Introduction
Neuroblastoma is one of the most common childhood
tumors, usually diagnosed at a median age of 17 months (1). The tumor originates from neural-crest
tissues, deep in the adrenal medulla and paraspinal ganglia, with
no specific clinical presentations (2). Approximately 50–60% patients are
diagnosed with neuroblastoma at advanced stages (3). In spite of multidisciplinary care,
even the introduction of dose intensive chemotherapy (4), the outcome of high risk neuroblastoma
has achieved no significant improvement (5), with a 5-year survival rate of <50%
(6).
Brain-derived neurotropic factor (BDNF), a member of
the neurotropin family, plays a critical role in neuronal survival,
differentiation and axon wiring through binding to its preferred
receptor TrkB (tyrosine kinase receptor B) (7–9).
Research has demonstrated that BDNF-TrkB signals do not only
regulate the growth of nerve neutrons, but also affect the
development, invasion and outcome of many types of tumors,
including lung (10), bladder
(11) and pancreatic cancer
(12). A recent study showed that
BDNF enhanced the proliferation and survival of transitional cell
carcinoma (13). High expression
levels of BDNF are associated with more aggressive malignant
behavior in human cancers, including pancreatic (14) and breast cancer (15).
microRNAs (miRNAs) are small non-coding RNAs,
involved in the post-transcriptional regulation of gene expression.
Through binding to the imperfect sequence of target mRNAs, miRNAs
result in translational inhibition or destabilization of target
mRNAs. miRNAs can act as tumor suppressors or as oncogenes
(16). miR-106a was shown to be
upregulated in gastric cancer, having pro-tumorigenic effects
(17). miR-16 functions as a tumor
suppressor and was found to be downregulated in mantle cell
lymphoma SP cells by regulating Bmi1, leading to reduction in tumor
size in lymphoma xenografts (18).
Cisplatin is an effective drug in the in vivo
treatment of neuroblastoma. It also can inhibit the growth of
neuroblastoma cells and reduce cell viability (19). In our previous study, we found that
miR-21 expression was decreased after treatment with cisplatin,
while MSH2 expression was enhanced (20). In the present study, we further
explored the mechanism of cisplatin in suppressing the
proliferation of neuroblastoma cells and found that cisplatin
downregulated the expression of BDNF through upregulation of miR-16
to inhibit SH-SY5Y cell proliferation.
Materials and methods
Cell culture
The neuroblastoma cell line SH-SY5Y, obtained from
the Shanghai Institute of Biochemistry and Cell Biology (Shanghai,
China), was cultured in DMEM/F12 (1:1) (HyCLone, Logan, UT, USA)
with 10% FCS (HyClone), 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C in 5% CO2. Cells were detached from
cell culture flasks with 0.25% trypsin when they grew and spread in
the bottom of the flasks (at 48 h) and were subcultured in fresh
culture medium.
Cisplatin treatment
SH-SY5Y cells in logarithmic phase were detached,
counted and planted into cell culture plates. On the following day,
different concentrations of cisplatin (0, 1.5, 3, 4.5, 6 and 12
μg/ml, separately) (QiLu Pharmaceutical Co., Ltd., Jinan, China)
were dissolved in the culture medium. Cells were collected for
subsequent experiments at 48 h after cisplatin treatment.
MTT detection
SH-SY5Y cells were planted into a 96-well plate at a
density of 1×104 cells/well. The following day, each
group was treated with corresponding concentrations of cisplatin
according to the experimental design. MTT (10 μl) (5 mg/ml; Sigma,
St. Louis, MO, USA) was added into each well after 48 h. Then, 100
μl DMSO (Sigma) was added to each well. The plate was shaken until
the MTT was fully dissolved. After that, the OD value was measured
with an enzyme-linked immunosorbent assay reader (ELx800, USA) at
570 nm. Cell growth inhibition rate was calculated as follows: Cell
growth inhibition rate = (ODcontrol −
ODsample)/ODcontrol × 100 (%).
Flow cytometric analysis
SH-SY5Y cells (8×104) were treated with
cisplatin for 48 h, the culture medium was discarded, and the
SH-SY5Y cells were collected. Binding buffer (500 μl) (KeyGen
Biotech Co., Ltd., Nanjing, China) was added to the collected cells
to suspend the cells. Annexin V-FITC (5 μl) was added with gently
mixing. After that, 5 μl PI was added to the cells. The cells were
incubated for 15 min and measured by flow cytometry
(Beckman-Coulter, Inc., Brea, CA, USA) with a 488-nm exciting
wavelength and a 530-nm emitting wavelength.
miRNA transfection
miR-16 and NC (control oligos) were chemically
synthesized (GenePharma Co., Ltd., Shanghai, China). The sense
sequence of the miR-16 was 5′-UAGCAGCACGUAAAUAUUGGCG-3′; the
antisense was 5′-CCAAUAUUUACGUGCUGCUAUU-3′. The sense sequence of
NC was 5′-CAGUACUUUUGUGUAGUACAA-3′; the antisense was
5′-GUACUACACAAAAGUACUGUU-3′. The cells were treated with 1 μg
oligos and 2.5 μl Lipofectamine 2000 (Invitrogen, Carlsbad, CA,
USA) as introductions. Cells were collected at 48 h after
transfection.
SH-SY5Y cell xenografts and cisplatin
interference
BALB/C-nu mice (nude mice) were obtained from HFK
Bio-Technology Co., Ltd. (Beijing, China). The nude mice were
narcotized with 1.5% pentobarbital sodium and were injected
subcutaneously with 1×107 SH-SY5Y cells. Tumors appeared
3 days after cell xenograft. When the tumor volume (V = length ×
width2/2, length>width) grew to 100 mm3,
the mice were treated with 3 mg/kg cisplatin by intraperitoneal
injection once every 4 days, the treatment was carried out 4 times
all together. The control group was injected with the same volume
of saline solution. After the last treatment, the mice were
sacrificed to assess the tumors. The tumor weights were determined
with an electronic scale. Subsequently, the tumor tissues were
analyzed for the levels of miRNA or BDNF protein.
Western blotting
SH-SY5Y cells and xenograft tumors were collected
and lysed to extract the total protein following cisplatin
treatment. Protein (40 μg) of each sample was loaded respectively
on each lane of a polyacrylamide gel. Protein bands on the
separating gel were transferred to a PVDF membrane through a
transfer device. Then, rabbit anti-BDNF polyclonal antibody (1:200;
Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added to the
membrane for overnight incubation at 4°C. The following day, the
membrane was incubated in HRP-labeled goat anti-rabbit IgG for 2 h
(1:6,000; Beijing Zhongshan Golden Bridge Technology Co., Ltd.,
Beijing, China). Finally, a chemical spectral imager (Tanon, China)
was applied to observe the results.
Real-time PCR
RNAiso for small RNA (Takara, Shiga, Japan) was
applied to extract miRNA from SH-SY5Y cells or xenografts. Poly(A)
was added using poly(A) polymerase (Ambion, Foster City, CA, USA).
The cDNA was synthesized by RT primer
5′-AACATGTACAGTCCATGGATGd(T)30N (A, G, C or T)-3′. The
forward primer used to amplify miR-16 was
5′-TAGCAGCACATAAATATTGGCG-3′ and the reverse was
5′-AACATGTACAGTCCATGGATG-3′. Forward primer of 5S rRNA was
5′-GCCATACCACCCTGAACG-3′ and the reverse was
5′-AACATGTACAGTCCATGGATG-3′. SYBR® Premix Ex Taq™ kit
(Takara) is used to conduct qRT-PCR. The expression of miR-16 was
assessed using the RG3000 system (Corbett Research, Sydney,
Australia). Denaturing was carried out at 95°C for 3 min; 40 cycles
of 95°C for 20 sec; annealing at 60°C for 20 sec and extension at
72°C for 20 sec. At each extension step at 72°C, fluorescence was
detected at 585 nm. The human 5S rRNA served as the control.
Statistics
SAS software was used to analyze the significance of
all results. The Student’s t-test was used for inter-group
comparison. A P-value <0.05 was considered to indicate a
statistically significant result.
Results
Cisplatin inhibits the proliferation of
SH-SY5Y cells
To select an effective concentration of cisplatin
for suppressing the proliferation of SH-SY5Y cells, an MTT assay
was performed to determine the cell growth inhibition rate. The
cell growth inhibition rate reached ~50% in the 6 μg/ml
cisplatin-treated cultures (Fig.
1A). The number of live cells was obviously decreased with
increasing concentrations of cisplatin as noted under an inverted
microscope (Fig. 1B). The
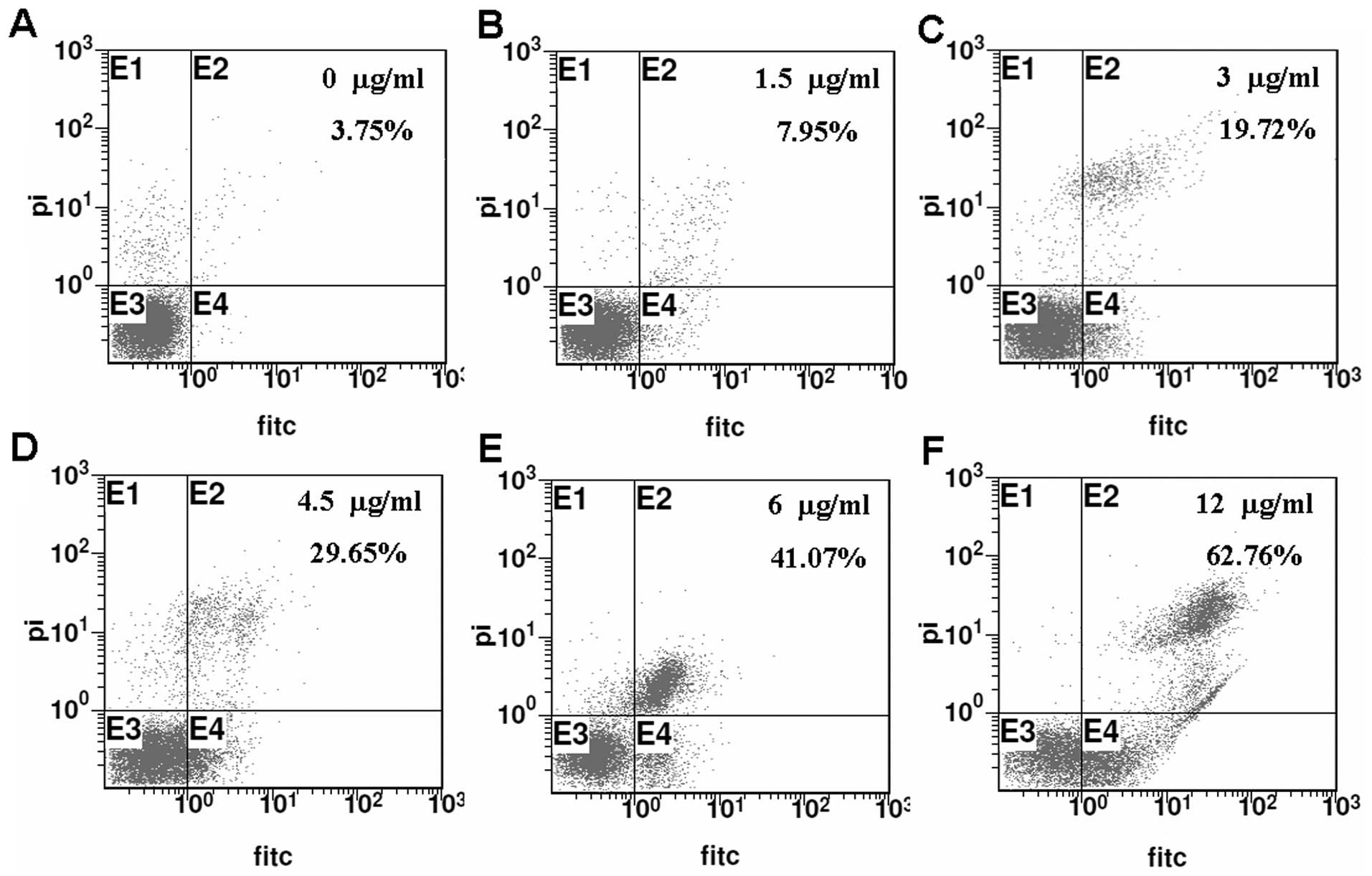
percentages of apoptotic cells were also increased with increasing
concentrations of cisplatin as detected by Annexin V-FITC/PI
analysis (Fig. 2). These results
showed that cisplatin inhibited SH-SY5Y cell proliferation
significantly with increasing concentrations.
Cisplatin decreases BDNF levels in
SH-SY5Y cells
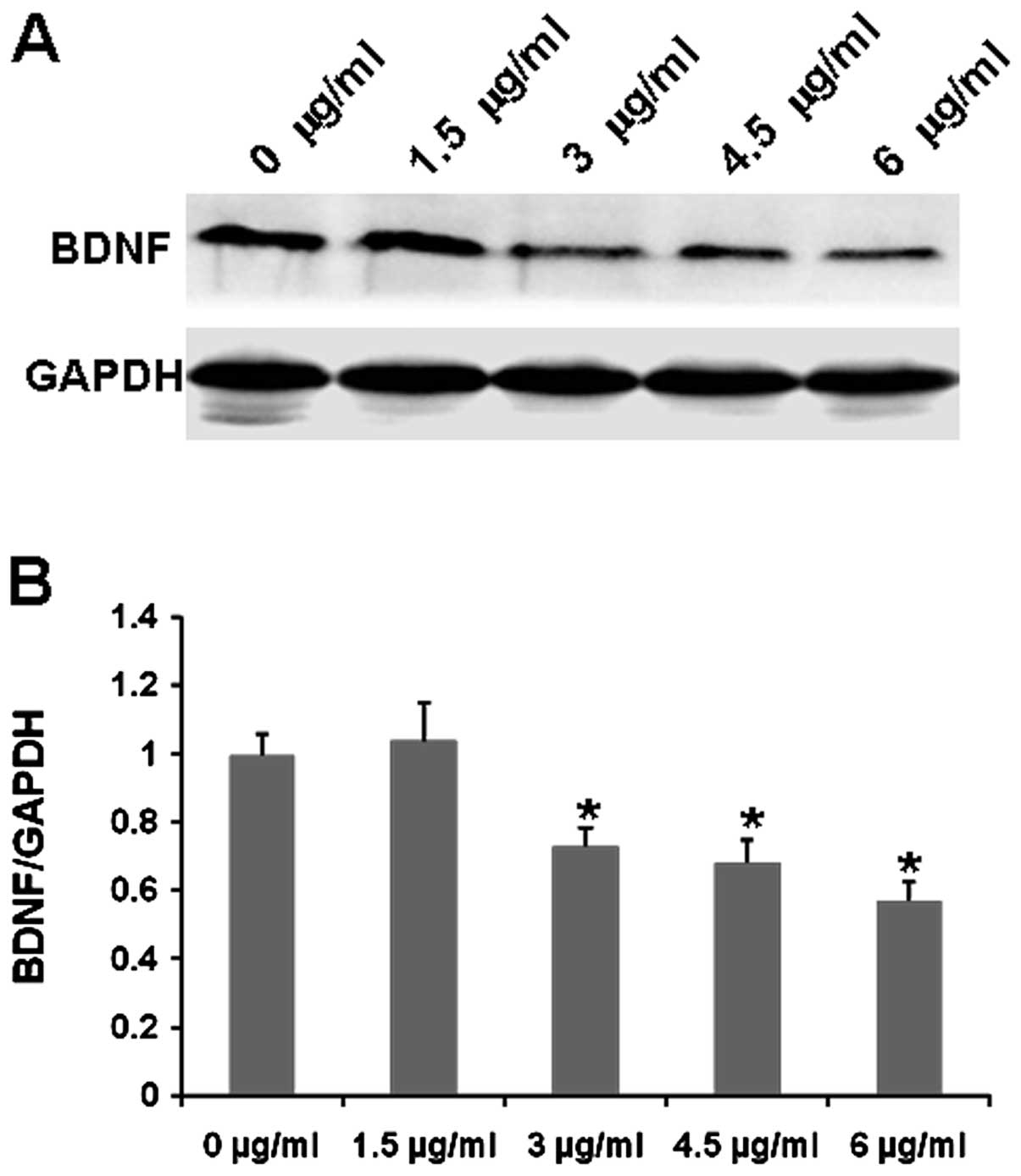
BDNF is a trophic factor for neuroblastoma cells
(21). To investigate whether
cisplatin inhibits SH-SY5Y cell growth by regulating BDNF levels,
the BDNF expression in SH-SY5Y cells was assessed following
cisplatin treatment. Western blotting showed that the expression of
BDNF was obviously decreased in the cisplatin-treated cells when
compared to that in the untreated controls, and the levels of BDNF
were significantly reduced following treatment with increasing
concentrations of cisplatin (Fig.
3). Thus, cisplatin suppresses SH-SY5Y cell growth by reducing
BDNF expression.
BDNF expression is regulated by
miR-16
miRNAs can play roles as tumor suppressors or as
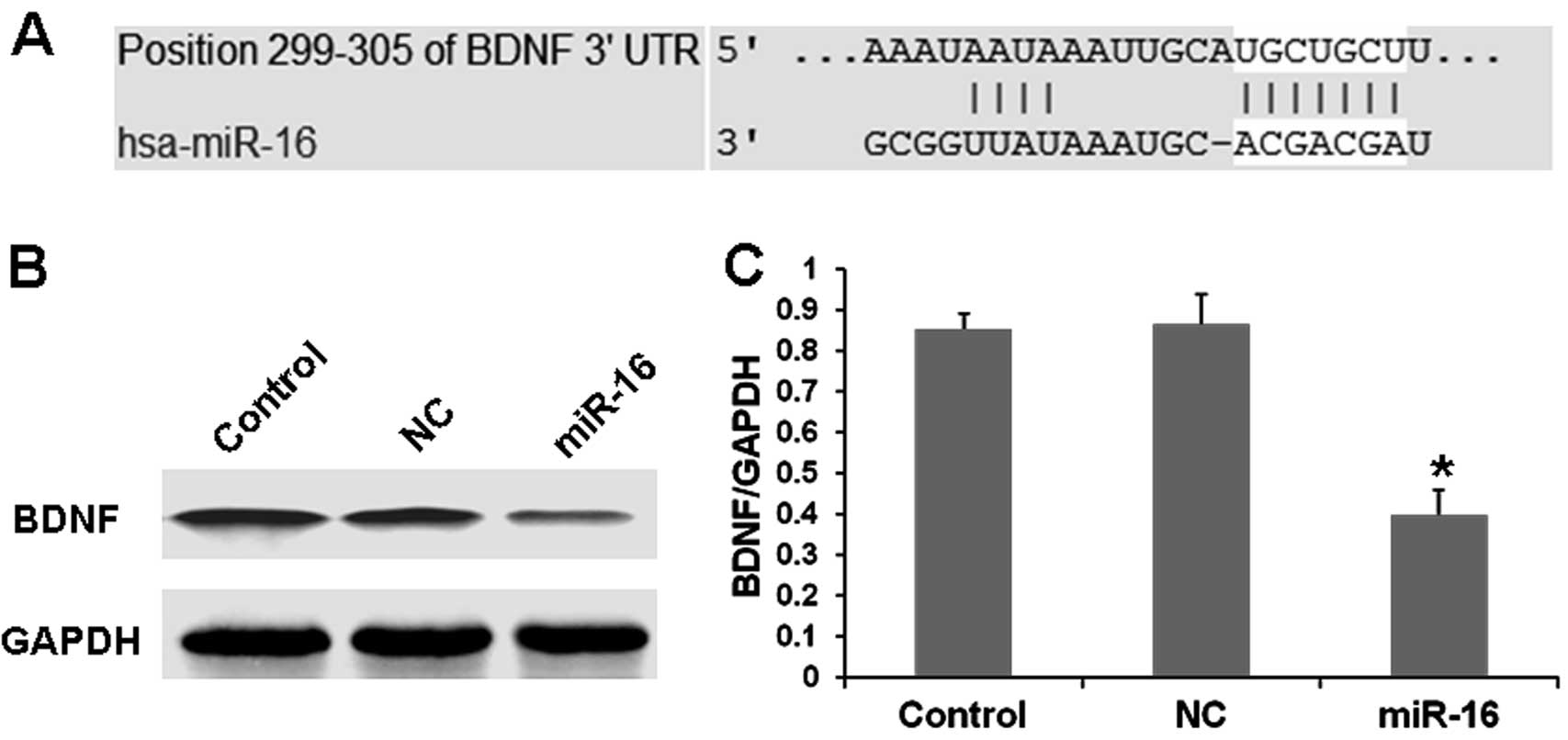
oncogenes by regulating their target genes (16). Yet, few studies have focused on
BDNF-related miRNAs. Based on microRNA analysis online software
(http://www.targetscan.org/ or http://www.microrna.org/microrna/home.do), we found
that BDNF-3′-UTR is targeted by miR-16 (Fig. 4A). Then, we synthesized miR-16 and
transfected the SH-SY5Y cells with miR-16. Our results showed that
the expression of BDNF was obviously decreased in the
miR-16-transfected cells when compared with that in the scrambled
oligos-treated cultures as determined by western blot analysis
(Fig. 4B and C). These results
showed that miR-16 negatively regulated BDNF expression. It is
known that miR-16 can function as a tumor suppressor in mantle cell
lymphoma cells (18). Then, we
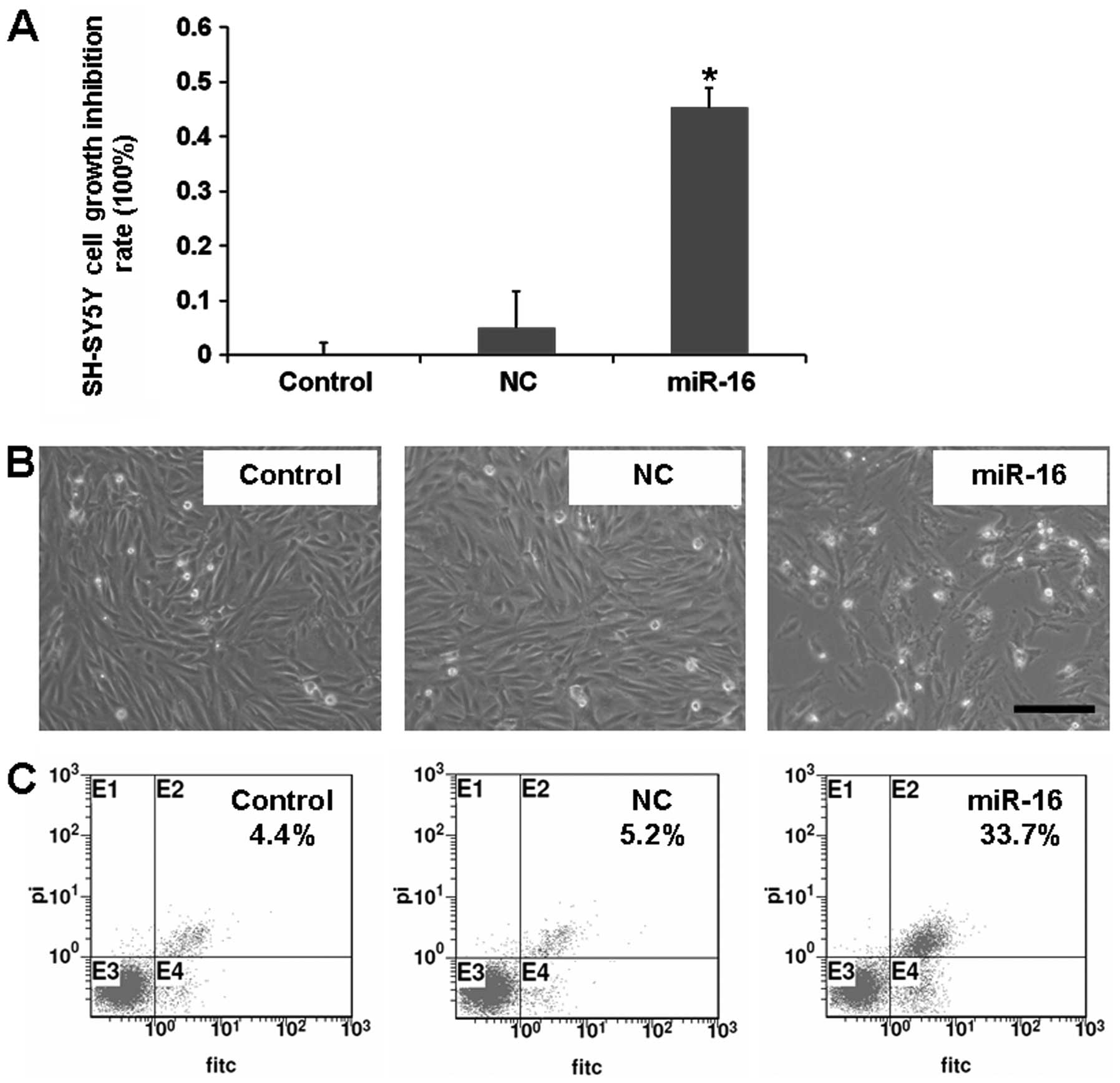
studied the effect of overexpression of miR-16 on SH-SY5Y cells. We
found that overexpression of miR-16 inhibited SH-SY5Y cell growth
and induced cell apoptosis (Fig.
5). The above findings revealed that miR-16 inhibits SH-SY5Y
cell growth by negatively regulating BDNF.
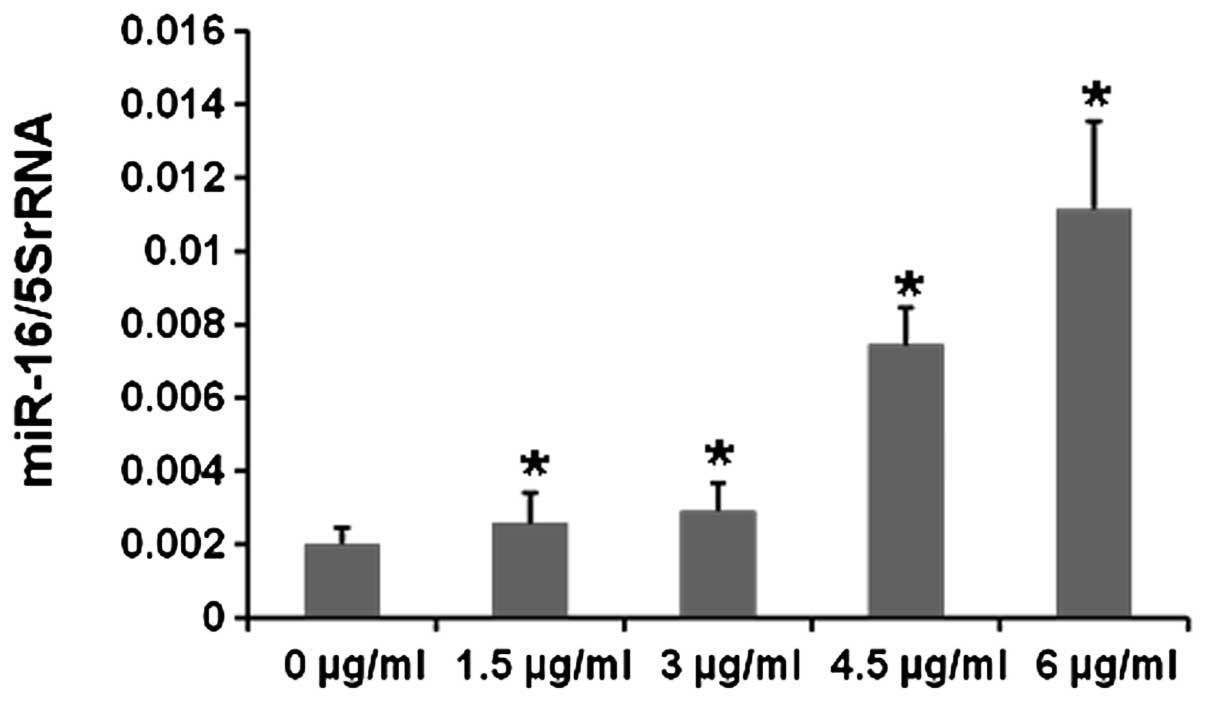
Cisplatin upregulates miR-16 levels in
SH-SY5Y cells
We demonstrated that BDNF is a new target of miR-16.
Since cisplatin can also downregulate the expression of BDNF, we
hypothesized that there may be a relationship between miR-16
expression and cisplatin treatment. To further test whether
cisplatin affects miR-16 expression, real-time PCR was performed to
analyze miR-16 levels following cisplatin treatment. After miRNA
isolation and reverse transcription, we found that the expression
level of miR-16 in cisplatin-treated cells increased to a greater
extent with increasing concentrations of cisplatin when compared
with the untreated controls (Fig.
6). Collectively, our results showed that cisplatin regulates
SH-SY5Y cell proliferation by upregulating miR-16 to inhibit BDNF
in vitro.
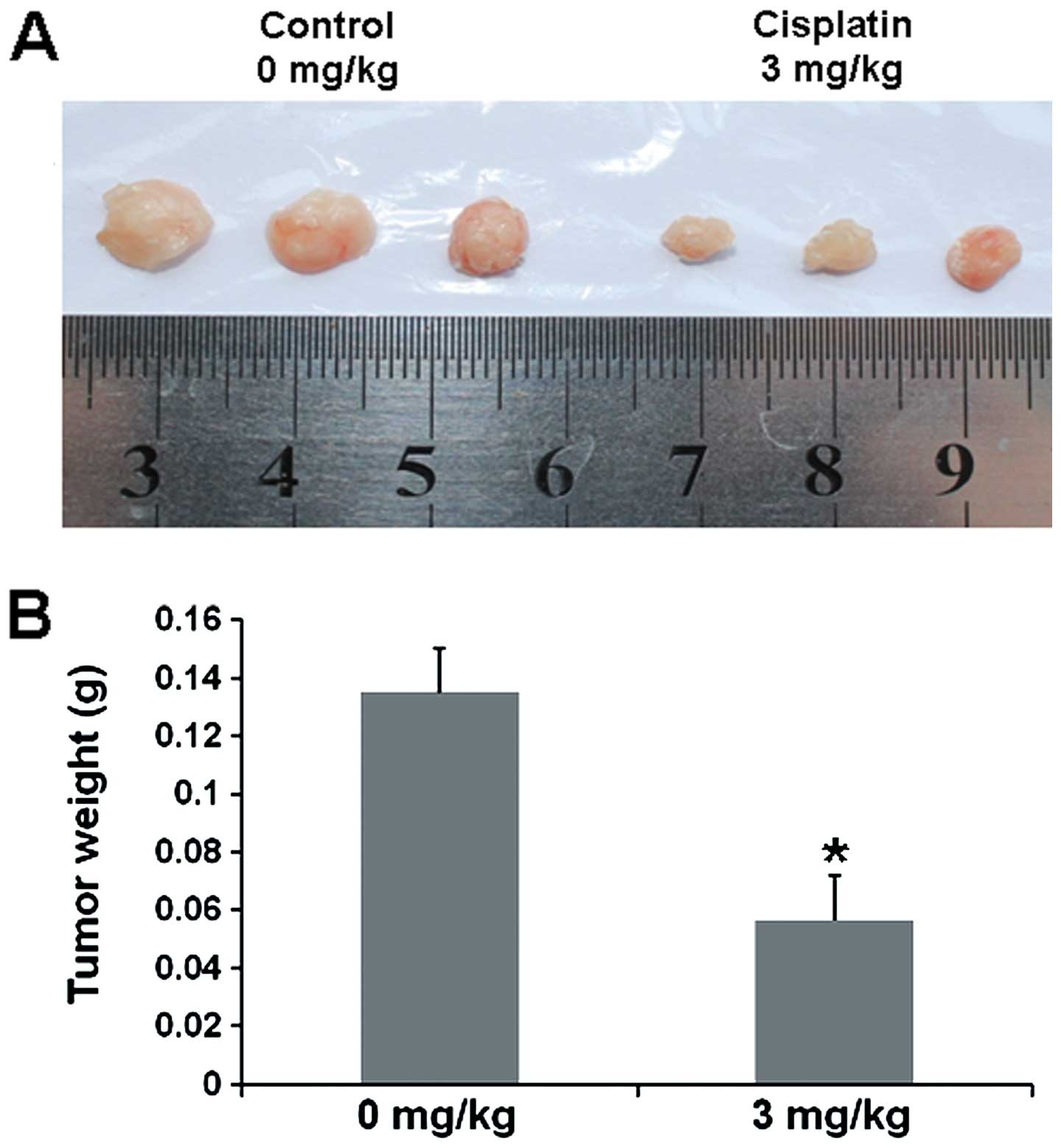
Cisplatin inhibits growth of SH-SY5Y cell
xenografts by decreasing BDNF through miR-16
To further investigate whether cisplatin inhibits
SH-SY5Y cell growth in vivo, nude mice xenografted with
SH-SY5Y cells were treated with cisplatin. We found that the tumor
volume of SH-SY5Y cell xenografts in the cisplatin-treated nude
mice was markedly reduced, and the weight of tumor xenografts was
decreased when compared with these values in the saline-treated
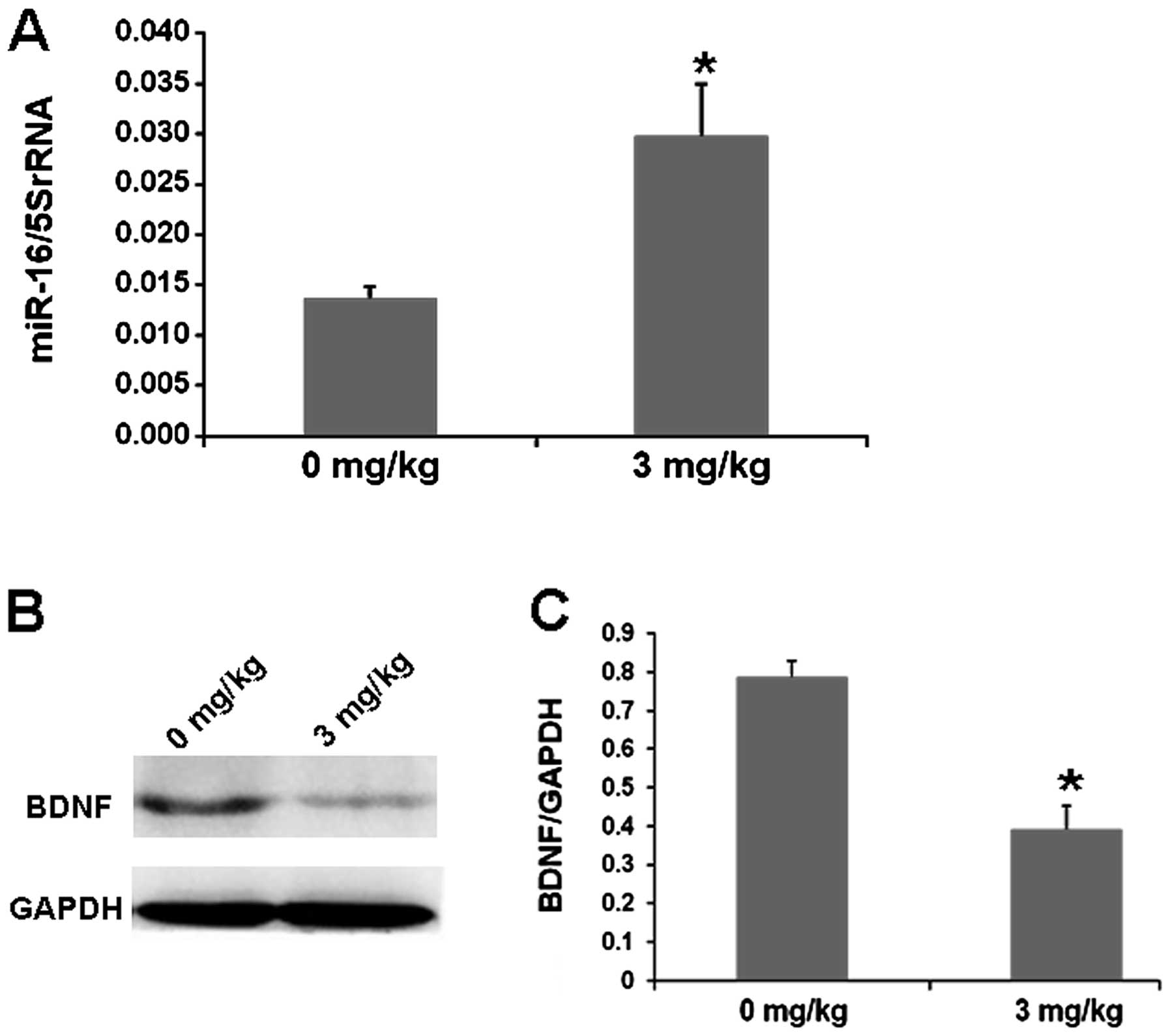
controls (Fig. 7). To study whether
cisplatin also affects miR-16 or BDNF expression in xenografts, we
analyzed the levels of miR-16 or BDNF by real-time PCR or western
blotting. Our result showed that the expression of miR-16 was
obviously increased in the cisplatin-treated cells (Fig. 8A), while BDNF levels were markedly
decreased in the cisplatin-treated cells when compared with these
levels in the saline-treated controls (Fig. 8B and C). Thus, cisplatin inhibits
SH-SY5Y cell proliferation by upregulating miR-16 to inhibit BDNF
in vivo.
Discussion
BDNF, initially identified as a neurotrophin, plays
an essential role in a variety of neuronal functions, including
proliferation, differentiation and survival in the central nervous
system (CNS). It also contributes to the regulation of synaptic
plasticity (22). In many cancer
studies, the expression of BDNF was found to be correlated with
tumor progression or poorer prognosis. The increased expression of
BDNF increased the risk of metastasis, regional invasion and
mortality. It was found that BDNF was overexpressed in gallbladder
adenocarcinoma compared with peritumoral tissues, adenoma, polyps
and chronic cholecystitis samples (23). In the present study, we also found
that the expression of BDNF was increased in the SH-SY5Y
neuroblastoma cells, and cisplatin inhibited the proliferation of
SH-SY5Y cells in vitro and in vivo by downregulating
BDNF expression.
Recent studies have shown that miRNAs are involved
in the initiation and progression of cancer (24). Approximately 17 differentially
expressed miRNAs, including miR-339-5p, miR-423-3p, miR-19a, were
reported to play important regulatory roles in the progression of
lung cancer (25). miR-7 was also
found to be a tumor-suppressor gene in glioblastoma, and was
associated with cancer cell proliferation, invasion and metastasis
(26). Overexpression of miR-7 not
only induced A549 cell apoptosis and inhibited cell migration in
vitro, but also reduced tumorigenicity in vivo(27). miR-16 was also found to be a tumor
suppressor and was downregulated in most solid tumors, such as
ovarian, prostate and colorectal cancer (28–30).
Overexpression of miR-16 inhibited cell proliferation and induced
cell apoptosis in CRC cells (30).
In the present study, our results demonstrated that overexpression
of miR-16 inhibited the proliferation of SH-SY5Y cells, which
further confirmed the suppressor role of miR-16 in neuroblastoma
cells.
Recent studies have shown that the expression of
BDNF is regulated by miRNAs in many CNS diseases (31–33).
Lee et al(31) confirmed
that the expression of miR-206 was increased in AD mice and human
AD brains, and bioinformatics technology revealed that BDNF is a
potential target of miR-206. miR-206-neutralizing antagomir was
found to increase the brain levels of BDNF and improve the memory
function of AD mice. miR-30a-5p overexpression reduced the levels
of BDNF protein in rat forebrain neurons (32). BDNF levels were also found to be a
inversely correlated with miR-195 levels in a schizophrenic group
(33). Yet, few studies have
revealed the mechanism of BDNF-related miRNAs in regulating tumor
progression. BDNF was reported to be regulated by miR-204 in
ovarian and breast cancers (34).
In the present study, our results further showed that BDNF is a
target of miR-16, which induced the apoptosis of SH-SY5Y
neuroblastoma cells by regulating the level of BDNF in vitro
and in vivo.
Cisplatin is one of the first-line chemotherapeutic
drugs used for the treatment of many solid tumors (35,36).
Recent studies have indicated that miRNAs modulates the sensitivity
to various chemotherapeutic drugs (including cisplatin) in many
cancers by regulation of their target genes (37,38).
miR-302a expression levels were upregulated by cisplatin in a dose-
and time-dependent manner in NT2 cells, and miR-302a significantly
enhanced the sensitivity of NT2 cells to cisplatin through the
downregulation of p21 (37). Ryan
et al(38) found that
upregulation of miR-204 in neuroblastoma cell lines increased the
sensitivity to cisplatin and etoposide significantly by targeting
NTRK2 and BCL2 directly. Here, we found that miR-16 was upregulated
in cisplatin-treated neuroblastoma cells and xenografts, suggesting
that miR-16 is a target of cisplatin, and miR-16 overexpression may
enhance the anticancer effect of cisplatin. Further results
demonstrated that cisplatin downregulated BDNF through reducing
miR-16 in SH-SY5Y cells.
In summary, the present study demonstrated that the
expression of BDNF was regulated by miR-16 in SH-SY5Y cells, and
cisplatin inhibited SH-SY5Y cell proliferation in vitro and
in vivo by increasing miR-16 expression and downregulating
BDNF levels. These findings provide evidence for new gene targets
for neuroblastoma therapy.
Acknowledgements
The present study was supported by the NCET-10-0919,
National Natural Science Foundation (nos. 31371321, 81200601), the
Shandong Science and Technology Committee (no. ZR2012HQ035), the
Foundation of Shandong Educational Committee (nos. J10LC60,
J11LC01) and the Binzhou Science and Technology Committee of China
(no. 2011ZC0905).
Abbreviations:
|
miRNAs
|
microRNAs
|
|
BDNF
|
brain-derived neurotropic factor
|
|
3′-UTR
|
3′-untranslated region
|
|
TrkB
|
tyrosine kinase receptor B
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
DMSO
|
dimethyl sulfoxide
|
|
FACS
|
flow cytometry
|
|
HRP
|
horseradish peroxidase
|
|
AD
|
Alzheimer disease
|
References
|
1
|
London WB, Castleberry RP, Matthay KK, et
al: Evidence for an age cutoff greater than 365 days for
neuroblastoma risk group stratification in the Children’s Oncology
Group. J Clin Oncol. 23:6459–6465. 2005.PubMed/NCBI
|
|
2
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chu CM, Rasalkar DD, Hu YJ, Cheng FW, Li
CK and Chu WC: Clinical presentations and imaging findings of
neuroblastoma beyond abdominal mass and a review of imaging
algorithm. Br J Radiol. 84:81–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pavlidis N, Stahel R, Clarke M and
Djulbegovic B: Cancer treatment reviews welcomes submission of the
Cochrane Reviews. Cancer Treat Rev. 32:243–244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verissimo CS, Molenaar JJ, Fitzsimons CP
and Vreugdenhil E: Neuroblastoma therapy: what is in the pipeline?
Endocr Relat Cancer. 18:R213–R231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hara J: Development of treatment
strategies for advanced neuroblastoma. Int J Clin Oncol.
17:196–203. 2012. View Article : Google Scholar
|
|
7
|
Arévalo JC and Wu SH: Neurotrophin
signaling: many exciting surprises! Cell Mol Life Sci.
63:1523–1537. 2006.PubMed/NCBI
|
|
8
|
Huang EJ and Reichardt LF: Trk receptors:
roles in neuronal signal transduction. Annu Rev Biochem.
72:609–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soppet D, Escandon E, Maragos J, et al:
The neurotrophic factors brain-derived neurotrophic factor and
neurotrophin-3 are ligands for the trkB tyrosine kinase receptor.
Cell. 65:895–903. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Odate S, Nakamura K, Onishi H, et al:
TrkB/BDNF signaling pathway is a potential therapeutic target for
pulmonary large cell neuroendocrine carcinoma. Lung Cancer.
79:205–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dionne CA, Camoratto AM, Jani JP, et al:
Cell cycle-independent death of prostate adenocarcinoma is induced
by the trk tyrosine kinase inhibitor CEP-751 (KT6587). Clin Cancer
Res. 4:1887–1898. 1998.PubMed/NCBI
|
|
12
|
Miknyoczki SJ, Lang D, Huang L,
Klein-Szanto AJ, Dionne CA and Ruggeri BA: Neurotrophins and Trk
receptors in human pancreatic ductal adenocarcinoma: expression
patterns and effects on in vitro invasive behavior. Int J Cancer.
81:417–427. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang YT, Lai PC, Wu CC, et al: BDNF
mediated TrkB activation is a survival signal for transitional cell
carcinoma cells. Int J Oncol. 36:1469–1476. 2010.PubMed/NCBI
|
|
14
|
Sclabas GM, Fujioka S, Schmidt C, et al:
Overexpression of tropomysin-related kinase B in metastatic human
pancreatic cancer cells. Clin Cancer Res. 11:440–449.
2005.PubMed/NCBI
|
|
15
|
Patani N, Jiang WG and Mokbel K:
Brain-derived neurotrophic factor expression predicts adverse
pathological and clinical outcomes in human breast cancer. Cancer
Cell Int. 11:232011. View Article : Google Scholar
|
|
16
|
Chen PS, Su JL and Hung MC: Dysregulation
of microRNAs in cancer. J Biomed Sci. 19:902012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou H, Guo JM, Lou YR, et al: Detection
of circulating tumor cells in peripheral blood from patients with
gastric cancer using microRNA as a marker. J Mol Med. 88:709–717.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teshima K, Nara M, Watanabe A, et al:
Dysregulation of BMI1 and microRNA-16 collaborate to enhance an
anti-apoptotic potential in the side population of refractory
mantle cell lymphoma. Oncogene. May 20–2013.(Epub ahead of print).
View Article : Google Scholar
|
|
19
|
Cece R, Barajon I and Tredici G: Cisplatin
induces apoptosis in SH-SY5Y human neuroblastoma cell line.
Anticancer Res. 15:777–782. 1995.PubMed/NCBI
|
|
20
|
Zhang YX, Yue Z, Wang PY, et al: Cisplatin
upregulates MSH2 expression by reducing miR-21 to inhibit A549 cell
growth. Biomed Pharmacother. 67:97–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Middlemas DS, Kihl BK, Zhou J and Zhu X:
Brain-derived neurotrophic factor promotes survival and
chemoprotection of human neuroblastoma cells. J Biol Chem.
274:16451–16460. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Numakawa T, Suzuki S, Kumamaru E, Adachi
N, Richards M and Kunugi H: BDNF function and intracellular
signaling in neurons. Histol Histopathol. 25:237–258.
2010.PubMed/NCBI
|
|
23
|
Xiong L, Deng X, Wen Y, Yang Z and Miao X:
Association of BDNF and BMPR1A with clinicopathologic parameters in
benign and malignant gallbladder lesions. World J Surg Oncol.
11:802013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar
|
|
25
|
Guo WG, Zhang Y, Ge D, et al:
Bioinformatics analyses combined microarray identify the
desregulated microRNAs in lung cancer. Eur Rev Med Pharmacol Sci.
17:1509–1516. 2013.PubMed/NCBI
|
|
26
|
Kefas B, Godlewski J, Comeau L, et al:
microRNA-7 inhibits the epidermal growth factor receptor and the
Akt pathway and is down-regulated in glioblastoma. Cancer Res.
68:3566–3572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhattacharya R, Nicoloso M, Arvizo R, et
al: MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer.
Cancer Res. 69:9090–9095. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonci D, Coppola V, Musumeci M, et al: The
miR-15a-miR-16-1 cluster controls prostate cancer by targeting
multiple oncogenic activities. Nat Med. 14:1271–1277. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma Q, Wang X, Li Z, et al: microRNA-16
represses colorectal cancer cell growth in vitro by
regulating the p53/survivin signaling pathway. Oncol Rep.
29:1652–1658. 2013.PubMed/NCBI
|
|
31
|
Lee ST, Chu K, Jung KH, et al: miR-206
regulates brain-derived neurotrophic factor in Alzheimer disease
model. Ann Neurol. 72:269–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mellios N, Huang HS, Grigorenko A, Rogaev
E and Akbarian S: A set of differentially expressed miRNAs,
including miR-30a-5p, act as post-transcriptional inhibitors of
BDNF in prefrontal cortex. Hum Mol Genet. 17:3030–3042. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mellios N, Huang HS, Baker SP, Galdzicka
M, Ginns E and Akbarian S: Molecular determinants of dysregulated
GABAergic gene expression in the prefrontal cortex of subjects with
schizophrenia. Biol Psychiatry. 65:1006–1014. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Imam JS, Plyler JR, Bansal H, et al:
Genomic loss of tumor suppressor miRNA-204 promotes cancer cell
migration and invasion by activating AKT/mTOR/Rac1 signaling and
actin reorganization. PLoS One. 7:e523972012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiang Q, Tang H, Yu J, Yin J, Yang X and
Lei X: MicroRNA-98 sensitizes cisplatin-resistant human lung
adenocarcinoma cells by up-regulation of HMGA2. Pharmazie.
68:274–281. 2013.PubMed/NCBI
|
|
36
|
Chauffert B, Dimanche-Boitrel MT, Garrido
C, et al: New insights into the kinetic resistance to anticancer
agents. Cytotechnology. 27:225–235. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu L, Lian J, Zhang H, et al:
MicroRNA-302a sensitizes testicular embryonal carcinoma cells to
cisplatin-induced cell death. J Cell Physiol. April 27–2013.(Epub
ahead of print). View Article : Google Scholar
|
|
38
|
Ryan J, Tivnan A, Fay J, et al:
MicroRNA-204 increases sensitivity of neuroblastoma cells to
cisplatin and is associated with a favourable clinical outcome. Br
J Cancer. 107:967–976. 2012. View Article : Google Scholar : PubMed/NCBI
|