Introduction
microRNAs (miRNAs) are non-coding single-stranded
RNA molecules that are 21–25 nucleotides in length and primarily
function as negative gene regulators (1). miRNAs are involved in several key
biological phenomena, including cell differentiation, proliferation
and apoptosis. There is increasing evidence that miRNAs play an
important role in cancer development (2,3).
During miRNA genesis, polymerase II transcribes an immature form of
miRNA that is referred to as pre-miRNA. This type of miRNA is
potentially as long as thousands of base pairs and contains both a
50-cap and a (poly)A tail (4).
Following the generation of the pri-miRNAs, two enzymes play
pivotal roles in the processing of these transcripts: Drosha
(RNASEN) and Dicer (DICER1). Drosha, a nuclear enzyme, cuts
pre-pri-mRNA segments into short double-stranded RNA precursors,
which are referred to as pre-miRNAs, that have an approximate
length of 60–70 nucleotides (5).
The pre-miRNAs are then cleaved in the cytoplasm by Dicer into
mature double-stranded miRNA fragments that are each ~15–30
nucleotides in length (6,7).
Drosha overexpression occurs frequently in cervical
cancer (8–10) and is associated with substantial
differences in miRNA profiles (11). However, the effects of Drosha
dysregulation on the proteomic profile of cervical cancer cells
following changes in their miRNA expression profile are
unknown.
Here, we used gene depletion experiments to
demonstrate that altered Drosha levels in cervical cancer cell
lines caused significant changes in the cellular phenotype and the
global differential expression of proteins in vitro.
Materials and methods
Cell culture
The following cervical cells were used: i) three
independent primary cultures of normal cervical epithelial cells
(12); ii) the normal cervical
epithelial cell line CRL2614 that was obtained from ATCC; and iii)
three cervical cancer cell lines HeLa, SiHa and C33a, which were
purchased from the China Center for Type Culture Collection
(CCTCC), Wuhan University, China. Standard protocols for cell
culture were used (13,14). The specimen collection and archiving
of patient data were performed with written informed consent and
were approved by the Ethics Committee of the Wuhan Union
Hospital.
RNA interference (RNAi) and stable
transfection
Four shRNAs (shDro homo 1614/570/1948/2936;
GenePharma, Shanghai, China) targeting Drosha were generated. Their
target sequences were as follows: shDro homo 1614, 5′-GGGA
GATTCTACAGTGGTTGG-3′; shDro homo 570, 5′-GCAGC CTCCTGTGCAATATCA-3′;
shDro homo 1948, 5′-GCAAGA CGCACAGGAATTAGG-3′; shDro homo 2936,
5′-GCTACC ACCAATGCCTAATCG-3′; and the negative control (shNC)
5′-GTTCTCCGAACGTGTCACGT-3′. The transfections were performed using
Lipofectamine 2000 reagent (Invitrogen, USA). Geneticin (G418)
selection was performed 24 h following transfection for 48 h at 800
ng/ml. The efficiency of transfection was evaluated by observing
the green fluorescence under an inverted fluorescence microscope
(Olympus, Japan), and the knockdown efficiency was assessed at the
mRNA level 72 h post-transfection.
For stable Drosha depletion, recombinant Lenti-virus
(GenePharma) was used to deliver shDro homo 2936 shRNA
(Lenti-shDro). GFP and anti-puromycin genes were incorporated into
this vector to permit the convenient monitoring of the infection
efficiency under the fluorescence microscope and screening of the
uninfected cells. The cervical cancer cells were seeded into 6-well
plates (cell line-dependent). The virus was added into the medium
at a MOI of 10 and co-cultured for 24 h. The cells that were
transduced with Lenti-shDro were screened using 2 μg/ml
puromycin.
RNA isolation and quantitative real-time
PCR (qRT-PCR)
Total RNA was isolated from cells using the TRIzol
reagent (Invitrogen, USA), and complementary DNA was synthesised
using a reverse transcription kit (Toyobo, Osaka, Japan) according
to the manufacturer′s protocol. The sequences of the primers for
Drosha mRNA detection were the following: forward,
5′-CATGTCACAGAATGTCGTTCCA-3′; and reverse,
5′-GGGTGAAGCAGCCTCAGATTT-3′. The PCR was performed on an ABI
StepOnePlus thermocycler (Applied Biosystems, USA) using the
SsoFast EvaGreen Supermix with Low ROX (Bio-Rad, USA). The
amplification protocols were as follows: 95ºC for 30 sec followed
by 40 cycles of 95ºC for 5 sec and 59ºC for 30 sec. After the PCR,
a melting curve was constructed by increasing the temperature from
65 to 95ºC with a temperature transition rate of 0.2ºC/sec. Each
sample was analysed in triplicate. The Drosha transcript level was
normalised to the β-actin amplification level and was calculated
using the comparative threshold cycle (Ct) method
(2−ΔΔCt).
Western blotting
SDS-PAGE and western blotting were performed as
previously described (16).
Briefly, the total protein in each sample was subjected to
Tris-glycine SDS-PAGE separation. After protein transfer, PVDF
membranes were incubated with a Drosha rabbit antibody (D30F3,
1:1,000; Cell Signaling Technology, USA) or a tubulin 5β mouse
polyclonal antibody (ab52837, 1:1,000; Abcam, USA) followed by
incubation with an HRP-conjugated secondary antibody (Amersham
Biosciences). The protein levels were visualised using the ECL
substrate kit (Amersham Biosciences). The protein expression of
β-actin was used as an internal control.
Wound-healing assay
Between 0.6 and 1.2×106 cells (cell
line-dependent) were seeded into 6-well plates, the under-surfaces
of which were marked with a horizontal line along the diameter, in
2 ml of DMEM with 10% fetal calf serum (both from HyClone, USA).
Twenty-four hours later, three parallel scratches, perpendicular to
the horizontal line, were made in the monolayer using a pipette
tip. The width of the scratches was measured over the following 24
h at three fixed distances from the line, and the mean values were
used to quantify wound healing.
Cell invasion assay
Between 2 and 4×103 transfected cells
(cell line-dependent) were seeded per well of an 8-μm Growth
Factor-Reduced invasion chamber (BD Biosciences, Oxford, UK) in 1
ml DMEM without serum. One milliliter of the DMEM (20% FBS) was
added below the chamber. Invasion proceeded for 24 h, after which
the membranes were fixed and Giemsa-stained. The cell numbers were
determined in three random microscopic fields of ×400 magnification
for each well, with the means of nine values from triplicate
experiments being used to quantify invasion.
Cell proliferation assessment using
MTT
The effect of Drosha knockdown (Drosha-KD) on cell
growth was examined using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. In total, 2×103 cells/well were plated in
triplicate into a 96-well plate, and cell proliferation was assayed
continuously for 5 days. At each time point, 20 μl MTT reagent (5
mg/ml in PBS) was added to the well, followed by 4 h of incubation
at 37ºC. The media were discarded, and 100 ml of DMSO was then
added to each well to dissolve the precipitate by incubating for 30
min. The absorbance was measured at a wavelength of 490 nm using a
Bio-Rad microplate reader.
Clone formation
For the colony-forming assay, 200 cells that were
infected with Lenti-shDro or Lenti-NC were seeded in a 6-well plate
and cultured for 2 weeks. Thereafter, the media was removed and
Giemsa-stained. The cell colonies with >1 mm diameter were
counted after staining. The experiments were performed in
triplicate using three cervical cancer cell lines (HeLa, SiHa and
C33a).
Two-dimensional electrophoresis (2-DE)
and mass spectrometry
2-DE was performed as previously described (17). Isoelectric focusing (IEF) was
performed using an IPGphor II apparatus (Amersham Biosciences,
Arlington Heights, IL, USA). IPG strips (24 cm, pH 4.0–10.0,
nonlinear) were used according to the manufacturer’s instructions.
Samples that contained 500 μg protein were diluted to 2 ml in a
rehydration solution (8 M urea, 2% CHAPS, 0.4% DTT, 0.5% IPG
buffer, 0.002% bromophenol blue). The rehydration step was
performed with 24-cm IPG strips for 12 h at room temperature. IEF
was run following a step-wise voltage increase procedure: 500 and
1,000 V for 1 h each followed by 8,000 V for 60 kVh. After IEF, the
IPG gel strips were placed in an equilibration buffer (6 M urea, 2%
SDS, 30% glycerol, 0.002% bromophenol blue, 50 mM Tris-HCl, pH 6.8)
that contained 1% DTT for 15 min under agitation. The IPG strips
were then transferred to an equilibration solution that contained
2.5% iodoacetamide and shaken for an additional 15 min before being
placed onto 12.5% uniform polyacrylamide gel slabs (1.5 mm).
Separation in the second dimension was performed in Tris-glycine
buffer (25 mM Tris, 0.2 M glycine, 0.1% SDS) at a constant current
setting of 15 mA/gel for 30 min and 30 mA/gel thereafter. SDS-PAGE
separation was terminated when the bromophenol dye front migrated
to the lower end of the gels.
After 2-DE, the gels were stained by a modified
Coomassie G-250 method that is compatible with downstream MS
analysis, as previously described (18). The entire staining procedure was
performed over three days at room temperature with the gels gently
shaken.
The raw images were digitised using a scanner
(Amersham Biosciences). The images were further analysed using
PDQuest (version 8.0; Bio-Rad). Twenty-one spots of interest were
manually excised from the 2-DE gels and de-stained by washing with
a mixture of 200 mM NH4HCO3/acetonitrile
(1:1). The proteins were reduced with DTT, alkylated with
iodoacetamide, and digested in-gel with trypsin (Promega, Madison,
WI, USA).
The peptides were lyophilised and subsequently
dissolved in 2% ACN/0.1% formic acid. Each fraction was subjected
to LC-MS analysis using a Nano HPLC (Eksigent) coupled to a Q-Star
Elite mass spectrometer (Applied Biosystems). The peptides were
first enriched using a CapTrap column (0.5×2 mm; Michrom
Bioresources, Inc.) followed by elution into an integrated
nanoscale analytical column (Magic C18AQ; Michrom Bioresources,
Inc.; 100 μm × 150 mm, 3-μm particle size, 200-Å pore size). We
conducted each MS scan from 400 to 1,800 amu, with 1 sec time
spans. For the MS/MS analysis, each scan cycle consisted of one
full-scan mass spectrum (with m/z ranging from 400 to 1,800 and
charge states from 2 to 5) followed by five MS/MS events. The
threshold count was set to 30, and the exclusion window was set at
90 sec. The mass tolerance was 50 mDa. The automatic collision
energy and automatic MS/MS accumulation settings were selected.
The raw data from the Q-Star Elite mass spectrometer
were analysed with Mascot Daemon software (version 2.2.2; Matrix
Science) using a local Mascot engine. The data were searched
against an NCBI database. Cysteine carbamidomethylation was set as
a fixed modification, and methionine oxidation as well as serine,
threonine, and tyrosine phosphorylation were set as variable
modifications. The peptide mass tolerance was set at 200 ppm and
0.4 Da. The peptide charge was set at 2+ and
3+, allowing for up to two missed cleavages, and the
significance threshold was set at P<0.05.
Statistical analysis
The data are expressed as the mean ± SD from at
least three separate experiments performed in triplicate, unless
otherwise noted. Significant differences between the groups were
compared using the Student’s t-test. A P-value <0.05 was
considered to indicate a statistically significant result.
Results
Lentiviral vector-mediated downregulation
of Drosha in cervical cancer cells
The expression levels of Drosha were determined
using qRT-PCR in the immortalised human normal cervical epithelial
cell line CRL2614, three primary cultured normal cervical squamous
cell lines and three cervical cancer cell lines (HeLa, SiHa and
C33a). Drosha mRNA expression was upregulated >60-fold in the
cervical cancer cell lines when compared to the normal cervical
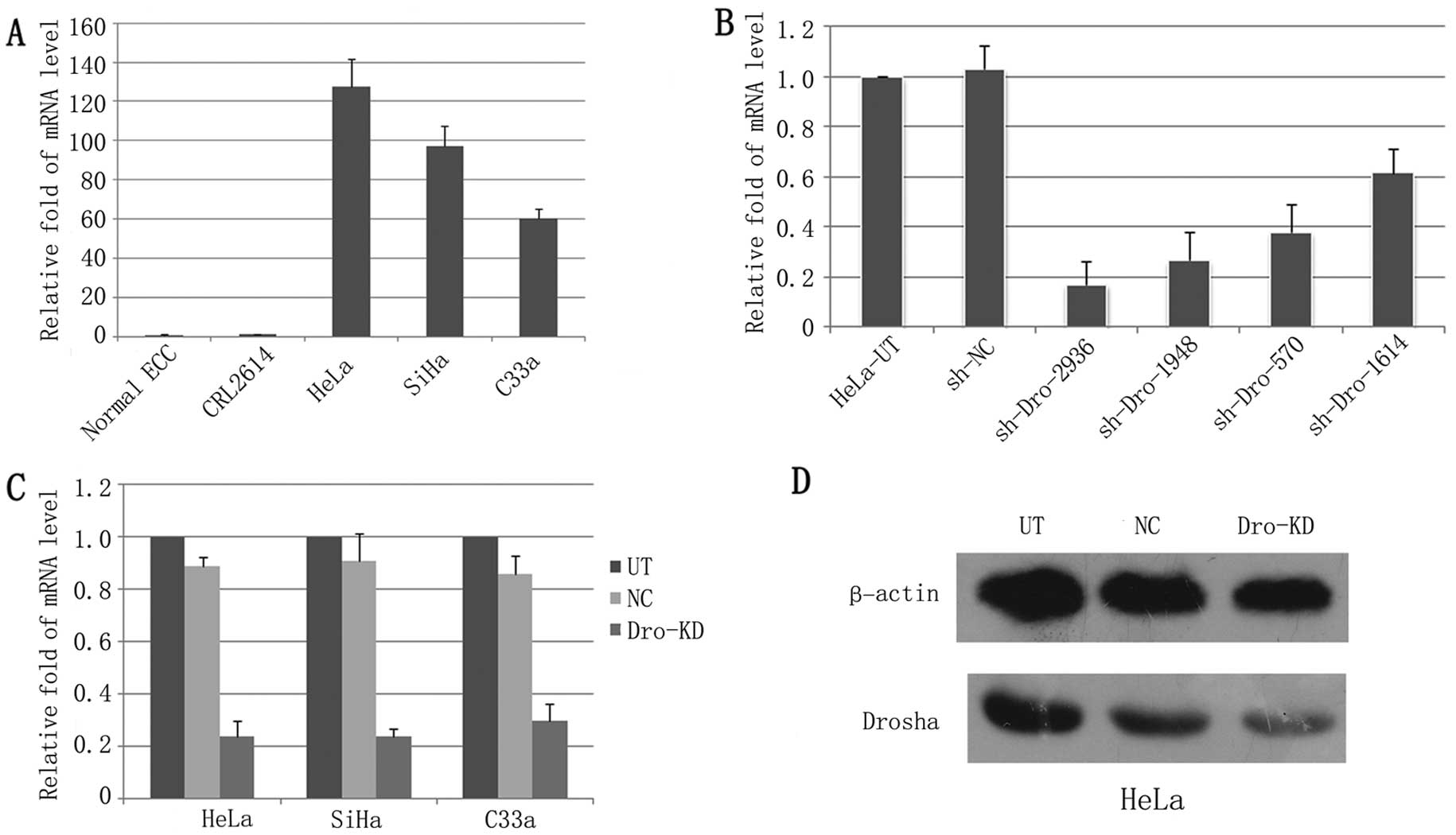
epithelial cells (Fig. 1A).
To identify an effective shRNA for Drohsa-KD, 4
shRNAs targeting Drosha were transfected into HeLa cells. Among
these shDros, shDro homo 2936 downregulated Drosha the most
significantly, with Drosha mRNA levels being reduced by ~83%
(Fig. 1B). We then established a
Lenti-shDro using this shRNA. The lentiviral vector expressing shNC
was used as a control. Over 95% of the cervical cancer cells that
were transduced with Lenti-shDro or Lenti-shNC expressed GFP after
puromycin screening, and the Drosha mRNA level was dramatically
decreased (~80%) in cells that were infected with Lenti-shDro when
compared with the Lenti-shNC cells (Fig. 1C). The Drosha-KD in HeLa cells was
further confirmed by western blotting (Fig. 1D).
Downregulation of Drosha inhibits
cervical cancer cell growth and migration
To investigate the biological effects of Drosha-KD
in cervical cancer cells, we performed an MTT assay, a clone
formation assay, a wound-healing test and a Transwell migration
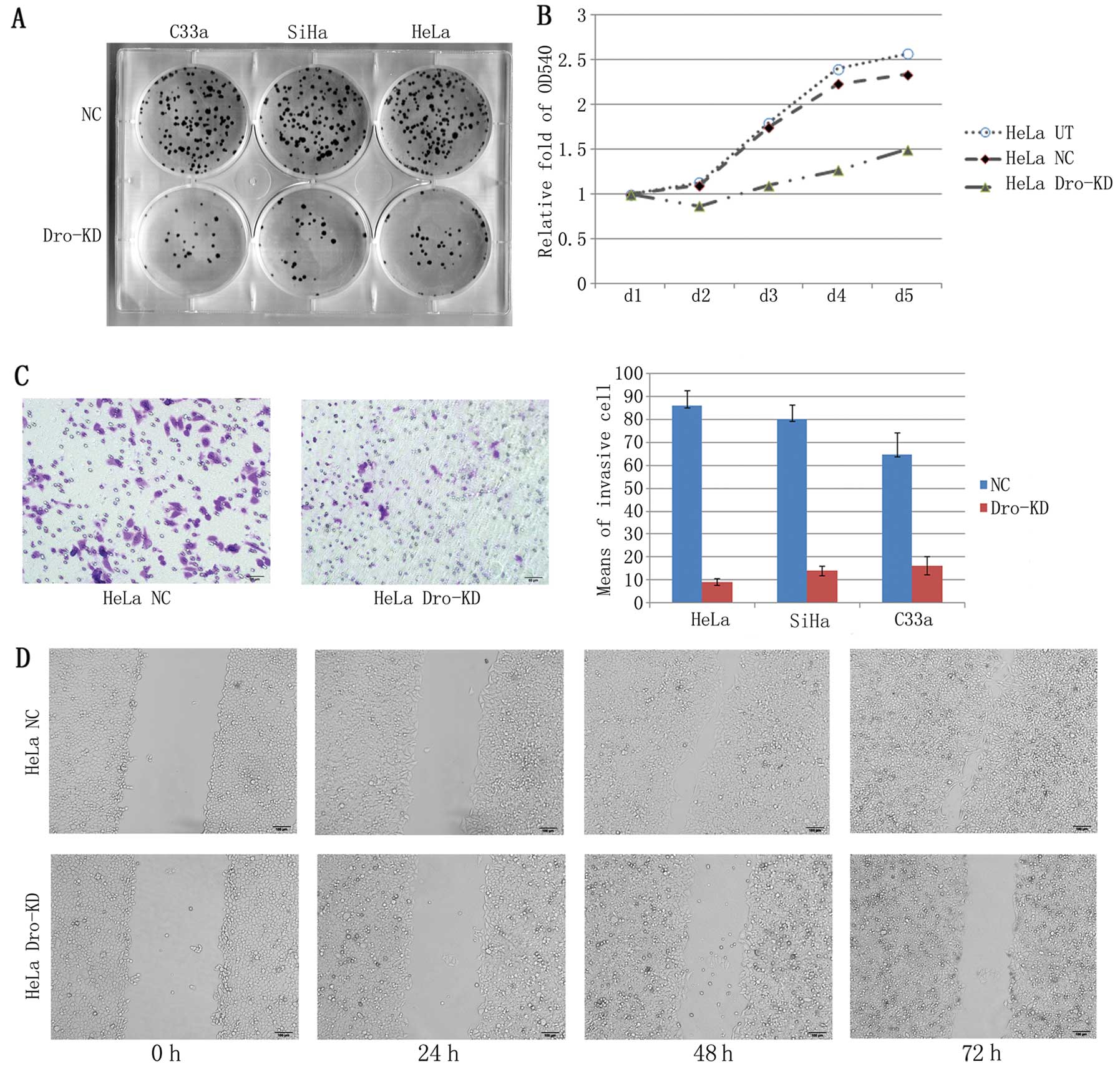
assay. The MTT assay revealed a significant inhibition of cell
proliferation in HeLa cells with Drosha-KD (Fig. 2A). The cervical cancer cells lost
their enhanced clonogenicity following Drosha-KD (Fig. 2B). Additionally, the results from
the Transwell assay indicated that the migratory ability was
reduced in HeLa, SiHa and C33a cells that were infected with the
Lenti-shDro when compared with the NC cells (Fig 2C). The results of the wound-healing
tests confirmed that the reduction in migration was mediated by
Drosha-KD (Fig. 2D).
Drosha-KD alters the proteomic profile of
human cervical cancer
Total protein extracted from transduced HeLa cells
was used for 2-DE and subsequent mass spectrometry. PDQuest image
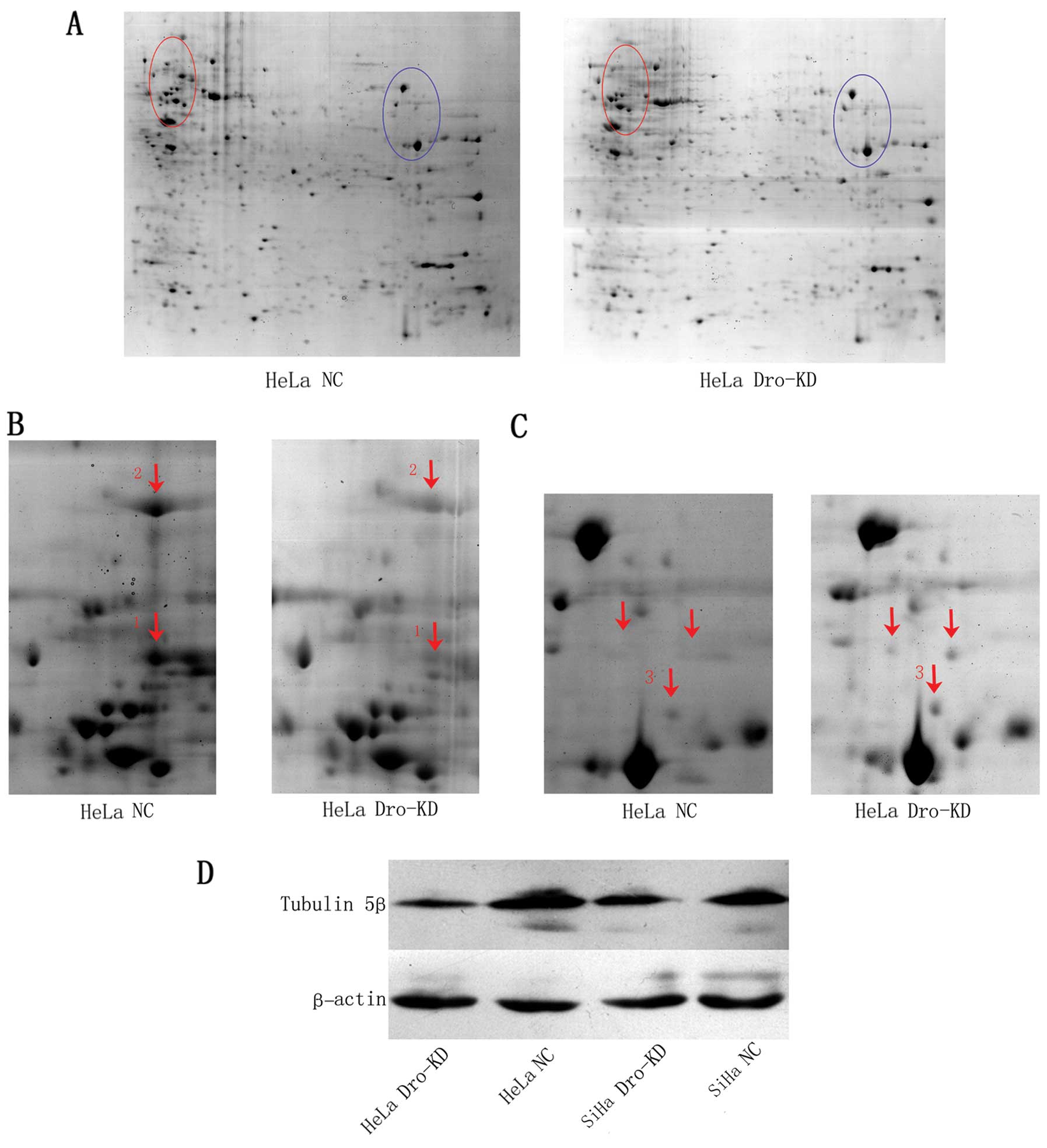
analysis revealed an increase in global protein expression in
Drosha-KD cells. Based on the data in the PDQuest report, the
number of detectable spots in the gels of the Drosha-KD cells was
significantly higher than the number in the NC cells (417±16.6 vs.
247±70.2; P=0.015). Sixty-nine spots were detected only in the
Drosha-KD gels, and only five spots were observed in the NC cell
gels.
Tubulin 5β is a potential target of
Drosha
Twenty-one protein spots with at least a 3-fold
change were chosen for protein identification by liquid
chromatography and tandem mass spectrometry technology (LC-MS/MS)
analysis. Eighteen proteins were ultimately identified (Table I). The associated diseases and
functions of these proteins are summarised in Table II. Twelve of these proteins are
associated with human tumours. As revealed by the 2-DE data, the
tubulin 5β spot had the highest-ranked change after Drosha-KD.
Functionally, as an important component of the cytoskeleton,
β-tubulin is associated with cell proliferation and the cell cycle
and is a target of chemotherapeutic agents, such as paclitaxol.
 | Table IThe proteins with differential
expression in HeLa cells infected with Lenti-shDro 2936 were
identified by MALDI-TOF MS. |
Table I
The proteins with differential
expression in HeLa cells infected with Lenti-shDro 2936 were
identified by MALDI-TOF MS.
| Spot no. | Accession no. | Protein name | MW (Da) | PI | Score | Additional names
for the protein | Up/downregulated
after Drosha knockdown | Tips |
|---|
| 1 | P04350 | Tubulin 5β | 50,095 | 4.78 | 630 | Tubulin β4A | Downregulated | |
| 2 | P11021 | 78 kDa
glucose-regulated protein | 72,402 | 5.07 | 1,482 | HSP A5 | Downregulated | |
| 3 | Q15365.2 | Poly(rC)-binding
protein 1 | 37,987 | 6.66 | 348 | | Upregulated | |
| 4 | P31949 | Protein
S100-A11 | 11,847 | 6.56 | 175 | Metastatic lymph
node gene 70 protein/S100 calcium-binding protein A11 | Upregulated | |
| 5,6 | P62937 | Peptidyl-prolyl
cis-trans isomerase A | 18,229 | 7.82 | 565 | Cyclosporin
A-binding protein/CYPA | Upregulated | |
| 7 | NP_001952 | Elongation factor
2 | 96,276 | 6.41 | 478 | | Upregulated | Fragment |
| 8 | NP_000282 | Phosphoglycerate
kinase 1 | 44,958 | 8.3 | 966 | | Upregulated | |
| 9 | P63241 | Eukaryotic
translation initiation factor 5A-1 | 17,049 | 5.07 | 245 | eIF-5A | Downregulated | |
| 10 | ACM51349 | Putative cytochrome
c oxidase subunit II | 25,565 | 4.67 | 63 | | Upregulated | |
| 11 | XP_003960565 | Predicted: putative
uncharacterized protein C12 or f63 | 135 k | 8.43 | 45 | | Upregulated | Fragment |
| 12–14 | P07910 | Heterogeneous
nuclear ribonucleoproteins C1/C2 | 33,707 | 4.95 | 342 | | Upregulated | |
| 15 | AAH66928 | Eukaryotic
translation initiation factor 4H | 25,261 | 7.79 | 79 | | Upregulated | |
| 16 | NP_036526 | Prefoldin subunit
2 | 16,695 | 6.2 | 291 | Genes involved in
microtubule biogenesis protein 4 | Upregulated | |
| 17 | NP_001257291 | UV excision repair
protein RAD23 homolog A | 39,642 | 4.54 | 119 | | Upregulated | |
| 18 | Q14980 | Nuclear mitotic
apparatus protein 1 | 238 k | 5.36 | 35 | NuMA protein/SP-H
antigen | Upregulated | Fragment |
| 19 | P33316 | Deoxyuridine
5′-triphosphate nucleotidohydrolase, mitochondrial | 26,975 | 9.46 | 153 | dUTP
pyrophosphatase | Upregulated | |
| 20 | P49773 | Histidine triad
nucleotide-binding protein 1 | 13,907 | 6.43 | 52 | Protein kinase C
inhibitor 1 | Upregulated | |
| P04406 |
Glyceraldehyde-3-phosphate
dehydrogenase | 36,201 | 8.57 | 129 | GAPDH | Upregulated | Fragment |
| 21 | O43150 | Arf-GAP with SH3
domain | 11,2835 | 6.24 | 38 | Development and
differentiation- enhancing factor 2 | Upregulated | Fragment |
 | Table IIAssociated disease and function of MS
identified proteins. |
Table II
Associated disease and function of MS
identified proteins.
| Spot no. | Protein name | Associated disease
(refs.) | Protein function
(refs.) |
|---|
| 1 | Tubulin 5β | Human cancer
(19,20) | Required for
mitosis, limited cell proliferation (21), increases following treatment with
chemotherapeutic agents (19,20) |
| 2 | 78 kDa
glucose-regulated protein | Breast cancer
(22), endometrial cancer (23) | Sensitises tumour
cells to chemotherapy (22,23) |
| 3 | Poly(rC)-binding
protein 1 | Viral infection
(24), human hepatoma (25) | Antiviral immunity
and prevention of inflammation (24) |
| 4 | Protein
S100-A11 | Lung cancer
(26), breast carcinoma (27) | Cell proliferation
(26), a diagnostic marker in
breast carcinoma (27) |
| 5,6 | Peptidyl-prolyl
cis-trans isomerase A | Viral infection
(28), hepato- cellular carcinoma
(29) | Diverse roles in
viral infection (28), promotes HCC
cell metastasis (29) |
| 7 | Elongation factor
2 | Lung adenocarcinoma
(30), gastrointestinal cancers
(31) | Anti-apoptotic
marker (30), promotes G2/M
progression and enhanced cell growth (31) |
| 8 | Phosphoglycerate
kinase 1 | Prostate tumour
(32), gastric cancer (33) | Promotes tumour
cell growth, supports the interactions between cancer and its
microenvironment (32), a potential
marker for peritoneal dissemination (33) |
| 9 | Eukaryotic
translation initiation factor 5A-1 | Lung cancer
(34), hepato- cellular carcinoma
(35) | Prognostic marker
(34,35) |
| 10 | Putative cytochrome
c oxidase subunit II | No report | No report |
| 11 | Predicted: putative
uncharacterized protein C12 or f63 | No report | No report |
| 12–14 | Heterogeneous
nuclear ribonucleoproteins C1/C2 | No report | Maintenance of
cellular homeostasis besides cellular differentiation and
proliferation (36) |
| 15 | Eukaryotic
translation initiation factor 4H | Colorectal cancer
(37) | Activates cyclin D1
(37) |
| 16 | Prefoldin subunit
2 | No report | No report |
| 17 | UV excision repair
protein RAD23 homolog A | Gene disease
(38) | Gene environment
interaction, cell cycle control and protein ubiquitination
(38) |
| 18 | Nuclear mitotic
apparatus protein 1 | Epithelial ovarian
cancers (39), breast cancer
(40) | Highly expressed in
EOC (39), spindle assembly
(41) |
| 19 | Deoxyuridine
5′-triphosphate nucleotidohydrolase | No report | No report |
| 20 | Histidine
mitochondrial nucleotide-binding protein 1 | Melanoma (42), gastric cancer (43) | Tumour suppressor,
inhibits the Wnt/β-catenin pathway (42) |
|
Glyceraldehyde-3-phosphate
dehydrogenase | Colon cancer
(44), ovarian cancer (45) | Associated with
energy metabolism and production, cell proliferation and
tumourigenesis (46) |
| 21 | Arf-GAP with SH3
domain colorectal cancer (48) | Breast cancer
(47), stimulates metastasis
formation (47,48) | Promotes tumour
cell motility and invasiveness, |
To confirm the regulation of tubulin 5β by Drosha,
we next performed western blotting to detect tubulin 5β protein
expression in HeLa and SiHa cells that were transduced with the
Lenti-shDro or Lenti-shNC. Our results found that Drosha-KD reduced
the expression of tubulin 5β in cervical cancer cells (Fig. 3D).
Discussion
It is well known that Drosha is one of the key
enzymes involved in the maturation of miRNA, and the expression
level of this gene is robustly associated with the miRNA profile
(11). In the present study, for
the first time, we demonstrated the significant influence of
Drosha-KD on the proteomic profile.
Since as many as 60% of the human protein-coding
genes are regulated by miRNA machinery (49), the last decade has seen an
increasing number of studies that have focused on the function of
miRNAs in cancer. For example, a global upregulation of miRNAs was
observed in cervical cancer. Hence, the enzymes that affect miRNA
genesis are becoming new and very popular areas of research. As a
nuclear enzyme that initiates miRNA processing, Drosha regulates
the expression of most miRNAs. In Drosha overexpressing cervical
cancer cells, 45 microRNAs exhibited a significant association with
Drosha levels. The majority of these miRNAs (n=40, 88.9%) were
upregulated, with only five (11.1%) being downregulated (11). In the present study, we determined
that Drosha-KD altered the entire cell proteomic profile, an effect
that can be explained by the potent biological functions of Drosha
and miRNAs. Theoretically, the depletion of Drosha will decrease
mature miRNA expression levels, reduce the suppression of proteins
by miRNAs, and consequently alter protein expression. Notably, we
observed that several proteins were upregulated after Drosha-KD,
although the majority of the proteins with significantly altered
abundance were downregulated. This result is well correlated with
the alteration in miRNAs that results from Drosha overexpression
that was observed by Balaji et al and suggests the
complexity of the regulation of miRNA and protein expression.
We observed that Drosha-KD led to the suppression of
cell proliferation, clonogenesis, and migration in cervical cancer,
suggesting that a higher level of Drosha is associated with a more
invasive tumour phenotype. This effect may potentially be due to
the Drosha-KD-induced alteration in the proteomic profile,
particularly in the expression of cancer-related proteins.
As a cornerstone of the microtubule system,
β-tubulin plays an important role in mitosis, intracellular
trafficking, migration and the maintenance of cell shape (50). The lack or alteration of β-tubulin
can inhibit mitosis and cause cells to be delayed in the G2/M phase
(51). Additionally, the importance
of microtubules in mitotic spindle formation and chromosome
movement during cell division makes microtubules a major target for
chemotherapeutic drugs that are used to halt the uncontrolled
division of cancer cells (52). Our
findings suggest that Drosha may influence the response of tumours
to drugs that target microtubules, such as paclitaxol, and could be
used to treat bulky cervical cancer in combination with platinum as
a neoadjuvant chemotherapy.
In conclusion, our study demonstrates that the
upregulation of Drosha alters the proteomic profile of cervical
cancer cells. Furthermore, tubulin-β and the microtubule system are
affected by Drosha expression.
Acknowledgements
This study was supported by the China Postdoctoral
Science Foundation (grant no. 20100480904).
References
|
1
|
Yang W, Lee DY and Ben-David Y: The roles
of microRNAs in tumorigenesis and angiogenesis. Int J Physiol
Pathophysiol Pharmacol. 3:140–155. 2011.PubMed/NCBI
|
|
2
|
Calin GA, Sevignani C, Dumitru CD, et al:
Human microRNA genes are frequently located at fragile sites and
genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krutzfeldt J, Rajewsky N, Braich R, et al:
Silencing of microRNAs in vivo with ‘antagomirs’. Nature.
438:685–689. 2005.
|
|
4
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
5
|
Lee Y, Ahn C, Han J, et al: The nuclear
RNase III Drosha initiates microRNA processing. Nature.
425:415–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bernstein E, Caudy AA, Hammond SM and
Hannon GJ: Role for a bidentateribonuclease in the initiation step
of RNA interference. Nature. 409:363–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Macrae IJ, Zhou K, Li F, et al: Structural
basis for double-stranded RNA processing by Dicer. Science.
311:195–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atkin NB: Cytogenetics of carcinoma of the
cervix uteri: a review. Cancer Genet Cytogenet. 95:33–39. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heselmeyer K, Macville M, Schrock E, et
al: Advanced-stage cervical carcinomas are defined by a recurrent
pattern of chromosomal aberrations revealing high genetic
instability and a consistent gain of chromosome arm 3q. Genes
Chromosomes Cancer. 19:233–240. 1997. View Article : Google Scholar
|
|
10
|
Kirchhoff M, Rose H, Petersen BL, et al:
Comparative genomic hybridization reveals a recurrent pattern of
chromosomal aberrations in severe dysplasia/carcinoma in situ of
the cervix and in advanced-stage cervical carcinoma. Genes
Chromosomes Cancer. 24:144–150. 1999. View Article : Google Scholar
|
|
11
|
Muralidhar B, Winder D, Murray M, et al:
Functional evidence that Drosha overexpression in cervical squamous
cell carcinoma affects cell phenotype and microRNA profiles. J
Pathol. 224:496–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muralidhar B, Goldstein LD, Ng G, et al:
Global microRNA profiles in cervical squamous cell carcinoma depend
on Drosha expression levels. J Pathol. 212:368–377. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pett MR, Alazawi WO, Roberts I, et al:
Acquisition of high-level chromosomal instability is associated
with integration of human papillomavirus type 16 in cervical
keratinocytes. Cancer Res. 64:1359–1368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pett MR, Herdman MT, Palmer RD, et al:
Selection of cervical keratinocytes containing integrated HPV16
associates with episome loss and an endogenous antiviral response.
Proc Natl Acad Sci USA. 103:3822–3827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boudreau RL, Spengler RM and Davidson BL:
Rational design of therapeutic siRNAs: minimizing off-targeting
potential to improve the safety of RNAi therapy for Huntington’s
disease. Mol Ther. 19:2169–2177. 2011.PubMed/NCBI
|
|
16
|
Cai J, Tang H, Xu L, Wang X, et al:
Fibroblasts in omentum activated by tumor cells promote ovarian
cancer growth, adhesion and invasiveness. Carcinogenesis. 33:20–29.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Diao S, Zhang JF, Wang H, et al: Proteomic
identification of microRNA-122a target proteins in hepatocellular
carcinoma. Proteomics. 10:3723–3731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dyballa N and Metzger S: Fast and
sensitive colloidal Coomassie G-250 staining for proteins in
polyacrylamide gels. J Vis Exp. 30:pii1431. 2009.PubMed/NCBI
|
|
19
|
Wiesen KM, Xia S, Yang CP and Horwitz SB:
Wild-type class I beta-tubulin sensitizes Taxol-resistant breast
adenocarcinoma cells harboring a beta-tubulin mutation. Cancer
Lett. 257:227–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmidt M, Schler G, Gruensfelder P and
Hoppe F: Differential gene expression in a paclitaxel-resistant
clone of a head and neck cancer cell line. Eur Arch
Otorhinolaryngol. 263:127–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akoumianaki T, Kardassis D, Polioudaki H,
et al: Nucleocyto plasmic shuttling of soluble tubulin in mammalian
cells. J Cell Sci. 122:1111–1118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li C, Harada A and Oh Y: IGFBP-3
sensitizes antiestrogen-resistant breast cancer cells through
interaction with GRP78. Cancer Lett. 325:200–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luvsandagva B, Nakamura K, Kitahara Y, et
al: GRP78 induced by estrogen plays a role in the chemosensitivity
of endometrial cancer. Gynecol Oncol. 126:132–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou X, You F, Chen H and Jiang Z:
Poly(C)-binding protein 1 (PCBP1) mediates housekeeping degradation
of mitochondrial antiviral signaling (MAVS). Cell Res. 22:717–727.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lian WX, Yin RH, Kong XZ, et al: THAP11, a
novel binding protein of PCBP1, negatively regulates CD44
alternative splicing and cell invasion in a human hepatoma cell
line. FEBS Lett. 586:1431–1438. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao J, Wang K, Yue Y, et al: Selective
expression of S100A11 in lung cancer and its role in regulating
proliferation of adenocarcinomas cells. Mol Cell Biochem.
359:323–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu XG, Wang XP, Li WF, et al:
Ca2+-binding protein S100A11: A novel diagnostic marker
for breast carcinoma. Oncol Rep. 23:1301–1308. 2010.
|
|
28
|
Zhou D, Mei Q, Li J and He H: Cyclophilin
A and viral infections. Biochem Biophys Res Commun. 424:647–650.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang M, Dai C, Zhu H, et al: Cyclophilin
A promotes human hepatocellular carcinoma cell metastasis via
regulation of MMP3 and MMP9. Mol Cell Biochem. 357:387–395. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen CY, Fang HY, Chiou SH, et al:
Sumoylation of eukaryotic elongation factor 2 is vital for protein
stability and anti-apoptotic activity in lung adenocarcinoma cells.
Cancer Sci. 102:1582–1589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura J, Aoyagi S, Nanchi I, et al:
Overexpression of eukaryotic elongation factor eEF2 in
gastrointestinal cancers and its involvement in G2/M progression in
the cell cycle. Int J Oncol. 34:1181–1189. 2009.PubMed/NCBI
|
|
32
|
Wang J, Ying G, Wang J, et al:
Characterization of phospho-glycerate kinase-1 expression of
stromal cells derived from tumor microenvironment in prostate
cancer progression. Cancer Res. 70:471–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zieker D, Königsrainer I, Tritschler I, et
al: Phosphoglycerate kinase 1 a promoting enzyme for peritoneal
dissemination in gastric cancer. Int J Cancer. 126:1513–1520.
2010.PubMed/NCBI
|
|
34
|
He LR, Zhao HY, Li BK, et al:
Overexpression of eIF5A-2 is an adverse prognostic marker of
survival in stage I non-small cell lung cancer patients. Int J
Cancer. 129:143–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee NP, Tsang FH, Shek FH, et al:
Prognostic significance and therapeutic potential of eukaryotic
translation initiation factor 5A (eIF5A) in hepatocellular
carcinoma. Int J Cancer. 127:968–976. 2010.PubMed/NCBI
|
|
36
|
Hossain MN, Fuji M, Miki K, Endoh M and
Ayusawa D: Downregulation of hnRNP C1/C2 by siRNA sensitizes HeLa
cells to various stresses. Mol Cell Biochem. 296:151–157. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu D, Matsushita K, Matsubara H, Nomura F
and Tomonaga T: An alternative splicing isoform of eukaryotic
initiation factor 4H promotes tumorigenesis in vivo and is a
potential therapeutic target for human cancer. Int J Cancer.
128:1018–1030. 2011. View Article : Google Scholar
|
|
38
|
Bailey SD, Xie C, Do R, et al: Variation
at the NFATC2 locus increases the risk of thiazolidinedione-induced
edema in the Diabetes REduction Assessment with ramipril and
rosiglitazone Medication (DREAM) study. Diabetes Care.
33:2250–2253. 2010. View Article : Google Scholar
|
|
39
|
Brüning-Richardson A, Bond J, Alsiary R,
et al: NuMA overexpression in epithelial ovarian cancer. PLoS One.
7:e389452012.
|
|
40
|
Kilpivaara O, Rantanen M, Tamminen A, et
al: Comprehensive analysis of NuMA variation in breast cancer. BMC
Cancer. 8:712008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chang P, Coughlin M and Mitchison TJ:
Interaction between Poly(ADP-ribose) and NuMA contributes to
mitotic spindle pole assembly. Mol Biol Cell. 20:4575–4585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Genovese G, Ghosh P, Li H, et al: The
tumor suppressor HINT1 regulates MITF and β-catenin transcriptional
activity in melanoma cells. Cell Cycle. 11:2206–2215.
2012.PubMed/NCBI
|
|
43
|
Huang H, Wei X, Su X, et al: Clinical
significance of expression of Hint1 and potential epigenetic
mechanism in gastric cancer. Int J Oncol. 38:1557–1564.
2011.PubMed/NCBI
|
|
44
|
Tang Z, Yuan S, Hu Y, et al:
Over-expression of GAPDH in human colorectal carcinoma as a
preferred target of 3-bromo-pyruvate propyl ester. J Bioenerg
Biomembr. 44:117–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang Q, Lan F, Zheng Z, et al: Akt2
kinase suppresses glyceraldehyde-3-phosphate dehydrogenase
(GAPDH)-mediated apoptosis in ovarian cancer cells via
phosphorylating GAPDH at threonine 237 and decreasing its nuclear
translocation. J Biol Chem. 286:42211–42220. 2011. View Article : Google Scholar
|
|
46
|
Nicholls C, Li H and Liu JP: GAPDH: a
common enzyme with uncommon functions. Clin Exp Pharmacol Physiol.
39:674–679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hashimoto S, Hirose M, Hashimoto A, et al:
Targeting AMAP1 and cortactin binding bearing an atypical src
homology 3/proline interface for prevention of breast cancer
invasion and metastasis. Proc Natl Acad Sci USA. 103:7036–7041.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Müller T, Stein U, Poletti A, et al: ASAP1
promotes tumor cell motility and invasiveness, stimulates
metastasis formation in vivo, and correlates with poor survival in
colorectal cancer patients. Oncogene. 29:2393–2403. 2010.PubMed/NCBI
|
|
49
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shibazaki M, Maesawa C, Akasaka K, et al:
Transcriptional and post-transcriptional regulation of βIII-tubulin
protein expression in relation with cell cycle-dependent regulation
of tumor cells. Int J Oncol. 40:695–702. 2012.
|
|
51
|
Nogales E: Structural insights into
microtubule function. Annu Rev Biochem. 69:277–302. 2000.
View Article : Google Scholar
|
|
52
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|