Introduction
Papillary thyroid carcinomas (PTCs) are the most
common types of thyroid cancer representing >70% of all thyroid
malignancies. PTC occurs more frequently in women, where the most
common etiologic factor is radiation, but genetic susceptibility
and other factors also contribute to its development (1,2). PTC
belongs to a class of well-differentiated thyroid cancers known for
a favourable prognosis when the diagnosis, staging, treatment and
risk of recurrence are carefully managed. It represents a large
group of thyroid carcinomas with several histologic variants
including microcarcinomas, follicular variants, encapsulated
variants, diffuse sclerosing variants, oxyphilic cell variants,
Warthin-like variant (WV), oncocytic variant (OV) and 2 more
aggressive variants, the tall-cell variant (TC) and the columnar
cell variant (2,3). While PTC is known for its favourable
prognosis, ~10% of patients develop recurrence in lymph node and/or
distant organs (4,5). One of the important diagnostic
characteristics of PTC is nuclear changes, which include subtle
irregularities in the nuclear contour and size, deep nuclear
grooves, and pseudo inclusions resulting from cytoplasmic
invaginations (6). PTC is known for
its tendency for multifocality, ranging from 18–46%, depending on
the series. Lymph node involvement is common, occurring in ~30% of
patients. Extrathyroidal extension ranges from 8–32% of cases. The
most common site of extrathyroidal extension is the surrounding
muscle (8%), followed by the recurrent laryngeal nerve (6%) and
trachea (5%) (7,8). Distant metastases are reported in only
1–25% of PTC patients, representing the lowest rate of all
well-differentiated thyroid carcinomas (2).
Both retinoids and rexinoids are known to affect a
broad spectrum of biochemical and molecular biology reactions in
organisms, but their associated effects are improbable without
fully functioning nuclear receptors. Hence, research on the role
and function of nuclear retinoid and retinoid X, which play a role
as biologically active ligand inducible transcription factors,
belongs to the dynamically developing branch of molecular
endocrinology. Thus, retinoids are involved in the complex
arrangements of physiological and developmental responses in
several tissues of higher vertebrates that include embryonic
development, vision, reproduction, bone formation, haematopoiesis,
metabolism, growth and differentiation of a variety of cell types,
apoptosis and processes of carcinogenesis (9–11). The
diversity of the retinoic acid-induced signalling pathway is
associated with at least 3 isotypes of nuclear receptors
[all-trans retinoic acid, RAR (α, β and γ)] and 3 isotypes
of nuclear receptors [9-cis retinoic acid, RXR or retinoid X
receptors (α, β and γ)]. Changes of expression of RARs and RXRs
during the dedifferentiation/redifferentiation and tumour
progression in thyroid carcinomas were first demonstrated by
Schmutzler et al(12).
Subsequently, changes in expression of RARs and RXRs during the
dedifferentiation and tumour progression in PTC were demonstrated
in patients treated in Japan by Tang et al(14) and Liu et al(13).
Thyroid hormone influences a wide variety of
biological events, including the balance between proliferation and
differentiation. The conversion of pro-hormone T4 into the
biologically active hormone T3 is catalyzed by iodothyronine
deiodinases [type 1 (hDIO1) and type 2 (hDIO2)] (15).
The expression status of RARs and RXRs and their
association with the clinicopathological parameters is clearly
requisite, hence the aim of this study was to investigate the
expression status of both RAR and RXR subtypes in 26 patients who
had undergone surgery for PTC.
Materials and methods
Human samples
Tumour and surrounding normal thyroid tissue were
randomly collected from 26 patients diagnosed with PTC at the
Endocrine or Head and Neck Surgery Divisions St. Elisabeth Oncology
Hospital in Bratislava, Slovakia. Sample tissues were immediately
frozen in liquid nitrogen and stored at −70°C. Surgery was
independently indicated by attending physicians. Tumours were
histologically classified and the clinical stage was determined by
the tumour, node, metastasis system (16). The study was approved by the Ethics
Committee of the St. Elisabeth Oncology Hospital in Bratislava,
Slovakia.
mRNA analyses
Total RNA was isolated using TRIzol reagent
according to the manufacturer's instructions. The concentration of
RNA was determined by spectrophotometry at 260 nm and the purity
assessed from the ratio of absorbance
A260nm/A280nm. Reverse transcription (RT) was
performed with 2 μg of total DNAse I-treated (Thermo Scientific,
Germany) RNA and the Ready-to-Go You-Prime First-Strand Beads
(Amersham Pharmacia Biotech, Inc., USA) according to the
manufacturer's protocol. PCR was performed in 25 μl total volume
comprising 2 μl RT mixture, 1X PCR buffer, 1.5/3
mmol.l−1 MgCl2 (RARs, RXRs and GAPDH/TRs,
SMRT, SRC-1 and hDIO1), 0.2 mmol.l−1 dNTP, 25 pmol of
each specific gene primer set and 0.6 U of DyNAzyme II DNA
polymerase (Finnzymes OY, Finland) in buffer provided by the
manufacturer. PCR was undertaken following treatment of samples at
94°C for 3 min to inactivate reverse transcriptase, which consisted
of 35 cycles of denaturing (95°C, 60 sec), annealing (60 sec),
extension (72°C, 60 sec), and a final extension at 72°C for 10 min.
The oligonucleotide of the primers employed in this study as well
as the corresponding annealing times are summarized in Table I(17). These conditions were proven to be in
the log phase for each amplified sequence. Triton tumour tissue was
used as a positive control (18). A
negative control without cDNA template was run with every assay
batch in order to assess overall specificity. The PCR products were
separated on 2% agarose gel and stained with GelRed™ dye (Biotium,
USA). The band intensities were measured using an STS 6220I
Documentation System (Ultralum, USA) and normalized to the band
intensity of the PCR product corresponding to the housekeeping gene
GAPDH.
 | Table IPrimers for semi-quantitative
RT-PCR. |
Table I
Primers for semi-quantitative
RT-PCR.
| Gene | Sequence | Annealing temp
(°C) | Product size
(bp) | Ref. |
|---|
| RARα |
5′-ACCCCCTCTACCCCGCATCTACAAG-3′
5′-CATGCCCACTTCAAAGCACTTCTGC-3′ | 60 | 226 | (17) |
| RARβ |
5′-ATTCCAGTGCTGACCATCGAGTCC-3′
5′-CCTGTTTCTGTGTCATCCATTTCC-3′ | 62 | 349 | (17) |
| RARγ |
5′-TACCACTATGGGGTCAGC-3′
5′-CCGGTCATTTCGCACAGCT-3′ | 60 | 195 | (17) |
| RXRα |
5′-TTCGCTAAGCTCTTGCTC-3′
5′-ATAAGGAAGGTGTCAATGGG-3′ | 58 | 113 | (17) |
| RXRβ |
5′-GAAGCTCAGGCAAACACTAC-3′
5′-TGCAGTCTTTGTTGTCCC-3′ | 58 | 111 | (17) |
| RXRγ |
5′-GCAGTTCAGAGGACATCAAGCC-3′
5′-GCCTCACTCTCAGCTCGCTCTC-3′ | 62 | 352 | (17) |
| GAPDH |
5′-TGAACGGGAAGCTCACTGG-3′
5′-TCCACCACCCTGTTGCTGTA-3′ | 60 | 307 | (17) |
| TRα |
5′-AGGAGAACAGTGCCAGGTCA-3′
5′-TCTTGAAGCGGCACAGCTGG-3′ | 60.4 | 297 | |
| TRβ |
5′-AACTACAGGTATAAGGCTGATTCAC-3′
5′-ATGCTTCTCTGCGTATATGCC-3′ | 59 | 295 | |
| SMRT |
5′-GACCCCACCTCCATACCCCG-3′
5′-GGGAGGTAGGCAAGGCGGTC-3′ | 64.5 | 392 | |
| SRC-1 |
5′-GCCCTGGGAGCTCCATGGTG-3′
5′-CTCTTCTGCTGGGCCTGGGG-3′ | 63 | 605 | |
| hDIO1 |
5′-GGACATCAGAAATCACCAGA-3′
5′-TTCCTCTGGGTTGTAGTTCC-3′ | 57 | 300 | |
Statistical analysis
Data are expressed as means (SD) or as medians
(range 5–95%). Statistical significance was assessed using an ANOVA
(Bonferroni) test.
Results
Ten males and 16 females were enrolled in this
study. Mean age at surgery was 48.9±15.6 years (means ± SD).
Clinicopathological parameters of the 26 cases of PTC are presented
in Table II. Various types of PTC
were recognized: 18 cases of classic variant (CV) (69.3%), 5 cases
of mixed type (MT) (19.3%), 1 case of TC (3.8%), 1 case of OV
(3.8%) and 1 case of WV (3.8%). The mean tumour size was 24.8±9.9
mm (means ± SD, median=25.5). Only 3 tumours were measured at ≥40
mm. Histologically confirmed lymph node metastasis (LNM) was
identified in 14 cases at surgery, but was absent in the other 12
cases.
 | Table IIClinicopathological parameters of the
26 cases of PTC. |
Table II
Clinicopathological parameters of the
26 cases of PTC.
| Patient | Gender | Age (years) | Type of PTC | Diameter (mm) | LNM (total/no. of
positive) |
|---|
| P1 | F | 59 | TC | 25 | 6/0 |
| P2 | F | 39 | OV | 30 |
18/1+ |
| P3 | F | 32 | WV | 22 | 8/0 |
| P4 | F | 81 | MT | 30 |
10/8+ |
| P5 | M | 27 | MT | 12 |
9/5+ |
| P6 | F | 70 | MT | 40 |
8/8+ |
| P7 | F | 62 | MT | 15 | 7/0 |
| P8 | F | 36 | MT | 45 |
14/7+ |
| P9 | F | 72 | CV | 20 | 3/0 |
| P10 | F | 56 | CV | 12 | 18/0 |
| P11 | F | 46 | CV | 15 | 4/0 |
| P12 | M | 59 | CV | 26 | 4/0 |
| P13 | F | 30 | CV | 28 | 11/0 |
| P14 | F | 70 | CV | 12 | 15/0 |
| P15 | M | 53 | CV | 35 | 12/0 |
| P16 | M | 34 | CV | 40 | 4/0 |
| P17 | M | 66 | CV | 40 | 7/0 |
| P18 | M | 39 | CV | 25 |
8/1+ |
| P19 | M | 33 | CV | 17 |
4/2+ |
| P20 | F | 36 | CV | 28 |
10/10+ |
| P21 | M | 29 | CV | 30 |
9/4+ |
| P22 | F | 33 | CV | 27 |
4/3+ |
| P23 | F | 57 | CV | 22 |
25/15+ |
| P24 | M | 47 | CV | 12 |
4/1+ |
| P25 | F | 51 | CV | 26 |
6/1+ |
| P26 | M | 55 | CV | 12 |
8/2+ |
The objective of this study was to investigate
all-trans retinoic acid/9-cis retinoic acid nuclear
receptor subtypes (RARα, RARβ, RARγ, RXRα, RXRβ, RXRγ), thyroid
hormone receptors (TRα, TRβ), nuclear receptor coregulators (SMRT,
SRC-1) and iodothyronine 5′-deiodinase, type I expression pattern
in papillary thyroid tumour tissue of patients in order to compare
with the expression pattern of the non-neoplastic thyroid tissue of
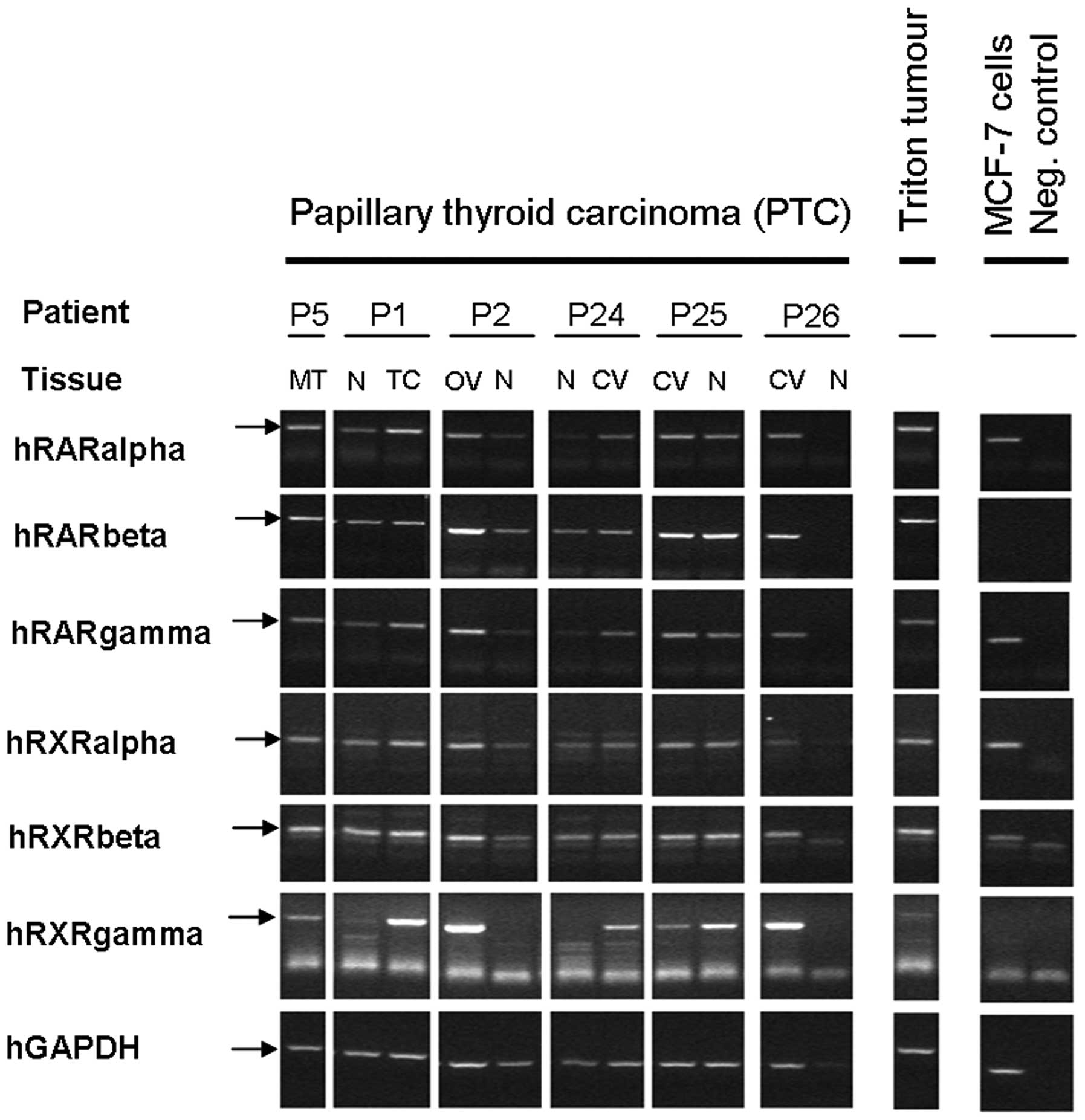
the corresponding patients. Fig. 1
shows representative expression pattern of selected parameters.
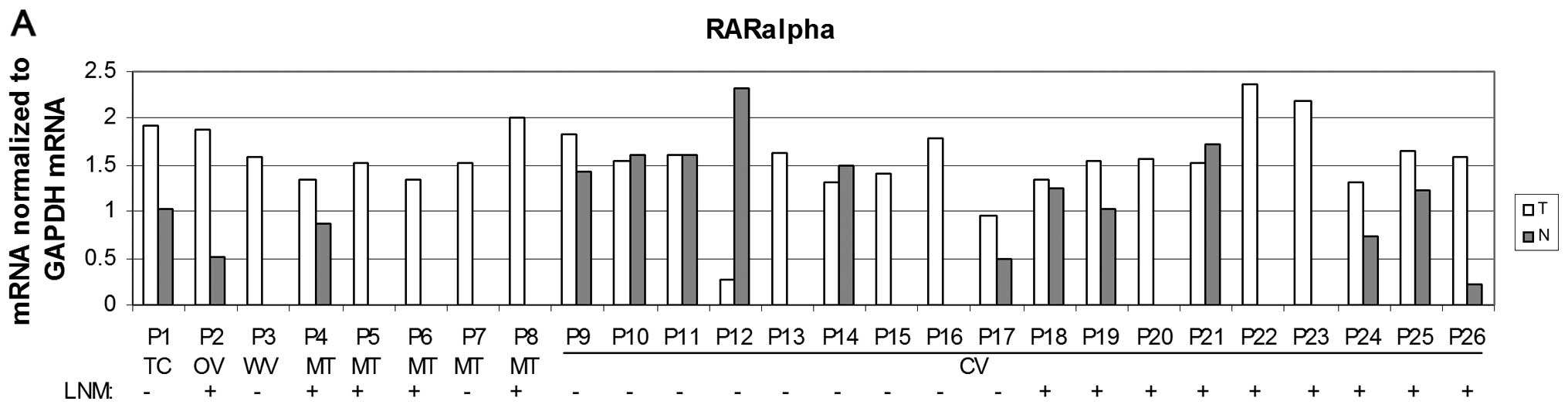
Statistically increased levels of RARα mRNA in
tumour tissue (P<0.05) were detected when compared to the
non-neoplastic tissue (Fig. 2A and
B). This increase was also detected in cases with positive LNM
but not in cases with negative LNM (Fig. 2C) when compared to corresponding
non-tumour tissue. However, there was no significant increase of
RARα mRNA levels in the subgroup of CV of PTC (data not shown).
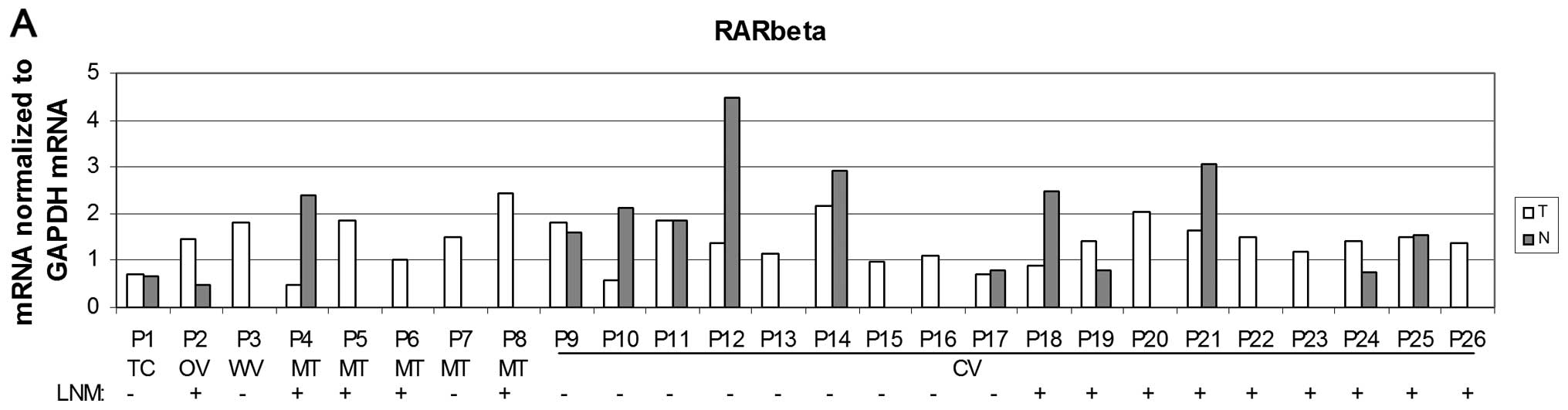
In this study of overall PTC cases, no significantly
altered expression of RARβ was detected when compared to non-tumour
thyroid tissue (Fig. 3) or when the
data were considered as positive or negative LNM cases (data not
shown). However, there were significantly (P<0.05) reduced RARβ
mRNA levels in the CV subgroup of PTC (Fig. 3B).
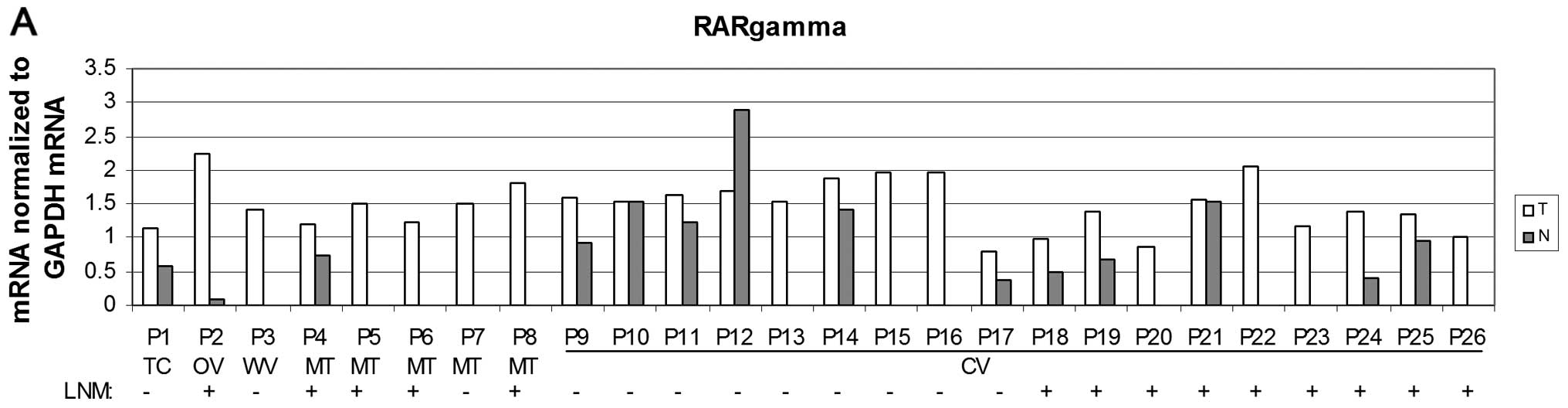
Normal tissue samples exhibited an overall lower
expression of RARγ mRNA when compared to PTC (P<0.01) (Fig. 4A and B). Significantly increased
levels were detected in cases with positive LNM, but not in PTC
with negative LNM (P<0.01 (Fig.
4C). However, we did not detect this increase in the CV
subgroup of PTC (data not shown).
Tumour tissue showed a significantly higher
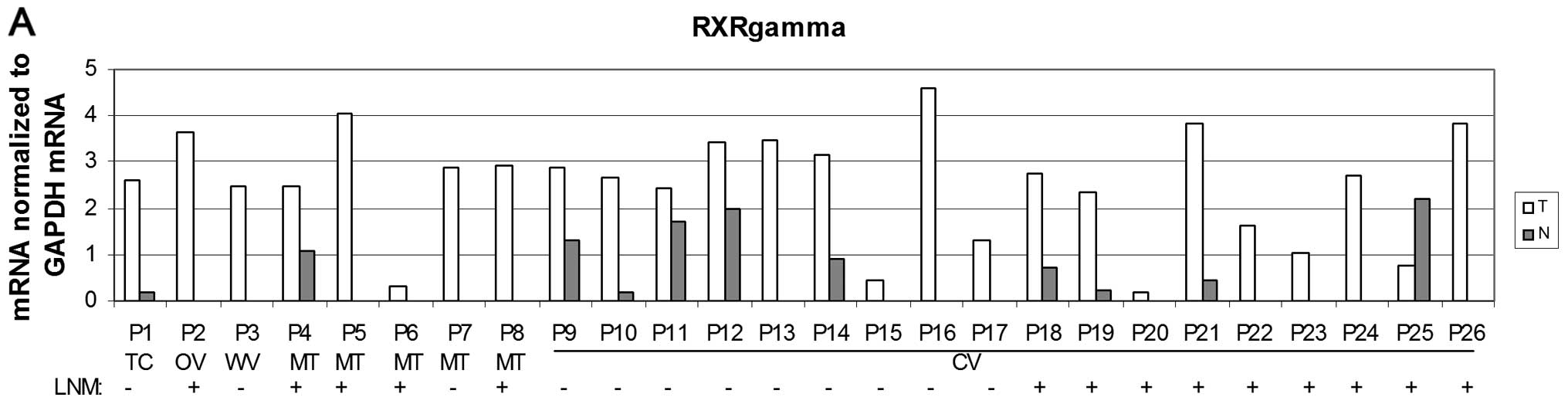
expression of RXRγ than non-neoplastic thyroid tissue (P<0.001)
in both overall PTC cases and in the CV subgroup (Fig. 5A and B). This increase was also
detected in cases with positive LNM and negative LNM (Fig. 5C) when compared to corresponding
non-tumour tissue (P<0.01). However, in the CV subgroup, the
significantly higher levels of RXRγ mRNA in PTC were detected only
in cases with negative LNM (P<0.01) but not in cases with
positive LNM (Fig. 5C).
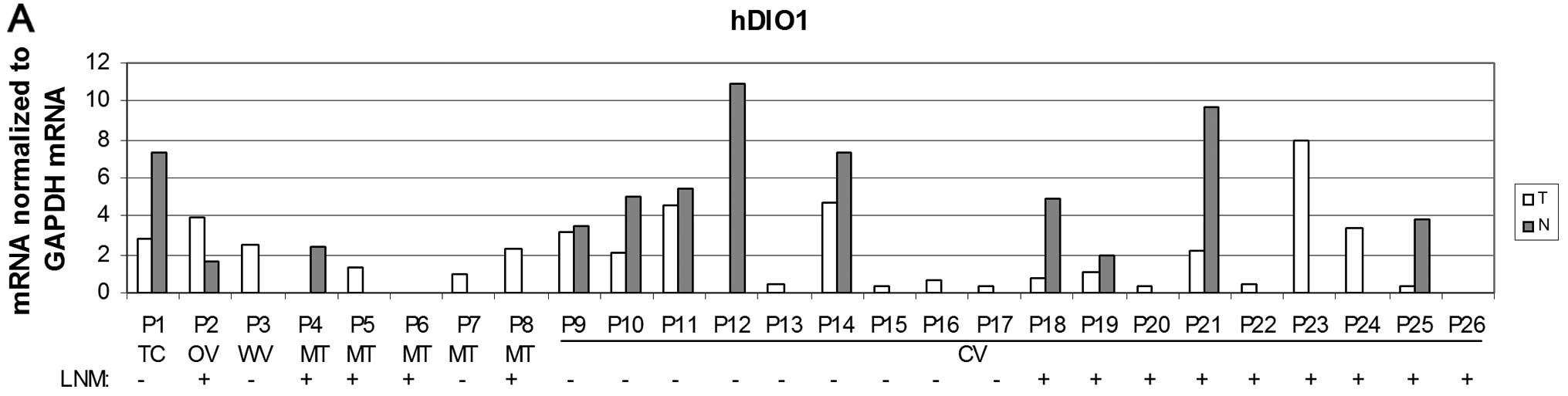
The expression of hDIO1 mRNA was subsequently
studied. The results were consistent with previous literature data
in that we detected either the absence or significantly lower
expression of this gene in tumour tissue when compared to
non-neoplastic tissue in both overall PTC cases and in the CV
subgroup (Fig. 6A and B). However,
these significantly decreased levels of hDIO1 mRNA were detected in
cases with negative LNM but not in cases with positive LNM
(Fig. 6C) when compared to
corresponding non-tumour tissue in both overall PTC cases and in
the CV subgroup (P<0.001 and P<0.05, respectively).
In the present study, the results showed that there
were no significant differences in expression of RXRα, RXRβ, TRα,
TRβ, coactivator SRC-1 and corepressor SMRT mRNA between tumour
thyroid tissue and normal tissue samples. However, higher
expression of SMRT mRNA in normal tissue samples of patients with
positive LNM was detected when compared to patients with negative
LNM (P<0.05) (data not shown).
Discussion
Papillary thyroid cancer (PTC) is the most frequent
histotype, accounting for >80% of all thyroid malignancies.
While PTC is generally associated with a favourable prognosis,
5–20% of patients develop tumour recurrence and 10% have distant
metastasis (19). PTC can be
further divided into several histotypes. Tall-cell variant (TC)
papillary carcinoma is characterized by tall columnar cells
eccentric nuclei located adjacent to the basement membrane
(20). Warthin-like papillary
thyroid carcinoma, a very rare variant of PTC, is histologically
characterized as a cystic or solid-cystic thyroid nodule with
large, polygonal cells with abundant eosinophilic, finely granular
cytoplasm lines on the papillae surrounded by dense lymphocytic
infiltrate (21,22). Five variants of PTC were detected in
this study: 18 cases of classic variant (CV; 69.3%), 5 cases of
mixed type (MT) (19.3%), 1 case of TC (3.8%), 1 case of oncocytic
variant (OV; 3.8%) and 1 case of Warthin-like variant (WV) (3.8%).
The mean tumour size was 24.8±9.9 mm. Only 3 tumours were ≥40 mm.
Histologically-confirmed lymph node metastasis (LNM) was identified
in 14 cases at surgery.
Hoftijzer et al(23) found an increased expression of the
cytoplasmic fraction of RARα, RARγ, RXRβ protein, and a decreased
expression of the nuclear fraction of RARβ, RARγ, and RARα protein
in PTCs compared with benign thyroid tissue. However, in this
study, increased expression of mRNA of RARα, RARγ and RXRγ in
overall PTC cases and/or in the CV subgroup of PTC cases was
detected using RT-PCR. Several studies reported reduced RARβ
expression in PTC using various techniques (immunohistochemistry,
RT-PCR), when compared to normal tissue (23). We found statistically decreased
levels of RARβ in the subgroup of CV of PTC.
Haugen et al(24) found very low levels of RXRγ mRNA in
all normal thyroid tissue tested, but expression was higher in
malignant thyroid tumours in comparison with matched normal tissue
stage. In our study, the expression levels of this nuclear receptor
were significantly increased in tumour tissue, compared to
non-neoplastic tissue. However, we detected 1 patient with
increased expression of RXRγ mRNA in normal tissue and 3 patients
with very low expression of this nuclear receptor subtype which is
in agreement with the study of Liu et al(13), who found no statistical significance
between RXRγ expression and patient age, gender or tumour size,
while, conversely, RXRγ upregulation mainly occurred in PTCs with
extrathyroid invasion, LNM or in advanced tumour. PTC with RXRγ
upregulation has been shown to have higher incidence of
extrathyroid invasion and LNM, and was more frequent in advanced
tumour stages (13). Expression of
RARβ and RXRγ in thyroid carcinomas appears to predict response to
retinoids and/or rexinoids in tumour redifferentiation therapy
(24). Recently, we showed that in
the thyroid non-Hodgkin's lymphoma tissue from all-trans
retinoic acid receptors, RARβ is the most expressed isoform of
RARs. In contrast to normal thyroid tissue lacking in RXRγ
expression, thyroid non-Hodgkin's lymphoma tissue was found to
express that isoform of RXRs at a high level (25). It has been shown that retinoids and
rexinoids are able to reduce tumour growth by inhibiting
angiogenesis, tumour invasion and metastasis in various human
cancer cells (26,27). In our animal MNU-induced mammary
gland carcinogenesis, the administration of TTNPB (selective ligand
for RAR subtypes) and/or Phytol (precursor of phytanic
acid-selective ligand for RXR) markedly reduced tumour progression,
as well as tumour incidence and tumour burden, and significantly
regulated expression of several nuclear receptor mRNA, coregulators
mRNA in tumour tissue or in selected organs (unpublished data).
Furthermore, in this study we found all RAR and RXR subtypes
differentially expressed in respective patient samples. We
conclude, therefore, that retinoids and rexinoids selectively bound
to their corresponding receptors may inhibit tumour
progression.
Our previous observations demonstrated that thyroid
status of animals can influence the progress of rat mammary gland
carcinogenesis (28). Thyroid
hormone is also able to block oncogenic Ras-mediated proliferation
and transcriptional induction of cyclin D1 in neuroblastoma cells
(29). Moreover, it has been shown
that thyroid carcinogenesis may be associated with mutations in
thyroid hormone receptor β (TRβ) gene (30). The conversion of the pro-hormone T4
into the biologically active hormone T3 is catalyzed by
iodothyronine deiodinases (type 1 and type 2) (15). However, in our study, the absence or
lower expression of hDIO1 mRNA in tumour tissue was detected when
compared to non-neoplastic tissue in both overall PTC cases and in
the CV subgroup. These significantly decreased levels of hDIO1 mRNA
were, however, detected in cases with negative LNM but not in cases
with positive LNM when compared to corresponding non-tumour tissue
in both overall PTC cases and in the CV subgroup. Moreover, several
studies have shown diminished or unaltered mRNA levels of
deiodinases of both type 1 and 2 in the majority of thyroid
neoplasias (reviewed in 15,29).
Inactivation of both T4 and T3 is catalysed by type 3 iodothyronine
deiodinase via inner-ring deiodination. Romitti et
al(29) observed increased
levels of activity of this enzyme in all analysed PTC samples and
mRNA levels were found to be significantly decreased in PTC when
compared to surrounding thyroid tissue. It has been shown that PTC
samples from patients with lymph node or distant metastasis
displayed significantly higher levels of hDIO3 activity than those
samples obtained from patients with intra-thyroidal disease
(29). Consequently, the reduction
of hDIO2 activity may further decrease levels of intracellular
thyroid hormone. These observations indicate a role of
intracellular hypothyroidism in the tumour cell proliferation
and/or differentiation (29).
It has been shown that Seocalcitol (EB1089),
synthetic analogue of vitamin D-ligand for vitamin D receptor
belonging to steroid/thyroid/retinoid nuclear receptors family,
significantly reduced KA and increased Bmax
of thyroid receptors in liver of rats with MNU-induced mammary
gland carcinomas when compared to healthy animals, treatment with
EB1089 or vitamin D significantly prolonged tumour latency,
markedly reduced tumour burden and volume (31). Vitamin D also reduced hDIO1 activity
in liver of rats with MNU-induced mammary gland carcinomas
(31). Treatment of MNU-treated
animals with EB1089 and Phytol resulted in markedly reduced mammary
gland tumour progression and significantly regulated expression of
several nuclear receptor mRNA, coregulators mRNA in tumour tissue
or in selected organs (unpublished data). Retinoid receptors (RARs)
and thyroid hormone receptors (TRs), as well as vitamin D receptor,
act as ligand-inducible transcription factors interacting as
heterodimers with retinoid X receptors (RXRs). Thus, these nuclear
receptors play a role as ligand-activated, DNA-binding,
trans-acting, transcription-modulating proteins involved in a
general molecular mechanism responsible (together with
coregulators) for transcriptional responses in target genes. RARs
exert both beneficial and detrimental activity; while they have
tumour-suppressive activity, they are also teratogenic. Several
ligands for RARs and RXRs inhibit carcinogenesis, suppress
premalignant epithelial lesions and tumour growth and invasion in a
variety of tissues. Novel synthetic retinoid and rexinoid analogues
acting through RARs as redifferentiation agents could have a
predominant role in treating patients with advanced thyroid
cancer.
Our data showed that PTC of investigated patients
expressed all subtypes of RARs and RXRs when compared to
non-neoplastic thyroid tissues of the corresponding patients that
were either lacking RXRγ expression or expression was very low. In
PTC, expression of RXRγ was enhanced in comparison with that of
RXRα or RXRβ.
In conclusion, the molecular mechanisms clearly
demonstrate differences in RAR and RXR subtype mRNA expression
patterns in PTC as studied by RT-PCR. These findings contribute to
this branch of immunochemistry, which offers possibilities for
exploitation in clinical oncology, predominantly in the
differential diagnosis of thyroid neoplasms.
Acknowledgements
The authors thank Dr Mike Scotter (The Food and
Environment Research Agency, UK) for editing the English language
of the original manuscript. This study was supported by APVV grants
(APVV-0120-07, APVV-0160-11), VEGA grant 2/0008/11 and the Centre
of Excellence CEMAN grant.
Abbreviations:
|
PTC
|
papillary thyroid carcinoma
|
|
CV
|
classic variant
|
|
MT
|
mixed type
|
|
OV
|
oncocytic variant
|
|
TC
|
tall-cell variant
|
|
WT
|
warthin-like type
|
|
LNM
|
lymph node metastasis
|
|
RAR
|
retinoic acid receptor
|
|
RXR
|
retinoid X receptor
|
|
TR
|
thyroid hormone receptor
|
|
SMRT
|
silencing mediator for retinoic acid
and thyroid hormone receptor
|
|
SRC-1
|
steroid receptor coactivator-1
|
|
hDIO1,2,3
|
3,5,3′-triiodo-L-iodothyronine
5′-deiodinase, type 1,2,3
|
References
|
1
|
Lloyd RV, Buehler D and Khanafshar E:
Papillary thyroid carcinoma variants. Head Neck Pathol. 5:51–56.
2011. View Article : Google Scholar
|
|
2
|
Slough CM and Randolph GW: Workup of
well-differentiated thyroid carcinoma. Cancer Control. 13:99–105.
2006.PubMed/NCBI
|
|
3
|
LiVolsi VA: Papillary thyroid carcinoma:
an update. Mod Pathol. 24(Suppl 2): S1–S9. 2011. View Article : Google Scholar
|
|
4
|
Bai Y, Kakudo K, Li Y, Liu Z, Ozaki T, Ito
Y, Kihara M and Miyauchi A: Subclassification of non-solid-type
papillary thyroid carcinoma identification of high-risk group in
common type. Cancer Sci. 99:1908–1915. 2008.PubMed/NCBI
|
|
5
|
Bai Y, Kakudo K, Nakamura M, Ozaki T, Li
Y, Liu Z, Mori I, Miyauchi A and Zhou G: Loss of cellular
polarity/cohesiveness in the invasive front of papillary thyroid
carcinoma and periostin expression. Cancer Lett. 281:188–195. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Lee MO, Wang HG, Li Y, Hashimoto Y,
Klaus M, Reed JC and Zhang X: Retinoic acid receptor β mediates the
growth-inhibitory effect of retinoic acid by promoting apoptosis in
human breast cancer cells. Mol Cell Biol. 16:1138–1149. 1996.
|
|
7
|
DeGroot LJ, Kaplan EL, McCormick M and
Straus FH: Natural history, treatment, and course of papillary
thyroid carcinoma. J Clin Endocrinol Metab. 71:414–424. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hay ID: Papillary thyroid carcinoma.
Endocrinol Metab Clin North Am. 19:545–576. 1990.
|
|
9
|
Brtko J: Retinoids, rexinoids and their
cognate nuclear receptors: character and their role in
chemoprevention of selected malignant diseases. Biomed Pap Med Fac
Univ Palacky Olomouc Czech Repub. 151:187–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brtko J and Dvorak Z: Role of retinoids,
rexinoids and thyroid hormone in the expression of cytochrome p450
enzymes. Curr Drug Metab. 12:71–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brtko J and Thalhamer J: Renaissance of
the biologically active vitamin A derivatives: established and
novel directed therapies for cancer and chemoprevention. Curr Pharm
Des. 9:2067–2077. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmutzler C, Brtko J, Bienert K and
Köhrle J: Effects of retinoids and role of retinoic acid receptors
in human thyroid carcinomas and cell lines derived therefrom. Exp
Clin Endocrinol Diabetes. 104(Suppl 4): S16–S19. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Zhou G, Nakamura M, Bai Y, Li Y,
Ozaki T, Mori I, Miyauchi A and Kakudo K: Retinoid X receptor γ
up-regulation is correlated with dedifferentiation of tumor cells
and lymph node metastasis in papillary thyroid carcinoma. Pathol
Int. 61:109–115. 2011.
|
|
14
|
Tang W, Nakamura Y, Zuo H, Yasuoka H, Yang
Q, Wang X, Nakamura M, Mori I, Miyauchi A and Kakudo K:
Differentiation, proliferation and retinoid receptor status of
papillary carcinoma of the thyroid. Pathol Int. 53:204–213. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Casula S and Bianco AC: Thyroid hormone
deiodinases and cancer. Front Endocrinol (Lausanne). 3:742012.
|
|
16
|
Greene FL and Sobin LH: The TNM system:
our language for cancer care. J Surg Oncol. 80:119–120. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kimura Y, Suzuki T, Kaneko C, Darnel AD,
Moriya T, Suzuki S, Handa M, Ebina M, Nukiwa T and Sasano H:
Retinoid receptors in the developing human lung. Clin Sci (Lond).
103:613–621. 2002.PubMed/NCBI
|
|
18
|
Brtko J, Sejnová D, Ondková S and Macejová
D: Malignant Triton tumour exhibits a complete expression pattern
of nuclear retinoid and rexinoid receptor subtypes. Gen Physiol
Biophys. 28:425–427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Franceschi S, Boyle P, Maisonneuve P, La
Vecchia C, Burt AD, Kerr DJ and MacFarlane GJ: The epidemiology of
thyroid carcinoma. Crit Rev Oncog. 4:25–52. 1993.
|
|
20
|
Clark DP and Faquin WC: Thyroid
Cytopathology. Springer Science and Business Media Inc.; New York,
NY: 2011
|
|
21
|
Amico P, Lanzafame S, Li Destri G, Greco
P, Caltabiano R, Vecchio GM and Magro G: Warthin tumor-like
papillary thyroid carcinoma with a minor dedifferentiated
component: report of a case with clinicopathologic considerations.
Case Report Med. 2010:4952812010.
|
|
22
|
Paker I, Kokenek TD, Yilmazer D, Seker GE
and Alper M: Oncocytic variant of papillary thyroid carcinoma with
lymphocytic stroma (Warthin-like variant): report of a case with
fine needle variant cytology and review of the literature.
Cytopathology. 23:408–410. 2012. View Article : Google Scholar
|
|
23
|
Hoftijzer HC, Liu YY, Morreau H, van Wezel
T, Pereira AM, Corssmit EP, Romijn JA and Smit JW: Retinoic acid
receptor and retinoid X receptor subtype expression for the
differential diagnosis of thyroid neoplasms. Eur J Endocrinol.
160:631–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haugen BR, Larson LL, Pugazhenthi U, Hays
WR, Klopper JP, Kramer CA and Sharma V: Retinoic acid and retinoid
X receptors are differentially expressed in thyroid cancer and
thyroid carcinoma cell lines and predict response to treatment with
retinoids. J Clin Endocrinol Metab. 89:272–280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brtko J, Macejova D and Galbavy S: Thyroid
non-Hodgkin's lymphoma expression pattern of nuclear retinoid and
rexinoid receptor subtypes. Gen Physiol Biophys. 29:411–413.
2010.
|
|
26
|
Papi A, Rocchi P, Ferreri AM and Orlandi
M: RXRγ and PPARγ ligands in combination to inhibit proliferation
and invasiveness in colon cancer cells. Cancer Lett. 297:65–74.
2010.
|
|
27
|
Yen WC, Prudente RY, Corpuz MR,
Negro-Vilar A and Lamph WW: A selective retinoid X receptor agonist
bexarotene (LGD1069, targretin) inhibits angiogenesis and
metastasis in solid tumours. Br J Cancer. 94:654–660.
2006.PubMed/NCBI
|
|
28
|
Macejova D, Radikova Z, Macho L, Liska J
and Brtko J: MNU-induced carcinogenesis of rat mammary gland:
effect of thyroid hormone on expression of retinoic acid receptors
in tumours of mammary gland. Mol Cell Endocrinol. 244:47–56. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Romitti M, Wajner SM, Zennig N, Goemann
IM, Bueno AL, Meyer EL and Maia AL: Increased type 3 deiodinase
expression in papillary thyroid carcinoma. Thyroid. 22:897–904.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weinert LS, Ceolin L, Romitti M, Camargo
EG and Maia AL: Is there a role for inherited TRβ mutation in human
carcinogenesis? Arq Bras Endocrinol Metabol. 56:67–71.
2012.PubMed/NCBI
|
|
31
|
Macejova D, Ondkova S and Brtko J: Vitamin
D3 affects expression of thyroid hormone receptor alpha and
deiodinase activity in liver of MNU-treated Sprague-Dawley rats.
Gen Physiol Biophys. 28:363–370. 2009. View Article : Google Scholar : PubMed/NCBI
|