Introduction
Liver cancer is the third leading cause of cancer
deaths worldwide and was responsible for 696,000 deaths in 2008
(1). HCC, which is the main type of
primary liver cancer, is more difficult to treat than many other
cancers because HCC is generally induced as a consequence of
underlying liver diseases, such as chronic hepatitis and liver
cirrhosis. Surgical resection and percutaneous local ablation are
curative treatments, but these applications are limited to HCC
patients with well-preserved liver function; metastasis is quite
common (2). In addition,
postoperative recurrence is a persisting issue (3). Liver transplantation is another
curative treatment of HCC with liver cirrhosis, but the lack of
donor organs is the main restricting factor for liver
transplantations and contributes to prolonged waiting time
(4). Systemic therapies have not
been shown to be effective for advanced HCC. Thus, there is a
strong demand for new curative approaches to HCC.
Angiogenesis plays an important role in the
proliferation and metastasis of solid tumors (5). HCC is a hypervascular tumor
characterized by vigorous neovascularization. Neovascularization is
pivotal in the growth and progression of HCC and increases during
the early phase of HCC development (6). Without neovascularization, tumors
cannot become larger than a few cubic mm in size, and they remain
in a state of tumor dormancy (7,8). Thus,
antiangiogenic treatments for solid tumors have been explored as a
new strategy to suppress tumor growth and progression.
Several studies have shown that the expression of
many angiogenic factors is closely related to the growth and
metastasis of HCC (9,10). However, the expression and
interaction of a wide range of angiogenic factors remains obscure
in HCC. In the present study, we examined the expression of various
angiogenic factors related to hepatocarcinogenesis.
Materials and methods
Tissue samples
Human tissue samples of HCC, cholangiocellular
carcinoma (CCC), colorectal cancer and esophageal cancer with the
adjacent tissues were obtained during surgery or liver biopsy from
9 patients (7 males and 2 females; mean age, 70.8±4.4 years; range,
51–92 years). None of the patients received any chemotherapy or
radiotherapy before surgery. The use of human specimens was
approved by the Human Subjects Committee of Kagawa University
School of Medicine.
Cell culture
PLC/PRF/5, Hep 3B, HuH7, HLE, HLF and Li-7 cells,
kind gifts from the Health Science Research Resource Bank (Osaka,
Japan) and the Cell Resource Center for Biomedical Research
(Institute of Development, Aging and Cancer, Tohoku University,
Miyagi, Japan), were used as human HCC cell lines. ACBRI3716 cells
were obtained from the Applied Cell Biology Research Institute
(Kirkland, WA, USA) and used as normal human hepatocytes. These
cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco
BRL, Tokyo, Japan) supplemented with 10% fetal calf serum (Gibco
BRL), 100 μg/ml penicillin and 100 μg/ml streptomycin at 37°C under
5% CO2 in air.
Preparation of protein from tissues and
cell lines
We thawed and homogenized tissue samples with
PRO-PREP™ (iNtRON Biotechnology, Inc., Seoul, Korea) solution
containing 1 mM of each of the protease inhibitors
phenylmethylsulfonyl fluoride and ethylenediaminetetraacetic acid,
1 μM each of pepstatin A and leupeptin and 0.1 μM aprotinin. The
homogenate was centrifuged at 13,000 × g for 5 min after incubation
for 20–30 min on ice. Equivalent amounts of the supernatant from
tissue samples were used for antibody arrays. Liver-infiltrating
mononuclear cells (LMNCs) were separated by Ficoll-Hypaque density
gradient centrifugation (Histopaque; Sigma-Aldrich Co., St. Louis,
MO, USA). We harvested the cell pellet by centrifuging at 13,000 ×
g for 10–20 sec. The pellet was mixed well with PRO-PREP solution
and incubated on ice for 10–20 min. The cell lysate was centrifuged
at 13,000 × g for 5 min after incubation. Equivalent amounts of the
supernatant from cell lines were used for antibody arrays.
Protein assay
The protein concentration was determined according
to the Bradford dye-binding assay (11).
Antibody array to screen angiogenic
factors
To detect angiogenic factors, we used the
TranSignal™ Angiogenesis Antibody Array (Panomics Inc., Redwood,
CA, USA), in which 19 different antibodies against angiogenic
factors are immobilized at predetermined positions on the membrane
(Table I). The angiogenesis
antibody array was based on the sandwich ELISA method for detecting
protein (12). The assay was
performed following the manufacturer’s protocol. The samples of
tissue extracts and cell lysates were incubated with the array
membranes at the same concentration of 4 mg/ml. Then,
biotin-labeled detection antibodies were added to the array
membranes. The antibody-protein complexes on the array membranes
were visualized using streptavidin-HRP to determine which active
angiogenic factors were present in the samples. Immunoreactive
proteins were visualized with an enhanced chemiluminescence
detection system (Amersham Japan Co., Tokyo, Japan) on a radiograph
film. The array data were normalized using positive control signal.
The average local background signal was subtracted from average
signal intensity of duplicated spots of each antibody.
 | Table IProfile of the TranSignal angiogenesis
antibody array. |
Table I
Profile of the TranSignal angiogenesis
antibody array.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|
| A | pos | pos | pos | pos | pos | pos | pos | pos |
| B | Ang | Ang | IL-1α | IL-1α | aFGF | aFGF | IFN-γ | IFN-γ |
| C | G-CSF | G-CSF | IL-1β | IL-1β | bFGF | bFGF | IL-12 | IL-12 |
| D | HGF | HGF | IL-6 | IL-6 | TNF-α | TNF-α | IP-10 | IP-10 |
| E | Leptin | Leptin | IL-8 | IL-8 | TGF-α | TGF-α | TIMP-1 | TIMP-1 |
| F | VEGF | VEGF | PIGF | PIGF | neg | neg | TIMP-2 | TIMP-2 |
Results
Expression of angiogenic factors in
normal liver and various liver diseases
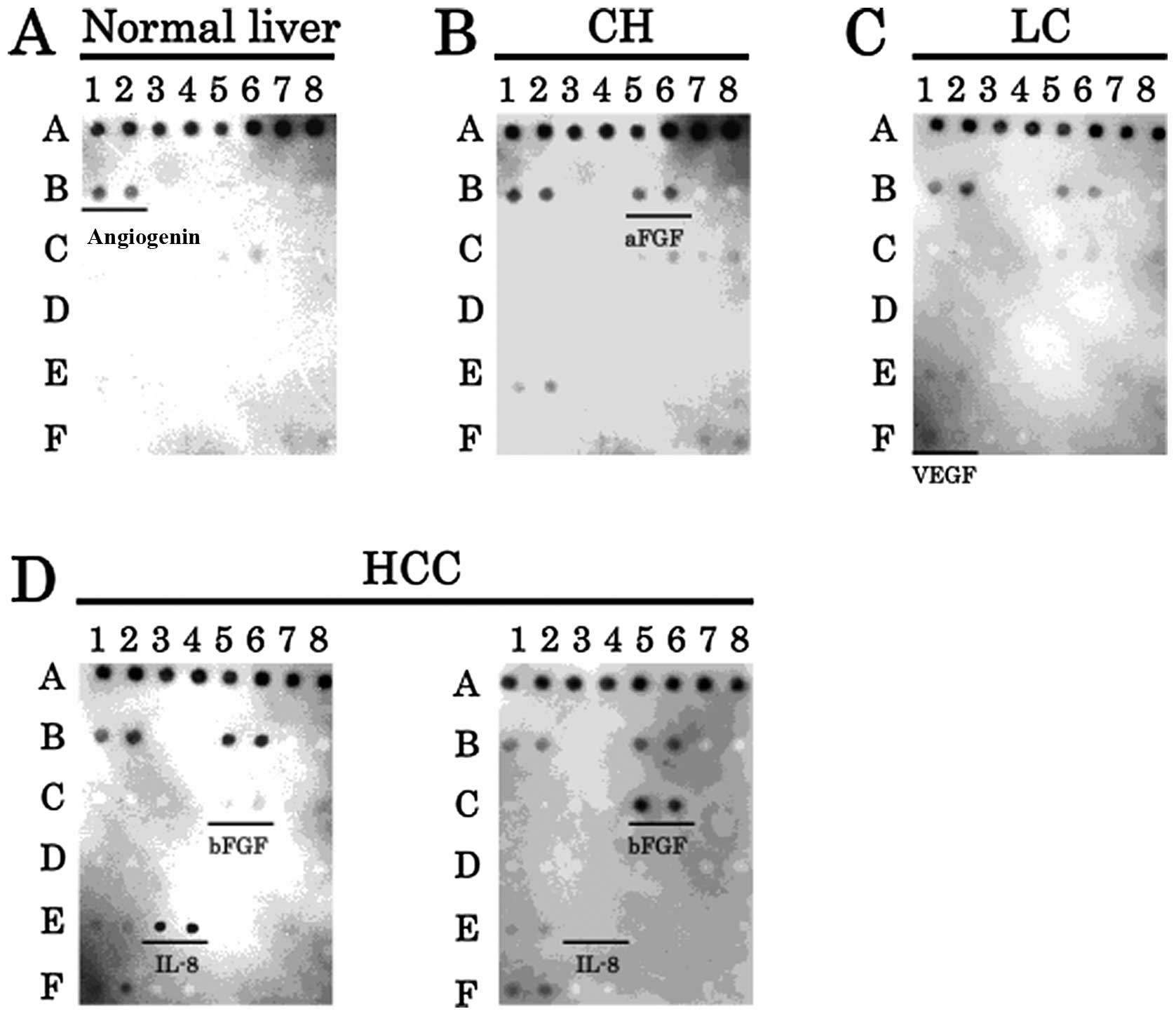
We used angiogenesis antibody arrays to investigate
which angiogenic factors were expressed in normal human liver and
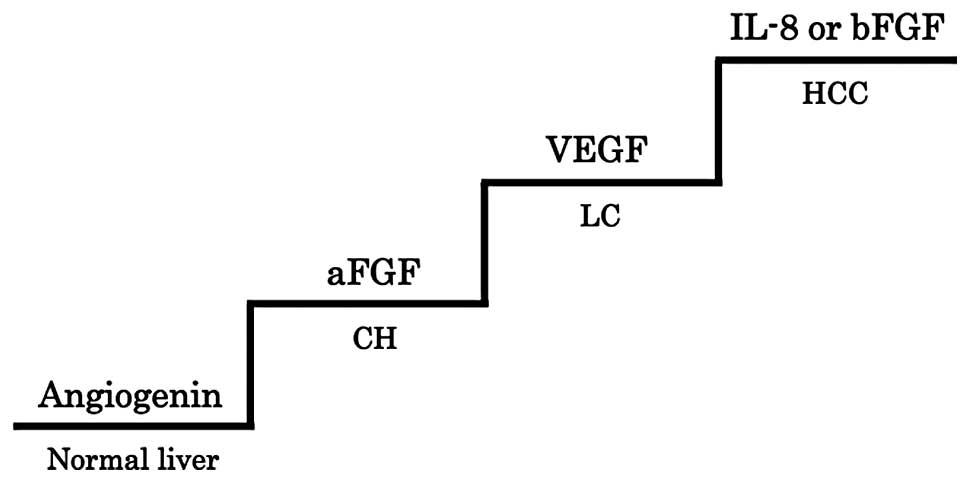
various liver diseases. The expression of angiogenin was detected
in normal liver (Fig. 1A). The
expression of aFGF was found to increase from normal liver to
chronic hepatitis (Fig. 1B).
Furthermore, VEGF was upregulated from chronic hepatitis to liver
cirrhosis (Fig. 1C). Noteworthy,
two different expression patterns of angiogenic factors were
detected in HCC. An IL-8 overexpression with weak bFGF expression
pattern, or a bFGF overexpression pattern was observed. Therefore,
IL-8 or bFGF was involved in the development of HCC from liver
cirrhosis (Fig. 1D).
Expression of angiogenic factors in
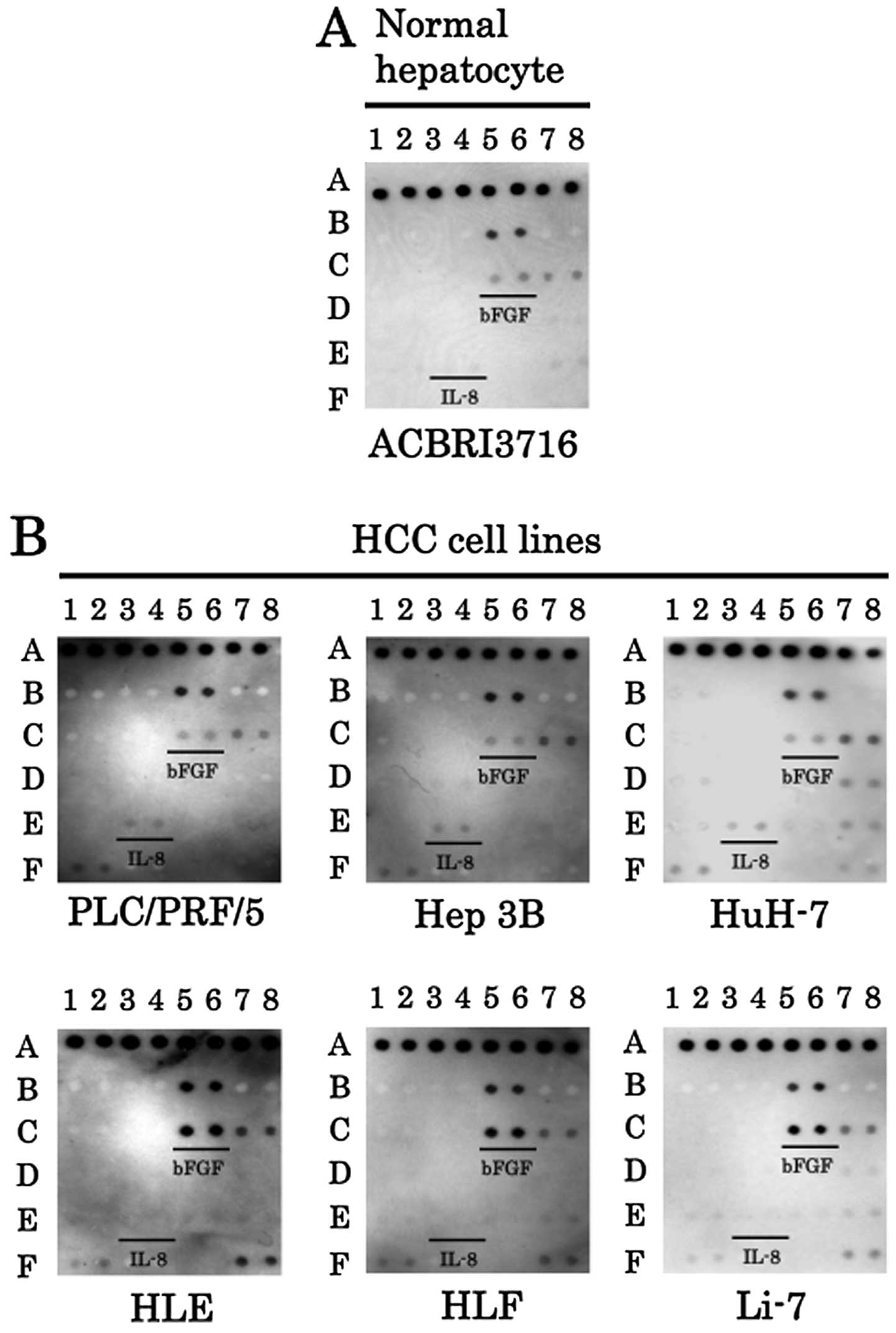
normal human hepatocyte and HCC cell lines
We also used angiogenesis antibody arrays to examine
which angiogenic factors were expressed in the normal human
hepatocyte and various HCC cell lines. The expression of angiogenin
was not detected from the normal hepatocyte cell line or from
various HCC cell lines (Fig. 2A).
The expression of IL-8 was elevated in the HCC cell lines
PLC/PRF/5, Hep 3B and HuH-7. In contrast, although the expression
of IL-8 was not detected, bFGF was strongly expressed in the other
HCC cell lines HLE, HLF, and Li-7, compared to the normal
hepatocyte cell line (Figs. 2A and
B). Thus, either IL-8 or bFGF was upregulated in HCC cell
lines.
Expression of angiogenic factors in HCC
with and without liver-infiltrating mononuclear cells (LMNCs)
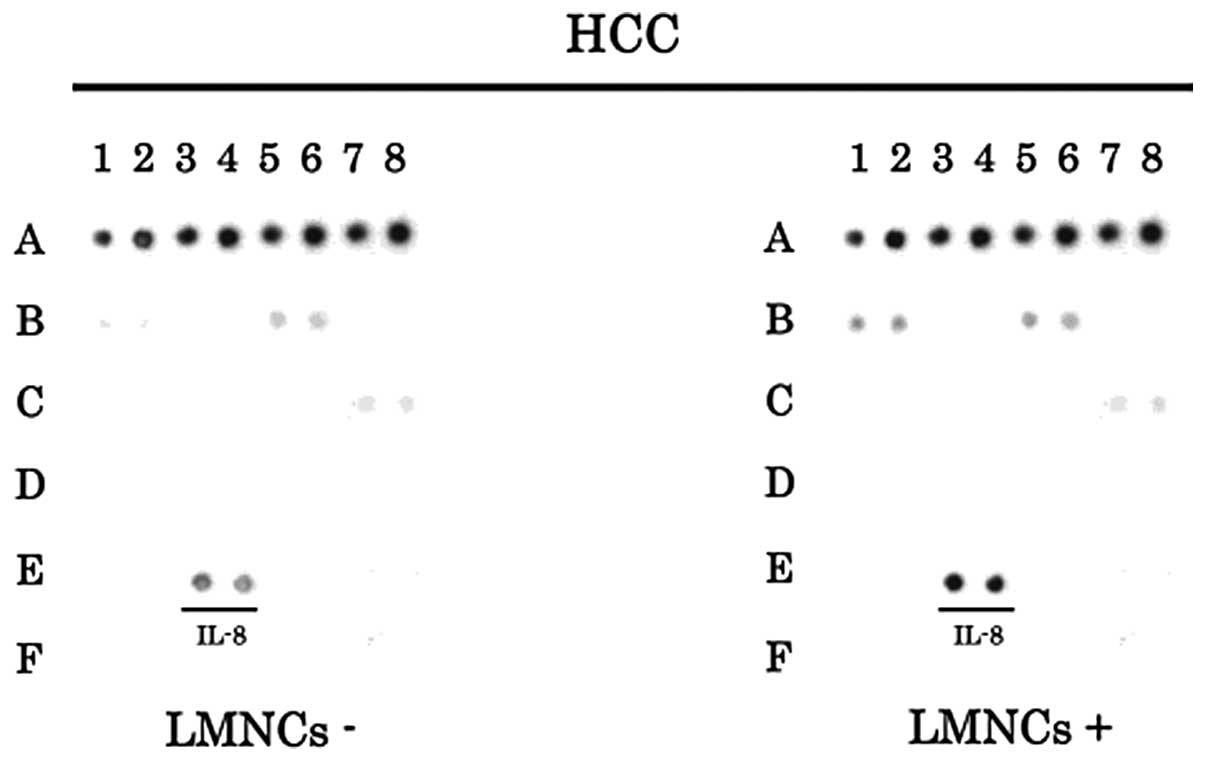
To evaluate the involvement of LMNCs in the
expression of angiogenic factors in HCC tissues, we examined the
samples of HCC tissues with or without LMNCs by angiogenesis
antibody arrays. The increased expression of IL-8 can be observed
in HCC samples with or without LMNCs (Fig. 3). These results suggested that IL-8
was actively produced not only in LMNCs but also in HCC cells.
Expression of angiogenic factors in
various malignant gastrointestinal tumors
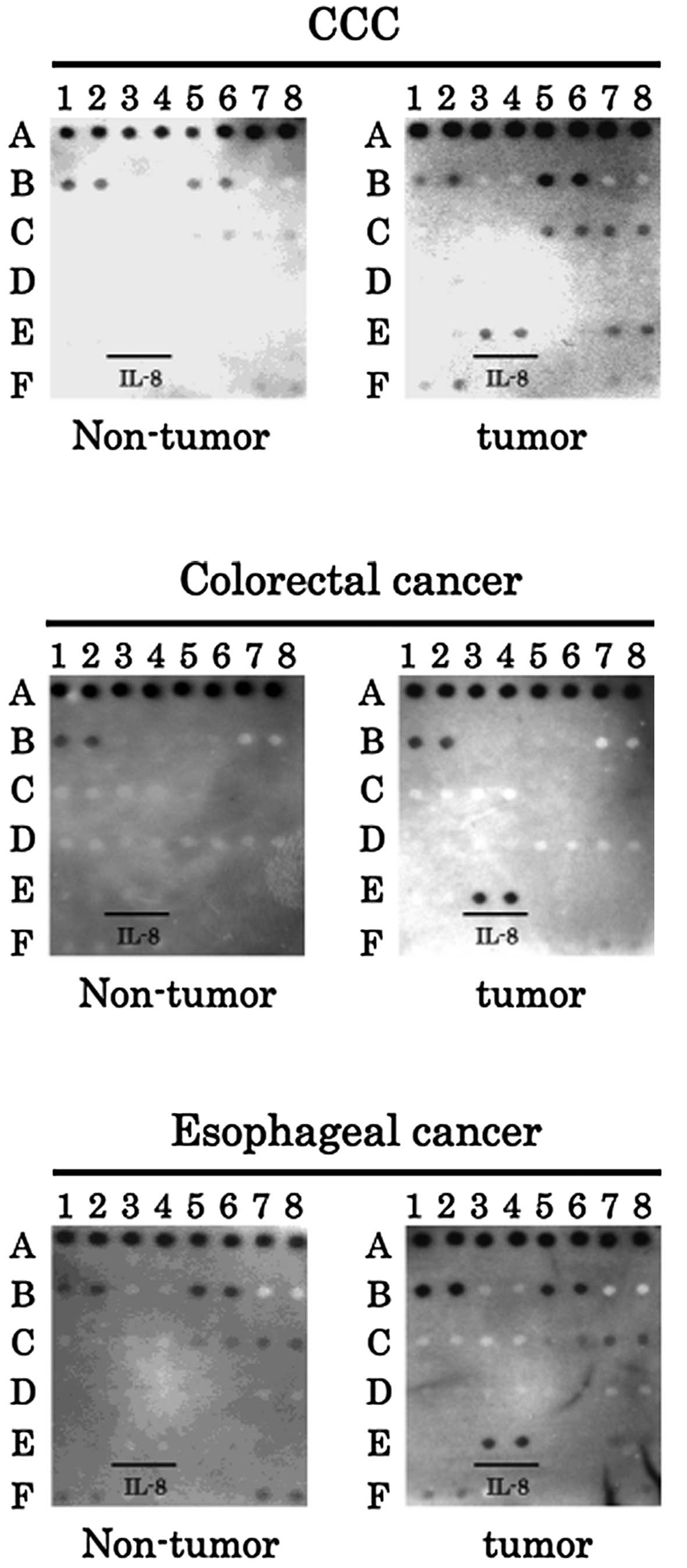
In order to determine angiogenic factors in other
malignant tumors, IL-8 was examined in cholangiocellular carcinoma
(CCC), colorectal cancer and esophageal cancer samples. Notably,
IL-8 was strongly expressed in the cancerous tissues, but not in
non-cancerous tissues from each organ (Fig. 4). These results were similar to the
data from the liver. Therefore, IL-8 is suggested to also play an
important role in gastrointestinal cancers (Fig. 5).
Discussion
Angiogenesis is a prime regulator of tumor growth,
and anti-angiogenic factors are likely to become an important
component of therapeutic strategies (13). It has been suggested that
angiogenesis is an early event in tumorigenesis (14). HCC is a typical hypervascular tumor
that is characterized by neovascularization. Many angiogenic
factors have been studied in HCC, and several anti-angiogenic
therapies have been tested in animals and patients (15,16).
Of note, in our present study, the expression of either IL-8 or
bFGF was enhanced in human HCC tissues and hepatocellular carcinoma
cell lines (Figs. 1 and 2). IL-8, a member of the CXC chemokine
family, is a potent angiogenic stimulator (17,18).
IL-8 has recently been shown to contribute to human cancer
progression through its potential functions as a mitogenic,
angiogenic, and motogenic factor (19). Angiogenesis and tumor growth are
inhibited by the downregulation or neutralization of IL-8 in
several tumor models (20,21). In addition, we demonstrated that
IL-8 was produced from human HCC tissues without LMNCs (Fig. 3). Moore et al have reported
that some HCC cell lines secrete IL-8 (21). In addition, the serum level of IL-8
increased prior to the development of HCCs and increased further
after the tumors appeared (22).
Surprisingly, IL-8 was also enhanced in other cancer tissues, such
as cholangiocarcinoma, colon cancer and esophageal cancer (Fig. 4). These results suggest that IL-8
may be closely involved in carcinogenesis and the progression of
various cancers, including HCC.
bFGF is a potentially important angiogenic
stimulator. The serum level of bFGF is correlated with tumor
invasiveness and postoperative recurrence in HCC (23). In the present study, our data also
demonstrated that bFGF was upregulated in human HCC samples and
some HCC cell lines (Figs. 1 and
2). Yoshiji et al have
reported that bFGF synergistically augments VEGF-mediated HCC
development and angiogenesis, partly by the induction of VEGF
through KDR/Flk-1 (24). These
studies strongly support our results that bFGF involvement in
angiogenesis is important for HCC development.
However, there is little documentation of the
relationship between IL-8 and bFGF to date. Our studies
demonstrated two patterns for the expression of angiogenic factors
in HCC cell lines and tissues: i) high IL-8 and low bFGF expression
and ii) high bFGF and no IL-8 expression. Angiogenic growth
factors, including VEGF, IL-8 and bFGF, are regulated in tumor
cells by epidermal growth factor receptor (EGFR) signaling, which
plays an important role in tumorigenesis (25,26).
In the EGFR family, the overexpression of ErbB-2 also leads to the
increased expression of angiogenic factors, whereas treatment with
anti-EGFR or anti-ErbB-2 agents produces a significant reduction in
the synthesis of these proteins by cancer cells (25). In our previous study, ErbB-2 was
found to be activated in HCC cell lines and tissues. We determined
that the inhibition of ErbB-2 by an ErbB-2 targeting drug,
trastuzumab, retarded the tumor development from HCC cells
(27). Therefore, these results
suggest that the identification of IL-8 and bFGF may be valuable as
downstream targets of EGFR for the treatment of individual patients
with HCC.
In conclusion, our findings showed that the
upregulation of either IL-8 or bFGF is closely related to HCC
development from liver cirrhosis. The results may be helpful in
studying whether IL-8 and bFGF could be promising targets for
anti-angiogenic therapies. The feasibility of utilizing protein
arrays in this study suggests that arrays can be a useful tool for
detecting the expression of angiogenic factors contributing to
hepatocarcinogenesis and thereby identifying novel therapies for
HCC.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blum HE: Hepatocellular carcinoma: therapy
and prevention. World J Gastroenterol. 11:7391–7400.
2005.PubMed/NCBI
|
|
3
|
Portolani N, Coniglio A, Ghidoni S, et al:
Early and late recurrence after liver resection for hepatocellular
carcinoma: prognostic and therapeutic implications. Ann Surg.
243:229–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poon RT and Fan ST: Resection prior to
liver transplantation for hepatocellular carcinoma: a strategy of
optimizing the role of resection and transplantation in cirrhotic
patients with preserved liver function. Liver Transpl. 10:813–815.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grepin R and Pages G: Molecular mechanisms
of resistance to tumour anti-angiogenic strategies. J Oncol.
2010:8356802010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanz-Cameno P, Trapero-Marugan M, Chaparro
M, Jones EA and Moreno-Otero R: Angiogenesis: from chronic liver
inflammation to hepatocellular carcinoma. J Oncol. 2010:2721702010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naumov GN, Folkman J and Straume O: Tumor
dormancy due to failure of angiogenesis: role of the
microenvironment. Clin Exp Metastasis. 26:51–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zappala G, McDonald PG and Cole SW: Tumor
dormancy and the neuroendocrine system: an undisclosed connection?
Cancer Metastasis Rev. 32:189–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hisai H, Kato J, Kobune M, et al:
Increased expression of angiogenin in hepatocellular carcinoma in
correlation with tumor vascularity. Clin Cancer Res. 9:4852–4859.
2003.PubMed/NCBI
|
|
10
|
Pang R and Poon RT: Angiogenesis and
antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett.
242:151–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crowther JR: ELISA. Theory and practice
Methods. Mol Biol. 42:1–218. 1995.PubMed/NCBI
|
|
13
|
Moserle L and Casanovas O:
Anti-angiogenesis and metastasis: a tumour and stromal cell
alliance. J Intern Med. 273:128–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rak J and Klement G: Impact of oncogenes
and tumor suppressor genes on deregulation of hemostasis and
angiogenesis in cancer. Cancer Metastasis Rev. 19:93–96. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun HC and Tang ZY: Angiogenesis in
hepatocellular carcinoma: the retrospectives and perspectives. J
Cancer Res Clin Oncol. 130:307–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwartz M, Roayaie S and Konstadoulakis
M: Strategies for the management of hepatocellular carcinoma. Nat
Clin Pract Oncol. 4:424–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qazi BS, Tang K and Qazi A: Recent
advances in underlying pathologies provide insight into
interleukin-8 expression-mediated inflammation and angiogenesis.
Int J Inflam. 2011:9084682011.PubMed/NCBI
|
|
18
|
Teicher BA: Antiangiogenic agents and
targets: A perspective. Biochem Pharmacol. 81:6–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hussain F, Wang J, Ahmed R, et al: The
expression of IL-8 and IL-8 receptors in pancreatic adenocarcinomas
and pancreatic neuroendocrine tumours. Cytokine. 49:134–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arenberg DA, Kunkel SL, Polverini PJ,
Glass M, Burdick MD and Strieter RM: Inhibition of interleukin-8
reduces tumorigenesis of human non-small cell lung cancer in SCID
mice. J Clin Invest. 97:2792–2802. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moore BB, Arenberg DA, Stoy K, et al:
Distinct CXC chemokines mediate tumorigenicity of prostate cancer
cells. Am J Pathol. 154:1503–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishii Y, Sakamoto T, Ito R and Yanaga K:
Anti-angiogenic therapy on hepatocellular carcinoma development and
progression. J Surg Res. 158:69–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poon RT, Ng IO, Lau C, Yu WC, Fan ST and
Wong J: Correlation of serum basic fibroblast growth factor levels
with clinicopathologic features and postoperative recurrence in
hepatocellular carcinoma. Am J Surg. 182:298–304. 2001. View Article : Google Scholar
|
|
24
|
Yoshiji H, Kuriyama S, Yoshii J, et al:
Synergistic effect of basic fibroblast growth factor and vascular
endothelial growth factor in murine hepatocellular carcinoma.
Hepatology. 35:834–842. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Luca A, Carotenuto A, Rachiglio A, et
al: The role of the EGFR signaling in tumor microenvironment. J
Cell Physiol. 214:559–567. 2008.PubMed/NCBI
|
|
26
|
Normanno N, Bianco C, De Luca A, Maiello
MR and Salomon DS: Target-based agents against ErbB receptors and
their ligands: a novel approach to cancer treatment. Endocr Relat
Cancer. 10:1–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu S, Gong J, Morishita A, et al: Use of
protein array technology to investigate receptor tyrosine kinases
activated in hepatocellular carcinoma. Exp Ther Med. 2:399–403.
2011.PubMed/NCBI
|