TNM stage (3,4).
However, only a limited number of molecules that have
clinicopathological significance in gastric cancer have been
discovered.
To date, several mechanisms that drive gastric
cancer development and progression have been discovered.
Wnt/β-catenin signaling is a well-known signaling pathway that is
activated and is critical in gastric cancer development and
progression. The Slit family of proteins were identified as
conserved targets of the Wnt/β-catenin signaling pathway (5). Slit2 is a secretory glycoprotein and a
ligand in the Slit/Robo system (6).
The physiological function of Slit2 is to maintain the development
of the neural system (7). Slit2 is
responsible for guiding neural cell migration by preventing
inappropriate midline axonal crossing events (8). Recently, Slit2 was found to contribute
to gut development (9). Slit2
protein was located in the outer gut mesenchyme in regions that
partially overlap with the secretion of netrin-1. Functionally,
vagal sensory axons are responsive to Slit proteins and are thus
repelled by Slits secreted in the gut wall and are prevented from
reaching inappropriate targets (9).
The discovery of Slit2 as a guidance cue across
multiple tissue types has prompted a number of studies to examine
its potential as a biomarker for cancer, with two contrasting roles
for Slit2 having been proposed; some suggest that it functions as a
tumor suppressor, whereas others propose that it plays a role in
oncogenesis. One of the mechanisms that support Slit2 as a tumor
suppressor is that Slit2 promoter is hypermethylated in several
types of human cancer. This methylation is found in many types of
malignancies including cervix cancer (10), ovarian cancer (11), breast cancer (12), hepatocellular cancer (13), colon cancer (14), lung cancer (14) and leukemia (15). On the other hand, Slit2 is found to
be pro-oncogenic. This notion is supported by the evidence that
link Slit2 expression to enhanced malignancy. Accordingly, the
clinical significance of Slit2 levels is highly case-dependent.
Loss of Slit2 was found to be associated with poor survival in
cervical cancer patients (16).
Conversely, the findings that Slit2 levels were higher in tumors of
patients with recurrent endometrial cancer compared to those
without recurrence supported its role as a recurrence biomarker for
endometrial cancer (17,18).
Mechanically, Slit2 regulates several signaling
pathways involved in cell growth and metastasis. During breast
development, Slit2 limits basal cell proliferation by inhibiting
canonical Wnt/β-catenin signaling, increasing the cytoplasmic and
membrane pools of β-catenin at the expense of its nuclear pool
(5). Hence, Slit2 may mediate its
function through modulating β-catenin signaling. In cancer, it was
reported that Slit2 suppressed β-catenin activity in breast cancer
(19). It remains unknown whether
this correlation is applied to gastric cancer. Other signaling
pathways or molecules are AKT/GSK3β (20), RhoA (21) and frizzled (22). Slit2 is a multi-functional molecule
and may play different roles in malignant and non-malignant
cells.
Despite the inconsistency between studies on Slit2,
its expression pattern and clinical significance in gastric cancer
remain unknown. Hence, we examined the expression pattern and
clinical correlations of Slit2 in human gastric cancer.
Materials and methods
Immunohistochemistry (IHC)
Gastric cancer tissues, confirmed by pathological
diagnosis, were obtained from 89 patients who underwent radical
resection for gastric cancer between 2006 and 2008 at the
Department of Surgery, Central Hospital of Shanghai Minhang
District, Shanghai, China. The corresponding non-tumor gastric
tissues were obtained at least 6 cm from the tumor. All tissue
samples were formalin-fixed and paraffin-embedded. TNM staging was
classified based on the criteria of the American Joint Committee on
Cancer (AJCC, 7th edition) for gastric cancer. The study was
approved by the Shanghai Jiaotong University Medical School
Institutional review board.
IHC staining was performed by using a highly
sensitive streptavidin-biotin-peroxidase detection system with
gastric cancer tissue microarrays. Rabbit monoclonal anti-Slit2
(working dilution 1:100) was purchased from Epitomics (Burlingame,
CA, USA) and rabbit anti-β-catenin (working dilution 1:100) was
purchased from Cell Signaling Technology (Danvers, MA, USA).
Immunolabeling was conducted using Dako EnVision + Rabbit Polymer
(cat. no. K4003) from Dako (Carpinteria, CA, USA). The slides were
counterstained with hematoxylin and coverslipped.
IHC scoring
The histology of the samples was examined by two
histopathologists independently without knowledge of
clinicopathological information. We scored the slides according to
a previous publication (23). The
percentage of positive tumor cells was assigned to 5 categories: ≤
5% (0), 5–25% (1), 25–50% (2), 50–75% (3), and ≥75% (4). Positive cells (≤5%) were used as the
cut-off to define negative tumors. The intensity of immunostaining
was scored as: weak (1), moderate (2), and strong (3). The
percentage of positivity of tumor cells and staining intensity were
multiplied to produce a weighted score for each tumor specimen. The
intensity scores were grouped as low (which included scores 0 to
+4) and high (which included scores +6 and +12).
Cell culture
Human gastric cancer cell lines SGC-7901, MKN28,
MKN45, and immortalized human gastric epithelial cell line GES-1,
were obtained from the Shanghai Institute of Cell Biology, Chinese
Academy of Sciences. KATO-III, SNU-16, NCI-N87 and AGS were
obtained from the American Type Culture Collection. The cells were
grown in RPMI-1640 medium containing 10% fetal bovine serum (FBS),
penicillin and streptomycin (Gibco-BRL, Gaithersburgh, MD,
USA).
Western blotting
Whole cell lysates were harvested using RIPA cell
lysis buffer supplemented with a protease inhibitor cocktail
(Sigma). A total of 50 μg protein was separated by SDS
polyacrylamide gel electrophoresis and blotted onto 0.22-μm
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). Antibodies against Slit2 (Epitomics) were used at 1:1,000
dilutions. Antibodies against GAPDH (Sigma) were used at a 1:5,000
dilution. The signals were visualized using Li-COR Odyssey Sa model
9260 (Li-COR Corp., Lincoln, NE, USA) and images were captured and
managed using Odyssey Sa Infrared Image System (Li-COR Corp.).
Relative density of Slit2 or β-catenin was measured by the
following equation: Relative density = density of Slit2 or
β-catenin band/density of GAPDH.
Immunofluorescence staining
Cells were fixed with 4% formaldehyde and then
permeabilized with PBS containing 0.2% Triton X-100. Slides were
blocked by 5% BSA and incubated with a primary antibody at room
temperature for 1 h followed by TRITC-labeled goat anti-rabbit IgG
(Sigma, St. Louis, MO, USA) for an additional hour. Nuclei were
counterstained using DAPI (Molecular Probes). Slides were washed by
PBS, mounted and observed under a microscope. Immunofluorescence
staining was visualized using an Olympus BX50 microscope (Olympus
Opticol Co., Tokyo, Japan), images were captured using Nikon
Digital Sight DS-U2 (Nikon, Tokyo, Japan) and NIS-Elements F3.0
software was used (Nikon).
Statistical analysis
For IHC staining, the differences in
clinicopathological features between the different groups were
determined using Pearson’s χ2 test. P<0.05 was
considered to indicate a statistically significant result.
Statistical Package for the Social Sciences version 13.0 (SPSS,
Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Expression of Slit2 in non-tumor gastric
tissues
We first examined the expression of Slit2 in human
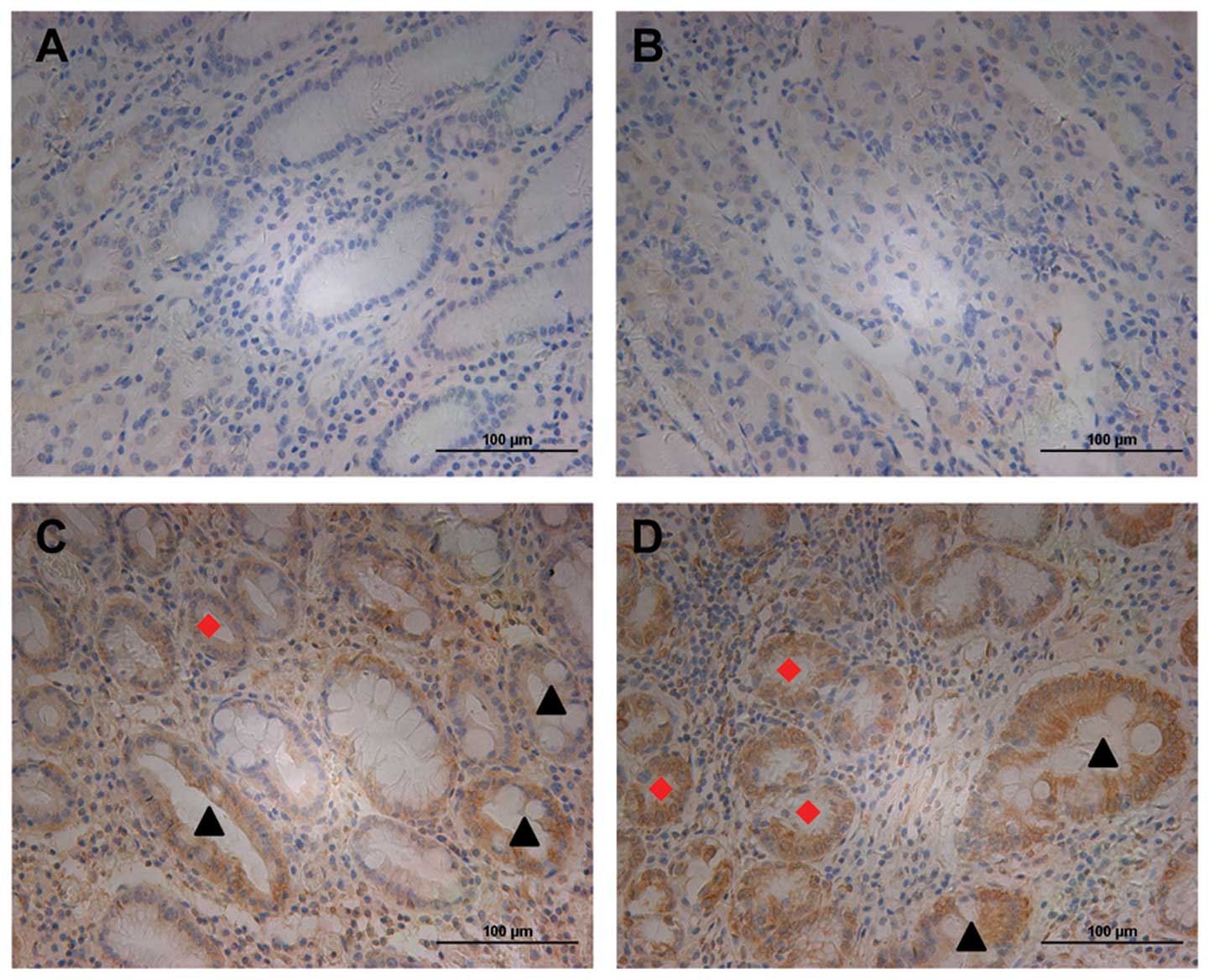
non-tumor gastric tissues. Slit2 did not express or was weakly
expressed in normal gastric epithelial cells (Fig. 1A and B). However, we observed
moderate staining of Slit2 in intestinal metaplasia (IM) (indicated
by black arrow heads) and dysplasia glands (indicated by red
diamonds) (Fig. 1A, C and D), two
types of precancerous lesions, suggesting its role in early gastric
tumorigenesis. These data suggested that Slit2 was not or was
weakly expressed in normal gastric tissues.
Expression of Slit2 in gastric cancer
tissues
We first discovered that Slit2 mRNA was upregulated
in gastric cancer tissues compared to normal gastric tissues
analyzed using publicly available datasets from the Oncomine
database (Fig. 2; indicated by
arrow). We then investigated the expression of Slit2 protein in
human gastric cancer tissues. The patients and pathological
features of gastric cancer tissues are described in Table I. In 89 cases, 14.5% (14/89) of
cases were Slit2 negative (including 8 cases with no staining
signal, 6 cases with staining cells ≤5% and staining intensity
scored 1), while the remaining 85.5% (75/89) showed variable levels
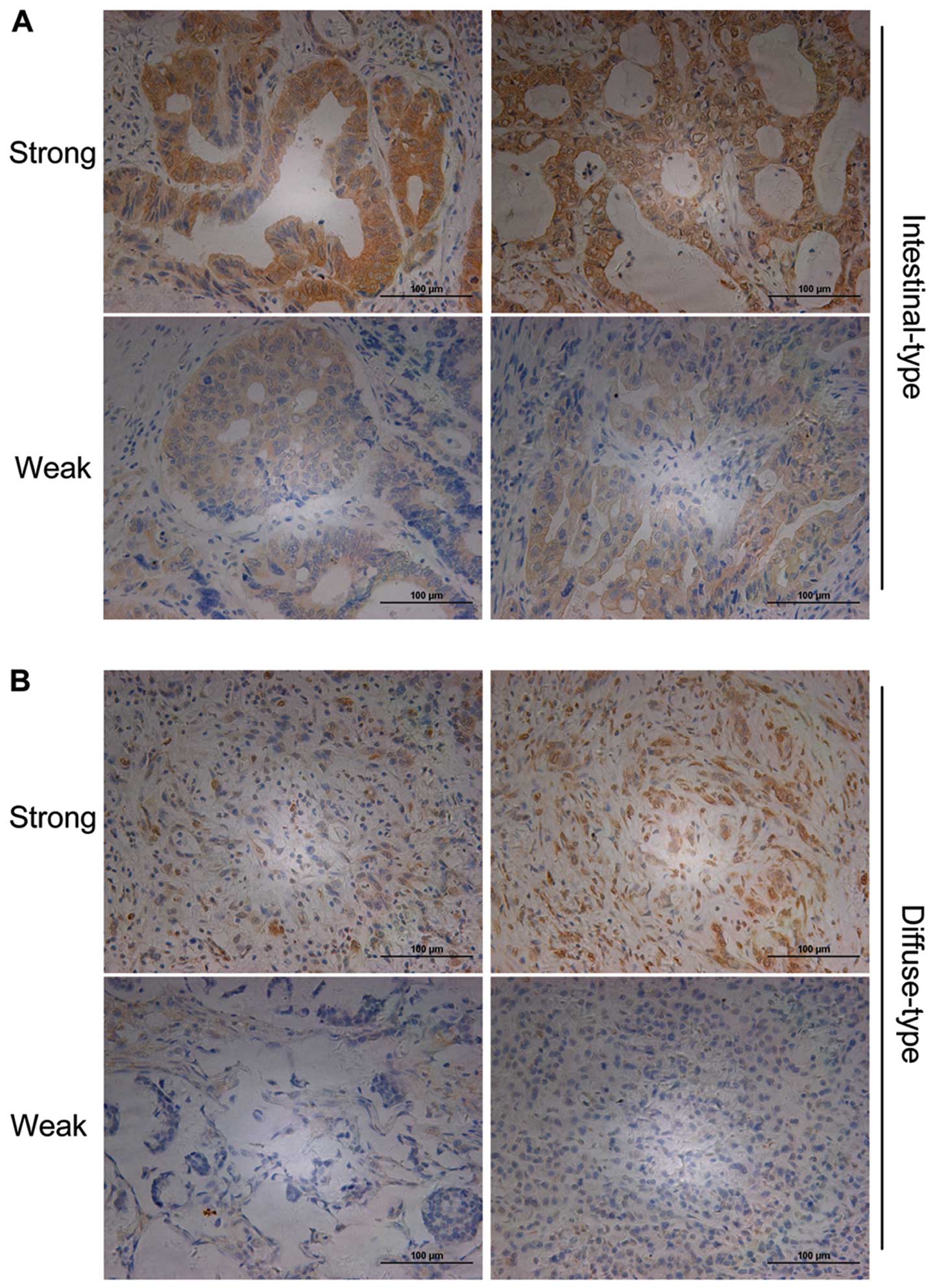
of Slit2 expression, with a medium-score of 4. The strong and weak
staining of Slit2 in intestinal-type (Fig. 3A) and diffuse-type (Fig. 3B) of gastric cancer tissues were
shown at the cell membrane and cytoplasm.
 | Table IPatients and tumor
characteristics. |
Table I
Patients and tumor
characteristics.
| Characteristics | N |
|---|
| Gender |
| Female | 23 |
| Male | 66 |
| Age (years) |
| Female (range) | 66.04±12.0
(34–83) |
| Male (range) | 65.27±10.3
(45–83) |
| Differentiation |
| High | 21 |
| Median | 24 |
| Low | 44 |
| Lauren’s
classification |
| Intestinal | 53 |
| Diffuse | 36 |
| T stage |
| T1 | 4 |
| T2 | 8 |
| T3 | 59 |
| T4 | 18 |
| N stage |
| N0 | 19 |
| N1 | 16 |
| N2 | 26 |
| N3a | 22 |
| N3b | 6 |
| M stage |
| M0 | 86 |
| M1 | 3 |
| TNM (AJCC) |
| I-A | 2 |
| I-B | 4 |
| II-A | 14 |
| II-B | 15 |
| III-A | 19 |
| III-B | 26 |
| III-C | 6 |
| IV | 3 |
The clinicopathological features of Slit2 in gastric
cancer tissues were also examined. All the cases were divided into
two groups according to the medium-score of Slit2; 44 cases were
considered as weakly (IHC score 0–4) and 45 cases were considered
as strongly (IHC score 6–12) expressed Slit2. As shown in Table II, Slit2 level decreased from
well-differentiated gastric carcinoma patients to moderately
differentiated and poorly differentiated gastric cancer tissues
(P=0.005). Slit2 levels were higher in intestinal-type gastric
cancer than that in diffuse-type gastric cancer (P=0.034). Higher
Slit2 expression levels were correlated with no lymph node
metastasis (P=0.005) and earlier TNM stage (stages I and II)
(P=0.021). Slit2 expression was not significantly affected by age
(P=0.700), gender (P=0.761), T stage (P=0.717) and M stage
(P=0.984). These data suggested that Slit2 was highly expressed in
gastric cancer tissues with less advanced clinicopathological
features.
 | Table IIClinicopathologic features of Slit2 in
gastric cancer. |
Table II
Clinicopathologic features of Slit2 in
gastric cancer.
| Slit2 | |
|---|
|
| |
|---|
| Features | Low (%) | High (%) | P-value |
|---|
| Age (years) |
| ≤60 | 13 (46.4) | 15 (53.6) | 0.700 |
| >60 | 31 (50.8) | 30 (49.2) | |
| Gender |
| Female | 12 (52.2) | 11 (47.8) | 0.761 |
| Male | 32 (48.5) | 34 (51.5) | |
| Differentiation |
| High | 5 (23.8) | 16 (76.2) | 0.005 |
| Median | 10 (43.5) | 13 (56.5) | |
| Low | 29 (65.9) | 15 (34.1) | |
| Lauren’s
classification |
| Intestinal | 20 (39.2) | 31 (60.8) | 0.034 |
| Diffusion | 23 (62.2) | 14 (37.8) | |
| T stage |
| T1–2 | 6 (54.5) | 5 (45.5) | 0.717 |
| T3–4 | 38 (48.7) | 40 (51.3) | |
| N stage |
| Negative | 4 (21.1) | 15 (78.9) | 0.005 |
| Positive | 40 (57.1) | 30 (42.9) | |
| M stage |
| Negative | 42 (48.8) | 44 (51.2) | 0.984 |
| Positive | 2 (66.7) | 1 (33.3) | |
| TNM stage |
| I–II | 12 (34.3) | 23 (65.7) | 0.021 |
| III–IV | 32 (59.3) | 22 (40.7) | |
Slit2 levels are correlated with
β-catenin in gastric cancer tissues
As demonstrated by previous studies, Slit2 regulates
the activity of β-catenin. To investigate the association of Slit2
and β-catenin, we performed IHC staining using the same cohort of
specimens as we used for Slit2 staining. As expected, we observed
the positive correlation between the expression levels of Slit2 and
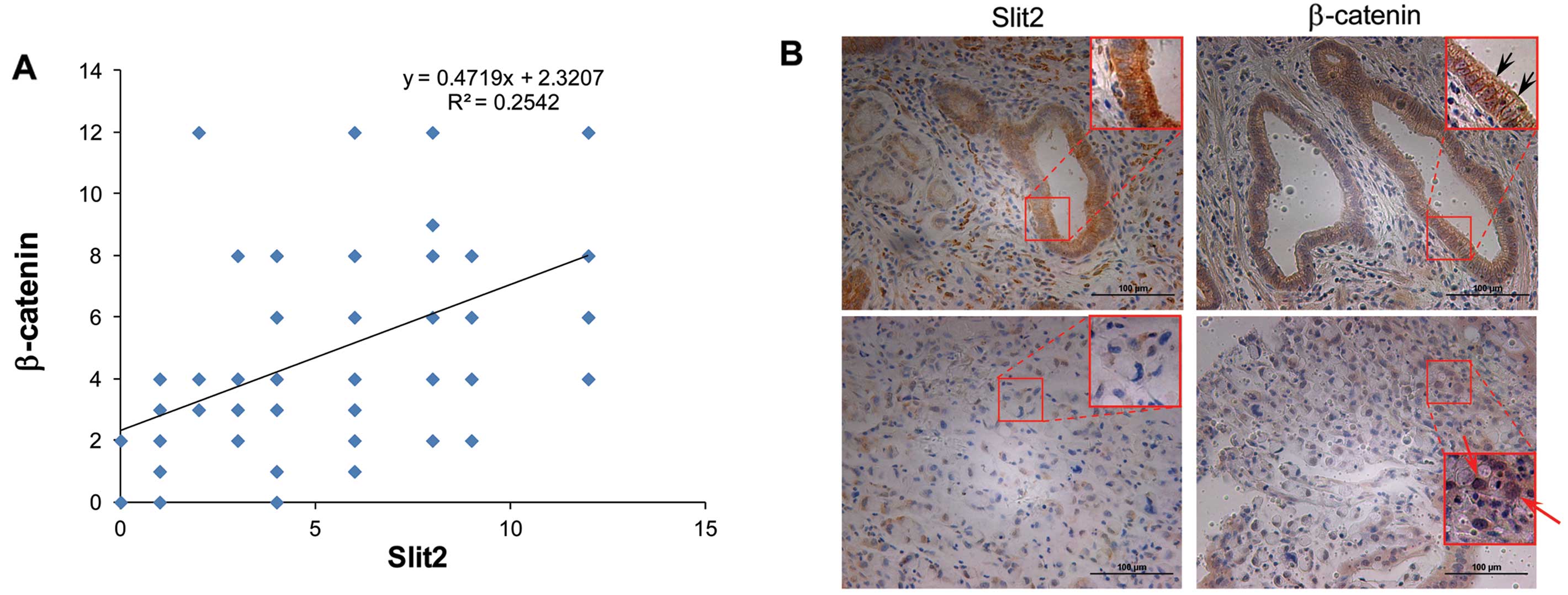
β-catenin in gastric cancer analyzed by IHC staining. As shown in
Fig. 4A, y-axis represented the IHC
scores of β-catenin and x-axis represented the Slit2 IHC scores
using the same cohort of gastric cancer specimens. Linear trend
line showed Slit2 levels were positively correlated with β-catenin
levels. Pearson correlation analysis showed the correlation
coefficient was 0.504 (P<0.001).
Moreover, we discovered that Slit2 was correlated
with the subcellular location of β-catenin, which might suggest the
activation of β-catenin related signaling pathway. Among these 89
cases, 24 cases displayed membrane staining of β-catenin, while 14
cases showed nuclear β-catenin. As shown in Table III, membrane β-catenin tended to
exist in the high Slit2 expression group (17/24 cases), while
nuclear β-catenin tended to exist in the low Slit2 expression group
(11/14 cases). As shown in Fig. 4B,
strong Slit2 expression was shown in an intestinal-type gastric
cancer specimen (upper left), and membrane β-catenin was also
observed in the same case (upper right, black arrows). While the
weak Slit2 expression was shown in a diffuse-type gastric cancer
specimen (lower left), and nuclear β-catenin translocation was
observed in the same case (lower right, red arrows). These data
suggested Slit2 expression levels were positively correlated with
β-catenin levels and its subcellular location.
 | Table IIIAssociations of Slit2 and subcellular
localization of β-catenin in gastric cancer. |
Table III
Associations of Slit2 and subcellular
localization of β-catenin in gastric cancer.
| Slit2 | |
|---|
|
| |
|---|
| Low (%) | High (%) | P-value |
|---|
|
β-catenin-menbrane |
| Negative | 37 (84.1) | 28 (62.2) | 0.02 |
| Positive | 7 (15.9) | 17 (37.8) | |
|
β-catenin-nuclear |
| Negative | 33 (75.0) | 42 (93.3) | 0.011 |
| Positive | 11 (27.2) | 3 (6.7) | |
Slit2 levels are correlated with
β-catenin in gastric cancer cell lines
We then examined whether this Slit2-β-catenin
expression pattern could also be observed in human gastric cancer
cell lines. To do so, we performed immunoblotting to examine the
expression of Slit2 and β-catenin using whole cell extracts
obtained from seven gastric cancer cell lines and one immortalized
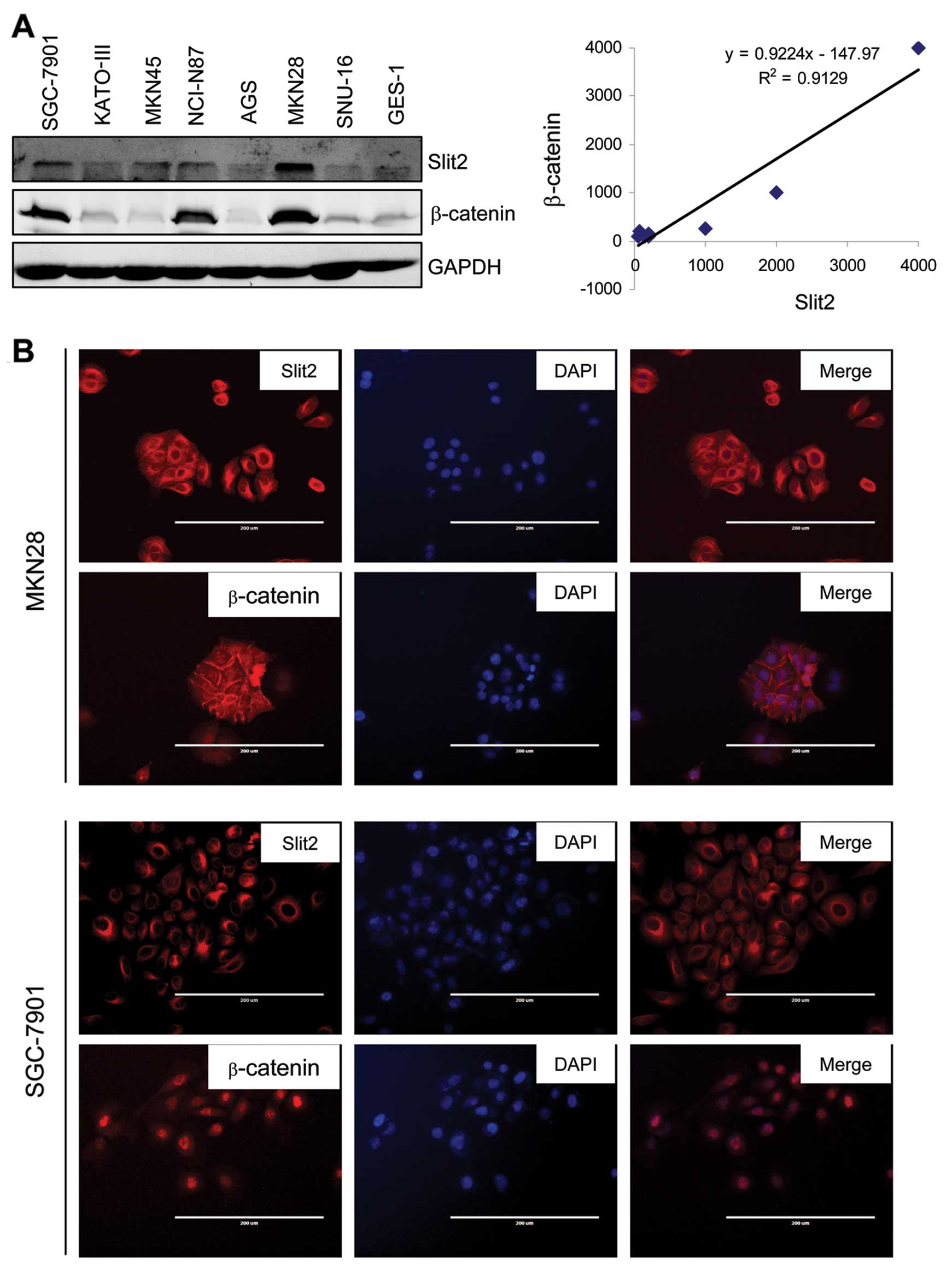
normal gastric epithelial cell line, GES-1. Expression of Slit2
protein was also elevated in all 7 gastric cancer cell lines
compared with immortalized normal gastric epithelial cell line
GES-1 (Fig. 5A, left panel). The
levels of β-catenin were correlated with Slit2 expression levels.
MKN28, a cell line generated from well-differentiated gastric
cancer tissue, showed the highest expression levels of Slit2 and
β-catenin. MKN45 and SGC-7901 showed moderate levels of Slit2 and
β-catenin. The other three cell lines KATO-III, AGS and SNU-16,
showed low levels of Slit2 and β-catenin. We then performed the
semi-quantitative densitometry analysis to calculate relative
expression levels of Slit2 and β-catenin in each cell line. Levels
of Slit2 and β-catenin were positively correlated, the correlation
coefficient was 0.95 (P<0.001). Linear trend line also showed
that Slit2 levels were positively correlated with β-catenin levels
(Fig. 5A, right panel).
We next performed immunofluorescence staining to
further observe the subcellular location of β-catenin. We employed
MKN28 and SGC-7901 cell lines to perform immunofluorescence
staining using anti-Slit2 and anti-β-catenin antibodies. As shown
in Fig. 4B, MKN28, which highly
expressed Slit2, showed the membrane β-catenin staining. On the
contrary, SGC-7901, which expressed lower Slit2 levels compared
with MKN28, showed nuclear β-catenin staining.
Discussion
Slit2 can positively and negatively regulate
tumorigenesis in different types of cancer. To our knowledge, the
immunoprofiles of Slit2 have not previously been studied in gastric
cancer. However, a few studies on Slit2 have been performed in
gastric cancer. Herein, we examined its expression levels in human
gastric cancer tissues and its correlations with
clinicopathological characteristics.
Using immunostaining, we showed that Slit2 was
negatively or weakly expressed in normal gastric epithelial cells.
We also noted that the positive staining of Slit2 was only observed
in cells that located at the neck and bottom part of the glands,
which were generally considered as normal gastric stem cells. As
indicated in previous findings that Slit2 is involved in gut
development (9), Slit2 may also
play roles in gastric stem cells, thus explaining why Slit2
staining was only shown in a specific part of normal gastric
glands. Dysplasia and intestinal metaplasia (IM) are two
pre-cancerous lesions. Slit2 was expressed in dysplasia and IM
glands, suggesting the initiation stage of gastric tumorigenesis
might be accompanied by the elevation of Slit2 expression.
We showed that Slit2 levels were higher in gastric
cancer tissues compared with normal gastric cancer tissues. The
correlations between Slit2 levels and clinicopathological
characteristics suggested gastric patients with high levels of
Slit2 tended to present less advanced cancer features. We also
discovered that Slit2 level was high in patients without lymph node
metastasis and in early TNM stage. Slit2 was reported to suppress
cancer cell growth in colon (14)
and breast cancer (19). However,
there is no study on the stage-wise correlation pattern of Slit2 in
gastric cancer. Our data suggested a correlation of high Slit2 with
less malignant features in gastric cancer. Considering the
inconsistency between results come from different types of cancer,
we concluded that the function of Slit2 in different types of
cancer may be different. This phenomenon is common. Other
well-studied molecules such as JNK, TGFβ1, and DDK, play different
or even controversial roles in different types of cancer or even in
different stages of the same cancer type.
Slit2 can also regulate other signaling pathways.
Slit2 can inhibit the AKT-GSK3β signaling pathway (20,24).
In the cytoplasm, serine and threonine phosphorylation regulated
the stability of β-catenin by targeting it to
GSK-3β/Axin/adenomatous polyposis coli complex-mediated proteasomal
degradation. Thus, the destabilization or stabilization of GSK3β
activity is a major factor which influences β-catenin amount and
subcellular location. Membrane β-catenin plays an important role in
cadherin-based cell-cell adhesion by indirectly linking cadherins
to the actin cytoskeleton. In the nucleus, β-catenin interacts with
members of the LEF/TCF family of transcriptional activators and
plays important roles in gastric cancer development and
progression. In agreement with previous findings, we show that high
Slit2 level is correlated with membrane location of β-catenin; on
the contrary, low Slit2 level can cause nuclear translocation of
β-catenin in gastric cancer specimens and gastric cancer cell
lines. Loss of Slit2 expression may stabilize catenin and
potentiate its nuclear translocation and thus activate β-catenin
mediated signaling pathway. Our data suggest that Slit2 is
associated with the function of β-catenin, a cell-cell adhesive
molecule or an oncogenic transcriptional factor.
In summary, our study reveal that Slit2 is highly
expressed in human gastric cancer with less advanced clinical
features, and Slit2 level is correlated with the expression and the
location of β-catenin. High Slit2 is associated with the integrity
of β-catenin mediated catenin-cadherin cell-cell adhesion. In
contrast, low Slit2 is associated with the activation of β-catenin
mediated signaling pathways. Our data suggest a possible mechanism
of the regulation of the subcellular location and activity of
β-catenin by Slit2 as well as a new role of Slit2 in gastric cancer
development and progression.
Acknowledgements
The present study is supported by a major program of
Minhang District Research Fund.
References
|
1
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar
|
|
2
|
Du C, Zhou Y, Cai H, Zhao G, Fu H and Shi
YQ: Poor prognostic factors in patients with stage I gastric cancer
according to the seventh edition TNM classification: a comparative
analysis of three subgroups. J Surg Oncol. 105:323–328. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lazar D, Taban S, Sporea I, et al: Gastric
cancer: correlation between clinicopathological factors and
survival of patients (III). Rom J Morphol Embryol. 50:369–379.
2009.PubMed/NCBI
|
|
4
|
Lazar D, Taban S, Sporea I, et al: Gastric
cancer: correlation between clinicopathological factors and
survival of patients. II. Rom J Morphol Embryol. 50:185–194.
2009.PubMed/NCBI
|
|
5
|
Macias H, Moran A, Samara Y, et al:
SLIT/ROBO1 signaling suppresses mammary branching morphogenesis by
limiting basal cell number. Dev Cell. 20:827–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Georgas K, Burridge L, Smith K, et al:
Assignment of the human slit homologue SLIT2 to human chromosome
band 4p15.2. Cytogenet Cell Genet. 86:246–247. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia L, Cheng L and Raper J: Slit/Robo
signaling is necessary to confine early neural crest cells to the
ventral migratory pathway in the trunk. Dev Biol. 282:411–421.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong K, Park HT, Wu JY and Rao Y: Slit
proteins: molecular guidance cues for cells ranging from neurons to
leukocytes. Curr Opin Genet Dev. 12:583–591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldberg D, Borojevic R, Anderson M, Chen
JJ, Gershon MD and Ratcliffe EM: Slit/Robo-mediated chemorepulsion
of vagal sensory axons in the fetal gut. Dev Dyn. 242:9–15. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitra S, Mazumder-Indra D, Mondal RK, et
al: Inactivation of SLIT2-ROBO1/2 pathway in premalignant lesions
of uterine cervix: clinical and prognostic significances. PLoS One.
7:e383422012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong R, Yu J, Pu H, Zhang Z and Xu X:
Frequent SLIT2 promoter methylation in the serum of patients with
ovarian cancer. J Int Med Res. 40:681–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alvarez C, Tapia T, Cornejo V, et al:
Silencing of tumor suppressor genes RASSF1A, SLIT2,
and WIF1 by promoter hypermethylation in hereditary breast
cancer. Mol Carcinog. 52:475–487. 2012.
|
|
13
|
Jin J, You H, Yu B, et al: Epigenetic
inactivation of SLIT2 in human hepatocellular carcinomas. Biochem
Biophys Res Commun. 379:86–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dallol A, Morton D, Maher ER and Latif F:
SLIT2 axon guidance molecule is frequently inactivated in
colorectal cancer and suppresses growth of colorectal carcinoma
cells. Cancer Res. 63:1054–1058. 2003.PubMed/NCBI
|
|
15
|
Dunwell TL, Dickinson RE, Stankovic T, et
al: Frequent epigenetic inactivation of the SLIT2 gene in chronic
and acute lymphocytic leukemia. Epigenetics. 4:265–269. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh RK, Indra D, Mitra S, et al:
Deletions in chromosome 4 differentially associated with the
development of cervical cancer: evidence of slit2 as a candidate
tumor suppressor gene. Hum Genet. 122:71–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma S, Liu X, Geng JG and Guo SW: Increased
SLIT immunoreactivity as a biomarker for recurrence in endometrial
carcinoma. Am J Obstet Gynecol. 202:68.e61–68.e11. 2010.PubMed/NCBI
|
|
18
|
Shen F, Liu X, Geng JG and Guo SW:
Increased immunoreactivity to SLIT/ROBO1 in ovarian endometriomas:
a likely constituent biomarker for recurrence. Am J Pathol.
175:479–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prasad A, Paruchuri V, Preet A, Latif F
and Ganju RK: Slit-2 induces a tumor-suppressive effect by
regulating β-catenin in breast cancer cells. J Biol Chem.
283:26624–26633. 2008.PubMed/NCBI
|
|
20
|
Chen WF, Gao WD, Li QL, Zhou PH, Xu MD and
Yao LQ: SLIT2 inhibits cell migration in colorectal cancer through
the AKT-GSK3β signaling pathway. Int J Colorectal Dis. Jan
13–2013.(Epub ahead of print).
|
|
21
|
Liu X, Lu Y, Zhang Y, et al: Slit2
regulates the dispersal of oligodendrocyte precursor cells via
Fyn/RhoA signaling. J Biol Chem. 287:17503–17516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hofmeister W, Devine CA, Rothnagel JA and
Key B: Frizzled-3a and slit2 genetically interact to
modulate midline axon crossing in the telencephalon. Mech Dev.
129:109–124. 2012. View Article : Google Scholar
|
|
23
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
24
|
Chang PH, Hwang-Verslues WW, Chang YC, et
al: Activation of Robo1 signaling of breast cancer cells by Slit2
from stromal fibroblast restrains tumorigenesis via blocking
PI3K/Akt/β-catenin pathway. Cancer Res. 72:4652–4661.
2012.PubMed/NCBI
|