Introduction
Pituitary adenomas comprise 10–15% of all
intracranial neoplasms (1). Despite
being benign tumors in most cases, nearly 35% of pituitary adenomas
display aggressive biological activity as demonstrated by
clinically evident local invasion (2). Although surgery, radiotherapy and
pharmaceutical therapy are routinely employed, tumor recurrence of
these aggressive adenomas still remains very high, resulting in
high morbidity and mortality (3).
Temozolomide (TMZ) has been shown to display a
promising antitumor effect in certain cases used as the last-line
treatment in aggressive pituitary adenomas (4–6). TMZ
is an alkylating chemotherapeutic drug capable of crossing the
blood-brain barrier, and has been widely used to treat refractory
glioblastoma multiforme and neuroendocrine tumors (7,8).
However, several clinical trials have shown that the efficacy of
TMZ may be dependent on O6-methylguanine-DNA
methyltransferase (MGMT) expression and is prone to be poor in
tumors with high MGMT expression (9). Since TMZ is only used as the last line
therapy for aggressive pituitary adenomas resistant to conventional
therapies, it is necessary to develop novel therapeutic approaches
to improve the TMZ efficiency in pituitary adenomas.
MGMT is a DNA repair kinase and can remove the
methyl/alkyl group from the O6-position of guanine,
thereby preventing TMZ-induced DNA damage (9). Modulation of MGMT expression has been
proposed as a mean to sensitize tumors to TMZ. Recent studies have
shown that suppression of hypoxia-inducible factor 1α (HIF-1α) can
down-modulate MGMT expression, which in turn overrides glioblastoma
multiforme resistance to TMZ (10).
We previously described that HIF-1α is expressed in all types of
pituitary adenomas, and HIF-1α knockdown inhibits hemorrhagic
transformation in pituitary adenomas (11). Based on these findings, we
hypothesized that HIF-1α knockdown may also down-modulate MGMT
expression in pituitary adenomas, which in turn enhances the
efficacy of TMZ in pituitary adenomas.
In order to verify this hypothesis, we enrolled two
patients with huge aggressive pituitary adenomas resistant to both
conventional and TMZ therapies. The adenoma tissue of the two
patients, being identified as high MGMT-expressing tissue after the
first operation, was obtained during the second surgery. The tissue
from one patient was primary cultured; the tissue from the other
patient was equally dissociated and implanted into nude mice. Then,
we employed HIF-1α knockdown strategy to determine the potential
regulating effect of HIF-1α on the MGMT expression in vitro.
Furthermore, the correlation between the HIF-1α-MGMT axis and TMZ
resistance in pituitary adenomas was also examined in vitro
and in vivo. Our results revealed that HIF-1α regulates MGMT
expression, and HIF-1α knockdown or inhibitor 2-methoxyestradiol
(2ME) can enhance the efficacy of TMZ in pituitary adenoma cells
via the down-modulation of MGMT expression.
Materials and methods
Patients and samples
The present study was approved by the Research
Ethics Committee of the Henan University of Science and Technology,
Luoyang, China. Prior informed consent was obtained from the
patients. Between January 2010 and January 2013, we enrolled two
patients harboring huge aggressive pituitary adenomas who had
previously undergone one transsphenoidal surgical procedure, one
radiation therapy and systemic chemotherapy. After tumor regrowth
was confirmed and informed consent from the patients was obtained,
treatment with TMZ was offered at the standard therapeutic dose 150
mg/m2 for 5 of every 28 days (5/28) as one cycle for 3
cycles (12). After 3 cycles, no
response was observed based on MRI. Then, the transsphenoidal
surgical procedure was eventually performed in the two patients.
The samples were also obtained. For each sample, a small section
was fixed with 10% formalin and embedded in paraffin for histology
and immunohistochemical staining, the other was prepared for
primary cell culture or tumor implantation in nude mice.
Histology, immunohistochemical staining,
cell viability assay, western blot analysis and RT-PCR
All the procedures and reagents employed in the
present study are described in our previous reports (11,13,14).
Cell viability assay was carried out using Cell Counting Kit-8
(Boster Biological Technology). The following primers for RT-PCR
were used: human HIF-1α F, GCAAGACTTTCCTCAGTCGACACA and R, GCATCC
TGTACTGTCCTGTGGTGA; human MGMT F, ATGGAT GTTTGAGCGACACA and R,
ATAGAGCAAGGGCAG CGTTA; human GAPDH F, ACGGATTTGGTCGTATTGGG and R,
TGATTTTGGAGGGATGTCGC. The primary antibodies used include
anti-HIF-1α (1:1,000; Cell Signaling Technology), anti-MGMT
(1:1,000; Santa Cruz Biotechnology), anti-PCNA (1:2,000; Santa Cruz
Biotechnology), anti-Rad51 (1:1,000; Santa Cruz Biotechnology) and
anti-GAPDH (1:3,000; Santa Cruz Biotechnology).
Primary cultures of pituitary adenoma
tissue
After the tissue was obtained, we placed it in
complete DMEM and dissociated it using mechanic and enzymatic
methods according to the standard protocols immediately. Then,
cells were dispersed and filtered through a magnetic bead column
coated with anti-fibroblast antibodies. Then, the cells were plated
in 16- (106 cells/well) or 96-well plates
(104 cells/well) in the complete DMEM. Twenty-four hours
later, a portion of the cells was transiently transfected with 2 μg
predesigned siRNAs targeting human HIF-1α or control siRNA duplex
via Lipofectamine 2000 (Invitrogen) according to the manufacturer’s
instructions. The HIF-1α siRNA sequences were as follows: F,
CUGAUGACCAGCAACUUGA and R, UCAAGUUGCUGGUCAUCAG; the mock siRNA
sequences were: F, CGUACGCGGAAUACUUCGA and R, UCGAAGUAUUCCGCGUACG;
the knockdown efficiency was confirmed by RT-PCR and western blot
analysis. The cells were then cocultured with TMZ (100 μM) and/or
an HIF-1α inhibitor 2-methoxyestradiol (2ME, 2 μM) for 72 h, then
the cell viability assay, RT-PCR and western blot analysis were
carried out.
Pituitary adenoma xenografts
In order to increase the tumor formation rate, the
tissue were dissociated equally using mechanic methods and were
implanted subcutaneously into the lower rear flank of 3 6-week-old
athymic nude mice. Two weeks later, tumors were formed. Then,
tumors were dissociated again, and were equally implanted
subcutaneously into the lower rear flank of 12 6-week-old athymic
nude mice. After two weeks, the mice were injected with saline, TMZ
(3 mg/kg), 2ME (25 mg/kg), TMZ (3 mg/kg) plus 2ME (25 mg/kg) for 5
days (each group, n=3). After another three weeks, tumors were
harvested. Half of each tumor was frozen in liquid nitrogen for
protein analysis and the other half was fixed in 10% formalin for
histology analysis.
Statistical analysis
Statistical software SPSS 13.0 was used for
statistical analyses in the present study. Data are expressed as
means ± SD. The Student’s t-test and variance analysis were used in
the present study. Probability values of <0.05 were considered
to indicate a statistically significant result.
Results
Clinical features of the patients
In the present study, two patients with aggressive
prolactin (PRL)-secreting adenomas were enrolled. Male patient no.
1 had undergone transsphenoidal surgery against prolactin
(PRL)-secreting adenomas in our hospital at the age of 43 in June
2008 following inefficient bromocriptine treatment. In October
2010, the recurrent pituitary adenoma was confirmed by MRI, and
external irradiation was carried out and showed poor efficiency.
Because the patient refused reoperation after the radiation
therapy, TMZ treatment was offered for three months, and no
response was observed. The patient then received another
transsphenoidal surgery in July 2012, and the tissue was obtained.
For male patient no. 2, the transsphenoidal surgery was performed
in August 2009 after the lack of efficacy of a half year
bromocriptine treatment for prolactin (PRL)-secreting adenomas.
Then, MRI showed the recurrent pituitary adenoma in January 2011
and the radiation was initiated without success. The patient denied
the operation and received TMZ treatment for three months with no
response. The transsphenoidal surgery was then performed in
September 2012 and the tissue was obtained.
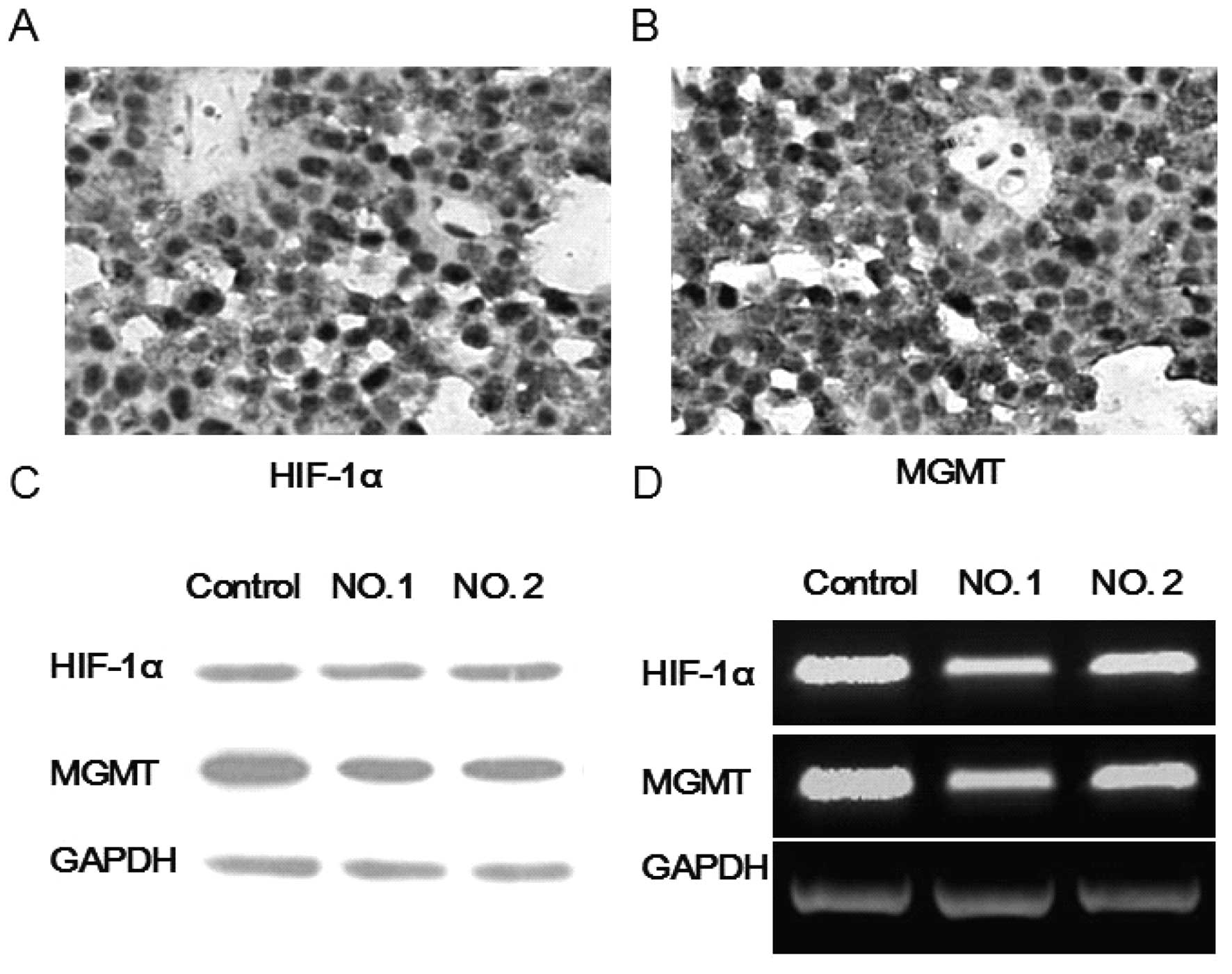
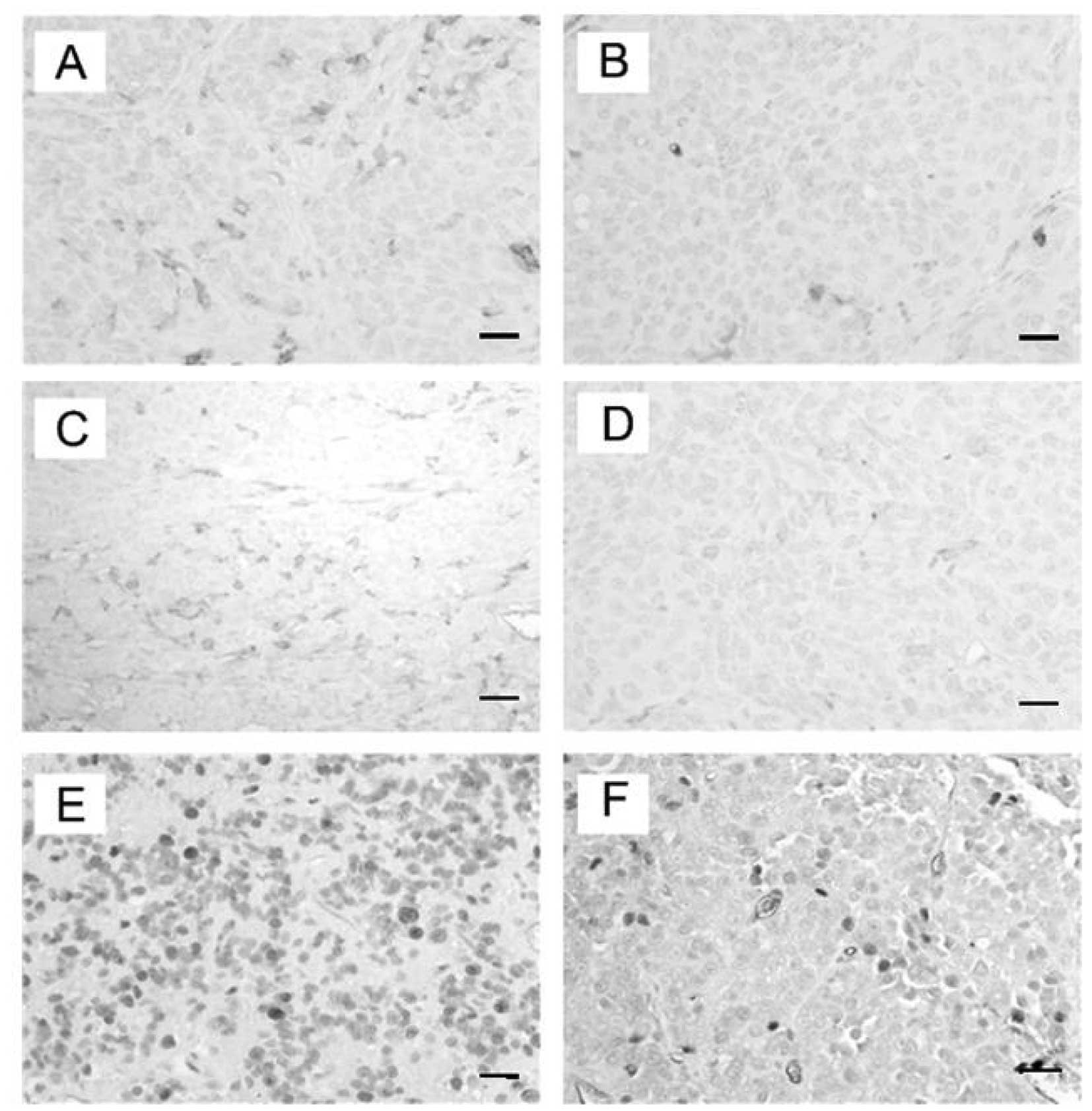
Characterization of HIF-1α and MGMT in
pituitary adenoma tissue
Although other studies reached an opposite
conclusion, MGMT expression has been shown to be responsible for
tumor resistance to TMZ in many reports (15–17).
Moreover, recent studies have shown that HIF-1α can modulate MGMT
expression and alter the tumor resistance to TMZ in gliomas
(10). Therefore, we investigated
the HIF-1α and MGMT expression in pituitary adenoma tissue via
RT-PCR, western blot analysis and immunohistochemistry. As
expected, we found high expression levels of HIF-1α and MGMT in the
pituitary adenoma tissues from the two patients (Fig. 1).
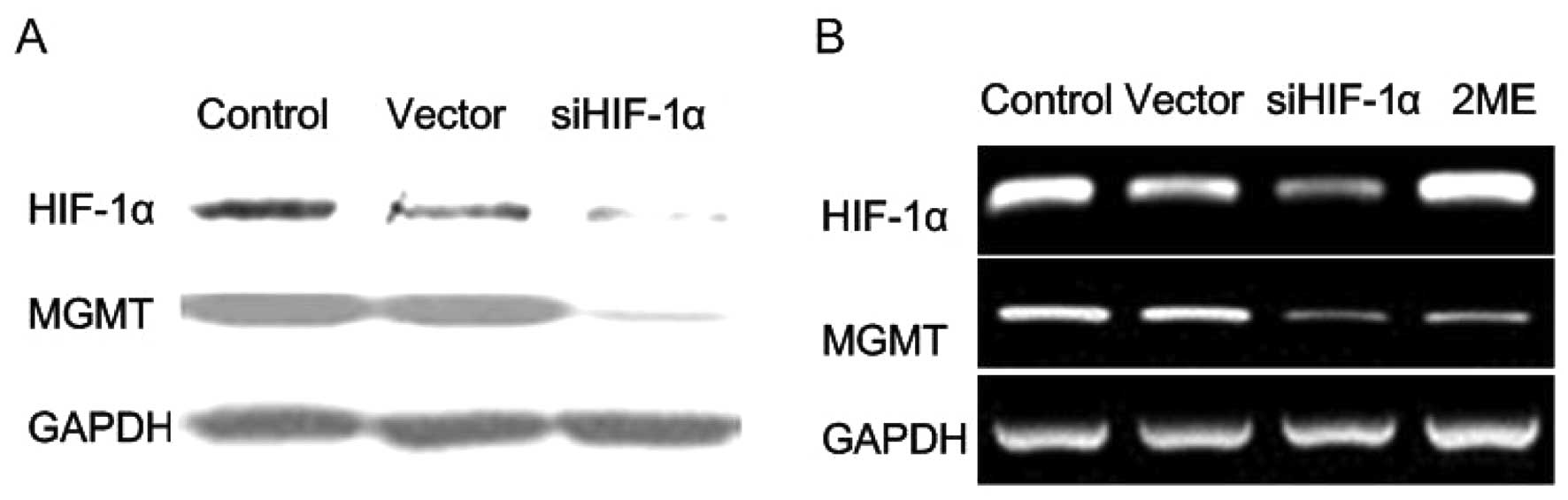
HIF-1α knockdown and 2ME downregulate
MGMT expression in vitro
HIF-1α has recently been shown to regulate MGMT
expression in gliomas (10,18), yet whether it exerts similar effects
in pituitary adenomas has not been examined. In our previous study,
we used HIF-1α knockdown strategy to inhibit hemorrhagic
transformation in pituitary adenomas (11). In the present study, the HIF-1α
knockdown system was employed for transient transfection in human
pituitary adenoma cells from primary culture, and the effect of
HIF-1α knockdown on the MGMT expression was examined. The pituitary
adenoma tissue from one patient was primarily cultured immediately
after dissection. After a 24-h primary culture, the cells were
plated in 16-well plates and were transiently transfected with
siHIF-1α or control siRNA. We found that HIF-1α knockdown
downregulated the MGMT expression in human pituitary adenoma cells
in primary culture (Fig. 2A). In
order to further clarify this issue, we cocultured human pituitary
adenoma cells with 2ME (2 μM) or saline for 72 h, and then the MGMT
expression was examined via RT-PCR. As expected, 2ME downregulated
the MGMT expression in vitro (Fig. 2B).
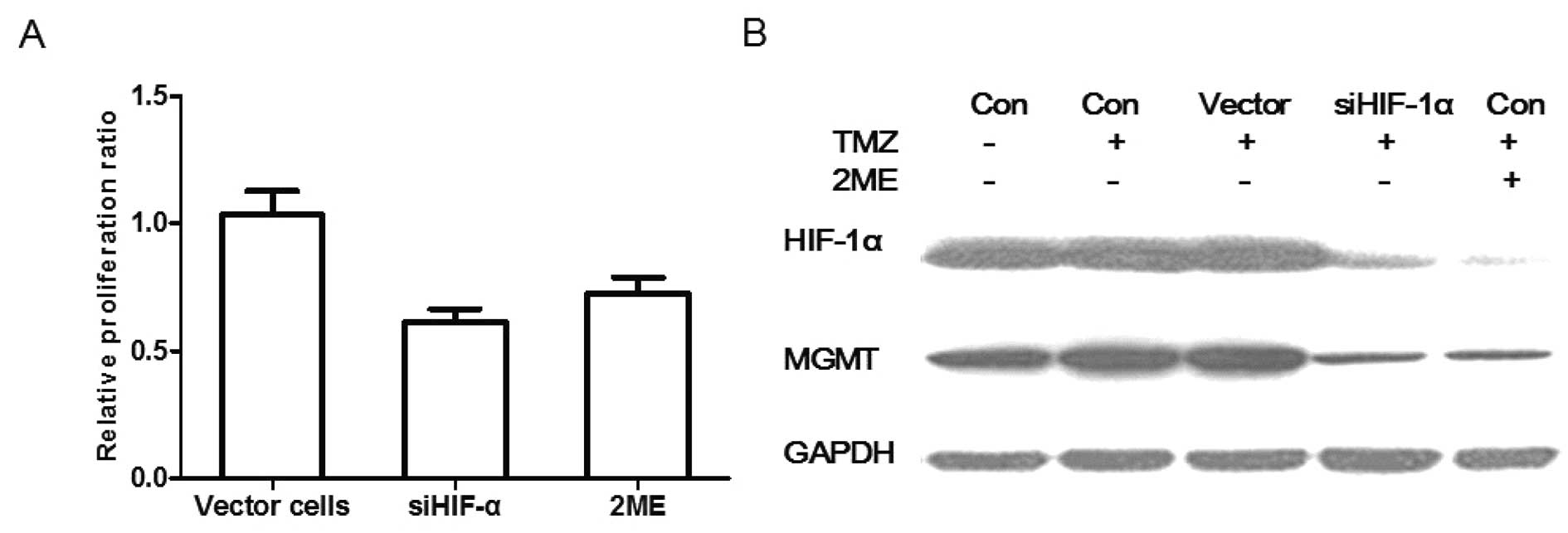
MGMT expression is correlated with TMZ
resistance in pituitary adenoma cells
To date, there are still several arguments over
whether TMZ efficacy is dependent on MGMT expression in pituitary
adenomas. Several clinical trials suggest a correlation between
MGMT expression and TMZ efficacy, while other researchers report an
opposite trend (5,19,20).
Experimental evidence clarifying this issue is lacking. In our
pre-experiment, we found that TMZ has no effect on the HIF-1α and
MGMT expression in human pituitary adenomas. In the present study,
we investigated the effect of HIF-1α knockdown and HIF-1α inhibitor
2ME on TMZ resistance. HIF-1α knockdown cells, vector cells and
control human pituitary adenoma cells were seeded into 96-well
flat-bottomed plates at a concentration of 1×104
cells/well. The cells were then cocultured with TMZ (100 μM) for 72
h. Another group of human pituitary adenoma cells was also seeded
and cocultured with TMZ (100 μM) plus 2ME (2 μM) for 72 h. Then,
the CCK-8 assays were carried out. We found that the relative
proliferation ratio of vector cells, siHIF-1α cells and 2ME treated
cells vs. control cells was 1.01±0.06, 0.62±0.03 and 0.72±0.05%,
respectively (P<0.05). TMZ had no obvious killing effect on
human pituitary adenoma cells and vector cells. Compared with the
cells treated with TMZ alone, HIF-1α knockdown and 2ME
significantly reduced the proliferation of cells when combined with
TMZ treatment (Fig. 3A). In order
to further clarify this issue, the HIF-1α and MGMT expression in
each group was also investigated. As shown in Fig. 3B, TMZ or vector siRNA displayed no
effect on the HIF-1α and MGMT expression, while TMZ plus HIF-1α
knockdown or TMZ plus 2ME significantly reduced the HIF-1α and MGMT
expression in human pituitary adenoma cells. These results indicate
that TMZ efficacy may be dependent on the MGMT expression in
pituitary adenomas.
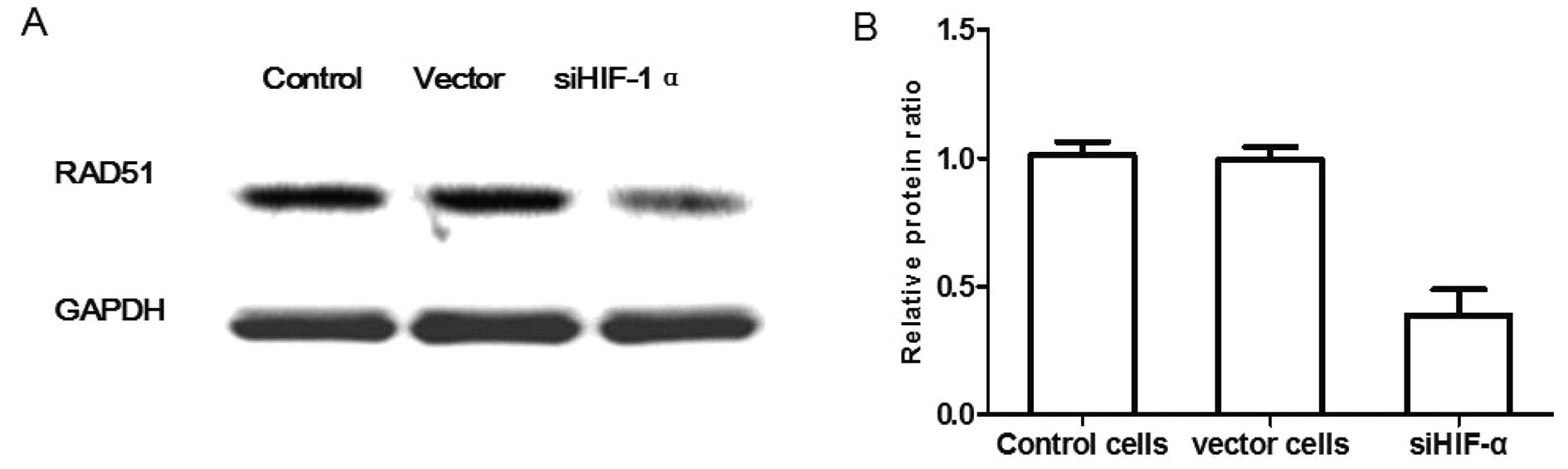
HIF-1α knockdown sensitizes pituitary
adenoma cells to TMZ via suppressing the repair ability of DNA
damage in vitro
Since MGMT expression has been associated with the
DNA damage repair ability (21), we
inferred that down-modulation of MGMT may also enhance TMZ efficacy
via suppression of DNA damage repair ability. As RAD51 is a protein
marker associated with DNA damage repair ability (22), the pituitary adenoma cells
(control), vector cells and HIF-1α knockdown cells were cultured
with TMZ for 72 h, then RAD51 protein expression was examined. As
shown in Fig. 4, the relative
protein ratio of vector cells, siHIF-1α cells vs. control cells was
0.99±0.03 and 0.38±0.05%, respectively (P<0.05), which showed
that HIF-1α knockdown of pituitary adenoma cells significantly
decreased the RAD51 protein expression compared with the control
group. These results indicate that MGMT may be involved in
sensitizing pituitary adenoma cells to TMZ via suppression of the
repair ability of DNA damage.
2ME sensitizes pituitary adenoma cells to
TMZ in vivo
To translate our above in vitro findings into
an in vivo model of pituitary adenoma, we evaluated the
effect of HIF-1α inhibitor 2ME on the MGMT expression and the tumor
xenograft proliferation of human pituitary adenoma cells in athymic
nude mice. Mice were randomized into four groups: control (saline)
group, 2ME group, TMZ group and 2ME plus TMZ group, with three mice
in each group. Mice were treated with 2ME (25 mg/kg), TMZ (3 mg/kg)
or 2ME plus TMZ for 5 days. Three weeks later, tumors were
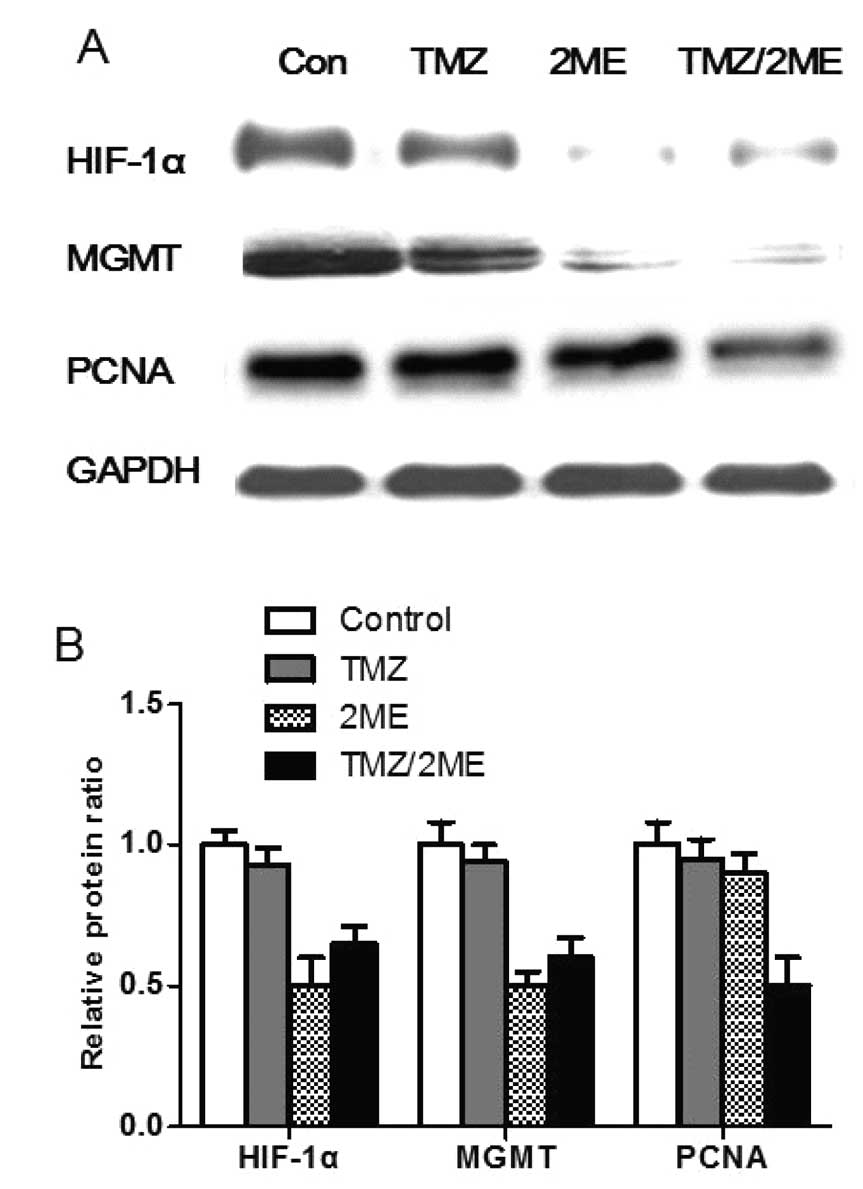
harvested for analysis. We found that 2ME treatment downregulated
HIF-1α and MGMT expression in the tumor xenografts, while no
similar effect was observed following TMZ treatment alone (Figs. 5 and 6). Since the human pituitary adenoma cells
from the primary culture grew very slowly in nude mice, we did not
measure a significant difference in tumor diameter among the
different groups. Since PCNA is a marker of tumor proliferation, we
examined the PCNA expression in tumors of the different groups. As
shown in Figs. 5 and 6, we found no significant difference in
the PCNA expression levels among the 2ME group, the TMZ group and
the control group. The xenografts of 2ME plus TMZ group displayed
significantly lower PCNA expression than the other groups. The
relative PCNA protein ratio of the vector cells, siHIF-1α cells vs.
control cells was 0.97±0.07, 0.90±0.06 and 0.49±0.11%, respectively
(P<0.05). These results suggest that 2ME sensitizes pituitary
adenoma cells to TMZ via the down-modulation of MGMT expression
in vivo.
Discussion
Our study, for the first time, presents experimental
evidence that TMZ resistance may be directly correlated with MGMT
expression in pituitary adenomas. Furthermore, the HIF-1α-MGMT
signaling pathway may be involved in the TMZ resistance of
pituitary adenoma cells. HIF-1α knockdown or inhibitor 2ME may be
applied as a mediator of TMZ resistance via the down-modulation of
MGMT expression in pituitary adenoma cells.
Invasive pituitary adenomas are very common in our
clinical practice and are characterized by a limited response to
standard therapies and poor prognosis (1). When conventional therapies including
radiation, surgery, pharmacological treatment fail, TMZ therapy is
often adopted as the last line treatment for these life-threatening
pituitary adenomas (23). Although
a previous report revealed a 60% response rate for TMZ treatment in
invasive pituitary adenomas (20),
at least in our center, TMZ exerts poor efficacy in many patients
with a much lower response rate. Furthermore, a poor response rate
was also observed in many published clinical trials. We believe
this discrepancy may be due to the following reasons: Most of the
authors tend to report successful cases, and different medical
centers may employ different enrollment criteria for TMZ treatment.
The schedule and dosing regimens of TMZ treatment varied in
different medical centers due to the lack of standard protocol in
the treatment of pituitary adenomas (23). Since TMZ is the last line treatment
and displays a low response rate in life-threatening pituitary
adenoma patients, seeking modulation of TMZ resistance is of high
value in our practice.
MGMT expression has been recently reported to be
associated with TMZ resistance in patients with gliomas (24). The findings of clinical studies
dealing with the association between MGMT expression and the
therapeutic response to TMZ in pituitary adenoma patients remain
controversial. Some studies report pituitary adenomas with low MGMT
expression tend to be responsive to TMZ, while others suggest that
no relation exist (5,19,20,25).
Moreover, all these previous reports are based on cohorts
consisting of a limited number of patients, and, to date, no
experimental evidence has been reported. In the present study, MGMT
expression was down-modulated by HIF-1α knockdown or inhibitor 2ME
in vitro and in vivo, suggesting that HIF-1α
inhibition can down-modulate MGMT expression in pituitary
adenomas.
There are numerous kinases that can regulate the
repair ability of DNA damage in chemotherapy. Checkpoint kinase 1
(Chk1) and MGMT are the key kinases in this process (26). However, in our pre-experiment,
inhibition of Chk1 did not lead to a preferential TMZ-induced
killing of pituitary adenoma cells. An explanation for this
inefficiency may be that most of the human pituitary adenoma cells
were wild-type p53 cells (27),
which are not hyper-dependent on the G2 damage-induced checkpoint
activity mediated by Chk1 activity. A recent report demonstrated
that MGMT expression is dependent on HIF-1α expression in glioma
cells (10), which promoted us to
infer that down-modulation of MGMT expression via HIF-1α inhibition
may sensitize pituitary adenoma cells to TMZ treatment. The present
study showed that HIF-1α knockdown or the HIF-1α inhibitor 2ME
down-modulated MGMT expression, thereby decreasing the
double-strand DNA repair capacity as evidenced by the decreased
RAD51 protein expression, which in turn sensitized pituitary
adenoma cells to TMZ in vitro and in vivo. These
results indicate that targeted modulation of MGMT via HIF-1α
inhibition may be a novel way to override TMZ resistance in
patients with pituitary adenomas.
There are several inherent limitations to our study.
We used cells from primary culture of human pituitary adenomas from
two patients in the present study. The cells were not from an
established cell line, which may have undermined the significance
of the results of the present study. The reason for this is that
there is no well-established human pituitary adenoma cell line
(11). Moreover, only a few
patients non-responsive to conventional and TMZ therapies in our
clinical practice were willing to receive a second operation.
Furthermore, due to the benignity of pituitary adenomas, the
limited imperfect framework for accessing tumor tissue samples and
primary culture technology, many cells from primary cell cultures
of human pituitary adenomas almost lose their capability for
duplication, and eventually die, which further wastes the valuable
but limited human pituitary adenoma tissue. In the present study,
HIF-1α knockdown was only used in vitro, as we employed the
HIF-1α inhibitor 2ME and obtained the expected results in
vivo. 2ME is an FDA approved anticancer drug which is more
likely to be used in our practice than the HIF-1α knockdown
strategy (28). Regarding the
mechanism, our results cannot confirm a direct regulatory role of
HIF-1α on MGMT expression as there may be other signaling pathways
between the HIF-1α and MGMT kinases, which require further
investigation.
In conclusion, the present study for the first time
demonstrates that HIF-1α modulates MGMT expression in pituitary
adenoma cells, and that the HIF-1α inhibitor 2ME can sensitize
pituitary adenoma cells to TMZ treatment via the down-modulation of
MGMT expression. Since both 2ME and TMZ are FDA approved anticancer
drugs and can cross the blood-brain barrier (29,30),
further research is warranted to define the underlying molecular
mechanisms and to optimize the protocol of the combined treatment
of TMZ and 2ME for pituitary adenomas refractory to conventional
therapies.
References
|
1
|
Selman WR, Laws ER Jr, Scheithauer BW and
Carpenter SM: The occurrence of dural invasion in pituitary
adenomas. J Neurosurg. 64:402–407. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scheithauer BW, Kovacs KT, Laws ER Jr and
Randall RV: Pathology of invasive pituitary tumors with special
reference to functional classification. J Neurosurg. 65:733–744.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kreutzer J and Fahlbusch R: Diagnosis and
treatment of pituitary tumors. Curr Opin Neurol. 17:693–703. 2004.
View Article : Google Scholar
|
|
4
|
Ekeblad S, Sundin A, Janson ET, Welin S,
Granberg D, Kindmark H, Dunder K, Kozlovacki G, Orlefors H, Sigurd
M, Oberg K, Eriksson B and Skogseid B: Temozolomide as monotherapy
is effective in treatment of advanced malignant neuroendocrine
tumors. Clin Cancer Res. 13:2986–2991. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCormack AI, McDonald KL, Gill AJ, Clark
SJ, Burt MG, Campbell KA, Braund WJ, Little NS, Cook RJ, Grossman
AB, Robinson BG and Clifton-Bligh RJ: Low
O6-methylguanine- DNA methyltransferase (MGMT)
expression and response to temozolomide in aggressive pituitary
tumours. Clin Endocrinol. 71:226–233. 2009.
|
|
6
|
Syro LV, Uribe H, Penagos LC, Ortiz LD,
Fadul CE, Horvath E and Kovacs K: Antitumour effects of
temozolomide in a man with a large, invasive prolactin-producing
pituitary neoplasm. Clin Endocrinol. 65:552–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Everhard S, Kaloshi G, Criniere E,
Benouaich-Amiel A, Lejeune J, Marie Y, Sanson M, Kujas M, Mokhtari
K, Hoang-Xuan K, Delattre JY and Thillet J: MGMT methylation: a
marker of response to temozolomide in low-grade gliomas. Ann
Neurol. 60:740–743. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stupp R, Hegi ME, Gilbert MR and
Chakravarti A: Chemoradiotherapy in malignant glioma: standard of
care and future directions. J Clin Oncol. 25:4127–4136. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Nifterik KA, van den Berg J, van der
Meide WF, Ameziane N, Wedekind LE, Steenbergen RD, Leenstra S,
Lafleur MV, Slotman BJ, Stalpers LJ and Sminia P: Absence of the
MGMT protein as well as methylation of the MGMT promoter predict
the sensitivity for temozolomide. Br J Cancer. 103:29–35.
2010.PubMed/NCBI
|
|
10
|
Persano L, Pistollato F, Rampazzo E, Della
PA, Abbadi S, Frasson C, Volpin F, Indraccolo S, Scienza R and
Basso G: BMP2 sensitizes glioblastoma stem-like cells to
temozolomide by affecting HIF-1alpha stability and MGMT expression.
Cell Death Dis. 3:e4122012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao Z, Liu Q, Zhao B, Wu J and Lei T:
Hypoxia induces hemorrhagic transformation in pituitary adenomas
via the HIF-1α signaling pathway. Oncol Rep. 26:1457–1464.
2011.PubMed/NCBI
|
|
12
|
Losa M, Mazza E, Terreni MR, McCormack A,
Gill AJ, Motta M, Cangi MG, Talarico A, Mortini P and Reni M:
Salvage therapy with temozolomide in patients with aggressive or
metastatic pituitary adenomas: experience in six cases. Eur J
Endocrinol. 163:843–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Ma Z, Xiao Z, Liu H, Dou Z, Feng X
and Shi H: Chk1 knockdown confers radiosensitization in prostate
cancer stem cells. Oncol Rep. 28:2247–2254. 2012.PubMed/NCBI
|
|
14
|
Yang J, Xiao Z, Li T, Gu X and Fan B:
Erythropoietin promotes the growth of pituitary adenomas by
enhancing angiogenesis. Int J Oncol. 40:1230–1237. 2012.PubMed/NCBI
|
|
15
|
Naumann SC, Roos WP, Jost E, Belohlavek C,
Lennerz V, Schmidt CW, Christmann M and Kaina B: Temozolomide- and
fotemustine-induced apoptosis in human malignant melanoma cells:
response related to MGMT, MMR, DSBs, and p53. Br J Cancer.
100:322–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sadones J, Michotte A, Veld P, Chaskis C,
Sciot R, Menten J, Joossens EJ, Strauven T, D’Hondt LA, Sartenaer
D, et al: MGMT promoter hypermethylation correlates with a survival
benefit from temozolomide in patients with recurrent anaplastic
astrocytoma but not glioblastoma. Eur J Cancer. 45:146–153. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Nifterik KA, van den Berg J, Stalpers
LJ, Lafleur MV, Leenstra S, Slotman BJ, Hulsebos TJ and Sminia P:
Differential radiosensitizing potential of temozolomide in MGMT
promoter methylated glioblastoma multiforme cell lines. Int J
Radiat Oncol Biol Phys. 69:1246–1253. 2007.PubMed/NCBI
|
|
18
|
Esteller M, Garcia-Foncillas J, Andion E,
Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB and Herman JG:
Inactivation of the DNA-repair gene MGMT and the clinical response
of gliomas to alkylating agents. N Engl J Med. 343:1350–1354. 2000.
View Article : Google Scholar
|
|
19
|
Neff LM, Weil M, Cole A, Hedges TR,
Shucart W, Lawrence D, Zhu JJ, Tischler AS and Lechan RM:
Temozolomide in the treatment of an invasive prolactinoma resistant
to dopamine agonists. Pituitary. 10:81–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raverot G, Sturm N, de Fraipont F, Muller
M, Salenave S, Caron P, Chabre O, Chanson P, Cortet-Rudelli C,
Assaker R, et al: Temozolomide treatment in aggressive pituitary
tumors and pituitary carcinomas: a French multicenter experience. J
Clin Endocrinol Metab. 95:4592–4599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma S, Salehi F, Scheithauer BW,
Rotondo F, Syro LV and Kovacs K: Role of MGMT in tumor development,
progression, diagnosis, treatment and prognosis. Anticancer Res.
29:3759–3768. 2009.PubMed/NCBI
|
|
22
|
Baumann P and West SC: Role of the human
RAD51 protein in homologous recombination and double-stranded-break
repair. Trends Biochem Sci. 23:247–251. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Syro LV, Ortiz LD, Scheithauer BW, Lloyd
R, Lau Q, Gonzalez R, Uribe H, Cusimano M, Kovacs K and Horvath E:
Treatment of pituitary neoplasms with temozolomide: a review.
Cancer. 117:454–462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brandes AA, Franceschi E, Tosoni A, Blatt
V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F,
Andreoli A, et al: MGMT promoter methylation status can predict the
incidence and outcome of pseudoprogression after concomitant
radiochemotherapy in newly diagnosed glioblastoma patients. J Clin
Oncol. 26:2192–2197. 2008. View Article : Google Scholar
|
|
25
|
Takeshita A, Inoshita N, Taguchi M, Okuda
C, Fukuhara N, Oyama K, Ohashi K, Sano T, Takeuchi Y and Yamada S:
High incidence of low O6-methylguanine DNA
methyltransferase expression in invasive macroadenomas of Cushing’s
disease. Eur J Endocrinol. 161:553–559. 2009.
|
|
26
|
Jackson SP and Bartek J: The DNA-damage
response in human biology and disease. Nature. 461:1071–1078. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Levy A, Hall L, Yeudall WA and Lightman
SL: p53 gene mutations in pituitary adenomas: rare events. Clin
Endocrinol. 41:809–814. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hagen T, D’Amico G, Quintero M,
Palacios-Callender M, Hollis V, Lam F and Moncada S: Inhibition of
mitochondrial respiration by the anticancer agent
2-methoxyestradiol. Biochem Biophys Res Commun. 322:923–929. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pribluda VS, Gubish ER Jr, Lavallee TM,
Treston A, Swartz GM and Green SJ: 2-Methoxyestradiol: an
endogenous antiangiogenic and antiproliferative drug candidate.
Cancer Metastasis Rev. 19:173–179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Verger E, Gil M, Yaya R, Vinolas N, Villa
S, Pujol T, Quinto L and Graus F: Temozolomide and concomitant
whole brain radiotherapy in patients with brain metastases: a phase
II randomized trial. Int J Radiat Oncol Biol Phys. 61:185–191.
2005. View Article : Google Scholar : PubMed/NCBI
|