Introduction
Despite the recent advance in therapeutic methods,
human cancer remains a leading cause of mortality worldwide
(1,2). Moreover, the incidence of many types
of cancers, including melanoma, prostate, breast, liver and lung
cancer, continues to increase (3,4). Human
lung cancer continues to be the leading cause of cancer-related
mortality among males in developing countries (5). There are two types of lung cancer:
small cell lung cancer and non-small cell lung cancer (NSCLC).
Among these, NSCLC (6,7) is aggressive and accounts for ~80–85%
(8,9) of all lung cancer cases. The current
5-year survival rate for NSCLC is <15% (10). Research on cancer chemotherapy has
focused on the handicaps of chemotherapy, including multi-drug
resistance caused by the extensive use of conventional
chemotherapeutic agents (9,10). Another handicap is that conventional
chemotherapeutic agents, which typically target rapidly dividing
cancer cells, are also associated with deleterious side-effects in
healthy cells and tissues (3).
Although treatment of NSCLC is guided by disease stage, most
patients with lung cancer are typically diagnosed at an advanced
stage when patients have limited treatment options (11). Thus, it is necessary to develop
novel anticancer drug candidates with lower toxicity than
conventional chemotherapeutic agents as new treatment
strategies.
Antimicrobial peptides (AMPs) have been isolated
from a wide range of organisms (12,13)
such as prokaryotes, insects, fish, amphibians and mammals
(including humans). Most AMPs are cationic and amphiphilic, but
they can differ greatly in regards to other characteristics such as
sequence, size, structural motifs and the presence of disulphide
bonds (14,15). AMPs possess broad antimicrobial
activity against bacteria, fungi and viruses. Certain AMPs also
present as the first line of defense in the innate immune system
(16–18). In addition to the activities
mentioned above, the anticancer activity of AMPs has attracted wide
attention in recent years. Recent studies have demonstrated that
cationic AMPs could play a promising role in fighting various
multi-drug resistant tumors as most types of cancer cells have more
anionic phospholipids on their external membranes (19).
Cathelicidin-BF (BF-30), isolated from the snake
venom of Bungarus fasciatus, is an antimicrobial peptide
that consists of 30 amino acids (19–21).
BF-30 was found to exert broad antimicrobial activity against
bacteria and to exhibit excellent inhibitory activity toward the
murine metastatic melanoma cell line B16F10, in vitro and
in vivo, as determined in our previous study (22). However, BF-30 had a negligible
effect on H460 and Lewis cells, with IC50 values of 45
and 40.3 μM, respectively.
Cbf-K16 is a mutant of BF-30 that was
generated by a Glu16 to Lys16 substitution,
which increases the positive charge of the molecule. In our
previous study, Cbf-K16 exhibited stronger antimicrobial
activity than BF-30, particularly against drug-resistant bacteria.
The minimum inhibitory concentration (MIC) of Cbf-K16
against E. coli BL21 (DE3)-NDM-1 was only 4 μg/ml while the
MBC was 8 μg/ml. Cbf-K16 displayed MICs of 32 μg/ml
against penicillin-resistant E. coli and 16 μg/ml against
S. aureus(21). Previously,
we found that Cbf-K16 exhibits selective anticancer
activity, particularly against lung cancer. Therefore, in the
present study, we investigated the anticancer activity of
Cbf-K16 against human lung cancer in vitro and
its molecular mechanisms.
Materials and methods
Peptide synthesis
Cbf-K16 (KFFRKLKKSVKKRAKKFFK KPRVIGVSIPF)
was synthesized by GL Biochem (Shanghai, China) via a stepwise
solid phase methodology. The resulting peptide was purified by a
Sephadex gel column and HPLC, and the homogeneity of the purified
peptide was >98.12%. The synthetic polypeptide was reconstituted
in phosphate-buffered saline (PBS, pH 7.4) for subsequent
experiments.
Cell lines and reagents
A series of human cancer cell lines [human lung
non-small cell carcinoma cell line (H460), human prostate cancer
cell line (PC-3), human breast cancer cell line (MCF-7), human
hepatocellular carcinoma cell line (HepG2) and human melanoma cell
line (A375)], mouse cancer cell lines [mouse lung cancer cell line
(Lewis), mouse melanoma cell line (B16) and mouse malignant
melanoma cell line (B16F10)] and Madin-Daby canine kidney (MDCK)
cells were used to investigate anticancer activity. All of the cell
lines were obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA). These cell lines were cultured in either
RPMI-1640 medium, DMEM or F12 medium supplemented with 10% fetal
bovine serum (FBS) provided by Gibco (Grand Island, NY, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
sodium pyruvate and dimethyl sulfoxide (DMSO) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The DNA extraction kit was
purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The
lactate dehydrogenase kit was purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Annexin V-fluorescein
(AV) and propidium iodide (PI) were purchased from Invitrogen
(Shanghai, China). Male ICR mice between 6 and 8 weeks of age
(weight, 18–22 g) were purchased from the Laboratory Animal Center
of Yangzhou University (Yangzhou, China) and acclimatized for 1
week prior to use in the experiment. Animals were provided with
continuous standard rodent chow and water and were housed in a
rodent facility at 22±1ºC with a 12-h light-dark cycle. All
procedures involving animals and their care in the present study
were in strict accordance with the protocols approved by the Ethics
Committee of the China Pharmaceutical University.
Assay of cell viability
To evaluate the effects of Cbf-K16 on
cell proliferation, MTT assays were conducted as previously
described (22–24). Spleens were collected from the ICR
mice under aseptic conditions in 0.1 M PBS, gently homogenized and
passed through a 200-mesh sieve to obtain single-cell suspensions
that were then treated with erythrocyte lysis buffer and washed
three times in PBS to remove the erythrocytes. The resulting
splenocytes were resuspended in RPMI-1640 medium containing 10% FBS
for further research.
Normal splenocyte and MDCK cells, as well as the
tumor cell lines, were collected at the logarithmic growth phase
(adherent cells were digested with trypsin) and centrifuged. Tumor
cells were seeded into 96-well plastic plates at a density of
5×105 cells/ml 24 h prior to peptide treatments. Cells
were then challenged with different doses of Cbf-K16 (0,
5, 10, 20, 40 and 80 μM) and cultured for 48 h at 37ºC in a
humidified 5% CO2 atmosphere. An additional 4-h
incubation was carried out with 5 mg/ml MTT solution (15 μl/well).
The supernatant was then discarded, and 150 μl DMSO was added to
each well to dissolve the formazan precipitate by gently shaking,
and the optical density at 570 nm was determined by
spectrophotometry using a microtiter plate reader. The cell
viability was calculated using the following formula: Cell
viability (%) = OD1/OD2 × 100%, where
OD1 is the absorbance at 570 nm of the experimental
group and OD2 is that of the control group.
Morphological changes in the human lung
non-small cell carcinoma cell line H460 as detected by transmission
electron microscopy (TEM)
Transmission electron microscopy (TEM) was conducted
to confirm changes in cellular and mitochondrial morphology, as
previously described (25–27). H460 cells were harvested after
exposure to Cbf-K16 (0, 20 and 40 μM) for 24 h.
Glutaraldehyde (2.5%) was added to pre-fix the H460 cells and
preserve morphological structure. The samples were washed twice
with PBS and post-fixed in 1% osmium tetroxide for 2 h. The cells
were then stained with 2% uranyl acetate and dehydrated with
ethanol before being embedded in LR White resin. After overnight
polymerization at 60ºC, embedded specimens were sectioned and
stained with uranyl acetate and lead citrate before examination
with a JEM-1011 electron microscope (Jeol, Tokyo, Japan).
Experiments were repeated three times.
Cytoplasmic membrane permeability assay
based on lactate dehydrogenase release
Increased release of lactate dehydrogenase (LDH)
into the medium supernatant occurs when plasma membranes are
injured in necrotic cells (28,29).
Based on this theory, the effect of Cbf-K16 on the
membrane integrity of H460 cells, splenocytes and MDCK cells were
evaluated using an LDH release assay. H460 and MDCK cells were
seeded in 96-well plastic plates at a density of 5×104
cells/well, while splenocytes were seeded at a density of
5×105 cells/well 24 h prior to Cbf-K16
treatment. The cells were cultured at 37ºC in the absence or
presence of different concentrations of Cbf-K16 (0, 20
and 40 μM) for 12, 24 or 48 h. Supernatants were collected at the
indicated times, and LDH activities were assessed according to the
kit protocols. Cells that had been ultrasonically disrupted were
used as a positive control. The reported results represent 3
independent repeats.
Cell apoptosis assay
Cell apoptosis assays (30–32)
were conducted by double staining with Annexin V-fluorescein (AV)
and propidium iodide (PI) to investigate whether Cbf-K16
induces apoptosis in H460 cells. H460 cells were seeded in 6-well
plastic plates (5×105 cells/well) 24 h prior to
Cbf-K16 treatments. The medium supernatant in the plates
was then replaced, and various concentrations of Cbf-K16
(0, 20 and 40 μM) diluted in PBS were added to the plates. After
incubation at 37ºC in a humidified atmosphere with 5%
CO2 for 24 or 48 h, the cells were harvested by
trypsinization and collected by centrifugation according to the
manufacturer’s specifications. Briefly, cells were washed three
times and then diluted in 100 μl reaction buffer containing 5 μl AV
and 1 μl PI, followed by a 15-min incubation. Binding buffer (400
μl) was added to each sample prior to the flow cytometric
analysis.
DNA retardation assay
A DNA retardation assay was used for quantitative
and qualitative evaluation of the degree of DNA binding by
Cbf-K16 in the H460 tumor cell line as previously
reported, with slight modifications (33). H460 cells were trypsinized and
collected at the logarithmic growth phase. According to the
manufacturer’s protocol, total DNA was isolated using a DNA
extraction kit, and the DNA concentration was measured using an
ELISA reader at 280 nm. Genomic DNA was mixed with different
concentrations of Cbf-K16 (0, 5, 10 and 20 μM) at a 1:1
(vol:vol) ratio for 30 min before electrophoretic analysis of DNA
ladder formation using a 0.8% agarose gel containing 0.1 mg/ml
ethidium bromide and visualized under UV light. DNA levels were
quantified based on density analysis using the ImageJ software, and
the DNA-binding rate (%) was calculated using the following
formula: DNA-binding rate (%) = [1 − (A/B)] × 100%, where A is the
average density of the electrophoretic band and B is the average
density of the total genomic DNA band.
Hemolysis assay
Hemolytic activity was investigated according to
previously reported methods, which were slightly modified (34). The sheep erythrocyte (SRBC) pellet
was gently washed three times with cold PBS buffer (pH 7.4), and
the erythrocytes were then resuspended in 10 volumes of the same
buffer (stock cell suspensions). The cell stock suspensions were
diluted 25-fold with the same buffer for a final erythrocyte
concentration of 0.4% (v/v). The SRBC suspension was then added to
a 96-well microtiter plate (100 μl/well), and increasing amounts of
the test samples (from 0 to 40 μM) were added to the erythrocyte
solution. After incubation for 1 h at 37ºC, samples were
centrifuged at 4,000 × g for 5 min and the absorbance of the
supernatant at 540 nm was determined.
Statistical analysis
All of the experiments described above were
performed in triplicate. The results were presented as the means ±
SD. The Student’s t-test was used for two-group comparisons, and a
one-way ANOVA was used for multiple comparisons to determine the
level of significance between the control and treated groups. A
P-value of <0.05 was considered to indicate a statistically
significant result.
Results
Cbf-K16 selectively inhibits
growth of the human lung non-small cell carcinoma cell line H460
and the mouse Lewis cell line in a dose- and time-dependent manner
in vitro
The effect of Cbf-K16 at various
concentrations on the growth of different human cancer cell lines
(H460, PC-3, MCF-7, HepG2 and A375) and mouse cancer cell lines
(B16F10, B16 and Lewis) was examined using an MTT assay. After
being exposed to Cbf-K16 for 48 h, the cell viability of
these tumor cell lines was determined, and the resulting
IC50 values are reported in Table I. These tumor cells exhibited
differing sensitivities to Cbf-K16. Among them, the
human non-small cell lung carcinoma cell line H460 and mouse lung
cancer Lewis cells were more sensitive, with IC50 values
of 16.5 and 10.5 μM, respectively. Although IC50 values
for Cbf-K16 against the mouse melanoma B16 and mouse
malignant melanoma B16F10 cell lines (0.4 and 7.3 μM, respectively)
were less than those against the Lewis mouse lung cancer cell line
(10.5 μM), the IC50 value for Cbf-K16 against
the human melanoma cell line A375 (70.3 μM) was much greater than
the value against the human non-small cell lung carcinoma cell line
H460 (16.5 μM). As shown in Fig.
1A, the viability of these tumor cell lines decreased in a
dose-dependent manner as the concentration of Cbf-K16
increased. Cbf-K16 significantly suppressed the
proliferation of all the tested cell lines. We observed that
certain cell lines, such as human melanoma cell A375, human breast
cell line MCF-7 and human hepatocellular carcinoma cell line HepG2,
showed a slowly descending tendency while the others such as human
non-small cell lung carcinoma H460 and the Lewis mouse lung cancer
cell line and the mouse melanoma B16 cell line showed the opposite
trend. In addition, there were significant changes in the
morphology and total number of H460 cells treated with
Cbf-K16 at doses of 20 and 40 μM in comparison to the
controls (Fig. 1B). The untreated
H460 cells showed a smooth, flattened morphology and a typical
growth pattern under phase contrast microscopy. In contrast, the
number of H460 cells decreased significantly and the morphology
became abnormal following Cbf-K16 treatment. The cells
displayed shrinkage, abnormal boundaries and cellular lysis as the
concentration of Cbf-K16 increased to 20 μM. As shown in
Fig. 1C, the cell viability of the
human non-small cell lung carcinoma H460 cells treated with 20 μM
Cbf-K16 for 12, 24 and 48 h was 75.4, 59.1 and 46.6%,
respectively. These results indicated that the effects of
Cbf-K16 on H460 cells were time-dependent. The same
phenomena were noted for Lewis cells (Fig. 1D). Cell viability decreased when
cells were treated with 40 μM Cbf-K16 for 12, 24 and 48
h, indicating that 40 μM Cbf-K16 could kill >80% of
the tumor cells. However, cell viability varied significantly, from
46.6 to 24.5%, when cells were treated with dosages of 20 and 40
μM, respectively. These data demonstrated that the
antiproliferative effects of Cbf-K16 on the lung cancer
cell lines H460 and Lewis were dose- and time-dependent and suggest
that Cbf-K16 selectively inhibits the proliferation of
cancer cells.
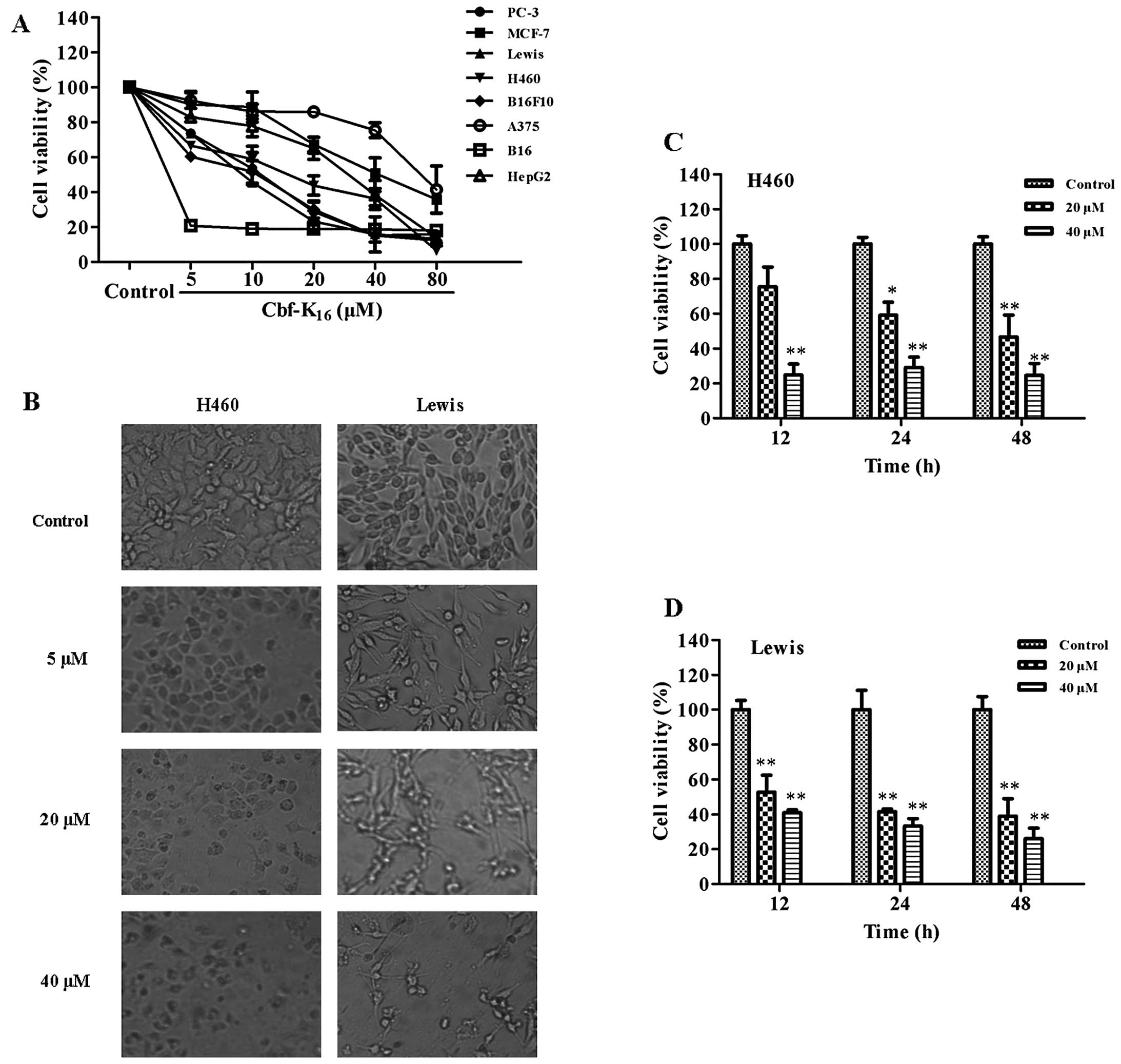 | Figure 1Effects of Cbf-K16 on the
proliferation of tumor cell lines. (A) The 8 tumor cell lines
(H460, PC-3, HepG2, MCF-7, A375, Lewis, B16 and B16F10) were
treated with different doses of Cbf-K16 (ranging from 0
to 80 μM) for 48 h, followed by an MTT assay. Cbf-K16
treatment affected cell viability in a dose-dependent manner. (B)
Optical micrographs of the treated H460 and Lewis cells. H460 and
Lewis cells treated with Cbf-K16 at different dosages
(0, 5, 20 and 40 μM) for 48 h were observed and photographed by
microscopy. The morphology of H460 and Lewis cells was altered, and
cell numbers were significantly reduced, following treatment with
Cbf-K16. Representative micrographs are shown
(magnification, ×200). Inhibition of proliferation of (C) H460 and
(D) Lewis cells by Cbf-K16 in a dose- and time-dependent
manner. H460 cells were treated with Cbf-K16 (0, 20 and
40 μM) for 12, 24 and 48 h. Cell viability was measured by an MTT
assay following Cbf-K16 treatment. Cbf-K16
suppressed the proliferation of H460 and Lewis in vitro in a
dose- and time-dependent manner (*P<0.05,
**P<0.01, compared with the untreated control at each
time point). |
 | Table IIC50 values of
Cbf-K16 against tumor cell lines. |
Table I
IC50 values of
Cbf-K16 against tumor cell lines.
| Sources | Tumor cell
lines | IC50
(μM) |
|---|
| Human | H460 | 16.5 |
| PC-3 | 12.5 |
| HepG2 | 35.7 |
| MCF-7 | 50.8 |
| A375 | 70.3 |
| Mouse | Lewis | 10.5 |
| B16 | 0.4 |
| B16F10 | 7.3 |
Cbf-K16 inhibits the human
lung non-small cell carcinoma cell line H460 by rupturing the
cytoplasmic membrane rather than by inducing cellular
apoptosis
To investigate the effect of Cbf-K16 on
the membrane integrity of H460 cells, a transmission electron
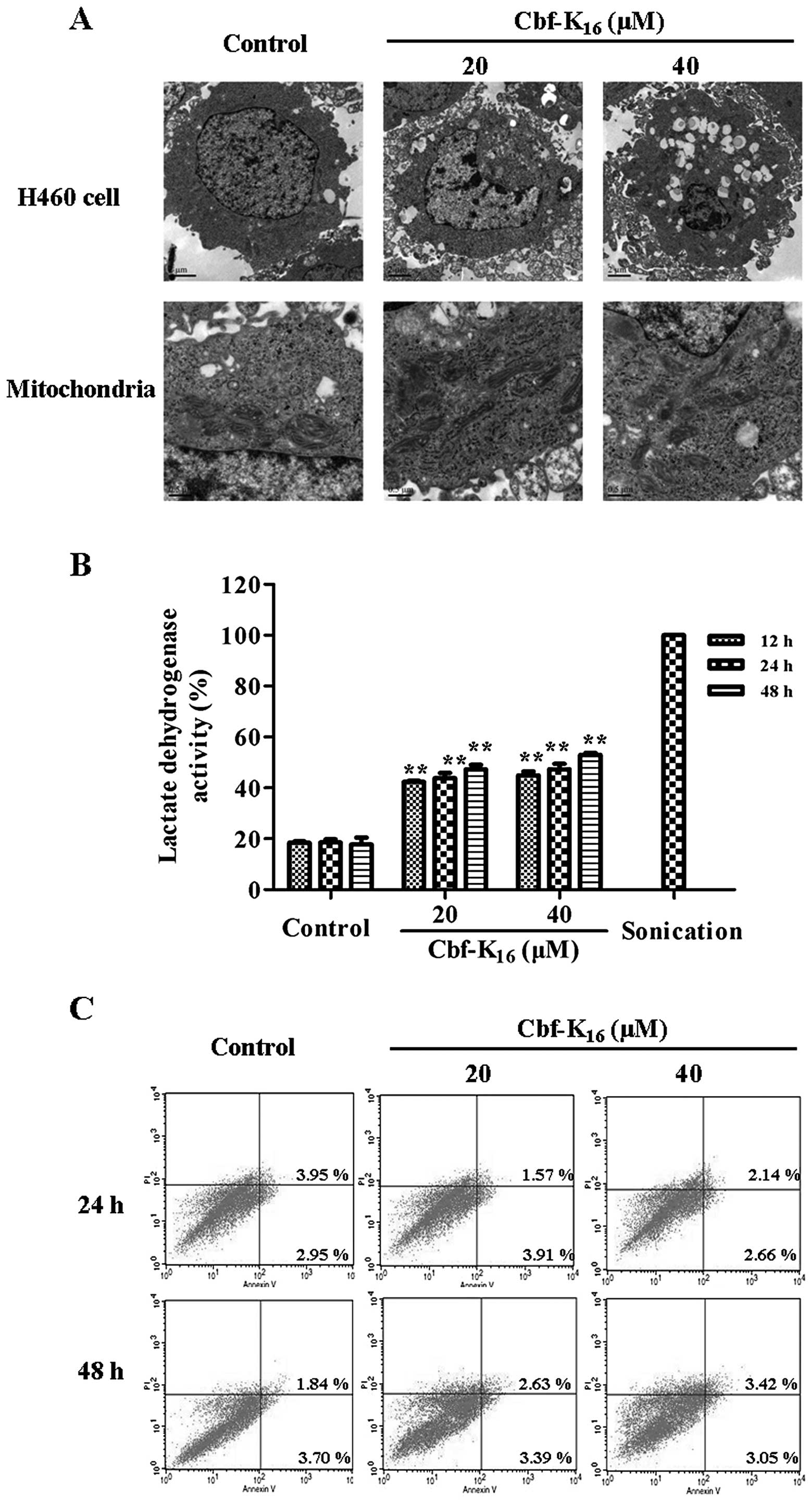
microscope assay was conducted. As shown in Fig. 2A, when treated with
Cbf-K16 at 20 and 40 μM doses, H460 cells exhibited
condensed and almost ruptured membranes, which resulted in the
leakage of the intracellular contents. Furthermore, the
mitochondria of H460 cells treated with Cbf-K16 were
swollen when compared with the control cells. These results
indicated that Cbf-K16 induced drastic changes in
cellular morphology and killed tumor cells via cytoplasmic membrane
permeabilization. Furthermore, the LDH activity of the H460 cells
increased from 42.4 to 52.9% following a 48-h Cbf-K16
treatment at 20 and 40 μM, respectively, which was significantly
different from the control (17.8%). These data indicate that the
H460 membrane was ruptured by Cbf-K16 treatment
(Fig. 2B) and suggest that the
anticancer mechanism of Cbf-K16 is partially due to
impaired cytoplasmic membrane integrity. AV/PI staining was further
conducted to investigate whether apoptosis plays a role in the
anti-H460 activity of Cbf-K16. As shown in Fig. 2C, there was no significant apoptosis
of H460 cells following Cbf-K16 treatment at 20 μM for
24 or 48 h; cells exhibited late apoptosis rates of 1.6 and 2.6%,
respectively. These results indicate that the molecular mechanism
of Cbf-K16 inhibition of H460 cell proliferation is a
loss of cytoplasmic membrane integrity rather than apoptosis.
Cbf-K16 bound genomic DNA of
the human lung non-small cell carcinoma cell line H460
Given that Cbf-K16 impairs the integrity
of the cytoplasmic membrane in H460 cells, we next sought to
determine whether Cbf-K16 interacts with genomic DNA
after the rupture of the cytoplasmic membrane. Using a gel
retardation assay with an ethidium bromide-stained agarose gel, the
electrophoretic mobility of genomic DNA was determined for a series
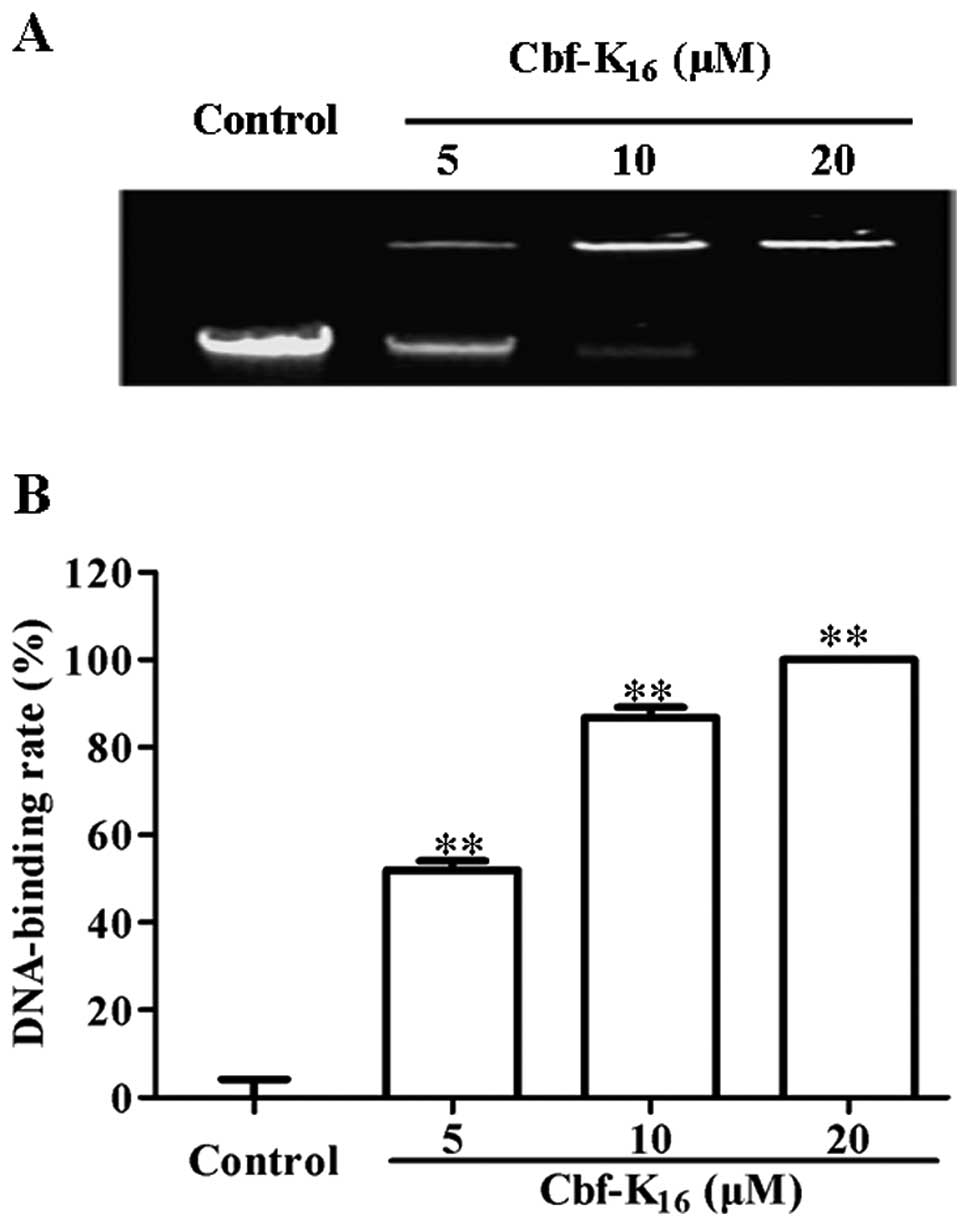
of Cbf-K16 concentrations. As shown in Fig. 3A, the forward motion of genomic DNA
extracted from H460 cells was inhibited in a dose-dependent manner
by Cbf-K16. The quantification of DNA levels by density
analysis using ImageJ software is depicted in Fig. 3B. The genomic DNA-binding rate in
H460 cells increased from 51.9 to 86.8% as the concentration of
Cbf-K16 increased from 5 to 10 μM. Additionally, the
genomic DNA-binding rate in H460 cells increased to 100% at 20 μM
Cbf-K16. This result was consistent with the
IC50 values listed in Table
I. The results detailed above indicate that Cbf-K16
selectively inhibits the proliferation of lung cancer cells by
rupturing the cytoplasmic membrane and by binding to genomic DNA
rather than by inducing cellular apoptosis.
Cbf-K16 exhibits hypotoxicity
against splenocytes and MDCK cells and limited hemolytic
activity
A series of assays were implemented to determine
whether Cbf-K16 induces cellular toxicity in normal
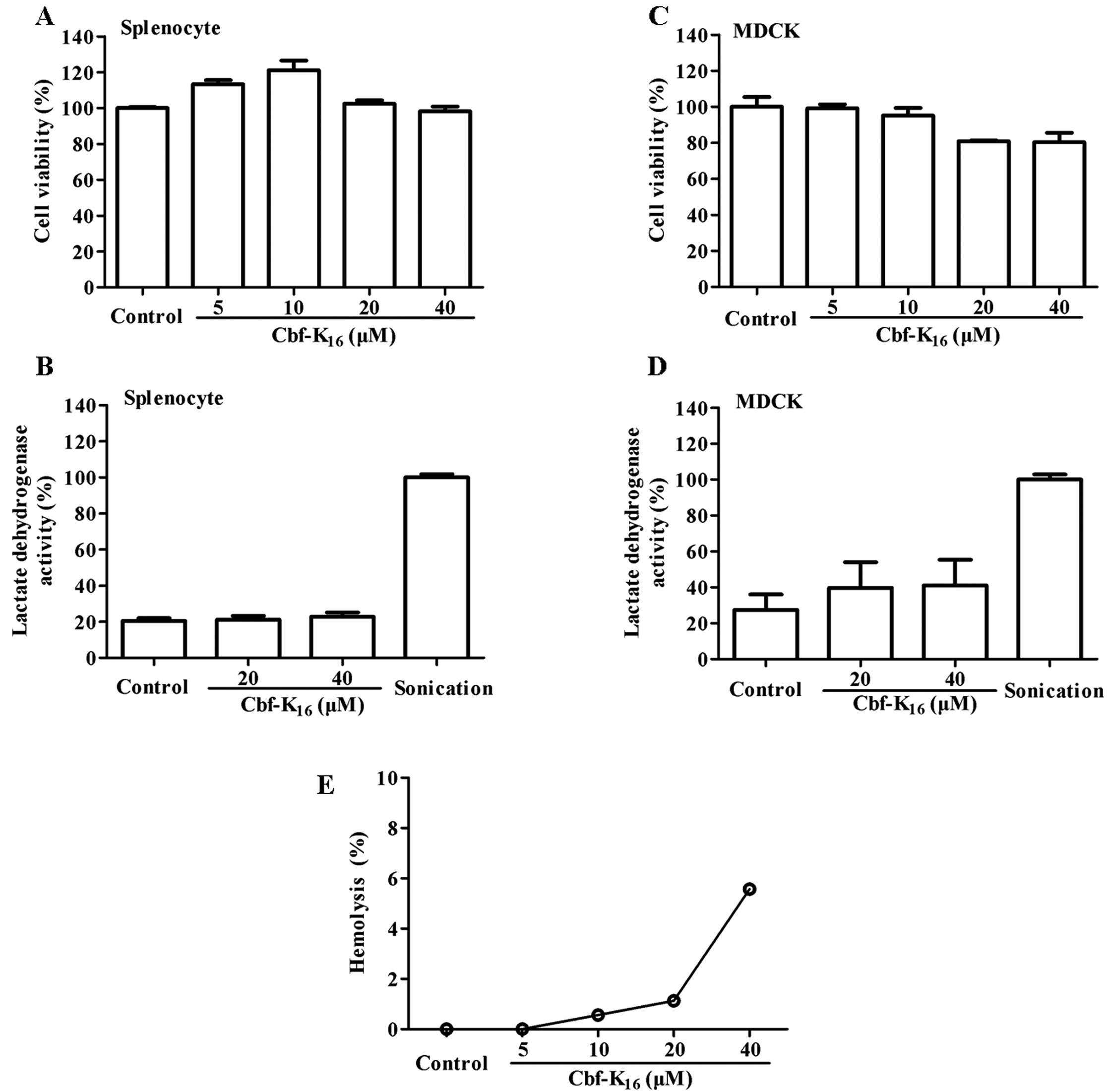
cells. As shown in Fig. 4A,
Cbf-K16 increased the proliferation of splenocytes to
113.3, 121.1 and 102.5% at doses of 5, 10 and 20 μM, respectively;
this may have been due to the immunoregulatory activity of
Cbf-K16. Compared with the significant cytotoxic
activity observed against tumor cells, 40 μM Cbf-K16
showed only a modest growth inhibition (<5%) of splenocytes.
Moreover, the LDH activities were 21.1 and 22.8% at 20 and 40 μM
Cbf-K16, respectively, compared to sonicated cells that
were used as a positive control. It should be noted that the LDH
activity in the untreated group was 20.5% (Fig. 4B). As shown in Fig. 4C, 20 and 40 μM Cbf-K16
resulted in only a modest inhibition of MDCK cells (<20%), while
these concentrations showed significant anticancer activity toward
lung cancer cells. These data were consistent with the results of
the LDH release assay shown in Fig.
4D. These results indicate that the Cbf-K16
polypeptide does not induce significant splenic or renal injury at
concentrations <40 μM. As shown in Fig. 4E, Cbf-K16 exhibited no
hemolytic activity at 5 to 20 μM, while 5.6% hemolysis was observed
with 40 μM Cbf-K16. In summary, the results above
indicate that the Cbf-K16 polypeptide (from 5 to 40 μM)
selectively inhibits the proliferation of lung cancer cells without
harming normal cells.
Discussion
Lung cancer remains the leading cause of
cancer-related mortality worldwide. Moreover, non-small cell lung
cancer (NSCLC) is a very lethal disease responsible for 80% of all
lung cancers. More than a million deaths worldwide are contributed
to NSCLC each year, and the 5-year survival rate for NSCLC patients
is <15% (35–37). Few treatments, including
chemotherapy and radiotherapy, are effective (38,39).
Additionally, conventional chemotherapy and radiotherapy are often
associated with severe side-effects to healthy cells and tissues.
Therefore, the most promising drugs are thought to be those with
better toxicity profiles, target selectivity and availability for
chronic treatment. Cbf-K16, a cationic amphiphilic
peptide, is a mutant of BF-30 that was generated by the
substitution of Glu16 with Lys16, which
results in an increase in net positive charge. In our recent study,
Cbf-K16 was also shown to possess broad-spectrum
antimicrobial activity, particularly against drug-resistant
bacteria (21); however, its
putative anticancer activity had not been elucidated.
In the search for new anticancer agents,
antimicrobial peptides and synthetic antimicrobials have recently
attracted significant attention owing to their novel mechanisms,
decreased likelihood of drug resistance, and low intrinsic
cytotoxicity (40,41). Our previous study indicated that
BF-30 could selectively inhibit the proliferation of the metastatic
melanoma cell line B16F10 without harming normal cells in
vitro or in vivo. Our results indicated that BF-30 had a
negative effect on human lung cells (22).
In the present study, we investigated the anticancer
activity and mechanism, as well as the toxicity of
Cbf-K16. Cbf-K16 demonstrated broad-spectrum
anticancer activity in vitro (Fig. 1). The viability of several tumor
cell lines gradually decreased as the concentration of
Cbf-K16 increased. Furthermore, different tumor cells
showed different sensitivities to Cbf-K16, and
differences in the membranes of these cancer cells may have
contributed to this selective permeability and toxicity, as
previously reported by Schweizer (41). Notably, human lung non-small cell
carcinoma H460 and Lewis cells were more sensitive to
Cbf-K16, with IC50 values of 16.5 and 10.5
μM, respectively. Cbf-K16 significantly suppressed the
proliferation of H460 and Lewis cells in a dose- and time-dependent
manner. Furthermore, H460 and Lewis cells displayed cell shrinkage,
abnormal boundaries and cell lysis after treatment with
Cbf-K16 at 20 μM. Thus, Cbf-K16 exhibited
enhanced cytotoxicity against H460 and Lewis cells compared with
BF-30 (45 and 40.3 μM, respectively) (22). Although the IC50 values
for Cbf-K16 against mouse melanoma B16 and mouse
malignant melanoma B16F10 cells (0.4 and 7.3 μM, respectively) were
less than those against mouse lung cancer Lewis cells (10.5 μM),
the IC50 value for Cbf-K16 against human
melanoma A375 cells (70.3 μM) was far greater than that for human
lung non-small cell carcinoma H460 cells (16.5 μM). Therefore,
considering a practical application in human cancer treatment, we
choose lung carcinoma H460 and Lewis cells for further study.
As previously reported, cationic antimicrobial
peptides exert their cytolytic activity by folding into an
amphipathic helix and inserting into the target membrane, leading
to the breakdown of membrane structure, leakage of cell contents,
and cell death (15). In the
present study, Cbf-K16 disrupted the integrity of the
cytoplasmic and mitochondrial membranes of lung non-small cell
carcinoma H460 cells. This disruption was corroborated by
transmission electron microscope images of H460 cells treated with
Cbf-K16 and by an LDH activity assay of the cell culture
supernatant following Cbf-K16 treatment (Fig. 2A and B). Cbf-K16 may
damage H460 cells by binding to the anionic cytoplasmic membrane
and spatially separating polar and hydrophobic residues. This
conformation facilitates the interaction of Cbf-K16 with
the membranes of lung non-small cell carcinoma H460 cells and
Cbf-K16 insertion into the cells, leading to membrane
rupture and release of LDH. However, it remains unclear whether
Cbf-K16 directly or indirectly penetrates the cell
membrane of H460 cells. Further experiments are in progress to
clarify this issue.
AV and PI staining were used to test whether
Cbf-K16 induces the apoptosis of lung non-small cell
carcinoma H460 cells. As shown in Fig.
2C, there was no significant apoptosis observed when the H460
cells were treated with either 20 or 40 μM (higher than
IC50) Cbf-K16 for 24 or 48 h. Therefore, the
molecular mechanism by which Cbf-K16 inhibits the
proliferation of H460 cells was not due to apoptosis. Instead,
membrane permeabilization and subsequent structural disruption may
be one of the main causes by which the Cbf-K16
polypeptide kills lung non-small cell carcinoma H460 cells.
Cbf-K16 also targeted additional anionic
constituents of lung non-small cell carcinoma H460 cells,
specifically genomic DNA. The results of our DNA retardation
experiment demonstrated that Cbf-K16 could bind to
genomic DNA from the H460 cells and suppress its electrophoretic
mobility in a dose-dependent manner (Fig. 3A). The genomic DNA-binding rate
increased to 86.8% as the concentration of Cbf-K16 increased to 10
μM, which was less than the IC50. The genomic
DNA-binding rate in H460 cells increased to 100% as the
concentration increased to 20 μM, which was higher than the
IC50. These data indicated that, at IC50,
Cbf-K16 significantly binds to genomic DNA. Therefore,
Cbf-K16 may exert its inhibitory effect on lung
non-small cell carcinoma H460 cells by binding to genomic DNA and
blocking gene expression. An overall positive charge favors the
binding of this antimicrobial peptide to negatively charged
membranes through electrostatic attraction, thereby functioning
selectivity. Although the precise mechanism of the selective
anticancer effect of Cbf-K16 has not yet been thoroughly
elucidated, our present study demonstrated that Cbf-K16
inhibits the proliferation of H460 cells by rupturing the plasma
membrane and binding to genomic DNA.
Unlike some antimicrobial peptides with greater
intrinsic cytotoxicity, for example NK-18 (11), Cbf-K16 exhibited no
significant inhibitory effect on the growth of normal cells,
including splenocytes and MDCK cells (Fig. 4A and C). As shown in Fig. 4A, Cbf-K16 increased the
proliferation of splenocytes at concentrations of 5, 10 and 20 μM
to 113.3, 121.1 and 102.5%, respectively. This may be due to
immunoregulatory activity, as previously reported (42), although the precise mechanism is
unknown. At 20 and 40 μM, Cbf-K16 showed significant
anticancer activity, but exhibited limited inhibition (<20%)
against splenocytes and MDCK cells. These data were supported by
the results of our LDH activity assays, which indicated that there
was no significant toxicity as the concentration of
Cbf-K16 increased from 0 to 40 μM. LDH activity data at
12 and 24 h are not shown. In addition, Cbf-K16 (from 0
to 40 μM) also exhibited no hemolytic activity (Fig. 4E). Taken together, these results
indicate that Cbf-K16 is not harmful to normal
cells.
The selective anticancer effect of
Cbf-K16 against human non-small cell lung carcinoma H460
cells that ultimately leads to H460 cell death is based on both the
formation of channels in the cell membrane and binding to genomic
DNA. Although further in vivo studies are required to
confirm the efficacy of Cbf-K16, our present research
initially suggests that Cbf-K16 may be a potential
candidate for the treatment of human NSCLC.
Acknowledgements
This research was financially supported by the
Scientific and Technological Support and Social Development Plan of
Jiangsu Province (SBE201270855), by the Six High Level Talent
Project from Jiangsu Province (no. 2011-WSN-048), the ‘111 Project’
from the Ministry of Education of China and the State
Administration of Foreign Expert Affairs of China (no. 111-2-07),
and the Project Program of the State Key Laboratory of Natural
Medicines, China Pharmaceutical University (no. SKLNMZZ201216).
References
|
1
|
Yan JX, Wang KR, Chen R, Song JJ, Zhang
BZ, Dang W, Zhang W and Wang R: Membrane active antitumor activity
of NK-18, a mammalian NK-lysin-derived cationic antimicrobial
peptide. Biochimie. 94:184–191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lehmann J, Retz M, Sidhu SS, Suttmann H,
Sell M, Paulsen F, Harder J, Unteregger G and Stöckle M: Antitumor
activity of the antimicrobial peptide magainin II against bladder
cancer cell lines. Eur Urol. 50:141–147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoskin DW and Ramamoorthy A: Studies on
anticancer activities of antimicrobial peptides. Biochim Biophysica
Acta. 1778:357–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vanajothi R, Sudha A, Manikandan R,
Rameshthangam P and Srinivasan P: Luffa acutangula and
lippia nodiflora leaf extract induces growth inhibitory
effect through induction of apoptosis on human lung cancer cell
line. Biomed Prev Nutr. 2:287–293. 2012. View Article : Google Scholar
|
|
5
|
Quadrelli S, Lyons G, Colt H, Chimondeguy
D and Silva C: Lung cancer as a second primary malignancy:
increasing prevalence and its influence on survival. Ann Surg
Oncol. 16:1033–1038. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim HM, Lim J, Park SK, Kang JS, Lee K,
Lee CW, Lee KH, Yun MJ, Yang KH, Han G, Kwon SW, Kim Y and Han SB:
Antitumor activity of cytokine-induced killer cells against human
lung cancer. Int Immunopharmacol. 7:1802–1807. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doroshow JH, Juhasz A, Ge Y, Holbeck S, Lu
J, Antony S, Wu Y, Jiang G and Roy K: Antiproliferative mechanisms
of action of the flavin dehydrogenase inhibitors diphenylene
iodonium and di-2-thienyliodonium based on molecular profiling of
the NCI-60 human tumor cell panel. Biochem Pharmacol. 83:1195–1207.
2012. View Article : Google Scholar
|
|
8
|
Maione P, Rossi A, Airoma G, Ferrara C,
Castaldo V and Gridelli C: The role of targeted therapy in
non-small cell lung cancer. Crit Rev Oncol Hematol. 51:29–44. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koh PK, Faivre-Finn C, Blackhall FH and
Ruysscher D: Targeted agents in non-small cell lung cancer (NSCLC):
clinical developments and rationale for the combination with
thoracic radiotherapy. Cancer Treat Rev. 38:626–640. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang W, Shi Y, Chen Y, Yu S, Hao J, Luo
J, Sha X and Fang X: Enhanced antitumor efficacy by
paclitaxel-loaded pluronic P123/F127 mixed micelles against
non-small cell lung cancer based on passive tumor targeting and
modulation of drug resistance. Eur J Pharm Biopharm. 75:341–353.
2010. View Article : Google Scholar
|
|
11
|
Dempke WC, Suto T and Reck M: Targeted
therapies for non-small cell lung cancer. Lung Cancer. 67:257–274.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang K, Yan J, Liu X, Zhang J, Chen R,
Zhang B, Dang W, Zhang W, Kai M, Song J and Wang R: Novel
cytotoxity exhibition mode of polybia-CP, a novel antimicrobial
peptide from the venom of the social wasp Polybia paulista.
Toxicology. 288:27–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zasloff M: Antimicrobial peptides of
multicellular organisms. Nature. 415:389–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen C, Hu J, Zhang S, Zhou P, Zhao X, Xu
H, Zhao X, Yaseen M and Lu JR: Molecular mechanisms of
antibacterial and antitumor actions of designed surfactant-like
peptides. Biomaterials. 33:592–603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou H, Dou J, Wang J, Chen L, Wang H,
Zhou W, Li Y and Zhou C: The antibacterial activity of BF-30 in
vitro and in infected burned rats is through interference with
cytoplasmic membrane integrity. Peptides. 32:1131–1138. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Almaaytah A, Zhou M, Wang L, Chen T,
Walker B and Shaw C: Antimicrobial/cytolytic peptides from the
venom of the North African scorpion, Androctonus amoreuxi:
biochemical and functional characterization of natural peptides and
a single site-substituted analog. Peptides. 35:291–299.
2012.PubMed/NCBI
|
|
17
|
Okumura K: Cathelicidins - Therapeutic
antimicrobial and antitumor host defense peptides for oral
diseases. Jpn Dental Sci Rev. 47:67–81. 2011. View Article : Google Scholar
|
|
18
|
Chan DI, Prenner EJ and Vogel HJ:
Tryptophan- and arginine-rich antimicrobial peptides: structures
and mechanisms of action. Biochim Biophys Acta. 1758:1184–1202.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Hong J, Liu X, Yang H, Liu R, Wu
J, Wang A, Lin D and Lai R: Snake cathelicidin from Bungarus
fasciatus is a potent peptide antibiotics. PLoS One.
3:e32172008.PubMed/NCBI
|
|
20
|
Wang Y, Zhang Z, Chen L, Guang H, Li Z,
Yang H, Li J, You D, Yu H and Lai R: Cathelicidin-BF, a snake
cathelicidin-derived antimicrobial peptide, could be an excellent
therapeutic agent for Acne Vulgaris. PLoS One. 6:e221202011.
View Article : Google Scholar
|
|
21
|
Hao Q, Wang H, Wang J, Dou J, Zhang M,
Zhou W and Zhou C: Effective antimicrobial activity of
Cbf-K16 and Cbf-A7A13 against
NDM-1-carrying Escherichia coli by DNA binding after
penetrating the cytoplasmic membrane in vitro. J Pept Sci.
19:173–180. 2013.PubMed/NCBI
|
|
22
|
Wang H, Ke M, Tian Y, Wang J, Li B, Wang
Y, Dou J and Zhou C: BF-30 selectively inhibits melanoma cell
proliferation via cytoplasmic membrane permeabilization and
DNA-binding in vitro and in B16F10-bearing mice. Eur J
Pharmacol. 707:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin WJ, Chien YL, Pan CY, Lin TL, Chen JY,
Chiu SJ and Hui CF: Epinecidin-1, an antimicrobial peptide from
fish (Epinephelus coioides) which has an antitumor effect
like lytic peptides in human fibrosarcoma cells. Peptides.
30:283–290. 2009.PubMed/NCBI
|
|
24
|
Chen JY, Lin WJ and Lin TL: A fish
antimicrobial peptide, tilapia hepcidin TH2-3, shows potent
antitumor activity against human fibrosarcoma cells. Peptides.
30:1636–1642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Li HB, Li S, Tian LL and Shang DJ:
Antitumor effects and cell selectivity of temporin-1CEa, an
antimicrobial peptide from the skin secretions of the Chinese brown
frog (Rana chensinensis). Biochimie. 94:434–441. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Qu Y, Zhang J and Wang X:
Ardipusilloside I purified from Ardisia pusilla
competitively binds VEGFR and induces apoptosis in NCI-H460 cells.
Phytomedicine. 17:519–526. 2010.
|
|
27
|
Bolt AM, Byrd RM and Klimecki WT:
Autophagy is the predominant process induced by arsenite in human
lymphoblastoid. Toxicol Appl Pharmacol. 244:366–373. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang WT, Pan CY, Rajanbabu V, Cheng CW
and Chen JY: Tilapia (Oreochromis mossambicus) antimicrobial
peptide, hepcidin 1–5, shows antitumor activity in cancer cells.
Peptides. 32:342–352. 2011.PubMed/NCBI
|
|
29
|
Hsu JC, Lin LC, Tzen JT and Chen JY:
Characteristics of the antitumor activities in tumor cells and
modulation of the inflammatory response in RAW264.7 cells of a
novel antimicrobial peptide, chrysophsin-1, from the red sea bream
(Chrysophrys major). Peptides. 32:900–910. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen JY, Lin WJ, Wu JL, Her GM and Hui CF:
Epinecidin-1 peptide induces apoptosis which enhances antitumor
effects in human leukemia U937 cells. Peptides. 30:2365–2373. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Paredes-Gamero EJ, Martins MN, Cappabianco
FA, Ide JS and Miranda A: Characterization of dual effects induced
by antimicrobial peptides: regulated cell death or membrane
disruption. Biochim Biophys Acta. 1820:1062–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu H, Zhou BH, Qiu X, Wang HS, Zhang F,
Fang R, Wang XF, Cai SH, Du J and Bu XZ: T63, a new 4-arylidene
curcumin analogue, induces cell cycle arrest and apoptosis through
activation of the reactive oxygen species-FOXO3a pathway in lung
cancer. Free Radic Biol Med. 53:2204–2217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang YQ, Su J, Wu F, Lu P, Yuan LF, Yuan
WE, Sheng J and Jin T: Biscarbamate cross-linked polyethylenimine
derivative with low molecular weight, low cytotoxicity, and high
efficiency for gene delivery. Int J Nanomedicine. 7:693–704.
2012.PubMed/NCBI
|
|
34
|
Hsu JC, Lin LC, Tzen JT and Chen JY:
Pardaxin-induced apoptosis enhances antitumor activity in HeLa
cells. Peptides. 32:1110–1116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim YS, Jin HO, Seo SK, Woo SH, Choe TB,
An S, Hong SI, Lee SJ, Lee KH and Park IC: Sorafenib induces
apoptotic cell death in human non-small cell lung cancer cells by
down-regulating mammalian target of rapamycin (mTOR)-dependent
survivin expression. Biochem Pharmacol. 82:216–226. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Custodio A, Méndez M and Provencio M:
Targeted therapies for advanced non-small-cell lung cancer: current
status and future implications. Cancer Treat Rev. 38:36–53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Wilt LH, Jansen G, Assaraf YG, van
Meerloo J, Cloos J, Schimmer AD, Chan ET, Kirk CJ, Peters GJ and
Kruyt FA: Proteasome-based mechanisms of intrinsic and acquired
bortezomib resistance in non-small cell lung cancer. Biochem
Pharmacol. 83:207–217. 2012.PubMed/NCBI
|
|
38
|
Leung HW, Yang WH, Lai MY, Lin CJ and Lee
HZ: Inhibition of 12-lipoxygenase during baicalein-induced human
lung non-small carcinoma H460 cell apoptosis. Food Chem Toxicol.
45:403–411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Silvestri GA and Rivera MP: Targeted
therapy for the treatment of advanced non-small cell lung cancer: a
review of the epidermal growth factor receptor antagonists. Chest.
128:3975–3984. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen YL, Li JH, Yu CY, Lin CJ, Chiu PH,
Chen PW, Lin CC and Chen WJ: Novel cationic antimicrobial peptide
GW-H1 induced caspase-dependent apoptosis of hepatocellular
carcinoma cell lines. Peptides. 36:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schweizer F: Cationic amphiphilic peptides
with cancer-selective toxicity. Eur J Pharmacol. 625:190–194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Y, Xia X, Xu L and Wang Y: Design of
hybrid β-hairpin peptides with enhanced cell specificity and potent
anti-inflammatory activity. Biomaterials. 34:237–250. 2013.
|