Introduction
The incidence of prostate cancer (PC) has increased
in recent years with the promotion of the prostate-specific antigen
(PSA) test and the increase in the growth of the elderly population
(1). The most important clinical
prognostic markers currently available for PC are the PSA serum
level and TNM staging including the Gleason score. Numerous studies
support the prognostic role of PSA, particularly the PSA doubling
time (2). In advanced disease, some
recent therapeutic progress has been made, such as the drug
abiraterone (3). However, the long
term outcome remains dismal and overall therapeutic options,
particularly in castration and hormone resistant cancer, remain
limited.
While PC is the most common male cancer, non-small
cell lung cancer (NSCLC) is among the most fatal ones. The outcome
following resection of early-stage NSCLC is poor, with 35–50%
recurrence rate resulting in mortality (1). Little progress has been made in the
past 30 years in the reduction of distant recurrence and subsequent
mortality (1), which remain
unacceptably high, even for patients with stage I disease in whom
no nodal or other metastatic involvement can be detected at the
time of surgery (4). Recent
improvements in diagnosis and treatment have been made, but they
only apply to a minority of lung cancer cases, particularly in
non-smokers. Thus, both epidermal growth factor receptor (EGFR)
mutations (5) and Alk gene
rearrangements (6) have become
important prognostic and predictive biomarkers. For most cases of
lung cancer, TNM classification, age, C-reactive protein (CRP) and
forcedexpiratory volume in one second (FEV1) significantly
influence the choice of treatment and strongly predict patient
overall survival (OS) (7–9). Due to the high unmet medical needs in
both types of cancer, it is critical to identify new prognostic
markers that may eventually also form the basis for novel
therapeutic approaches.
Attention has recently focused on the vagus nerve,
due to its role in three mechanisms essential for tumorigenesis and
progression in cancer. The first mechanism is oxidative stress
(10), which leads to both
DNA-damage, a central trigger of tumorigenesis, and to uncontrolled
cell proliferation (11).
Furthermore, DNA damage and oxidative stress are also predictive
factors of prognosis (12). The
second process is local excessive inflammation promoting
tumorigenesis in early stages (13), and disease progression in its later
stages (14,15). The inflammatory microenvironment,
normally recruited to help fight/eliminate the tumors, also
promotes tumor growth. Finally, the metastatic process is also
under the control of sympathetic neurotransmitters, which influence
the migratory ability of cancer cells and determine the direction
and the development of metastases (16). Vagal nerve activity is inversely
related to and inhibits all these three mechanisms (17–21).
Based on converging evidence, Gidron et al(22) hypothesized that vagal nerve activity
may modulate tumor growth, and if this model is valid, might become
a variable that could be used as a prognostic factor and could be
therapeutically manipulated.
The vagal nerve activity index, i.e., heart rate
variability (HRV), represents the time differences between
successive heartbeats (also known as the beat-to-beat intervals),
and is synonymous with RR variability. The R wave refers to the R
waves on the electrocardiogram corresponding to ventricular
depolarization (23). Analysis of
the time differences between successive heart beats can be
accomplished with reference to time (time domain analysis) or
frequency (frequency domain analysis). In this study, we focused on
the time domain analyses, i.e., standard deviation of normal beat
to beat intervals (SDNN), in msec, and root mean square successive
difference (RMSSD), in msec.
HRV is highly correlated with vagal nerve activity
(r=0.88) (24) and is mainly under
the control of and reflects efferent cardiac vagal nerve activity.
High HRV has been shown to predict longer cancer survival time and
reduced tumor burden (25–27). However, those studies had small
sample sizes and did not control for any confounder. A subsequent
study (28) showed that HRV was a
significant predictor of survival time in terminal cancer patients,
independent of confounders. However, that study did not control for
type of cancer. These studies also included patients with cardiac
diseases or other conditions known to affect HRV. We recently
showed that low HRV predicts increases in the colon marker
carcinoembryonic antigen (CEA), independent of confounders and
excluding cardiac patients (29).
However, the sample size of the latter study was also relatively
small (N=72), and baseline levels of the tumor marker were not
considered. Moreover, it remains unknown whether these findings may
be generalized to other types of cancer, and what the underlying
mechanisms may be. For this reason, we focused on two prognostic
and symptomatically different types of cancer.
The purpose of the present study was to test the
relationship between vagal nerve activity, indexed by HRV, and
prognosis in patients with PC and NSCLC, two types of cancer of
different severity. Furthermore, we tested whether inflammatory
factors, such as C-reactive protein (CRP), mediate, i.e., explain,
this relationship. Moreover, the predictors age and stage were
explored preliminarily as possible modulators (moderators) of the
HRV-prognosis relationship. These moderators were chosen since it
was expected that the role of new prognostic factors may differ
between age and stage. Age has been shown to be a major determinant
in multiple physiological systems (30). Furthermore, vagal nerve activity may
play different prognostic roles in different stages, due to the
possible different roles of its underlying mechanisms in different
stages. Additionally, by looking at multiple tumor marker outcomes
(in PC), we sought to determine at which time point vagal nerve
activity has prognostic value.
We hypothesized that lower HRV would predict
increased tumor marker and reduced OS and survival time. It was
also hypothesized that these relationships would be mediated by an
inflammatory marker and might be moderated by stage and age.
Materials and methods
Patients
Following approval of the Medical Ethics Committee,
UZ Brussels, medical records of 620 patients with PC and 650
patients with NSCLC, treated at UZ Brussels between January 2005
and December 2009, were reviewed. Patients diagnosed with NSCLC or
PC, with an intact ECG near diagnosis and available data for PSA
(PC) were included. Exclusion criteria included conditions known to
alter HRV or influence inflammation, such as inflammatory diseases
(such as, arthritis), cardiovascular disease, implanted pacemaker,
or prescribed cardiologic medication (β-blockers, anti-arrhythmic),
anemia, spleen and thyroid diseases.
Initially, we included 620 PC and 650 NSCLC
patients, of whom 113 PC and 133 NSCLC patients met our inclusion
criteria. Most patients were excluded due to the lack of ECG in
their medical files. Based on the effect sizes previously observed
with smaller samples (27,29), the sample sizes in both PC and NSCLC
were expected to be sufficient to detect statistically significant
relationships and to explore for moderators.
Design
The study included a historical prospective design,
formally known as a retrospective design. We collected archival
electronic patient records (historical) and examined the
prospective relationship between HRV measured at baseline (time of
diagnosis, Time 1), and prognosis at Time 2 (e.g., PSA at 6 months,
OS). This design is commonly used in the reanalysis of existing
data sets (31,32).
Measures
Confounders
Background information included patient age, gender
(NSCLC), treatments (radiotherapy, chemotherapy, surgery,
hormonal), body mass index (BMI), FEV1 (NSCLC), TNM staging in
NSCLC including Gleason score (PC), smoking history in units of
pack years (NSCLC), and baseline PSA (PC). Data on other
confounders, such as family history, were absent in several
cases.
Vagal nerve activity
This was measured by HRV, obtained from 10 sec ECGs
performed at diagnosis. The MUSE® cardiology information
system in the UZ Brussels hospital provided the distances (in msec)
between the RR peaks in each ECG, for determining HRV. Time domain
HRV parameters, i.e., SDNN in msec, and RMSSD in msec, were
derived. Power spectral analysis HRV parameters (such as HF and LF)
require longer recording periods, and thus, were not measured. Such
short ECGs have been found to correlate with ECGs of longer
durations (5 and 10 min), particularly with respect to RMSSD
(33,34), but also for SDNN (24 h) (35). Furthermore, 10 sec SDNN has been
shown to predict tumor marker levels in colon cancer (29). For the moderation analyses, a
cut-off of 20 msec was used, as in previous studies in cancer
(29), to distinguish between
patients with high vs. low SDNN and RMSSD.
Inflammatory marker
This included CRP, only for NSCLC.
Outcomes
Non-small cell lung cancer
OS (in the full sample) and duration of survival
time in the deceased patients.
Prostate cancer
PSA in ng/ml at 6 months and 2 years.
Statistical analysis
Univariate associations were tested between all
background variables and all outcomes and the HRV parameters, using
Pearson correlations for continuous variables and t-tests for
categorical data. A partial correlation tested whether HRV still
predicted survival time (in NSCLC) or tumor marker levels (in PC),
after controlling for all significant background factors. When
testing for moderation (interactions of HRV × age and HRV × stage),
the cut-off of 20 msec for SDNN and RMSSD to predict outcomes was
used, independent of confounders. For the main analyses, we used a
Bonferroni correction for both types of cancer. In the PC analyses,
we had two measurements of HRV and two outcomes of PSA, resulting
in 4 tests, requiring a significance level α of 0.05/4=0.0125. In
the lung cancer analyses, we had two measurements of HRV and two
outcomes (OS and survival time), resulting in four tests, requiring
a significance level α of 0.05/4=0.0125. Since the moderation part
is only an exploratory analysis, we did not use a corrected α for
these additional tests.
Results
Prostate cancer
Of the 620 reviewed PC patients, only 113 met our
inclusion criteria (for the majority of the excluded patients, no
ECG or PSA levels were available). The descriptive statistics of
the study variables are shown in Table
I, however the continuous variables are not log transformed for
purpose of clarity.
 | Table IDescriptive statistics of the
prostate cancer sample. |
Table I
Descriptive statistics of the
prostate cancer sample.
| A. Categorical
variables |
|---|
|
|---|
| Variable | (%) |
|---|
| Radiotherapy | 42.5 |
| Hormonal
therapy | 40.7 |
| Chemotherapy | 1.8 |
| Surgery | 62.8 |
| Stage |
| Stage 2 | 64.5 |
| Stage 3 | 9.7 |
| Stage 4 | 25.8 |
|
| B. Continuous
variables |
|
| Variable | (Mean ± SD) |
|
| Age (years) | 65.06±8.87 |
| Heart rate | 73.32±14.00 |
| HRV variables |
| SDNN | 31.24±30.27 |
| RMSSD | 32.34±40.25 |
| PSA |
| Baseline PSA | 130.00±520.67 |
| PSA 6 months | 9.44±36.24 |
| PSA 2 years | 2.84±10.50 |
As shown in Table I,
various treatments were used, and the percentages exceeded a 100%
since a multi-modality therapeutic approach was used for several
patients. Due to the lack of normal distributions, the scores of
PSA, SDNN and RMSSD were log transformed, on which the following
analyses were performed. Furthermore, this was carried out due to
the large SD, reflecting large inter-patient variability, in PSA
levels. In the following analyses, for purposes of brevity, we used
the terms SDNN, RMSSD, CRP and HRV, which refer to the log
transformed measurements of these parameters.
The relationship between each measured confounder
and both the predictor HRV (SDNN for this analysis), and PSA levels
at 6 months, was tested. Among all confounders, stage (including
Gleason score), radiotherapy, hormonal treatment, age and baseline
PSA were significantly related to PSA levels at 6 months. Surgery
was not related to either PSA or HRV. None of the confounders were
correlated with SDNN. Concerning the confounders, in patients who
did not receive radiotherapy or hormonal treatment, PSA levels at 6
months were significantly lower than in patients who received
either treatment (p=0.018 and p=0.02, respectively). Age and
baseline PSA were significantly positively correlated with PSA
levels at 6 months (r=0.414; p=0.001 and r=0.48; p<0.001,
respectively); stage was also associated with PSA levels at 6
months (p=0.001).
With regard to vagal nerve activity and PSA, in
univariate analyses, SDNN significantly inversely predicted PSA
levels at 6 months (r=−0.399; p=0.002). Furthermore, RMSSD also
significantly inversely predicted PSA levels at 6 months (r=−0.345;
p=0.009) and tended to significantly predict PSA levels at 2 years
(r=−0.304; p=0.018), considering the Bonferroni corrected p-value
of 0.0125. Of note, in a multivariate partial correlation, baseline
SDNN remained a significant predictor of PSA levels at 6 months
from diagnosis, controlling for tumor stage, age, baseline PSA, as
well as hormonal treatment and radiotherapy (r=−0.434; p=0.004)
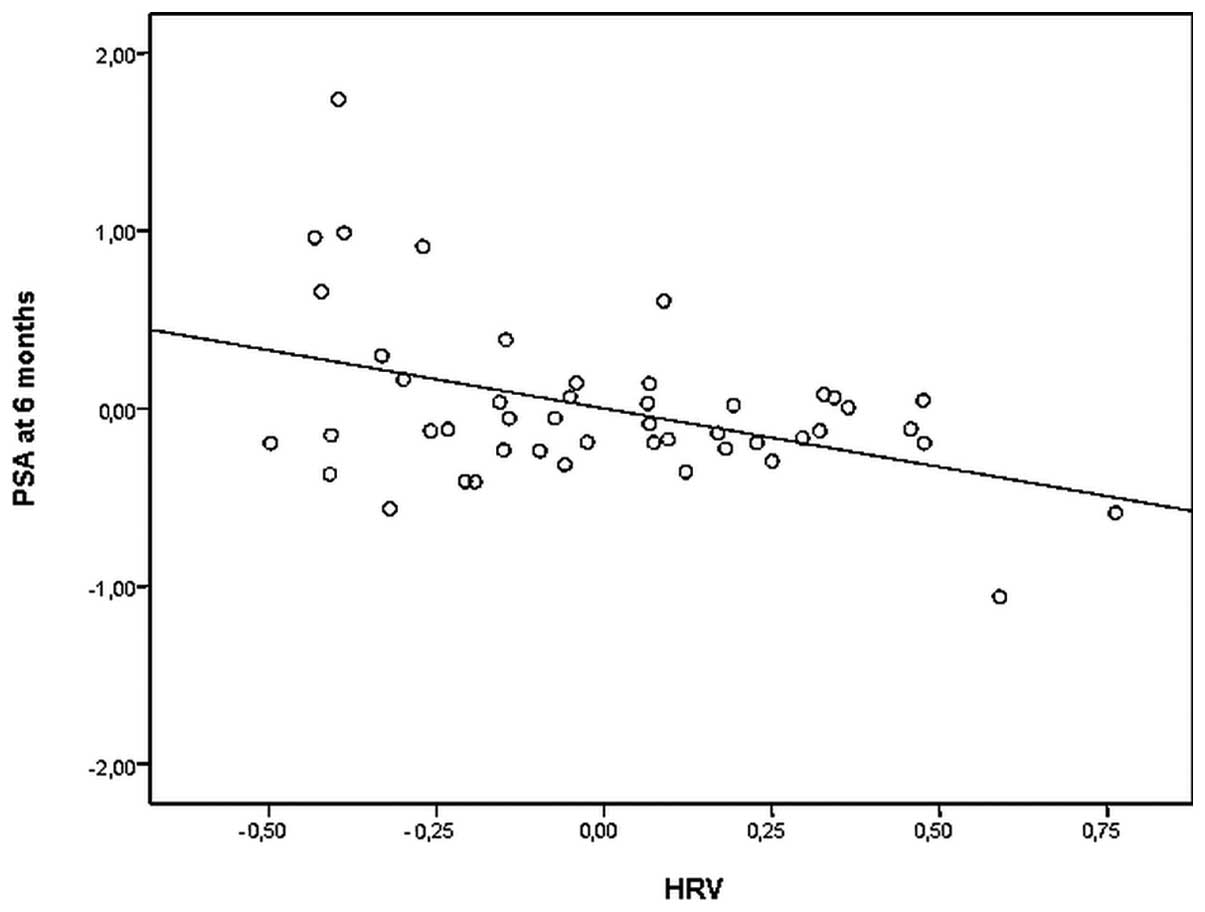
(Fig. 1). Furthermore, baseline
RMSSD was a significant predictor of PSA levels at 6 months from
diagnosis (r=−0.437; p=0.004), as well as of PSA levels at 2 years
(r=−0.381; p=0.0125), independent of all confounders.
Moderation
Using a cut-off of 20 msec for SDNN (29), a significant interaction was found
between SDNN and stage [F(2,41)=15.262; p<0.001] in relation to
PSA levels at 6 months. The same significant interaction was
observed between the cut-off for RMSSD (also 20 ms) and stage
[F(2,37)=13.194; p<0.001] in relation to PSA levels at 6 months.
The two interactions were independent of confounders. Following
this interaction, we examined the correlation between SDNN and
RMSSD with PSA levels at 6 months, in the 3 stages separately,
independent of confounders. In stages 2 and 3, we did not find a
significant correlation between either SDNN or RMSSD and PSA levels
at 6 months and 2 years (p>0.05). In stage 4, however, we found
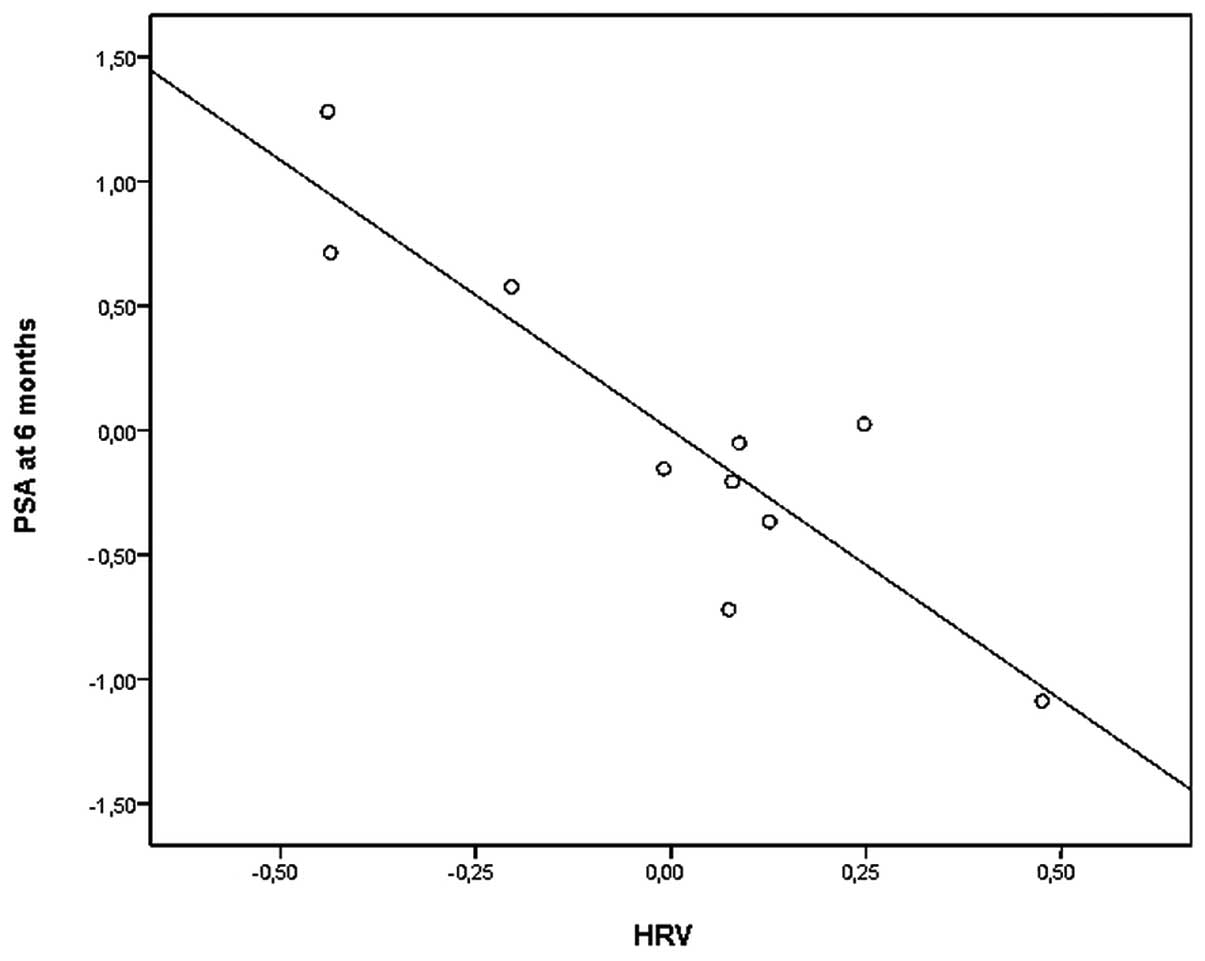
a significant inverse correlation between SDNN and PSA levels at 6
months (r=−0.895; p<0.05) (Fig.
2). RMSSD was also significantly inversely correlated with PSA
levels at 6 months (r=−0.804; p=0.05). No significant correlations
were found for PSA levels at 2 years in stage 4 (p>0.05). Age
did not significantly interact with SDNN in relation to PSA levels
at 6 months [F(1,43)=0.686; p=0.412], independent of
confounders.
Non-small cell lung cancer
Of the 650 initially reviewed medical charts of
NSCLC, only 133 met our inclusion criteria (for the majority of the
excluded patients, no ECG was available). The descriptive
statistics of the study variables of this sample are shown in
Table II, however they are not log
transformed. As shown in Table II,
various treatments were used, and the percentages exceeded a 100%
since a multi-modality therapeutic approach was used for several
patients.
 | Table IIDescriptive statistics of the NSCLC
patient sample. |
Table II
Descriptive statistics of the NSCLC
patient sample.
| A. Categorical
variables |
|---|
|
|---|
| Variable | (%) |
|---|
| Deceased
patients | 84.2 |
| Radiotherapy | 67.7 |
| Chemotherapy | 74.4 |
| Surgery | 22.6 |
| Stage |
| Stage 1 | 15.9 |
| Stage 2 | 9.8 |
| Stage 3 | 19.7 |
| Stage 4 | 54.5 |
|
| B. Continuous
variables |
|
| Variable | (Mean ± SD) |
|
| Age (years) | 62.2±10.2 |
| Heart rate | 82.0±17.3 |
| HRV variables |
| SDNN | 17.0±14.6 |
| RMSSD | 19.1±21.1 |
| Survival days | 422.6±391.2 |
Due to the lack of normal distributions, the scores
of CRP, SDNN, RMSSD and survival time (in days) were log
transformed. We then tested the relationship between each measured
confounder and OS, and chemotherapy, surgery and stage were found
to be significant predictors. Similarly, we tested the covariates
for survival time. Among all confounders, radiotherapy, surgery and
stage were correlated with survival time. These confounders were
considered for their respective analyses. CRP also correlated with
OS (p=0.007). However, we treated CRP as a variable which may
explain (mediate) the relationship between vagal activity and
prognosis, thus we did not consider it as a covariate.
Overall survival as outcome
Concerning OS, a multivariate Cox regression
analysis was conducted and revealed no relationship between SDNN or
RMSSD and OS (p=0.357 and p=0.364, respectively). In this
multivariate analysis, only stage and surgery significantly
predicted OS (p<0.0125) (Table
III).
 | Table IIICox regression analysis for the
relationship between cancer stage, treatments and SDNN with OS. |
Table III
Cox regression analysis for the
relationship between cancer stage, treatments and SDNN with OS.
| Predictor | B | Sig | R.R. | 95% CI |
|---|
| Stage | 0.621 | 0.000 | 1.861 | 1.484–2.333 |
| Surgery | −0.948 | 0.002 | 0.388 | 0.215–0.698 |
| SDNN | −0.176 | 0.357 | 0.838 | 0.576–1.22 |
| Chemotherapy | −0.667 | 0.014 | 0.513 | 0.302–0.873 |
We then tested whether there was an interaction
between age and SDNN in relation to OS. This interaction was not
significant (B=0.152; p=0.703) and thus, no subsequent analyses
were conducted. Similarly, there was no interaction between stage
and SDNN in relation to OS (B=−0.068; p=0.737).
We initially hypothesized that CRP may mediate the
relation between SDNN and OS. However, since no significant
relationship was found between SDNN and OS, as mentioned above, a
mediation analysis was not appropriate. However, CRP on its own
significantly predicted OS [t(74)=2.799; p=0.007], without
controlling for confounders, and this relationship remained
significant after controlling for stage, chemotherapy and surgery
as well (B=0.447; p=0.038).
Survival time as outcome
The following analyses were conducted in the
deceased NSCLC patients only. In univariate analyses, SDNN and
RMSSD did not significantly predict survival time (p=0.24 and
p=0.486, respectively). In a multivariate partial correlation,
baseline SDNN and RMSSD were not significantly predictive of
survival time, controlling for tumor stage, surgery and
radiotherapy (p=0.435 and p=0.34, respectively).
Regarding the moderators, we tested the interaction
between stage and SDNN in relation to survival time, however, no
interaction was observed [F(1,106)=0.005; p=0.943]. By contrast,
age and SDNN significantly interacted in relation to survival time
[F(1,105)=5.577; p=0.02]. In the group under the age of 65 (N=73),
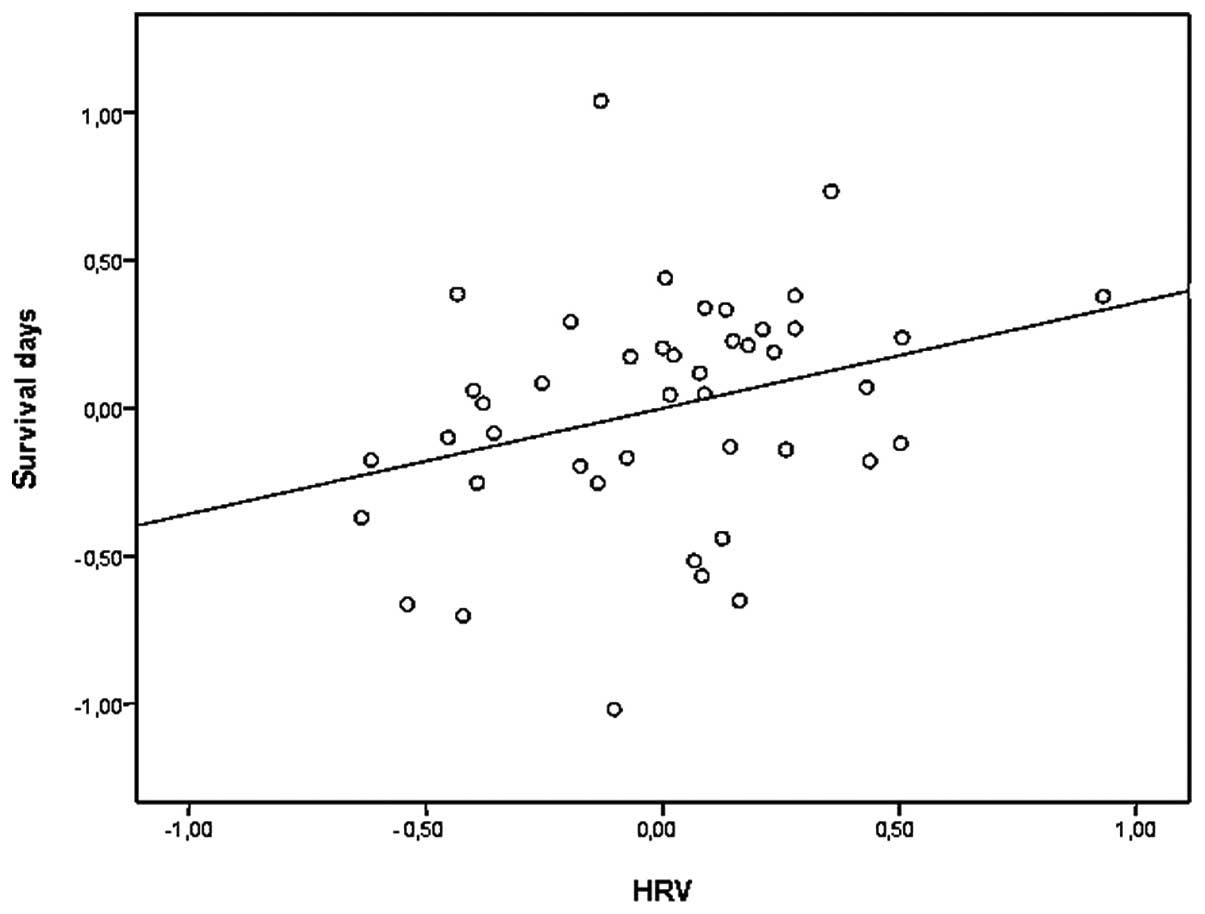
SDNN (and RMSSD) significantly predicted survival time, independent
of confounders (r=0.278; p=0.032 and r=0.282; p=0.029,
respectively) (Fig. 3). By
contrast, in the group over the age of 65, SDNN and RMSSD did not
predict survival time (r=−0.174; p=0.247 and r=−0.144; p=0.340,
respectively).
Finally, since no significant correlation was found
between SDNN and survival time, as mentioned above, a mediation
analysis of CRP on the relationship between SDNN and survival time
was not appropriate. However, CRP alone significantly predicted
survival time (r=−0.298; p=0.012), without controlling for
confounders, and this correlation remained significant after
controlling for stage, radiotherapy and surgery as well (r=−0.263;
p=0.03).
Discussion
The present study tested the potential prognostic
role of vagal nerve activity in two different but common types of
cancer, i.e., prostate cancer (PC) and non-small cell lung cancer
(NSCLC), excluding patients on cardiac medication or with cardiac
disease. The effects of vagal nerve activity on clinical outcomes
were hypothesized to occur as a result of the known inhibitory
roles of this nerve on three mechanisms promoting tumor onset and
progression (excessive inflammation, excessive sympathetic activity
and oxidative stress) (21). Vagal
nerve activity was indexed by heart rate variability (HRV) obtained
from a 10 sec ECG at approximately the time of cancer diagnosis. We
used a historical prospective design to predict PSA tumor marker
levels (in PC) as well as overall survival (OS) and survival time
(in NSCLC), by HRV levels measured at approximately the time of
diagnosis. In PC, HRV parameters (SDNN and RMSSD) were found to be
significantly and inversely correlated with levels of the tumor
marker PSA at 6 months and 2 years, independent of confounders
including tumor stage and treatment. Moreover, we observed that HRV
predicted PSA levels in patients with metastatic prostate cancer,
but not in less severe disease stages, emphasizing the moderating
role of stage. These findings corroborated with previous studies
showing HRV to predict cancer prognosis in several types of cancer
(25–29). However, unlike previous studies, the
present study used a more rigorous control of confounders,
including baseline tumor marker, among the other crucial
confounders. Although our strict exclusion criteria reduced the
generalizability of our findings, this serves to enhance the
ability to interpret our findings and to attribute them to vagal
nerve activity, unaffected by interfering variables.
The prognostic role of vagal nerve activity
specifically in the metastatic stage of PC remains to be
elucidated. It is possible that in the earlier tumor stages,
commonly provided treatments such as surgery and radiotherapy are
successful in reducing the tumor burden, possibly leaving less of a
margin for vagal nerve activity to contribute to the process. By
contrast, these treatments may have less impact in later,
metastatic stages, where vagal activity could possibly be of more
importance. In addition, during the metastatic stage, all three
mechanisms considered to underlie the effects of the vagus on tumor
burden, i.e., inflammation, oxidative stress and sympathetic
activation (10,13,16),
may bear a greater role on prognosis. This would then potentially
enable greater impact of the vagal nerve on these three processes
and on tumor burden in the metastatic stage.
In patients with NSCLC, no correlation was found
between SDNN and RMSSD and OS. Furthermore, age and stage did not
moderate this relationship. Concerning survival time in the
deceased patients, no correlation between SDNN and RMSSD and
survival time was observed. However, in patients under the age of
65, higher HRV did significantly predict longer survival time,
independent of confounders. The lack of correlation between HRV and
prognosis in NSCLC in the elderly may result from the cumulative
effects of this cancer with comorbidities (such as cardiac disease)
and age on the parasympathetic system. Several respiratory
parameters are known to affect HRV, such as respiratory frequency
(36,37), tidal volume (37), the time ratio of
expiration/inspiration (38),
restricted breathing, pulmonary function, functional capacity, and
chronic obstructive pulmonary disease (COPD) (39). These respiratory parameters, as well
as multiple regulatory systems, are likely to be impaired in older
patients with NSCLC (30,40–42).
Another reason why we did not observe a relationship
could be explained by the mean SDNN in the NSCLC patients. The
NSCLC patients in general had a low SDNN, leading to less variation
and hence to a restricted range. Contrasting the two types of
cancer we investigated, the mean SDNN of the NSCLC patients was
significantly lower than the mean of the PC patients (SDNN, 17
msec; SDNN, 31.2 msec; p<0.005), possibly due to the
cancer-induced respiratory problems and the severity of the cancer
burden in NSCLC. In addition, younger people in a general
population have more variations in their vagal nerve activity and,
similarly, more variations were found in this sample of the younger
NSCLC patients. This could explain the correlation in the younger
subgroup with survival time, as a result of the broader range of
HRV parameters and a potentially better regulatory functioning of
the autonomic nervous system. Finally, in the NSCLC sample, CRP
significantly predicted survival time and OS, as expected, since
CRP is a known prognostic parameter in cancer in general, and in
NSCLC specifically (43).
The present study had several limitations. First, in
both samples, HRV measurements were based on ECGs of 10 sec.
However, several studies have suggested that HRV indices, obtained
from 10 sec ECG, are correlated with 5 and 20 min ECGs (34,44).
Such brief HRV data have also been shown to predict prognosis in
cardiac disease (45) and in cancer
(29). Another potential limitation
could be that the ECG was obtained at approximately the time of
diagnosis. Patients had just been informed about their diagnosis
and this may have impacted their sympathetic nervous system
activity and, consequently, their HRV (46). This may result in an underestimation
of the full relationship between HRV and cancer prognosis. Future
studies should assess the levels of stress at the moment of ECG
measurement. In addition, both sample sizes, although larger than
in previous studies, were limited in size for establishing with
adequate statistical power the effects of moderators and mediators.
Finally, a historical prospective design was used in which a priori
control over confounders, such as performance status (including
Karnofsky), sampling and ECG measures, was lacking. It is suggested
that future studies should replicate these results using a
prospective design, with larger samples and longer ECG
measurements.
Since our design is correlational, we cannot infer
any causal relationship between HRV and tumor burden. It may be
that vagal nerve activity is also affected by cancer (De Couck and
Gidron, unpublished data). However, two experimental studies in
mice showed that vagotomy or capsaicin-induced denervation enhanced
cancer metastasis (47,48), demonstrating experimentally the
protective effects of the vagus nerve on cancer progression.
Furthermore, a recent study found an anti-inflammatory drug that
activates the vagus nerve (CNI-1493) to reduce tumor burden in a
breast cancer murine model (49).
Collectively, these results support the theory that this nerve
plays a pivotal homeostatic role whose function may be disease
prevention or protection against poor prognosis in certain types of
cancer.
Acknowledgements
The authors thank Professor D. Schoors and Sven
D’haese for their contribution to this research. This study was
supported by grants from the Reliable Cancer Therapies and the
Willy Gepts Foundation provided to Professor Yori Gidron.
References
|
1
|
Global cancer statistics. CA Cancer J
Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Eastham JA: Prostate-specific antigen
doubling time as a prognostic marker in prostate cancer. Nat Clin
Pract Urol. 2:482–491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng
T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL,
Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D,
Loriot Y, Chieffo N, Kheoh T, Haqq CM and Scher HI; COU-AA-301
Investigators. Abiraterone and increased survival in metastatic
prostate cancer. N Engl J Med. 364:1995–2005. 2011.
|
|
4
|
Kelsey CR, Marks LB, Hollis D, Hubbs JL,
Ready NE, D’Amico TA and Boyd JA: Local recurrence after surgery
for early stage lung cancer: an 11-year experience with 975
patients. Cancer. 115:5218–5227. 2009.PubMed/NCBI
|
|
5
|
Rosell R, Molina MA, Costa C, Simonetti S,
Gimenez-Capitan A, Bertran-Alamillo J, Mayo C, Moran T, Mendez P,
Cardenal F, Isla D, Provencio M, Cobo M, Insa A, Garcia-Campelo R,
Reguart N, Majem M, Viteri S, Carcereny E, Porta R, Massuti B,
Queralt C, de Aguirre I, Sanchez JM, Sanchez-Ronco M, Mate JL,
Ariza A, Benlloch S, Sanchez JJ, Bivona TG, Sawyers CL and Taron M:
Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in
erlotinib-treated advanced non-small-cell lung cancer patients with
EGFR mutations. Clin Cancer Res. 17:1160–1168. 2011. View Article : Google Scholar
|
|
6
|
Gandhi L and Janne PA: Crizotinib for
ALK-rearranged non-small cell lung cancer: a new targeted therapy
for a new target. Clin Cancer Res. 18:3737–3742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brundage MD, Davies D and Mackillop WJ:
Prognostic factors in non-small cell lung cancer: a decade of
progress. Chest. 122:1037–1057. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marijon H, Bouyon A, Vignot S and Besse B:
Prognostic and predictive factors in lung cancer. Bull Cancer.
96:391–404. 2009.PubMed/NCBI
|
|
9
|
Lee JH, Song EM, Sim YS, Ryu YJ and Chang
JH: Forced expiratory volume in one second as a prognostic factor
in advanced non-small cell lung cancer. J Thorac Oncol. 6:305–309.
2011.PubMed/NCBI
|
|
10
|
Valko M, Izakovic M, Mazur M, Rhodes CJ
and Telser J: Role of oxygen radicals in DNA damage and cancer
incidence. Mol Cell Biochem. 266:37–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Faux SP, Tai T, Thorne D, Xu Y, Breheny D
and Gaca M: The role of oxidative stress in the biological
responses of lung epithelial cells to cigarette smoke. Biomarkers.
14:90–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maki A, Kono H, Gupta M, Asakawa M, Suzuki
T, Matsuda M, Fujii H and Rusyn I: Predictive power of biomarkers
of oxidative stress and inflammation in patients with hepatitis C
virus-associated hepatocellular carcinoma. Ann Surg Oncol.
14:1182–1190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Voronov E, Shouval DS, Krelin Y, et al:
IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl
Acad Sci USA. 100:2645–2650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar
|
|
16
|
Entschladen F, Drell TL IV, Lang K, Joseph
J and Zaenker KS: Tumour-cell migration, invasion, and metastasis:
navigation by neurotransmitters. Lancet Oncol. 5:254–258. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tracey KJ: Reflex control of immunity. Nat
Rev Immunol. 9:418–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ek M, Kurosawa M, Lundeberg T and Ericsson
A: Activation of vagal afferents after intravenous injection of
interleukin-1beta: role of endogenous prostaglandins. J Neurosci.
18:9471–9479. 1998.PubMed/NCBI
|
|
19
|
Pavithran P, Nandeesha H, Sathiyapriya V,
Bobby Z and Madanmohan T: Short-term heart variability and
oxidative stress in newly diagnosed essential hypertension. Clin
Exp Hypertens. 30:486–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vlcek M, Radikova Z, Penesova A, et al:
Heart rate variability and catecholamines during hypoglycemia and
orthostasis. Auton Neurosci. 143:53–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Couck M, Mravec B and Gidron Y: You may
need the vagus nerve to understand pathophysiology and to treat
diseases. Clin Sci. 122:323–328. 2012.PubMed/NCBI
|
|
22
|
Gidron Y, Perry H and Glennie M: Does the
vagus nerve inform the brain about preclinical tumours and modulate
them? Lancet Oncol. 6:245–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Task Force of the European Society of
Cardiology and the North American Society of Pacing and
Electrophysiology. Heart rate variability: standards of
measurement, physiological interpretation and clinical use.
Circulation. 93:1043–1065. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuo TB, Lai CJ, Huang YT and Yang CC:
Regression analysis between heart rate variability and
baroreflex-related vagus nerve activity in rats. J Cardiovasc
Electrophysiol. 16:864–869. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoffmann J, Grimm W, Menz V, Wied M,
Sprenger A, Arnold R and Maisch B: Prognostic value of heart rate
variability analysis in patients with carcinoid syndrome.
Digestion. 63:35–42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fadul N, Strasser F, Palmer LJ, Yusuf SW,
Guo Y, Li Z, Allo J and Bruera E: The association between autonomic
dysfunction and survival in male patients with advanced cancer: a
preliminary report. J Pain Symptom Manage. 39:283–290. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiang JK, Koo M, Kuo TB and Fu CH:
Association between cardiovascular autonomic functions and time to
death in patients with terminal hepatocellular carcinoma. J Pain
Symptom Manage. 39:673–679. 2010. View Article : Google Scholar
|
|
28
|
Kim do H, Kim JA, Choi YS, Kim SH, Lee JY
and Kim YE: Heart rate variability and length of survival in
hospice cancer patients. J Korean Med Sci. 25:1140–1145.
2010.PubMed/NCBI
|
|
29
|
Mouton C, Ronson A, Razavi D, Delhaye F,
Kupper N, Paesmans M, Moreau M, Nogaret JM, Hendlisz A and Gidron
Y: The relationship between heart rate variability and time-course
of carcinoembryonic antigen in colorectal cancer. Auton Neurosci.
166:96–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crimmins EM, Johnston M, Hayward M and
Seeman T: Age differences in allostatic load: an index of
physiological dysregulation. Exp Gerontol. 38:731–734. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pfleger CC, Flachs EM and Koch-Henriksen
N: Social consequences of multiple sclerosis: clinical and
demographic predictors - a historical prospective cohort study. Eur
J Neurol. 17:1346–1351. 2010.PubMed/NCBI
|
|
32
|
Lerner-Geva L, Keinan-Boker L, Blumstein
T, Boyko V, Olmar L, Mashiach S, Rabinovici J, Potashnik G,
Lunenfeld E, Schenker JG, Shushan A, Fishman A, Cohen I, Vagman I
and Lunenfeld B: Infertility, ovulation induction treatments and
the incidence of breast cancer - a historical prospective cohort of
Israeli women. Breast Cancer Res Treat. 100:201–212. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thong T, Li K, McNames J, Aboy M and
Goldstein B: Accuracy of ultra-short heart rate variability
measures. Engineering in Medicine and Biology Society. Proc 25th
Annual Int Conference of the IEEE. 3:2424–2427. 2003.
|
|
34
|
Nussinovitch U, Elishkevitz KP, Katz K,
Nussinovitch M, Segev S, Volovitz B and Nussinovitch N: Reliability
of ultra-short ECG indices for heart rate variability. Ann
Noninvasive Electrocardiol. 16:117–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hodgart E and Macfarlane PW: 10 second
heart rate variability. Comp Cardiol. 31:217–220. 2004.
|
|
36
|
Schipke JD, Pelzer M and Arnold G: Effect
of respiration rate on short-term heart rate variability. J Clin
Basic Cardiol. 2:92–94. 1999.
|
|
37
|
Poyhonen M, Syvaoja S, Hartikainen J,
Ruokonen E and Takala J: The effect of carbon dioxide, respiratory
rate and tidal volume on human heart rate variability. Acta
Anaesthesiol Scand. 48:93–101. 2004.PubMed/NCBI
|
|
38
|
Strauss-Blasche G, Moser M, Voica M,
McLeod DR, Klammer N and Marktl W: Relative timing of inspiration
and expiration affects respiratory sinus arrhythmia. Clin Exp
Pharmacol Physiol. 27:601–606. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brown JK, Cooley ME, Chernecky C and Sarna
L: A symptom cluster and sentinel symptom experienced by women with
lung cancer. Oncol Nurs Forum. 38:E425–E435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Johannessen A, Lehmann S, Omenaas ER, Eide
GE, Bakke PS and Gulsvik A: Post-bronchodilator spirometry
reference values in adults and implications for disease management.
Am J Respir Crit Care Med. 173:1316–1325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Katona PG and Jih F: Respiratory sinus
arrhythmia: noninvasive measure of parasympathetic cardiac control.
J Appl Physiol. 39:801–805. 1975.PubMed/NCBI
|
|
42
|
Cassart M, Pettiaux N, Gavenois PA, Pavia
M and Estenne M: Effect of chronic hyperinflation on diaphragm
length and surface area. Am J Respir Crit Care Med. 156:504–508.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alifano M, Falcoz PE, Seegers V, Roche N,
Schussler O, Younes M, Antonacci F, Forgez P, Dechartres A, Massard
G, Damotte D and Régnard JF: Preresection serum C-reactive protein
measurement and survival among patients with resectable non-small
cell lung cancer. J Thorac Cardiovasc Surg. 142:1161–1167. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hamilton RM, McKechnie PS and Macfarlane
PW: Can cardiac vagal tone be estimated from the 10-second ECG? Int
J Cardiol. 95:109–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dekker JM, Schouten EG, Klootwijk P, Pool
J, Swenne CA and Kromhout D: Heart rate variability from short
electrocardiographic recordings predicts mortality from all causes
in middle-aged and elderly men. The Zutphen Study. Am J Epidemiol.
145:899–908. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
O’Donnell K, Brydon L, Wright CE and
Steptoe A: Self-esteem levels and cardiovascular and inflammatory
responses to acute stress. Brain Behav Immun. 22:1241–1247.
2008.PubMed/NCBI
|
|
47
|
Erin N, Akdas Barkan G, Harms JF and
Clawson GA: Vagotomy enhances experimental metastases of 4THMpc
breast cancer cells and alters substance P level. Regul Pept.
151:35–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Erin N, Boyer PJ, Bonneau RH, Clawson GA
and Welch DR: Capsaicin-mediated denervation of sensory neurons
promotes mammary tumor metastasis to lung and heart. Anticancer
Res. 24:1003–1009. 2004.PubMed/NCBI
|
|
49
|
Erin N, Duymuş O, Oztürk S and Demir N:
Activation of vagus nerve by semapimod alters substance P levels
and decreases breast cancer metastasis. Regul Pept. 179:101–108.
2012. View Article : Google Scholar : PubMed/NCBI
|