Introduction
Gastric cancer is the fourth leading cause of
cancer-related death in the world (1). To date, chemotherapy is the most
frequently used fundamental treatment for gastric cancer. However,
the main barrier to successful chemotherapy is MDR caused by
insensitivity to multiple chemotherapeutic agents after exposure to
a single chemotherapeutic drug (2).
For decades, the most representative consensus is the
overexpression of drug efflux pumps (such as P-gp) occupying a
critical position in development of MDR. Consequently, several
innovative chemosensitizers against P-gp, such as verapamil,
trifluoperazine and cyclosporine, have been found to enhance
chemosensitivity of MDR phenotype cancer cells (3). However, the clinical application of
these chemosensitizers showed disappointing results (4,5),
indicating that there are some unknown molecules and mechanisms
also responsible for MDR. Recent studies have verified that some
novel factors including defective apoptosis pathway, enhanced DNA
repair activity, or altered metabolism of drugs play a critical
role in promoting formation of MDR (6). Though the underlying mechanisms have
been deeply studied in vitro and in vivo(6–9), the
precise mechanisms involved in MDR have not been fully
characterized. Evidence so far suggest that mechanisms responsible
for MDR in gastric cancer are likely to be multifaceted and
extremely intricate. Thus, further investigation of the MDR
mechanisms to find acceptable chemosensitizers for clinical
application in human cancers, including gastric cancer is
required.
Interleukin-24 (IL-24), a novel member of
interleukin-10 family of cytokines, also known as melanoma
differentiation associated gene-7 (mda-7), was first identified by
using subtraction hybridization of cDNA libraries from actively
proliferating human HO-1 melanoma cells versus interferon-β and
mezerein-treated HO-1 cells (10).
It can specifically induce apoptosis in a wide variety of malignant
tumor cells exerting no discernible toxic effects towards normal
cells by eliciting potent ‘antitumor bystander activity’ as a
consequence of autocrine secretion (11), which has attracted particular
attention from researchers worldwide. Subsequently studies
demonstrated that expression of IL-24 was lost in a broad spectrum
of malignant tumors including gastric cancer, whereas ectopic
expression of this gene performed a ubiquitous growth inhibition,
apoptosis induction, reversal malignant phenotype and terminal
differentiation in a variety of cancers (10,12–17).
These gratifying findings have led to the development of INGN241, a
replication-incompetent IL-24-expressing adenovirus, which is
currently in phase II/III clinical trials (18).
Previous studies showed that combination of
chemotherapy, radiotherapy and other conventional therapies with
gene therapy is a promising practice in cancer treatment (19–21).
Furthermore, adenoviral p53 gene was in adjuvant use with
conventional chemotherapy, radiation therapy, and surgery of lung
and head and neck cancers (22).
IL-24 has been reported to sensitize human colorectal cancer cells
to doxorubicin and 5-fluorouracil, human melanoma cells to
dacarbazine, and human hepatocellular carcinoma cells to
5-fluorouracil, respectively (3,17,23,24).
In addition, our laboratory demonstrated that adenovirus-mediated
IL-24 gene therapy could enhance chemosensitivity of MDR phenotype
colon cancer cells to oxaliplatin (25). However, its chemosensitizing effects
for human MDR phenotype gastric cancer cells so far have not been
reported. Given this, we successfully established the CDDP-induced
MDR phenotype gastric cancer cell subline and hypothesized that
IL-24 gene may sensitize these human gastric cancer cells to
cisplatin therapy. In this study, we also investigated the possible
role of IL-24 in chemosensitizing of human gastric cancer cells and
its underlying mechanisms.
Materials and methods
Adenoviral vectors, cell lines, reagents
and mice
The Ad-IL-24 and Ad-GFP adenoviral vectors were
constructed in our laboratory (26). The human embryonic kidney cell line
QBI-293A was kindly provided by Professor Jiang Zhong of Fudan
University (Shanghai, China). The human gastric cancer cell line
SGC7901 was purchased from the American Type Culture Collection
(ATCC, Rockville, MD, USA). The QBI-293A and SGC7901 cell lines
were cultured in RPMI-1640 medium (Gibco, Shanghai, China)
supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT,
USA). The TRIzol reagent and the reverse transcriptase MuMLV were
purchased from Invitrogen (Shanghai, China). The cell counting
kit-8 was purchased from the subsidiary of Dojindo Laboratories
(Shanghai, China). The Annexin V-PE/7-AAD apoptosis detection kit
was purchased from BD Biosciences (Shanghai, China). The in
situ cell death detection kit was purchased from Roche Applied
Science (Shanghai, China). The monoclonal anti-IL-24 antibody and
human IL-24 enzyme-linked immunosorbent assay (ELISA) kit were
purchased from R&D Systems (Shanghai, China). The antibodies
specific for P-gp, Bax, Bcl-2 were from Cell Signaling Technology
(Boston, MA, USA). The SuperEnhanced chemiluminescence detection
kit was from Applygen Technologies Inc. (Beijing, China). The
UltraSensitive™ SP kit was obtained from Maixin (Fuzhou, China).
Chemotherapeutical drugs cisplatin (CDDP), 5-fluorouracil (5-FU),
adriamycin (ADM) and methotrexate (MTX) were kindly provided by The
First Hospital Affiliated of Soochow University (Suzhou, China).
Additionally, female athymic nude mice were purchased from Shanghai
Experimental Animal Center (Shanghai, China) and maintained in the
animal facility at Soochow University according to the animal
research committee’s guidelines of Soochow University.
Development of the MDR phenotype human
gastric cancer cell subline SGC7901/CDDP
Gastric cancer SGC7901 cells were cultured in
RPMI-1640 supplemented with 10% FBS overnight and then changed to
cisplatin-containing medium to induce MDR by repeated selection of
resistant clones of parental sensitive SGC7901 cells to stepwise
increasing concentrations of cisplatin. After 4 months, the SGC7901
cells could stably grow in 1 μg/ml cisplatin-containing medium. To
maintain the MDR phenotype, the 1 μg/ml cisplatin-containing medium
was used in later MDR phenotype cell culture.
Transfection
To assess the optimal multiplicity of infection
(MOI) for a maximal infection and transgene expression, human
gastric cancer cells SGC7901/CDDP were infected with Ad-IL-24 and
Ad-GFP at various MOIs (0, 1, 10, 25, 50, 100 and 200) for 24 h.
The adenoviral infection efficiency was analyzed according to GFP
expression by fluorescence microscopy. Furthermore, the IL-24
transgene expression mediated by adenoviral infection in
SGC7901/CDDP cells was determined by using RT-PCR and western blot
analysis.
RT-PCR analysis
Total RNA was extracted from Ad-IL-24- or
Ad-GFP-infected and uninfected SGC7901/CDDP cells using TRIzol and
then reversely transcribed into cDNA using Oligo d(T)18 as primer
according to the manufacturer’s protocol. PCR amplification was
carried out using these cDNA samples as templates and IL-24 primers
(5′-GCACTCGAGCCATGAATTTTCAACAGAGGCTGCA-3′ and
5′-GCTTCTAGATCAGAGCTTGTAGAATTTCTG-3′) under conditions of an
initia1 cycle at 94°C for 2 min and 72°C for 10 min followed by 35
cycles at 94°C for 50 sec, 58°C for 50 sec and 72°C for 55 sec and
a final extension at 72°C for 10 min. The PCR products were
separated in 1% agarose gels using electrophoresis with ethidium
bromide staining. Subsequently, The GAPDH as well as multidrug
resistant- and apoptosis-related genes MDR1, Bax and Bcl-2 were
detected as the above protocols, the PCR reaction was carried out
using the following primers (5′-GCGGCTCCGATACATGGTT-3′ and
5′-TGGCGAGCCTGGTAGTCAAT-3′ for MDR1; 5′-GGA TGCGTCCACCAAGAA-3′ and
5′-GCACTCCCGCCACAAAGA-3′ for Bax; 5′-TGTGGCCTTCTTTGAGTTCG-3′ and
5′-CTACCCAGCCTCCGTTATCC-3′ for Bcl-2;
5′-TTACTCCTTGGAGGCCATGTGGGCC-3′ and 5′-ACT
GCCACCCAGAAGACTGTGGATGG-3′ for human GAPDH).
Western blot analysis
Total proteins was isolated from Ad-IL-24- or
Ad-GFP-infected and uninfected SGC7901/CDDP cells and resolved in
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and subsequently transferred onto a polyvinylidene
difluoride membrane. After that, the membrane was incubated in 5%
(w/v) non-fat dry milk in Tris-buffered saline containing 0.05%
Tween-20 (TBST) for 1 h at 37°C and then further with a panel of
primary antibodies specific for IL-24, P-gp, Bax, Bcl-2 and GAPDH
(an internal control) in blocking solution for 1 h at 37°C. The
membrane was then washed with TBST and incubated with a peroxidase
horseradish peroxidase-conjugated secondary antibody in blocking
solution for another 1 h at 37°C. After three washes with TBST, the
positive bands were developed by using a SuperEnhanced
chemiluminescence detection kit and visualized after exposure of
the membranes to Kodak X-ray film.
ELISA analysis
The adenovirus-mediated secretory expression of
IL-24 in SGC7901/CDDP cells was detected by ELISA analysis.
Briefly, the SGC7901/CDDP cells (2.5×106) were infected
with 100 MOI Ad-IL-24, Ad-GFP or without adenovirus (PBS) in 10 ml
medium, respectively. After 24 h of treatment, the cellular culture
supernatants generated from the three groups were collected, and
the amount of IL-24 in above culture supernatants was analysed by
ELISA using human IL-24 ELISA kit according to the manufacturer’s
instructions.
CCK-8 assay
The in vitro resistance index of SGC7901/CDDP
cells were analyzed by CCK-8 assay. Briefly, SGC7901 and
SGC7901/CDDP cells (1×104 per well) were seeded in
96-well culture plates and incubated for 24 h at 37°C and then
treated with CDDP, 5-FU, ADM and MTX in seven different
concentrations for 48 h (see Results). The viability of SGC7901 and
SGC7901/CDDP cells were then analyzed by using CCK-8 kit according
to the manufacturer’s protocol. Similarly, the cells infected with
Ad-IL-24 and Ad-GFP were also included for CCK-8 assay. The
SGC7901/CDDP cells were infected with 100 MOI Ad-IL-24 or Ad-GFP or
without adenovirus (PBS) for 24 h and then treated with CDDP, 5-FU,
ADM and MTX for 48 h and then subjected to CCK-8 assay. Inhibitory
rate (%) was calculated using the formula: 1 −
(ODexperiments/ODcontrols) ×100%; Resistance
index: IC50(SGC7901/CDDP)/IC50(SGC7901) and
Reversion index:
IC50(PBS)/IC50(Ad-IL-24).
Flow cytometric analysis of cell cycle
alteration
The SGC7901/CDDP human gastric cancer cells
(1×106) were cultured with 100 MOI Ad-IL-24, Ad-GFP or
without adenovirus (PBS), respectively. After 48 h, the infected
and uninfected SGC7901/CDDP cells were trypsinized and washed in
cold PBS, then subjected to cold 70% ethanol for 12 h, and the
cells were stained with propidium iodide for cell cycle analysis by
flow cytometry. All experiments were repeated three times.
Analysis of in vitro and in vivo
chemosensitizing effects
To test the chemosensitizing effects of Ad-IL-24
in vitro and in vivo, the following groups were
studied: PBS+SGC7901/CDDP (PBS), Ad-GFP+SGC7901/CDDP (Ad-GFP),
Ad-IL-24+SGC7901/CDDP (Ad-IL-24), CDDP+SGC7901/CDDP (CDDP),
Ad-GFP+CDDP+SGC7901/CDDP (Ad-GFP+CDDP) and
Ad-IL-24+CDDP+SGC7901/CDDP (Ad-IL-24+CDDP). Firstly, we
investigated in vitro effects. The SGC7901/CDDP cells
(2.5×105) were cultured in 6-well culture plates (marked
A, B, C, D, E and F, respectively), After 24 h, 100 MOI Ad-IL-24
was added in A and B well, 100 MOI Ad-GFP was added in C and D
well, equivalent PBS was added in E and F well as controls. After
next 24 h, 2.5 μg/ml CDDP was added in A, C and E wells. Two days
later, all treatment groups were harvested and washed in cold PBS,
the apoptosis rate was assessed by flow cytometry using the Annexin
V-PE/7-AAD apoptosis detection kit according to the manufacturer’s
protocol. Briefly, the SGC7901/CDDP cells (2.5×105) were
incubated with 5 μl of Annexin V-PE and 5 μl 7-AAD in 100 μl of 1X
Annexin V-binding buffer at room temperature. After incubating for
15 min, 400 μl of 1X binding buffer was added, and the apoptotic
cells were analyzed by flow cytometry.
Secondly, we investigated in vivo effects.
The female athymic nude mice were subcutaneously (s.c.) inoculated
into the armpits of their right anterior limbs with
2×106 human SGC7901/CDDP cells. After the tumor mass
reached a mean tumor volume of ~100 mm3, Ad-IL-24,
Ad-GFP or PBS were given once every 3 days by intratumoral
injection for 36 days. From days 7–14 and 21–28, 4.5 mg/kg of CDDP
was given via tail vein injection weekly. Tumor progression and
regression were monitored and tumor volume was measured with a
caliper every four days. The tumor volume was calculated by a
formula, i.e., ab2/2, where a is the larger and b is the
smaller of the two dimensions. The tumor-bearing mice were then
sacrificed at day 36 after the treatments and tumor xenograft
tissues were removed, weighed, fixed by 10% neutral formalin, and
then embedded in paraffin for hematoxylin and eosin staining and
immunohistochemical analysis.
Immunohistochemistry
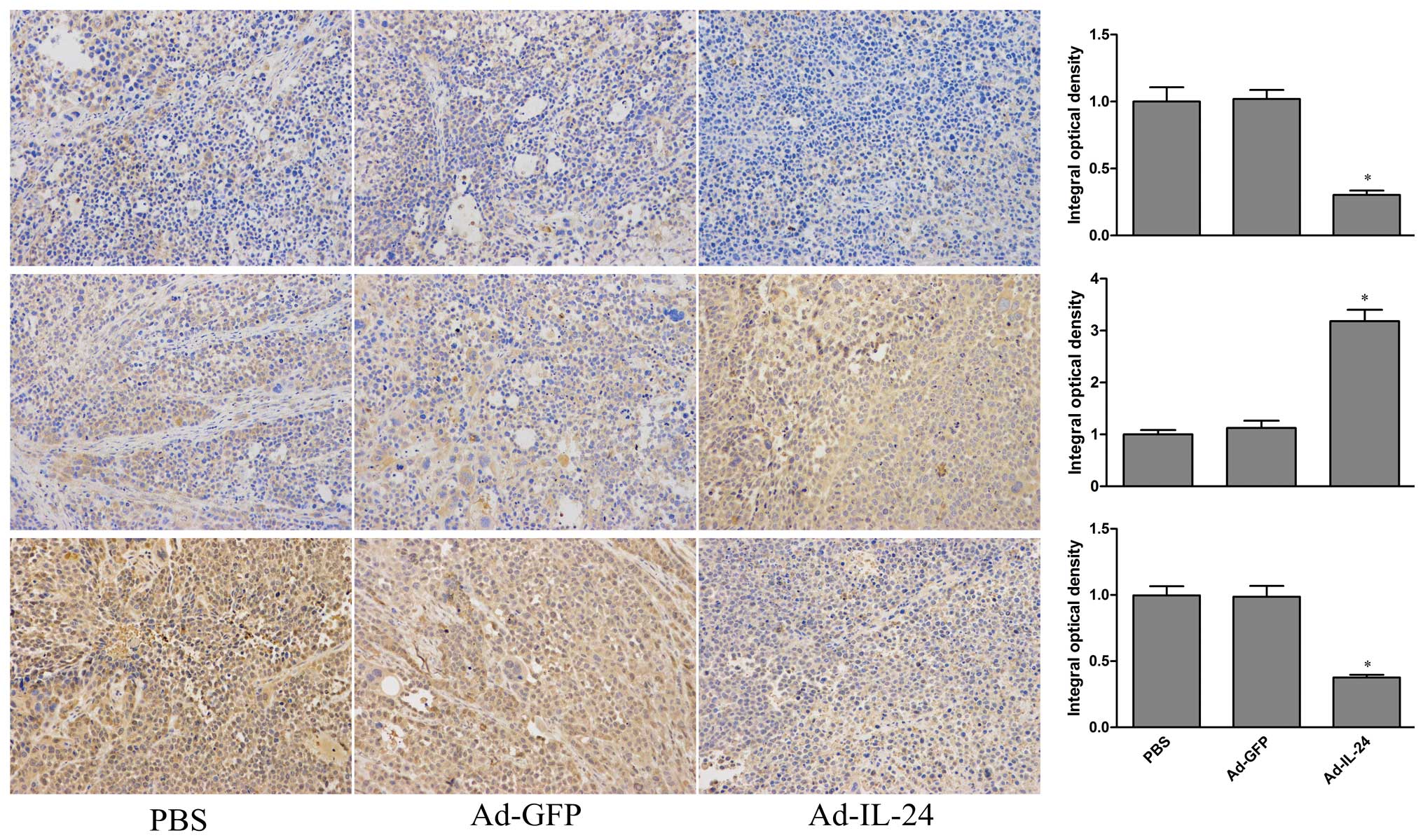
Expression of P-gp, Bax and Bcl-2 proteins of PBS,
Ad-GFP and Ad-IL-24 groups in human gastric cancer xenograft
tissues was analyzed by using immunohistochemistry with an
UltraSensitive SP kit according to the manufacturer’s instructions.
The presence of buffy or brown diaminobenzidine precipitates is
indicative of positive reactivity. The integral optical density
(IOD) of immunohistochemical intensity was analyzed by Image-Pro
Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA).
Statistical analysis
All data were presented as the mean ± SD. The
significant difference between two groups was evaluated by using
Student’s t-test and one-way or two-way repeated measures analysis
of variance and multiple comparisons with SPSS 10.0 software (SPSS,
Chicago, IL, USA). A value of P<0.05 was considered to be
significant.
Results
Development of the CDDP-induced MDR
phenotype gastric cancer cell subline SGC7901/CDDP
To obtain CDDP-induced MDR phenotype gastric cancer
cell subline SGC7901/CDDP, we selected the SGC7901 cells repeatedly
under increased cisplatin concentrations from 0.04 μg/ml to 1 μg/ml
in a 4 months period. The MDR phenotype gastric cancer cells could
grow stably in 1 μg/ml cisplatin-containing medium. To maintain the
MDR phenotype, 1 μg/ml cisplatin-containing medium was used in
later MDR phenotype cell cultures. We first performed CCK-8 assay
to detect the changed viability in SGC7901/CDDP cells. The data
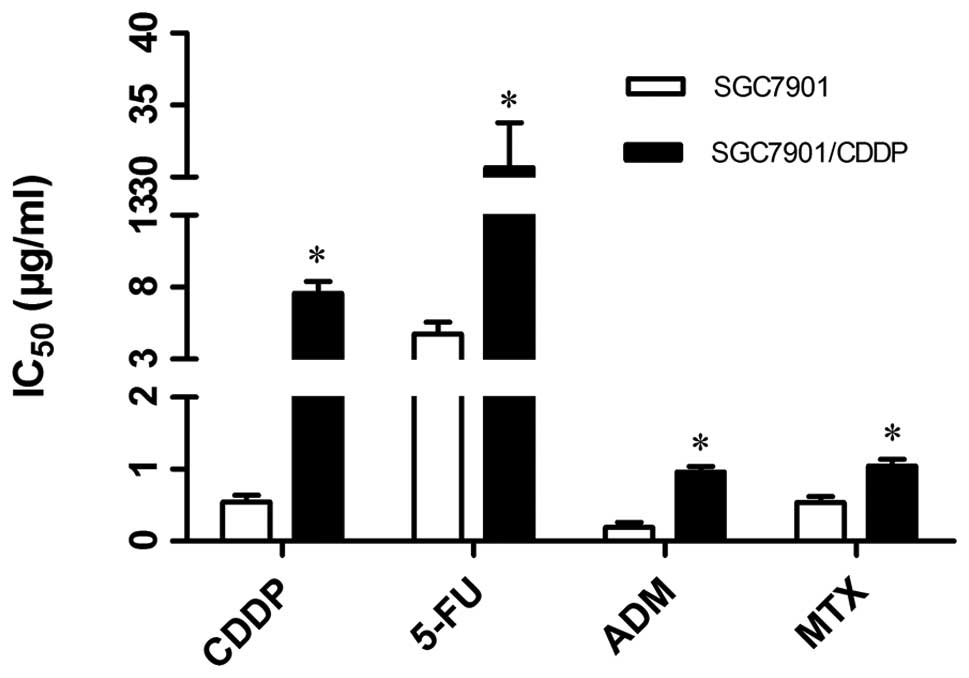
showed that SGC7901/CDDP cells acquired 14.03-, 6.47-, 5.06- and
1.94-fold resistance to CDDP, 5-FU, ADM and MTX compared to
parental SGC7901 cells, respectively (P<0.05; Fig. 1).
Stable expression of IL-24 in vitro
To assess the optimal MOI for a maximal transgene
expression with minimal adenovirus itself-caused cytotoxicity,
SGC7901/CDDP cells were infected with Ad-IL-24 or Ad-GFP at
different MOIs (see Materials and methods) and examined under
fluorescence microscopy. More than 90% of GFP expression was found
in the Ad-IL-24- or Ad-GFP-infected SGC7901/CDDP cells at MOI of
100 or above, whereas the GFP expression was not found in
uninfected SGC7901/CDDP cells. Additionally, there was rarely
adenovirus-elicited cytotoxic effect in 100 MOI blank
Ad-GFP-infected SGC7901/CDDP cells (data not shown). Furthermore,
adenovirus-mediated exogenous IL-24 tumor suppressor gene and
protein was significant expressed at 100 MOI in Ad-IL-24-infected
SGC7901/CDDP cells but not in Ad-GFP-infected and uninfected
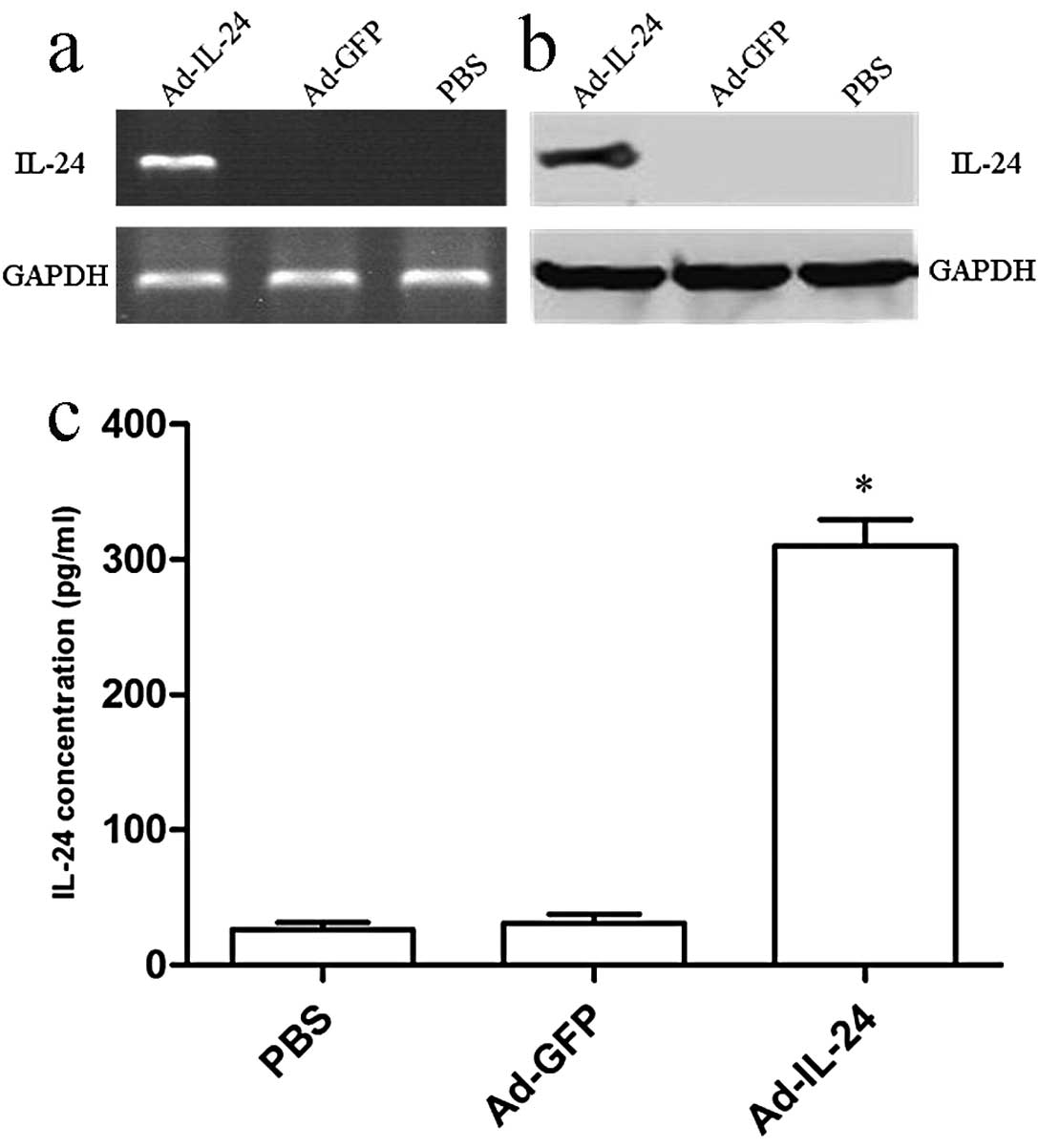
SGC7901/CDDP cells (Fig. 2a and b),
indicating that IL-24 is expressed in Ad-IL-24-transfected
SGC7901/CDDP cells at transcriptional and translational levels. In
addition, a significant amount of secreted IL-24 was found in the
culture supernatants of Ad-IL-24 infected SGC7901/CDDP cells but
not in the Ad-GFP infected or uninfected SGC7901/CDDP cells
(P<0.05; Fig. 2c). These results
suggested that 100 MOI can be used as an optimal dose for the
adenovirus-mediated IL-24 gene induction and transgene expression
in human gastric cancer SGC7901/CDDP cells.
Ad-IL-24 significantly enhances
chemosensitivity of SGC7901/CDDP cells to cisplatin in vitro and in
vivo
Based on the development of MDR phenotype
SGC7901/CDDP cells, we further explored whether Ad-IL-24 had
chemosensitizing effects on SGC7901/CDDP cells. In vitro,
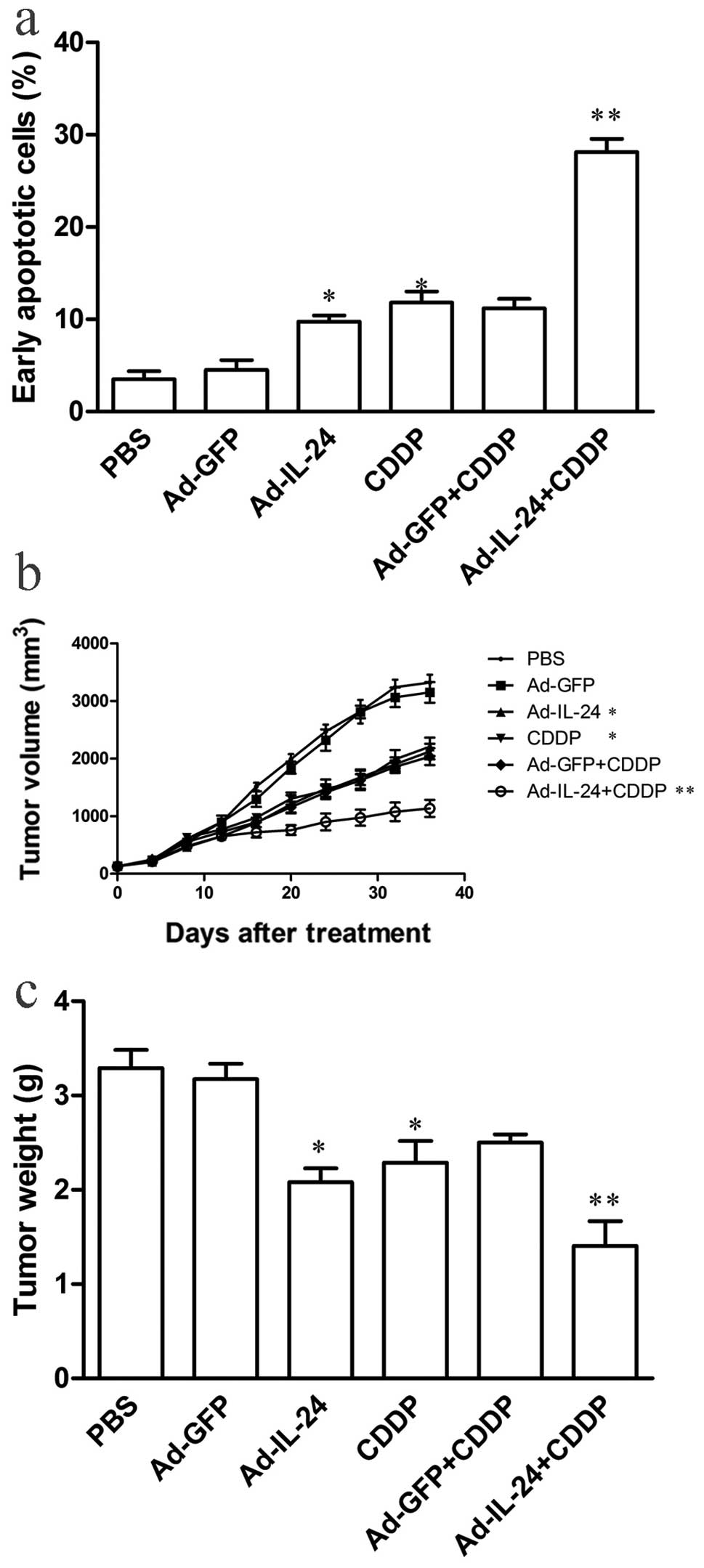
Annexin V-PE and 7-AAD double-positive staining by flow cytometry
showed that Ad-IL-24 plus CDDP could induce greater early apoptosis
(28.13%) than Ad-IL-24 (9.77%) or CDDP (10.83%) alone (P<0.05
Fig. 3a), indicating that Ad-IL-24
treatment could significantly enhance the chemosensitivity of
SGC7901/CDDP cells to cisplatin. To further address the potential
chemosensitizing effects of Ad-IL-24 on SGC7901/CDDP cells, in
vivo, we injected SGC7901/CDDP cells into athymic nude mice and
then injected Ad-IL-24, Ad-GFP or PBS and treatment continued with
or without CDDP. The data showed that Ad-IL-24 plus CDDP
significantly reduced tumor volume from days 12–36 compared to the
other groups (P<0.05; Fig. 3b).
Similarly, the tumors weight also showed a difference in Ad-IL-24
plus CDDP treatment group (P<0.05; Fig. 3c), indicating that Ad-IL-24 also has
a robust chemosensitizing effect on gastric cancer SGC7901/CDDP
cell xenografts in vivo in an athymic nude mouse model.
Ad-IL-24 elicits chemosensitizing effect
by induction of cell cycle alteration and reversal of expression of
multidrug resistant- and apoptosis-related proteins
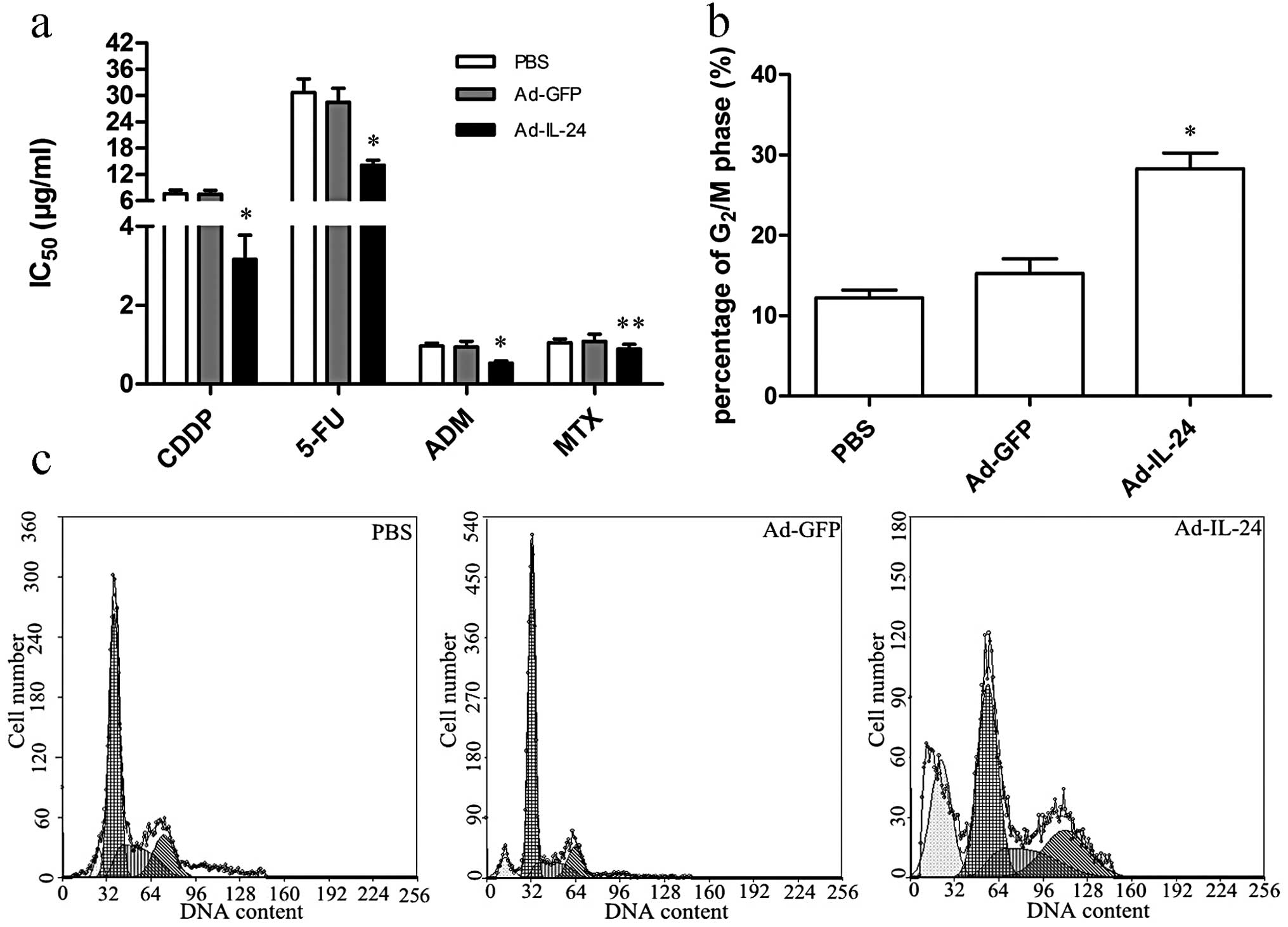
We first investigated the changes in IC50
elicited by Ad-IL-24 in MDR phenotype gastric cancer cells. The
SGC7901/CDDP cells were infected with Ad-IL-24 or Ad-GFP at 100
MOI, cell viability was determined on the fourth day by using CCK-8
assay. Compared with Ad-GFP and PBS treatment groups, the
IC50 (Fig. 4a) of the
Ad-IL-24-infected SGC7901/CDDP cells to CDDP, 5-FU, ADM (not MTX)
was significantly decreased. To further address the underlying
mechanisms that may be responsible for Ad-IL-24-mediated
chemosensitizing effect. We analyzed the cell cycle distribution
and the changes of multidrug resistant- and apoptosis-related
protein expression. The cell cycle alteration of SGC7901/CDDP cells
in Ad-IL-24 or Ad-GFP or PBS treatment group was analyzed by flow
cytometry.
As shown in Fig. 4b and
c, the proportion of SGC7901/CDDP cells in the G2/M
phase was significant increased in Ad-IL-24 treatment group
compared with Ad-GFP and PBS groups (P<0.05). Subsequently, the
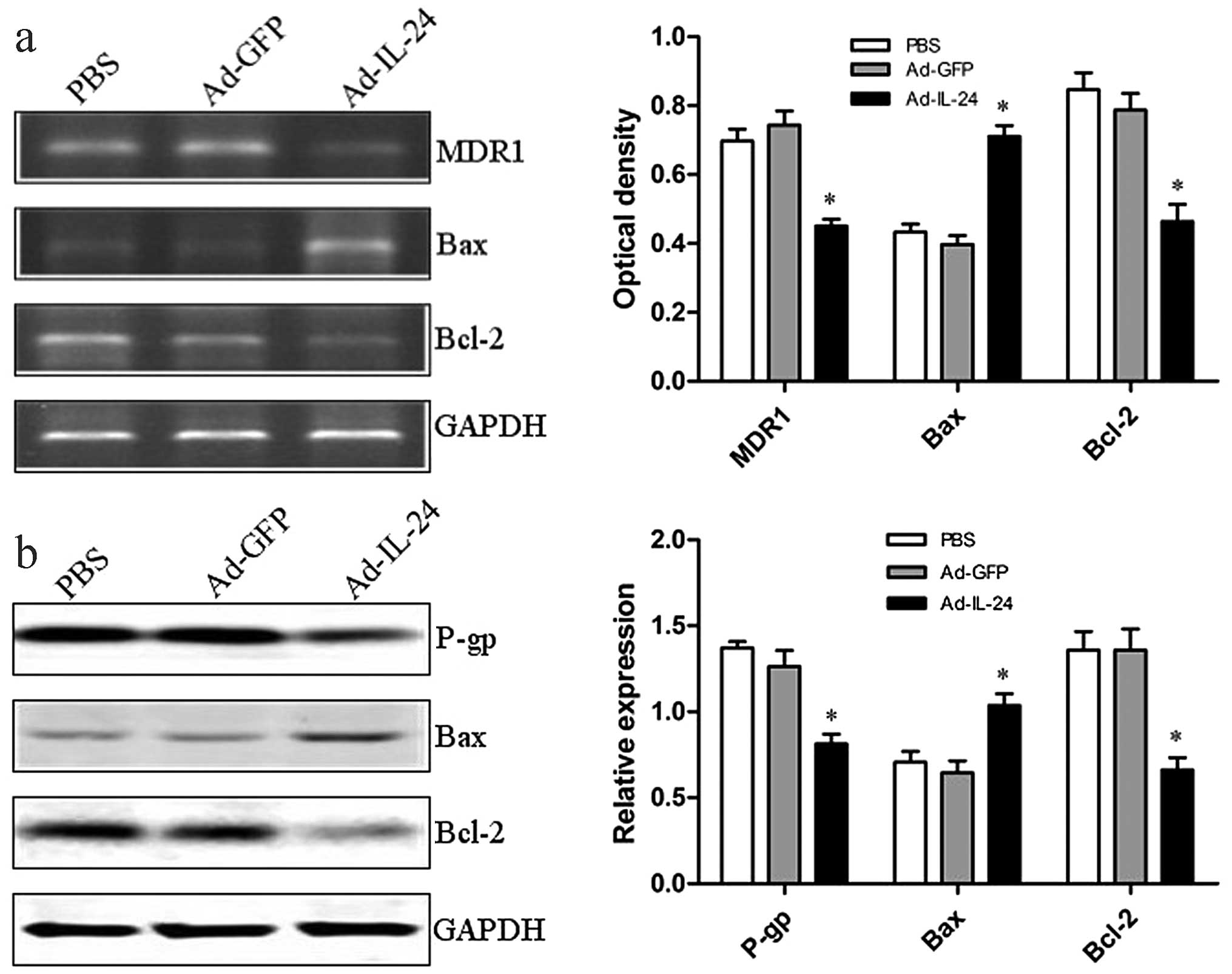
expression of MDR- and apoptosis-related genes/proteins (MDR1/P-gp,
Bax/Bax and Bcl-2/Bcl-2) were detected by RT-PCR and western blot
analysis in these cells. Our results showed that MDR1/P-gp and
Bcl-2/Bcl-2 were downregulated, whereas Bax/Bax was upregulated in
Ad-IL-24-treated SGC7901/CDDP cells compared to PBS and Ad-GFP
groups (P<0.05; Fig. 5a and b).
Furthermore, expression of multidrug resistant-related proteins
P-gp and apoptosis-related proteins Bax and Bcl-2 was also
modulated in nude xenograft tissues (P<0.05; Fig. 6). Those results indicate that
Ad-IL-24 is a strong chemosensitizer for MDR phenotype gastric
cancer SGC7901/CDDP cells.
Discussion
Chemotherapy is one of the most conventional
therapeutic strategies for human cancers, but MDR is frequently a
major impediment to the successful use of cancer chemotherapy
(27). MDR refers to phenomenon by
which cancer cells that have been exposed to a certain
chemotherapeutic drug, develop cross-resistance to a variety of
functionally unrelated reagents. Naturally, MDR plays a mainstay
role in chemoresistance of gastric cancer treatment. Different
drugs may finally induce the MDR phenotype but through various
signaling pathways, consequently leading to apparently different
expression or functional changes of involved molecules. Previous
studies have shown that several known proteins (such as
P-glycoprotein, RhoE, GAS1, thymidylate synthase Bax and Bcl-2)
play an important role in development of VCR-, 5-fluorouracil- or
doxorubicin-based MDR phenotype gastric cancer cells (6,28–31),
therefore, we speculate that different chemosensitizers may work
through these known or some unknown ways to restore
chemosensitivity of tumor MDR cells. Cisplatin,
cis-diamminedichloroplatinum (CDDP), is deemed to be the
‘penicillin of cancer drugs’ due to its universal, early, and
effective treatment for many cancers, including gastric cancer
(32). However, the inherent or
acquired resistance of tumors to CDDP therapy is a major clinical
problem. The dose that is necessary to overcome even a small
increase in cellular resistance can result in severe cytotoxicity
in normal cells. Given this, it is imperative to explore novel
chemosensitizers to reduce drug dosage, minimize side effects,
enhance the efficacy of therapy, and promote the application of
cisplatin in MDR phenotype cancer therapy.
Gene therapy provides a viable option, especially
when used with a cancer-selective apoptosis-inducing gene, such as
interleukin-24 (IL-24), a unique member of the IL-10-related
cytokine gene family, exhibits nearly ubiquitous antitumor
properties in vitro and in vivo(11). Unlike other tumor suppressors, the
IL-24 unique aspects lie in its selective induction of apoptosis in
cancer cells, profound ‘bystander’ activity by the secreted IL-24
protein, potent antitumor immune response. Some studies
demonstrated that the cytotoxic activity of IL-24 gene did not
depend on the status of other tumor suppressor genes, such as p53,
Rb and ras (33). Furthermore,
IL-24 exhibits strong chemosensitizing effects in broad-spectrum
MDR phenotype cancer cells (3,17,23,24)
and promising results in patients with multiple solid tumors in
phase I clinical trials (34).
These exciting findings indicate that IL-24 is likely to be a
potent nontoxic chemosensitizer for eliminating MDR in tumor
cells.
In the current study, we first established a
CDDP-induced MDR phenotype gastric cancer subline SGC7901/CDDP by
repeated selection and found that MDR promoted resistance of
gastric cancer cells to 20 times CDDP treatment or other anticancer
drugs, such as 5-FU, ADM or MTX. Then, we assessed the
chemosensitizing effects of Ad-IL-24 gene in gastric cancer cells
in vitro and in vivo. The results demonstrated that
ectopic IL-24 expression decreased the IC50 of CDDP,
5-FU, and ADM (not MTX). Generally, cellular apoptosis often occurs
after cell cycle arrest (35), and
the anticancer and chemosensitizing mechanism of many treatments is
mediated by the induction of cell cycle-specific apoptosis. In our
study, we found that Ad-IL-24 significantly increased the
percentage of SGC7901/CDDP cells in G2/M phase. Ad-IL-24
plus CDDP enhanced the induction of apoptosis in SGC7901/CDDP cells
in vitro, and elicited a significant tumor suppression
effect in vivo further indicating that Ad-IL-24 could be a
potent model of adjuvant chemotherapy for gastric cancer.
Of the 48 human ATP-binding cassette transport
proteins (36), P-glycoprotein
(P-gp) is the best-known and principal mediator of MDR (37,38).
It extrudes the chemotherapeutic agents out of cancers by using the
energy of ATP hydrolysis, resulting in lower intracellular drug
concentration and the decline of drug efficacy (39), therefore, is a thorny problem in
cancer chemotherapy and inhibition of P-gp expression may restore
chemosensitivity of cancer cells to anticancer drugs. In our study,
overexpression of IL-24 downregulated P-gp expression, and Ad-IL-24
decreased the IC50 of gastric cancer cells SGC7901/CDDP
to CDDP, 5-FU, ADM (not MTX). These results demonstrated that
decreased expression of P-gp could be a mainstay chemosensitizing
pathway. Additionally, Bcl-2 family is a key player in the
mitochondrial pathway of apoptosis, which consists of more than 20
members of pro-apoptotic proteins and anti-apoptotic proteins
(40). A previous study showed that
apoptosis-related proteins (such as Bax and Bcl-2) are also key
factors responsible for MDR (30,41–43).
The ratio of Bcl-2 family molecules, such as Bcl-2/Bax constitutes
the threshold of susceptibility to apoptosis, which promotes pore
formation in the mitochondrial outer membrane, loss of
mitochondrial integrity, and the release into the cytosol of
cytochrome c followed by the cleavage of caspase-9, leading to the
activation of intrinsic apoptotic pathway. Our current study
demonstrated that Ad-IL-24 significantly induced expression of
pro-apoptotic Bax protein, but inhibited expression of
apoptosis-suppressing proteins Bcl-2 in vitro and in
vivo. Furthermore, Ad-IL-24 plus CDDP enhanced induction of
apoptosis in vitro SGC7901/CDDP cells and elicited
significant tumor suppression of in vivo of SGC7901/CDDP
xenograft tissues. These results demonstrated that intrinsic
apoptotic pathway could be another key pathway for
Ad-IL-24-elicited chemosensitizing in gastric cancer.
Recent evidence has shown that PI3K/Akt, a crucial
effectors of the oncogenic signaling pathway, plays a key role in
the MDR of gastric cancer cells, and altered expression of P-gp,
Bax, and Bcl-2 might be responsible for the PI3K/Akt-induced drug
resistance in AGS cells (44–46).
Coincidentally, our investigation demonstrated that Ad-IL-24
restores chemosensitivity via modulating the expression of P-gp,
Bax, and Bcl-2 in MDR phenotype gastric cancer cells. On the basis
of the above evidence, it is believed that PI3K/Akt pathway is very
likely to be involved in the Ad-IL-24-mediated chemosensitizing
effects for gastric cancer. However, our study is just a
proof-of-principle and further study will be performed to verify
whether PI3K/Akt or some other unknown signaling pathways
participate in IL-24-enhanced chemosensitivity in gastric cancer
cells.
Acknowledgements
This work was supported by grants from Medical
Science and Technology Development Foundation, Suzhou Department of
Technology Bureau (no. YJS0916), Medical Science and Technology
Development Foundation, Jiangsu, Department of Health (no. H 201013
and H 201209).
Abbreviations:
|
MDR
|
multidrug resistance
|
|
P-gp
|
P-glycoprotein
|
|
RT-PCR
|
reverse transcriptase polymerase chain
reaction
|
|
IC50
|
50% inhibitory concentration
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Szakacs G, Paterson JK, Ludwing JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Emdad L, Lebedeva IV, Su ZZ, Sarkar D,
Dent P, Curiel DT and Fisher PB: Melanoma differentiation
associated gene-7/interleukin-24 reverses multidrug resistance in
human colorectal cancer cells. Mol Cancer Ther. 6:2985–2994. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di Nicolantonio F, Knight LA, Glaysher S,
Whitehouse PA, Mercer SJ, Sharma S, Mills L, Prin A, Johnson P,
Charlton PA, Norris D and Cree IA: Ex vivo reversal of
chemoresistance by tariquidar (XR9576). Anticancer Drugs.
15:861–869. 2004.PubMed/NCBI
|
|
5
|
Robert J and Jarry C: Multidrug resistance
reversal agents. J Med Chem. 46:4805–4817. 2003. View Article : Google Scholar
|
|
6
|
Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun
S, Hong L, Liu J and Fan D: miR-15b and miR-16 modulate multidrug
resistance by targeting BCL-2 in human gastric cancer cells. Int J
Cancer. 123:372–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Filipits M: Mechanisms of cancer:
multidrug resistance. Drug Discov Today Dis Mech. 1:229–234. 2004.
View Article : Google Scholar
|
|
8
|
Shi Y, Zhai H, Wang X, Han Z, Liu C, Lan
M, Du J, Guo C, Zhang Y, Wu K and Fan D: Ribosomal proteins S13 and
L23 promote multidrug resistance in gastric cancer cells by
suppressing drug-induced apoptosis. Exp Cell Res. 296:337–346.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y, You H, Liu F, An H, Hi Y, Yu Q and
Fan D: Differentially expressed gene profiles between multidrug
resistant gastric adenocarcinoma cells and their parental cells.
Cancer Lett. 185:211–218. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang H, Su ZZ, Lin JJ, Goldstein NI,
Young CS and Fisher PB: The melanoma differentiation associated
gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA.
93:9160–9165. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dash R, Bhutia SK, Azab B, Su ZZ, Quinn
BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, Yacoub A, Rahmani
M, Emdad L, Dmitriev IP, Wang XY, Sarkar D, Grant S, Dent P, Curiel
DT and Fisher PB: Mda-7/IL-24: A unique member of the IL-10 gene
family promoting cancer-targeted toxicity. Cytokine Growth Factor
Rev. 21:381–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gopalan B, Litvak A, Sharma S, Mhashilkar
AM, Chada S and Ramesh R: Activation of the Fas-FasL signaling
pathway by mda-7/IL-24 kills human ovarian cancer cells. Cancer
Res. 65:3017–3024. 2005.PubMed/NCBI
|
|
13
|
Li YJ, Liu G, Li Y, Vecchiarelli-Federico
LM, Liu JC, Zacksenhaus E, Shan SW, Yang BB, Li Q, Dash R, Fisher
PB, Archer MC and Ben-David Y: mda-7/IL-24 expression inhibits
breast cancer through up-regulation of growth arrest-specific
gene-3 (gas 3) and disruption of β1 integrin function. Mol Cancer
Res. 11:593–603. 2013.PubMed/NCBI
|
|
14
|
Jiang H, Lin JJ, Su ZZ, Goldstein NI and
Fisher PB: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.
|
|
15
|
Xu S, Oshima T, Imada T, Masuda M, Debnath
B, Grande F, Garofalo A and Neamati N: Stabilization of MDA-7/IL-24
for colon cancer therapy. Cancer Lett. 335:421–430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pataer A, Chada S, Roth JA, Hunt KK and
Swisher SG: Development of Ad-mda-7/IL-24-resistant lung cancer
cell lines. Cancer Biol Ther. 7:103–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang G, Liu YQ, Wei ZP, Pei DS, Mao LJ
and Zheng JN: Enhanced anti-tumor activity by the combination of a
conditionally replicating adenovirus mediated interleukin-24 and
dacarbazine against melanoma cells via induction of apoptosis.
Cancer Lett. 294:220–228. 2010.
|
|
18
|
Tong AW, Nemunaitis J, Su D, Zhang Y,
Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K,
Coffee K, Ramesh R, Ekmekcioglu S, Grimm EA, van Wart Hood J,
Merritt J and Chada S: Intratumoral injection of INGN241, a
nonreplicating adenovector expressing the melanoma-differentiation
associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer
patients. Mol Ther. 11:160–172. 2005. View Article : Google Scholar
|
|
19
|
Xie Y, Sheng W, Miao J, Xiang J and Yang
J: Enhanced antitumor activity by combining an adenovirus harboring
ING4 with cisplatin for hepatocarcinoma cells. Cancer Gene Ther.
18:176–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishikawa T, Ramesh R, Munshi A, Chada S
and Meyn RE: Adenovirus-mediated mda-7(IL-24) gene therapy
suppresses angiogenesis and sensitizes NSCLC xenograft tumors to
radiation. Mol Ther. 9:818–828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Cheung RM, Komaki R, Fang B and
Chang JY: Radiotherapy sensitization by tumor-specific TRAIL gene
targeting improves survival of mice bearing human non-small cell
lung cancer. Clin Cancer Res. 11:6657–6668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moon C, Oh Y and Roth JA: Current status
of gene therapy for lung cancer and head and neck cancer. Clin
Cancer Res. 9:5055–5067. 2003.PubMed/NCBI
|
|
23
|
Xu J, Mo Y, Wang X, Liu J, Zhang X, Wang
J, Hu L, Yang C, Chen L and Wang Y: Conditionally replicative
adenovirus-based mda-7/IL-24 expression enhances sensitivity of
colon cancer cells to 5-fluorouracil and doxorubicin. J
Gastroenterol. 48:203–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang P, Zhang X, Gao Y, Ding CR, Cui F and
Jiao SC: Reversal effect of melanoma differentiation associated
gene-7/interleukin-24 on multidrug resistance in human
hepatocellular carcinoma cells. Anat Rec. 295:1639–1646. 2012.
View Article : Google Scholar
|
|
25
|
Chang S, Yang J, Cheng W, Xie Y and Sheng
W: Antitumor activity of an adenovirus harboring human IL-24 in
colon cancer. Mol Biol Rep. 38:395–401. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Ye Z, Zhong J, Xiang J and Yang J:
Adenovirus-mediated IL-24 expression suppresses hepatocellular
carcinoma growth via induction of cell apoptosis and cycling arrest
and reduction of angiogenesis. Cancer Biother Radiopharm. 22:56–63.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi JH, Lim HY, Joo HJ, Kim HS, Yi JW,
Kim HC, Cho YK, Kim MW and Lee KB: Expression of multidrug
resistance-associated protein1, P-glycoprotein, and thymidylate
synthase in gastric cancer patients treated with 5-fluorouracil and
doxorubicin-based adjuvant chemotherapy after curative resection.
Br J Cancer. 86:1578–1585. 2002. View Article : Google Scholar
|
|
29
|
Nakamura A, Nakajima G, Okuyama R,
Kuramochi H, Kondoh Y, Kanemura T, Takechi T, Yamamoto M and
Hayashi K: Enhancement of 5-fluorouracil-induced cytotoxicity by
leucovorin in 5-fluorouracil-resistant gastric cancer cells with
upregulated expression of thymidylate synthase. Gastric Cancer.
Mar..15–2013.(Epub ahead of print).
|
|
30
|
Li K, Lu Y, Liang J, Luo G, Ren G, Wang X
and Fan D: RhoE enhances multidrug resistance of gastric cancer
cells by suppressing Bax. Biochem Biophys Res Commun. 379:212–216.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao L, Pan Y, Gang Y, Wang H, Jin H, Tie
J, Xia L, Zhang Y, He L, Yao L, Qiao T, Li T, Liu Z and Fan D:
Identification of GAS1 as an epirubicin resistance-related gene in
human gastric cancer cells with a partially randomized small
interfering RNA library. J Biol Chem. 284:26273–26285. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chada S, Mhashilkar AM, Liu Y, Nishikawa
T, Bocangel D, Zheng M, Vorburger SA, Pataer A, Swisher SG, Rmamesh
R, Kawase K, Meyn RE and Hunt KK: mda-7 gene transfer sensitizes
breast carcinoma cells to chemotherapy, biologic therapies and
radiotherapy: Correlation with expression of bcl-2 family members.
Cancer Gene Ther. 13:490–502. 2006. View Article : Google Scholar
|
|
34
|
Eager R, Harle L and Nemunaitis J:
Ad-MDA-7; INGN 241: a review of preclinical and clinical
experience. Expert Opin Biol Ther. 8:1633–1643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fotedar R, Diederich L and Fotedar A:
Apoptosis and the cell cycle. Prog Cell Cycle Res. 2:147–163. 1996.
View Article : Google Scholar
|
|
36
|
Dean M, Rzhetsky A and Allikmets R: The
human ATP-binding cassette (ABC) transporter superfamily. Genome
Res. 11:1156–1166. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Juliano RL and Ling V: A surface
glycoprotein modulating drug permeability in chinese hamster ovary
cell mutants. Biochim Biophys Acta. 455:152–162. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ueda K, Cardarelli C, Gottesman MM and
Pastan I: Expression of a full-length cDNA for the human ‘MDR1’
gene confers resistance to colchicine, doxorubicin, and
vinblastine. Proc Natl Acad Sci USA. 84:3004–3008. 1987.
|
|
39
|
Gottesman MM and Ling V: The molecular
basis of multidrug resistance in cancer: the early years of
P-glycoprotein research. FEBS Lett. 580:998–1009. 2006.PubMed/NCBI
|
|
40
|
Antonsson B and Martinou JC: The Bcl-2
protein family. Exp Cell Res. 256:50–57. 2000. View Article : Google Scholar
|
|
41
|
Eberle J, Kurbanov BM, Hossini AM, Trefzer
U and Fecker LF: Overcoming apoptosis deficiency of melanoma-hope
for new therapeutic approaches. Drug Resist Updat. 10:218–234.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang L, Ming L and Yu J: BH3 mimetics to
improve cancer therapy: mechanisms and examples. Drug Resist Updat.
10:207–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu W, Shan X, Wang T, Shu Y and Liu P:
miR-181b modulates multidrug resistance by targeting BCL2 in human
cancer cell lines. Int J Cancer. 127:2520–2529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Michl P and Downward J: Mechanisms of
disease: PI3K/AKT signaling in gastrointestinal cancers. Z
Gastroenterol. 43:1133–1139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han Z, Hong L, Han Y, Wu K, Han S, Shen H,
Li C, Yao L, Qiao T and Fan D: Phospho Akt mediates multidrug
resistance of gastric cancer cells through regulation of P-gp,
Bcl-2 and Bax. J Exp Clin Cancer Res. 26:261–268. 2007.PubMed/NCBI
|
|
46
|
Yu HG, Ai YW, Yu LL, Zhou XD, Liu J, Li
JH, Xu XM, Liu S, Chen J, Liu F, Qi YL, Deng Q, Cao J, Liu SQ, Luo
HS and Yu JP: Phosphoinositide3-kinase/Akt pathway plays an
important role in chemoresistance of gastric cancer cells against
etoposide and doxorubicin induced cell death. Int J Cancer.
122:433–443. 2008. View Article : Google Scholar : PubMed/NCBI
|