Introduction
During the past 20 years, various therapeutic
approaches based on the p53 pathway have been developed (1,2). Among
them, therapeutic application of adenoviral-mediated p53 gene
transfer has been most widely investigated (1). Although efficacy without non-specific
toxicity is noted in both tissue culture and animal models,
responses of adenoviral-mediated p53 gene transfer in early human
trials have not been spectacular. This is due, in part, to the
observation that a substantial fraction of tumor cells,
particularly tumor cells exhibiting wild-type (wt) p53, are
resistant to p53 gene transfer (3,4).
A large body of evidence has established murine
double minute 2 (MDM2) (also termed HDM2 for its human equivalent)
as a crucial negative regulator of p53 and the major suppressor of
p53 function in tumors with wt p53. MDM2 binds p53 and functions as
a ubiquitin E3 ligase to promote p53 ubiquitination and degradation
by proteasomes (5–7). As the MDM2 gene itself is a downstream
target of p53, induction of MDM2 establishes a negative feedback
loop to limit the action of p53, in which p53 itself initiates its
own destruction (8,9). Therefore, it has been suggested that
MDM2 may be potentially responsible for the resistance of tumors to
p53 gene transfer, in which, overexpression of MDM2 or exogenous
p53-induced overexpression of MDM2, leads to rapid degradation of
the p53 protein (10–13).
Ribosomal protein L23 (RPL23) was reported to have
the capability to inhibit MDM2-mediated p53 ubiquitination and
degradation through direct binding to MDM2 (14,15).
In addition, ectopically expressed RPL23 was found to interact with
MDM2 in both the cytoplasm and the nucleus, which indicates that
MDM2 was retained in the cytoplasm and the nucleus as a complex,
and this complex formation represents one more mechanism by which
RPL23 indirectly inhibits MDM2-p53 binding (14). A previous study by our group showed
that, through inhibition of MDM2-p53 interaction,
adenoviral-mediated expression of RPL23 displayed modest
tumor-suppressor activity in vitro and in vivo in
human gastric cancer MKN45 cells with endogenous wild-type p53,
which were initially resistant to the p53 gene transfer (12,16,17).
In the present study, using a RPL23/p53 bicistronic adenovirus, we
aimed to determine whether co-transduction of the RPL23 gene
enhances the therapeutic efficacy of adenoviral-mediated p53 gene
transfer through protecting exogenous p53 protein from
MDM2-mediated inactivation in human gastric cancer cells.
Materials and methods
Cell lines and reagents
Two human gastric cancer cell lines, MKN45
(endogenous wt p53 gene) and MGC803 (endogenous mutant p53 gene),
were used in this study (18,19).
Both cells lines were maintained in RPMI-1640 supplemented with 10%
fetal calf serum (FCS) (Invitrogen Life Technologies) at 37°C in a
humidified atmosphere containing 5% CO2. Mouse
monoclonal antibodies specific for p53 (ab-4, specific for
wild-type p53), MDM2, p21 and PUMA were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA), and mouse monoclonal
β-actin antibody was obtained from Sigma-Aldrich Chemical Co. (St.
Louis, MO, USA). All reagents were used in different concentrations
as indicated.
Construction of recombinant
adenovirus
A replication defective adenoviral vector, deleted
in E1A, E1B and E3, was constructed in which the viral E1A and E1B
genes were replaced with a bicistronic cassette encoding RPL23 and
p53 under the control of the CMV promoter (Ad-RPL23/p53), using the
AdEasy vector system (Wuhan Genesil Biotechnology Co., Ltd.,
China), following the manufacturer’s protocol. The bicistronic
cassette was obtained from the pIRES vector of Clontech
Laboratories, Inc. (Carlsbad, CA, USA). The p53 coding sequence was
placed downstream of the internal ribosome entry site. Viral
packaging and expansion were accomplished by transfection of human
kidney 293 cells. Virus was purified from cell lysates according to
standard protocol. Adenoviral vector encoding RPL23 (Ad-RPL23) was
prepared similarly except that the coding sequence for RPL23 was
directly inserted into the multicloning site of pSHUTTLE-CMV.
Adenoviral vector encoding human wild-type p53 (Ad-p53) was kindly
provided by Professor Shanwen Zhang and Professor Shaowen Xiao
(Department of Radiotherapy, Beijing University School of Oncology,
Beijing, China). The titer of the stock virus was assessed by a
plaque formation assay using 293 cells. The virus solutions were
stored at −80°C. The transduction efficiency of the adenoviral
vectors in gastric cancer cells was evaluated by flow cytometry
(Coulter Epics-XL; Beckman Coulter, Miami, FL, USA) using
GFP-expressing adenovirus (Ad-GFP) (20). Ad-null was used as the control virus
in this study.
Western blot analysis
Cells infected with adenoviral vectors at a
multiplicity of infection (MOI) of 50 for 48 h were collected,
washed twice in cold PBS, and lysed at 4°C in lysis buffer using
protease and phosphatase inhibitors as described previously
(16). Cell lysates containing 50
μg total protein were resolved in 10% SDS-PAGE, transferred
to PVDF membranes (Amersham Pharmacia Biotech), and probed with
primary antibodies. The antibodies used included p53, MDM2, p21,
PUMA and β-actin. The signals for the immunoreactive proteins were
visualized on the ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA)
using an enhanced chemiluminescence system (Santa Cruz
Biotechnology, Inc.).
Quantitative RT-PCR analysis
For quantitative real-time PCR (qRT-PCR) analysis,
total RNA was extracted with TRIzol, and cDNA was prepared using
the PrimerScript reverse transcriptase kit (both from Takara Bio,
Inc.). Expression levels of targeted genes were analyzed by qRT-PCR
using Applied Biosystems 7500 Real-Time PCR and the
SYBR®-GreenER™ reagent system from Invitrogen Life
Technologies as described previously. The primers used in this
study are listed as follows: p53 (exon 9) forward, 5′-TATCC
AGCCCTCACTCCTTC-3′ and reverse, 5′-CACGGATCTGA AGGGTGAAA-3′
(21); MDM2 forward, 5′-CCGGATGATC
GCAGGTG-3′ and reverse, 5′-AAAAGCTGAGTCAACCTG CCC-3′; p21 forward,
5′-GCGATGGAACTTCGACTTTG-3′ and reverse, 5′-CAGGTCCACATGGTCTTCCT-3′;
PUMA forward, 5′-GACCTCAACGCACAGTACGA-3′ and reverse,
5′-CTAATTGGGCTCCATCTCG-3′; β-actin forward, 5′-GG
ACTTCGAGCAAGAGATGG-3′ and reverse, 5′-AGGAAGG AAGGCTGGAAGAG-3′.
Assay of cell growth and viability
Cells were plated at a density of 5×103
cells/100 μl in 96-well plates (Corning Incorporated), and
were infected with adenoviral vectors at the indicated MOI 24 h
later. Three days after adenoviral infection, cell viability was
evaluated using trypan blue exclusion cell counts, and 5 days after
adenoviral infection, cell growth was assessed by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay.
Analysis of cell apoptosis
Analysis of apoptosis was performed as described
previously (16). Briefly, cells
cultured in 6-well plates (Corning Incorporated) were infected with
various vectors at an MOI of 50 for 48 h, and then the cells were
stained with PI/Annexin V and analyzed by flow cytometry (Coulter
Epics-XL). Each analysis was performed in triplicate.
Adenovirus treatment in vivo
The protocol for the animal experiments was approved
by the Institutional Animal Care and Use Committee at the Wuhan
General Hospital of Guangzhou Command, People’s Liberation Army and
followed guidelines set forth in the National Institutes of Health
Guide for the Care and Use of Laboratory Animals. MKN45 and MGC803
cells were used to establish subcutaneous xenograft tumors in
BALB/c athymic nude mice (Shanghai Laboratory Animal Center of the
Chinese Academy of Sciences). Briefly, MKN45 and MGC803 cells
(5×106 each) were respectively inoculated into the left
and right flanks of a single nude mouse. Seven days after tumor
inoculation, 24 mice bearing tumors with a diameter of 3–7 mm were
randomized into 3 treatment groups: Ad-RPL23/p53, Ad-p53 and
Ad-null. Mice were then treated according to the following
schedule: at days 8, 15, 22 and 29 after inoculation, each mouse
was injected intratumorally with adenoviral vectors (Ad-RPL23/p53,
Ad-p53 and Ad-null) at a dose of 5×107 viral
particles/tumor in a volume of 50 μl. Serial changes in
tumor volume were estimated weekly after the start of the
adenovirus treatments. On day 36, after 5 measurements, animals
were sacrificed by cervical dislocation under methoxyflurane
anesthesia. The volume of the tumors was calculated according to
the formula: Tumor volume = (length × width2)/2.
Furthermore, to closely resemble the human patient, another animal
model of human gastric cancer constructed orthotopically from
histologically intact patient specimens was established according
to a method described previously (22,23).
Briefly, a fresh surgical specimen derived from one patient with
advanced gastric cancer was cut into pieces of ~2–3 mm3
in size, and then the pieces of the tumor tissues were implanted
orthotopically into the gastric wall of nude mice. Seven days after
surgical orthotopic implantation, the mice were randomized into 3
groups. The grouping and treatments were performed using the same
protocol as the subcutaneous xenograft model, except that the
method of adenovirus administration was by intravenous injection
instead of intratumoral injection. Subsequently, a long-term
survival study was performed.
Statistical analysis
Quantitative results are expressed as the means ±
SD. Statistical analysis was performed using ANOVA and LSD t-test.
The survival rates were estimated using the Kaplan-Meier method,
and the differences were analyzed using the log-rank test to
compare the resulting curves of the treatment groups. P<0.05 was
considered to indicate a statistically significant result. All
statistical analyses were performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA).
Results
Effective infection of the adenoviral
vector in gastric cancer cells
To determine the infection efficiency of the
adenoviral vector in gastric cancer cells, we infected MKN45 and
MGC803 cells with Ad-GFP at a MOI ranging from 10 to 200.
Preliminary titration revealed a high transfer efficiency of the
recombinant adenovirus in gastric cancer cells and an optimal
expression at 48 h postinfection. At a MOI of 50, >85% gastric
cells were GFP-positive without obvious adenoviral toxicity.
Therefore, we performed most of our studies at 48 h of incubation
with the optimal MOI of 50.
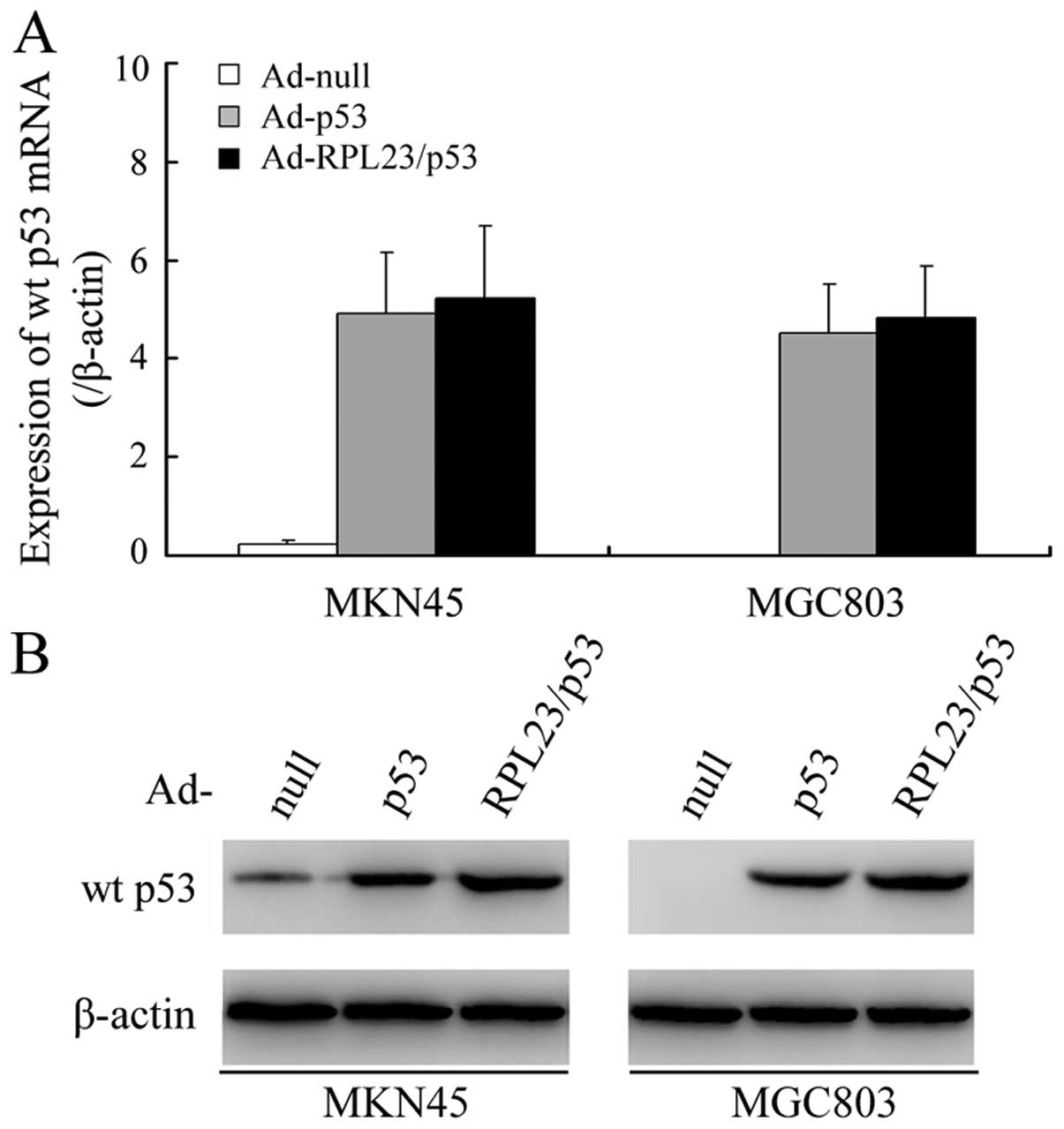
Ad-RPL23/p53 induces greater accumulation
of p53 protein compared to Ad-p53
We initially studied the effects of ectopically
expressed RPL23 on the accumulation and stability of exogenous wt
p53 protein in MKN45 cells (endogenous wt p53) and MGC803 cells
(endogenous mutant p53) using western blot analysis. A modest
increase in wt p53 protein was noted in both the Ad-p53-transduced
MKN45 and MGC803 cells, independent of the endogenous p53 status,
whereas, cells treated with Ad-RPL23/p53 at the same MOI had an ~4-
and 3-fold higher wt p53 signal intensity than that in the
Ad-p53-treated MKN45 and MGC803 cells, respectively (Fig. 1B). Since the Ad-RPL23/p53 and Ad-p53
vectors produced similar levels of p53 message when each was
administered at a MOI of 50 (Fig.
1A), these results suggest involvement of post-transcriptional
or post-translational control over the wt p53 protein
accumulation.
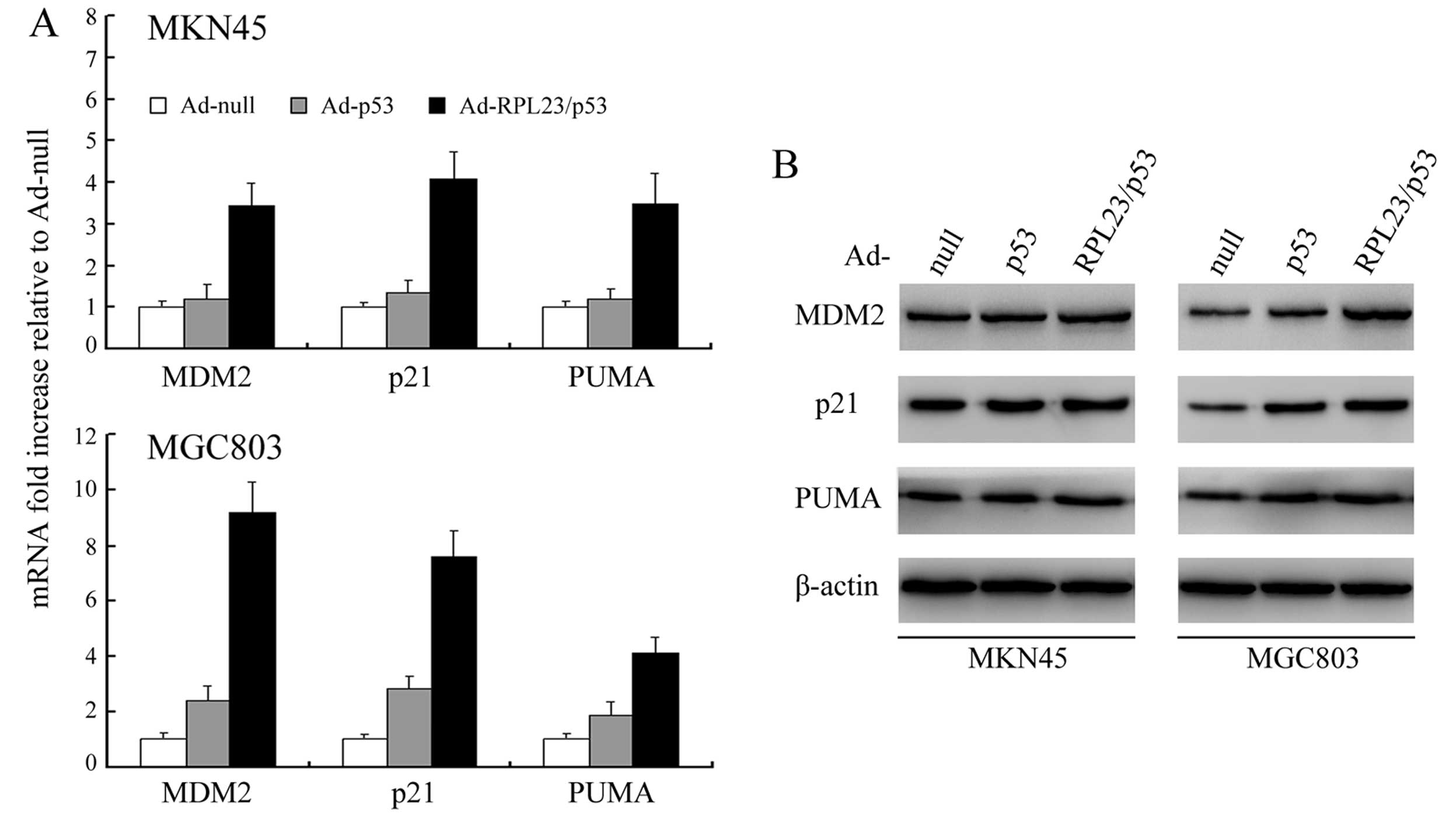
Ad-RPL23/p53 is more efficient than
Ad-p53 at inducing p53 target gene expression
We used qRT-PCR and western blot analysis,
respectively, to analyze the mRNA and protein expression of MDM2,
the cell cycle inhibitor p21, and the pro-apoptotic PUMA, all of
which have p53-responsive promoters (24). Consistent with the higher levels of
induced p53 protein achieved with Ad-RPL23/p53, we observed
enhanced transcription of MDM2, p21 and PUMA in both MKN45 and
MGC803 cells by qRT-PCR analysis after treatment with Ad-RPL23/p53
but no detectable or slight enhancement of transcription of these
genes after similar treatment with Ad-p53 (Fig. 2A). As indicated by western blot
analysis (Fig. 2B), levels of the
MDM2, p21 and PUMA gene products also increased in both MKN45 and
MGC803 cells after treatment with Ad-RPL23/p53, whereas similar
Ad-p53 treatment resulted in no change or slight change in
expression of these gene products.
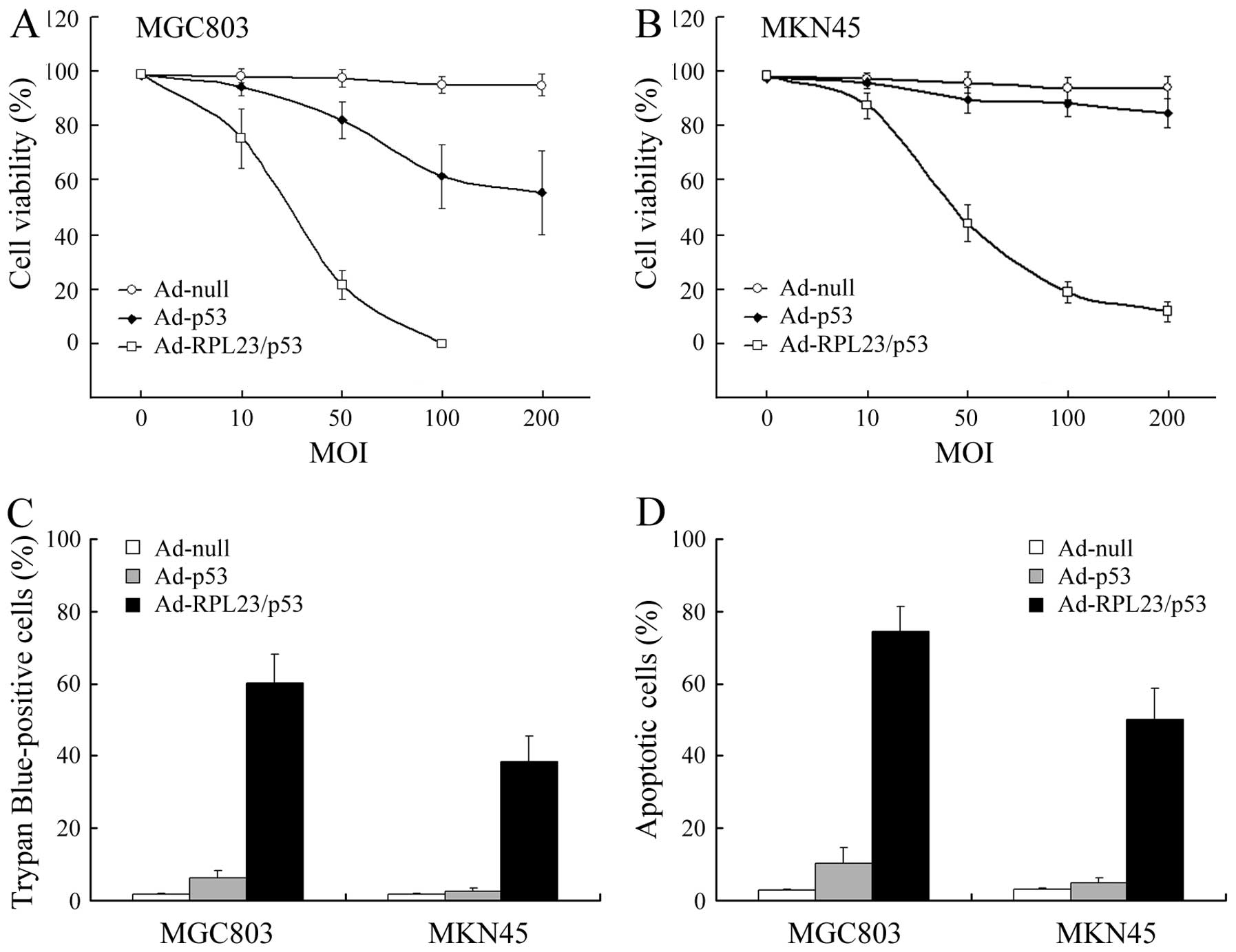
Ad-RPL23/p53 displays more marked
tumor-suppressor activity in vitro relative to Ad-p53
Next, we evaluated the in vitro
tumor-suppressor activity of Ad-RPL23/p53 relative to Ad-p53 in
MKN45 and MGC803 cells using MTT assay. In mutant p53 MGC803 cells,
Ad-RPL23/p53 suppressed growth to a greater extent than Ad-p53,
achieving complete suppression at an MOI of 100, obviously less
than the required doses of Ad-p53 to achieve a similar effect
(Fig. 3A). In wt p53 MKN45 cells,
despite the efficient gene transfer, the p53-specific suppression
of cell growth by Ad-p53 was minimal, which has been previously
reported due to the high expression of MDM2 (12). As for Ad-RPL23/p53, infection at a
MOI of 50 caused a significant inhibition of cell growth
(P<0.05) when compared with Ad-p53, which was consistent with
the marked accumulation of wt p53 protein in MKN45 cells after
Ad-RPL23/p53 treatment (Fig. 3B).
Single ectopically expressed RPL23 protein had the capacity to
induce the accumulation and stability of endogenous wt p53 protein;
therefore, we also compared the in vitro tumor-suppressor
activity of Ad-RPL23/p53 relative to Ad-RPL23 in wt p53 MKN45
cells. As predicated, Ad-RPL23/p53 was also more effective than
Ad-RPL23 in the MKN45 cells (P<0.05, compared with the data
obtained in our previous study) (16), suggesting that the combined gene
therapy strategy was more efficient at achieving tumor growth
suppression.
Suppression of growth after treatment
with Ad-RPL23/p53 correlates with increased cell death, as
determined by trypan blue exclusion assay
Fig. 3C shows the
results of the trypan blue staining carried out 72 h after
treatment of MGC803 or MKN45 cells with Ad-null, Ad-p53 or
Ad-RPL23/p53 at an MOI of 50. In the case of Ad-RPL23/p53, trypan
blue-positive cells (dead cells) accounted for ~60.1% (MGC803) and
~38.7% (MKN45) of the cells, consistent with the marked reduction
in cell viability relative to the control (Fig. 3A and B). Regarding Ad-p53, the
percentage of trypan blue-positive cells remained low and accounted
for ~6.2% (MGC803) and ~2.5% (MKN45) of the cells, consistent with
the minimal reduction in cell viability relative to the control
observed after this treatment.
Apoptosis induction is firmly established as a
central mechanism of p53 cancer gene therapy (25). In this study, apoptosis of gastric
cancer cells treated with the various vectors at a MOI of 50 was
evaluated by FCM analysis. As shown in Fig. 3D, in MGC803 and MKN45 cells, Ad-p53
treatment at a MOI of 50 had slight or little effect on apoptosis,
respectively. However, at the same dose, treatment with
Ad-RPL23/p53 caused more obvious apoptosis in both cell lines
(P<0.01, compared with Ad-p53).
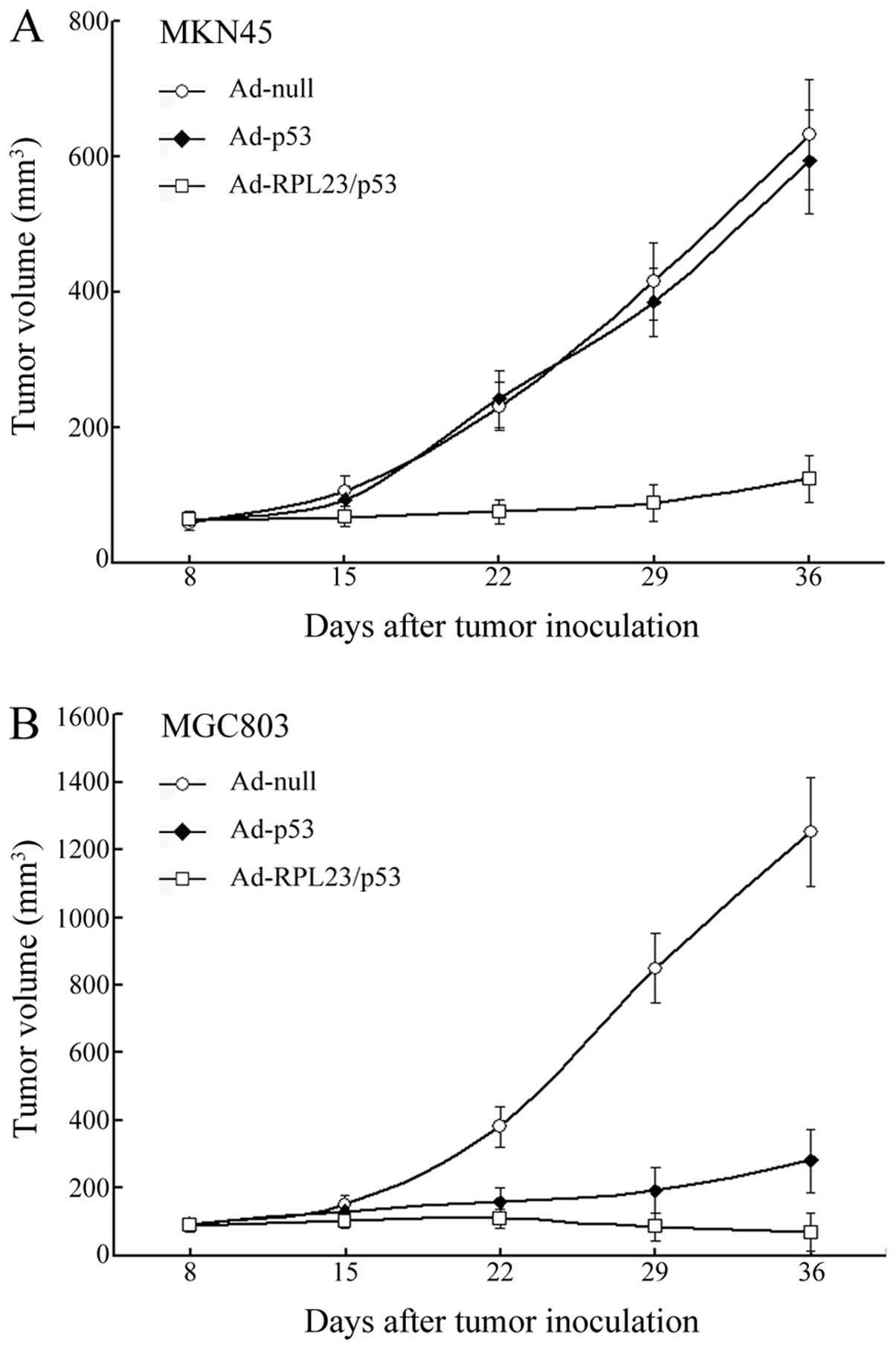
Ad-RPL23/p53 treatment achieves a more
potent therapeutic effect than Ad-p53 in a nude mouse subcutaneous
xenograft model using human gastric cancer cells
Results of the in vitro experiments led us to
further investigate the in vivo effect of administration of
Ad-RPL23/p53 by injection. As shown in Fig. 4A, the volumes of the MKN45
cell-derived tumors in the Ad-null and Ad-p53 groups increased
~10.6- and 9.4-fold, respectively, over a period of 5 weeks,
whereas in the Ad-RPL23/p53 treated mice the tumors barely
progressed during that time, suggesting that the combined gene
therapy displayed a significant tumor growth inhibition in
vivo in the wt p53-expressing tumors which were initially
resistant to gene therapy using the single p53 gene. In the case of
MGC803 cell-derived tumors, treatment of Ad-p53 displayed an
obvious tumor growth inhibition relative to Ad-null. However,
treatment of Ad-RPL23/p53 in the same manner resulted in a more
marked tumor growth inhibition relative to Ad-p53 (Fig. 4B). In the Ad-RPL23/p53 group, the
MGC803 tumors began to shrink after 3 injections and disappeared in
3 of the 8 mice by the end of the experiment. The average volume of
the MGC803 tumors in the Ad-RPL23/p53-treated mice (including the 3
vanishing tumors) on day 36 was 68±57.7 mm3, which was
significantly less than the average tumor volume of 281±91.5
mm3 for the Ad-p53 treated mice (P<0.01).
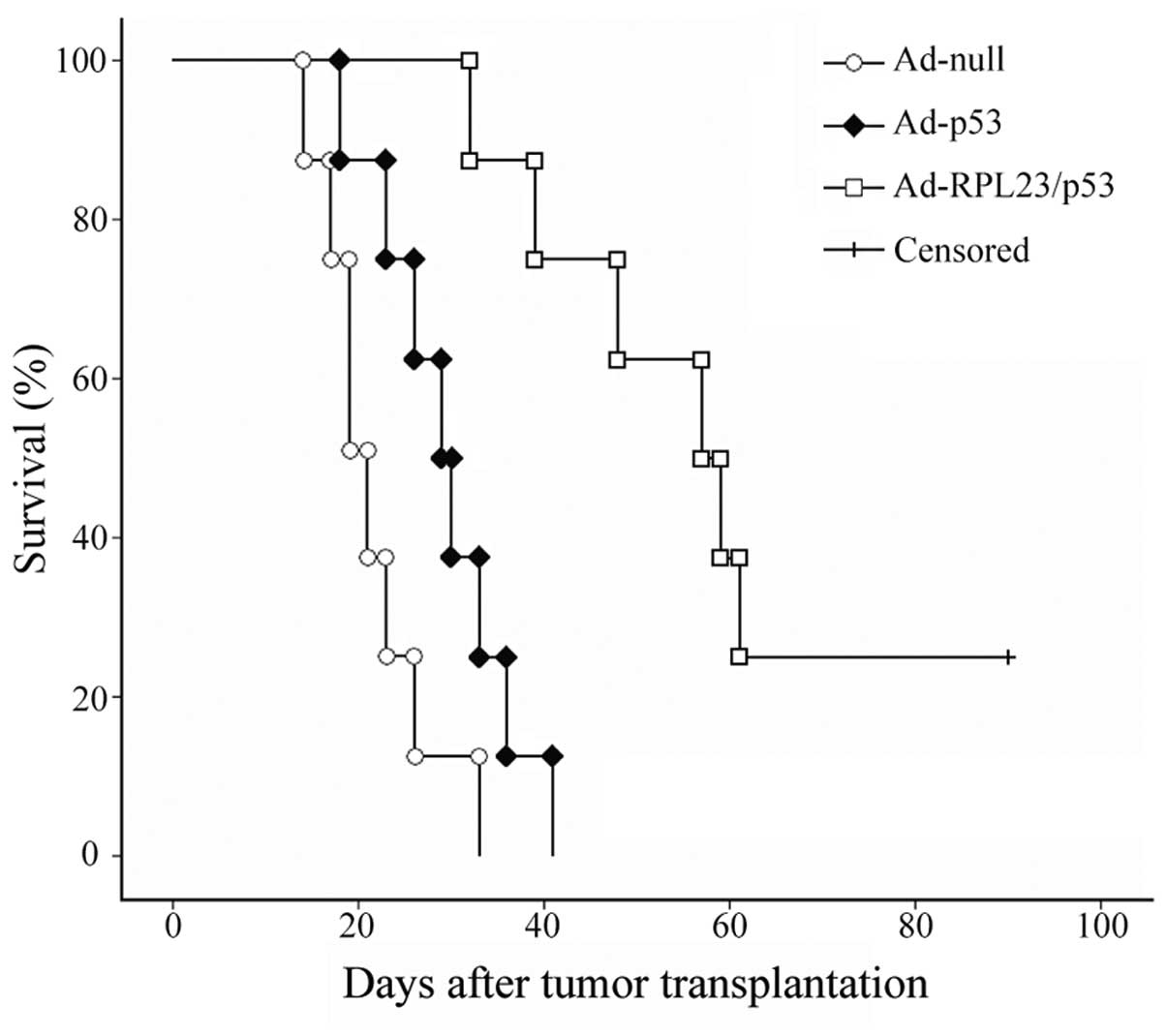
Ad-RPL23/p53 treatment results in
significantly longer survival than Ad-p53 treatment in an
orthotopic nude mouse model constructed by using histologically
intact human gastric cancer specimens
Most human gastric cancers show heterogeneous p53
expression; therefore, the animal model constructed above using
established gastric cancer cell lines did not closely resemble the
human patient. Therefore, we established another animal model of
human gastric cancer using orthotopic transplantation of
histologically intact patient specimens, and investigated the
effect of Ad-RPL23/p53 treatment on the survival times of the
tumor-bearing mice. In this study, control mice (Ad-null-treated)
rapidly succumbed to the tumor burden, with a median survival of
21.5 days. Ad-p53 treatment mildly prolonged the survival of the
mice, and the median survival was 29.5 days (P<0.05, compared
with Ad-null). However, as revealed in Fig. 5, relative to Ad-p53, the survival
benefit was greater for the mice receiving Ad-RPL23/p53 treatment.
The median survival for the 8 Ad-RPL23/p53-treated mice was 59.5
days (P<0.01, compared with Ad-p53), and among them, 2 mice
survived >90 days. These data suggest that the combined therapy
with the RPL23 and p53 genes significantly increases patient
survival even in the event of tumors with heterogeneous p53
expression.
Discussion
Repair of specific genetic defects responsible for
the aberrant biological behavior of cancer cells is a fascinating
approach to cancer treatment. p53, due to its essential role as a
tumor-suppressor, has been established as ‘the gatekeeper for cell
growth and division’ (26).
Functional integrity of the p53 gene is an essential cellular
defense against neoplastic transformation. In fact, nearly all of
the different types of human malignancies analyzed to date were
shown to contain mutations of the p53 gene or alterations in the
p53 regulating pathway (1–4). Thus, it is believed that p53 is an
attractive target for gene replacement therapy for cancer.
Transfer of the wt p53 gene has proven effective in
suppressing the proliferation of tumor cells bearing mutant p53 as
noted in vitro, in animal models, and in patients with
cancer. However, as many cancer cells, including gastric cancer
cells, express wt p53 and most tumors are also heterogeneous with
respect to their p53 status, p53 gene transfer alone may be
insufficient to suppress tumor cell growth due to the general
resistance of the wt p53-expressing tumor cells to p53 gene
transfer. It is currently known that MDM2, the key negative
regulator of p53, is found to be overexpressed in a variety of
tumors, resulting in the inactivation of wt p53 protein, with an
effect similar to that of mutations in the p53 gene (4). Thus, it is proposed that tumors with
wt p53 may avoid p53-mediated enhancement of oncolysis due to a
high MDM2 expression by which exogenous p53 protein is rapidly
degraded. In fact, human cancer cell lines with endogenous wt p53
which are poorly responsive to the oncolysis-enhancing effect of
p53 almost exclusively express high levels of MDM2 (12,13,17).
Furthermore, the MDM2 gene itself has been a downstream target of
p53; it is proposed that induction of MDM2 expression by exogenous
p53 may also limit the responsiveness of cancer cells to p53 gene
transfer, in which the introduction of exogenous p53 induces the
overexpression of endogenous MDM2, which, in turn, results in rapid
degradation of the p53 protein.
It thus becomes clear that one way to enhance the
tumor-suppression activity of p53 gene transfer is by abrogating
MDM2-mediated p53 inactivation. Nutlin, the first identified
small-molecule MDM2 antagonists that inhibits MDM2-p53 interaction,
has been demonstrated to enhance tumor cell killing following
adenoviral-mediated p53 gene transfer (27,28).
Combined delivery of one addition gene with a product that could
inhibit the MDM2-p53 feedback loop is another flexible strategy.
Because of its well-characterized ability to inhibit MDM2-mediated
p53 degradation, Tango et al(29) and Huang et al(30) hypothesized that co-transduction of
p14ARF may enhance the tumor-suppressive activity of p53
gene transfer by increasing p53 protein stability. By using in
vitro and in vivo approaches, they demonstrated that the
therapeutic efficacy of the combined transduction of p53 with
p14ARF was superior to transduction with p53 alone.
Since the RPL23 protein has a similar function with
p14ARF(14,15), we hypothesized that co-transduction
of RPL23 could also enhance the therapeutic efficacy of the
adenoviral-mediated p53 gene transfer through protecting exogenous
wt p53 from MDM2-mediated inactivation. Using an in vitro
system with cultured cells, we observed that gastric cancer MKN45
and MGC803 cells, regardless of the p53 status, showed marked
growth arrest and apoptosis after treatment with a bicistronic
adenovirus expressing both the RPL23 and p53 genes (Ad-RPL23/p53),
while, at the same infectious dose, the efficacies of the single
gene vector for p53 (Ad-p53) were not detectable or minimal.
Ad-RPL23/p53 and Ad-p53 induced similar levels of p53 message under
similar treatment conditions, while, consistent with the function
of ectopic RPL23 protein in stabilizing p53, the accumulation of
p53 protein in cells treated with Ad-RPL23/p53 was more significant
than that achieved by a similar treatment with Ad-p53. Accompanied
with this, increased RNA and protein expression of the p53
downstream target genes MDM2, p21 and PUMA was observed. The in
vivo data further indicated that co-expression of RPL23 and p53
may have greater antitumor activity than p53 alone. In a
subcutaneous xenograft model established from MKN45 (endogenous wt
p53) and MGC803 cells (endogenous mutant p53), we found that
Ad-RPL23/p53 treatment achieved a more potent therapeutic effect
relative to Ad-p53. To better estimate the usefulness of
Ad-RPL23/p53 for patients with gastric cancer which usually exhibit
heterogeneous p53 expression, we established another animal model
of human gastric cancer using orthotopic transplantation of
histologically intact patient specimens. Ad-RPL23/p53 treatment led
to prolonged survival when compared to that obtained by Ad-p53.
Since the orthotopic transplantation model showed various
manifestations similar to the tumor behavior in patients, including
metastasis, the prolonged survival after Ad-RPL23/p53 treatment
indicated that the combined gene therapy suppressed not only tumor
growth but also metastasis.
In conclusion, the present study suggests that
combination gene therapy with adenoviral vector-mediated RPL23 and
p53 is feasible and more effective than p53 alone. More
importantly, therapeutic efficacy of the bicistronic adenovirus can
be achieved in both p53 mutant cancer cells but also in p53
wild-type cancer cells. Therefore, this strategy has broad
application for cancer treatment by ensuring the induction of p53
protein accumulation in malignant cells regardless of the p53
genotypes.
Acknowledgements
The present study was supported by the National
Foundation of Natural Sciences, China (no. 81101533) and the China
Postdoctoral Science Foundation (nos. 201104755 and
20100481468).
References
|
1
|
Cheok CF, Verma CS, Baselga J and Lane DP:
Translating p53 into the clinic. Nat Rev Clin Oncol. 8:25–37. 2011.
View Article : Google Scholar
|
|
2
|
Brown CJ, Lain S, Verma CS, Fersht AR and
Lane DP: Awakening guardian angels: drugging the p53 pathway. Nat
Rev Cancer. 9:862–873. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lane DP and Lain S: Therapeutic
exploitation of the p53 pathway. Trends Mol Med. 8(Suppl 4):
S38–S42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lane DP, Cheok CF and Lain S: p53-based
cancer therapy. Cold Spring Harb Perspect Biol.
2:a0012222010.PubMed/NCBI
|
|
5
|
Momand J, Zambetti GP, Olson DC, George D
and Levine AJ: The mdm-2 oncogene product forms a complex with the
p53 protein and inhibits p53-mediated transactivation. Cell.
69:1237–1245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kubbutat MH, Jones SN and Vousden KH:
Regulation of p53 stability by Mdm2. Nature. 387:299–303. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marine JC and Lozano G: Mdm2-mediated
ubiquitylation: p53 and beyond. Cell Death Differ. 17:93–102. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moll UM and Petrenko O: The MDM2-p53
interaction. Mol Cancer Res. 1:1001–1008. 2003.PubMed/NCBI
|
|
10
|
Shangary S and Wang S: Targeting the
MDM2-p53 interaction for cancer therapy. Clin Cancer Res.
14:5318–5324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiman KG: Strategies for therapeutic
targeting of the p53 pathway in cancer. Cell Death Differ.
13:921–926. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Beusechem VW, van den Doel PB and
Gerritsen WR: Conditionally replicative adenovirus expressing
degradation-resistant p53 for enhanced oncolysis of human cancer
cells overexpressing murine double minute 2. Mol Cancer Ther.
4:1013–1018. 2005.
|
|
13
|
Nishizaki M, Sasaki J, Fang B, Atkinson
EN, Minna JD, Roth JA and Ji L: Synergistic tumor suppression by
coexpression of FHIT and p53 coincides with FHIT-mediated MDM2
inactivation and p53 stabilization in human non-small cell lung
cancer cells. Cancer Res. 64:5745–5752. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai MS, Zeng SX, Jin Y, Sun XX, David L
and Lu H: Ribosomal protein L23 activates p53 by inhibiting MDM2
function in response to ribosomal perturbation but not to
translation inhibition. Mol Cell Biol. 24:7654–7668. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin A, Itahana K, O’Keefe K and Zhang Y:
Inhibition of HDM2 and activation of p53 by ribosomal protein L23.
Mol Cell Biol. 24:7669–7680. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Shi Y, Li X, et al: Inhibition of
the p53-MDM2 interaction by adenovirus delivery of ribosomal
protein L23 stabilizes p53 and induces cell cycle arrest and
apoptosis in gastric cancer. J Gene Med. 12:147–156.
2010.PubMed/NCBI
|
|
17
|
Ohashi M, Kanai F, Ueno H, et al:
Adenovirus mediated p53 tumour suppressor gene therapy for human
gastric cancer cells in vitro and in vivo. Gut. 44:366–371. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang XH, Wong BC, Lin MC, et al:
Functional p53 is required for triptolide-induced apoptosis and
AP-1 and nuclear factor-κB activation in gastric cancer cells.
Oncogene. 20:8009–8018. 2001.PubMed/NCBI
|
|
19
|
Sun S, Li XM, Li XD and Yang WS: Studies
on inducing apoptosis effects and mechanism of CIK cells for
MGC-803 gastric cancer cell lines. Cancer Biother Radiopharm.
20:173–180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HJ, Cho HI, Han YH, et al: Efficient
transduction with recombinant adenovirus in EBV-transformed B
lymphoblastoid cell lines. J Biochem Mol Biol. 37:376–382. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang HY, Zhang WG, Ma LP, Wang SW and
Zhang ZY: An approach to enhancing the phototoxicity of a novel
hypocrellin congener to MGC803 cells. Dyes Pigments. 51:103–110.
2001. View Article : Google Scholar
|
|
22
|
Furukawa T, Kubota T, Watanabe M, Kitajima
M and Hoffman RM: Orthotopic transplantation of histologically
intact clinical specimens of stomach cancer to nude mice:
correlation of metastatic sites in mouse and individual patient
donors. Int J Cancer. 53:608–612. 1993. View Article : Google Scholar
|
|
23
|
Shi J, Wei PK, Zhang S, et al: OB glue
paste technique for establishing nude mouse human gastric cancer
orthotopic transplantation models. World J Gastroenterol.
14:4800–4804. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozaki T and Nakagawara A: p53: the
attractive tumor suppressor in the cancer research field. J Biomed
Biotechnol. 2011:6039252011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amaral JD, Xavier JM, Steer CJ and
Rodrigues CM: Targeting the p53 pathway of apoptosis. Curr Pharm
Des. 16:2493–2503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tisato V, Norcio A, Voltan R, Celeghini C,
Zella D and Secchiero P: MDM2 non-genotoxic inhibitors as
innovative therapeutic approaches for the treatment of pediatric
malignancies. Curr Med Chem. 20:2226–2236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Graat HC, Carette JE, Schagen FH, et al:
Enhanced tumor cell kill by combined treatment with a
small-molecule antagonist of mouse double minute 2 and adenoviruses
encoding p53. Mol Cancer Ther. 6:1552–1561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tango Y, Fujiwara T, Itoshima T, et al:
Adenovirus-mediated p14ARF gene transfer cooperates with
Ad5CMV-p53 to induce apoptosis in human cancer cells. Hum Gene
Ther. 13:1373–1382. 2002.PubMed/NCBI
|
|
30
|
Huang Y, Tyler T, Saadatmandi N, Borgstrom
P and Gjerset RA: Enhanced tumor suppression by a p14ARF/p53
bicistronic adenovirus through increased p53 protein translation
and stability. Cancer Res. 63:3646–3653. 2003.PubMed/NCBI
|