Introduction
Gastric cancer (GC) is a world health burden and is
the second leading cause of cancer-related mortality worldwide,
despite improvements in prognosis as a result of early diagnosis
(1). Only slight progress has been
made in the treatment strategies for GC during the past 30 years
(2). Accumulating evidence in
recent years strongly indicates the existence of cancer stem cells
(CSCs) in solid tumours of a wide variety of organs, including GC
(3). Therefore, we searched for new
effective treatment methods for GC.
RegIV, a member of the regenerating gene family, is
involved in digestive tract malignancies, including the pancreas,
colorectum and stomach, as well as in benign diseases such as
ulcerative colitis (4–6). RegIV overexpression in tumour cells
has been associated with cell growth, survival, adhesion and
resistance to apoptosis and can even predict the intrinsic
chemoresistance in advanced GC (7).
A few research groups recently investigated its possible
applications for cancer biomarkers and acquired marked results.
RegIV was found in the serum of patients with GC which could
predict the peritoneal dissemination in gastric adenocarcinoma
(8–10). However, the relationship between
CSCs and RegIV in human GC has yet to be reported. Thus, a better
understanding of the relationship between CSC and RegIV may help to
improve early diagnosis and may aid in identifying a new molecular
therapeutic target for GC.
Materials and methods
Cells and animals
The human poorly differentiated GC cell line MKN45
was from the Cell Bank of the Chinese Academy of Sciences. Cells
were cultured in log growth phase in Dulbecco’s modified Eagle’s
medium (DMEM), supplemented with 10% heat-inactivated fetal calf
serum and 0.01 mg/ml bovine insulin at 37°C in a humidified
atmosphere of 5% CO2. We used 30 4-week-old Balb/cA
nu/nu female mice from the Shanghai Experimental Animal Centre of
the Chinese Academy of Sciences (Shanghai, China), they were
maintained in plastic cages (5 mice/cage) in a room with constant
temperature (22±1°C) with a dark-light cycle (12 h/12 h). Animal
experiments were performed in accordance with the ethics code by
the Ethics Committee of the Chongqing Medical University.
Cancer stem cell culture
To obtain CSCs and to propagate them as
mammospheres, cells floating in the supernatant of 4-day-old
cultures were collected by centrifugation for 5 min at 500 g,
washed in Hank’s buffered salt solution, and resuspended in phenol
red-free DMEM supplemented with 0.4% bovine serum albumin (BSA,
Sigma), 5 μg/ml bovine insulin, 20 ng/ml basic fibroblast growth
factor (bFGF, Sigma), 10 ng/ml epidermal growth factor (EGF, Sigma)
at a density of 1,000 cells/ml. Growth factors were added to the
mammosphere cultures every 3 days.
Flow cytometry of CD44, CD24 and CD133
expression
Expression of CD44, CD24 and CD133 was analysed in
cells derived from monolayer cultures or in 15-day-old mammospheres
following incubation in trypsin-EDTA dissociation with a Pasteur
pipette and passage through a 40-μm sieve. At least 105
cells were pelleted by centrifugation at 500 g for 5 min at 4°C,
resuspended in 10 μl of monoclonal mouse anti-human
CD44-phycoerythrin (PE) antibody, monoclonal mouse anti-human
CD24-PE antibody and a monoclonal mouse anti-human CD133-PE
antibody, respectively, and incubated for 20 min at 4°C.
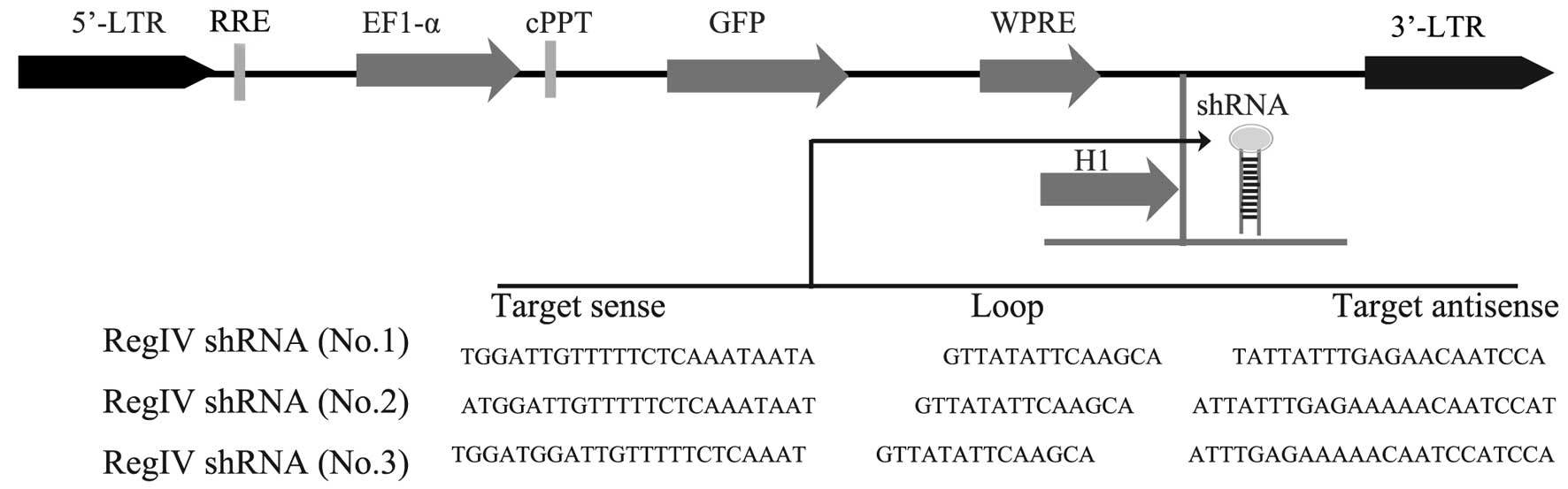
RegIV knockdown by lentivirus-mediated
short hairpin RNA
RegIV knockdown in mammospheres was performed by
infection with a lentivirus that expresses human RegIV-specific
short hairpin RNA (shRNA) and contains a green fluorescent protein
gene under a separate promoter for tracking the transfection
efficiency. Briefly, the lentivirus vector plasmid encoding human
RegIV-specific shRNA (Sigma-Aldrich) was transfected with capsule
and packaging plasmids using SuperFect (Qiagen, Valencia, CA, USA)
into HEK293T cells, and after 48 h, supernatant was collected and
used as infection solution without enrichment. The three
predesigned target sequences for human RegIV (GeneBank accession:
NM_001159352) are, 5′-TGGATTGTTTTTCTCAAA TAATA-3′,
5′-ATGGATTGTTTTTCTCAAATAAT-3′, and 5′-TGGATGGATTGTTTTTCTCAAAT-3′.
The following sequence was used in this experiment: 5′-TGGATTGTTT
TTCTCAAATA ATA-3′. The scramble shRNA obtained from Addgene
(Cambridge, MA, USA) was used as control. Forty-eight hours after
viral infection, RegIV knockdown was confirmed by real-time-PCR
analysis.
In vivo xenograft assay
Cells were derived from RegIV-KD or control
mammospheres by incubation in trypsin-EDTA dissociation with a
Pasteur pipette. We adjusted the concentration of the cell
suspension to be inoculated to 5×104/ml in PBS. Then,
0.2 ml of the cell suspension was subcutaneously injected in the
right hind limb of the mice, respectively. Fifteen mice were
injected in each group. Mice were observed daily and inspected for
tumour growth each week for 8 weeks.
Plate clone formation assays
RegIV-KD and control mammospheres were incubated in
trypsin-EDTA dissociation with a Pasteur pipette. They were seeded
at 1,000 cells in each 6-well plate and cultured in DMEM medium
containing 10% FCS for ~14 days. When most cell clones reached
>50 cells, they were fixed with 4% paraformaldehyde for 15 min
and stained with 1% crystal violet at room temperature. Each
experiment was repeated three times.
Drug sensitivity and apoptosis
analysis
The RegIV-KD and control mammospheres were used to
determine the cell growth inhibition ability of 5-FU and cisplatin.
Cells were re-inoculated into 96-well plates (5,000 cells/well) in
triplicate on the day prior to testing. Each well was supplemented
with medium containing 10% FCS, 20 ng/ml bFGF and 10 ng/ml EGF and
the next day, cells were incubated with a chemotherapy reagent 100
μM 5-fluorouracil (5-FU) and 100 μM of cisplatin (both
Sigma-Aldrich) or no drug as control. After 2 days, 20 μl of MTT
solution (5 mg/ml in PBS) was added to each well and cells were
incubated for 4 h at 37°C. Then, 50 μl DMSO was added to each well
and plates were incubated at 37°C overnight. The optical absorbance
at wavelength 450 nm was measured for the supernatant of each well
using the plate reader.
To determine the extent of cellular apoptosis
following drug treatments, both cells were plated into 6-well
plates (5×105 cells/well). After 24 h, the media was
removed and fresh media containing 100 μmol of 5-FU or cisplatin
were added. The cells were then stained with Annexin V and
propidium iodide (PI). Annexin V-FITC Apoptosis Detection kit used
in this experiment was purchased from Beyotime Biotechnology
(Beijing, China). The protocol supplied by the manufacturer was
strictly followed. Briefly, cells were trypsinized, washed twice
with cold PBS and pelleted by centrifugation at 800 rpm for 5 min.
The pellets were resuspended in 100 μl of 1X Annexin binding buffer
and 5 μl fluorescein isothiocyanate (FITC)-Annexin V. Propidium
iodide (100 μg/ml) was added to each 100 μl of cell suspension. The
stained cells were immediately analysed by flow cytometry.
Radioresistance experiments
In radioresistance experiments, the RegIV knockdown
and control mammospheres were inoculated into 6-well plates (100
cells/well) in triplicate on the day prior to testing. To mimic the
monolayer cultures, cells were plated in DMEM media containing 10%
FCS and irradiated with 2 and 4 Gy of radiation, respectively,
using Varian Clinac iX linear accelerator (Varian, Palo Alto, CA,
USA). The single cell gel electrophoresis (SCGE)/comet assay was
used for detecting DNA single strand breaks in both groups 2 h
after the irradiation. CASP image analysis system was adopted for
the quantitation of SCGE data by measuring the length of DNA
migration (Tail length). Generally, 30 randomly selected cells were
analysed per slide. The performance of the comet assay was mainly
based on the method described by Olive et al(11).
Real-time PCR
Total RNA was isolated using the TRIzol reagent
according to the manufacturer’s instructions. Quantitative
real-time RT-PCR was performed using the Maxima SYBR Green/ROX qPCR
Master Mix (2X) (both from Fermentas, Burlington, Canada).
Reactions were carried out using iCycler (Bio-Rad Laboratories,
Hercules, CA, USA) and the results were evaluated with the iCycler
Real-Time Detection System software. Relative quantitation of
target gene expression was evaluated by the comparative Ct
method.
Statistical analyses
All data are represented as means and differences of
the means with 95% confidence intervals (CIs). P-values ≤0.05,
calculated using a paired two-sided Student’s t-test, were
considered to indicate statistically significant differences.
Results
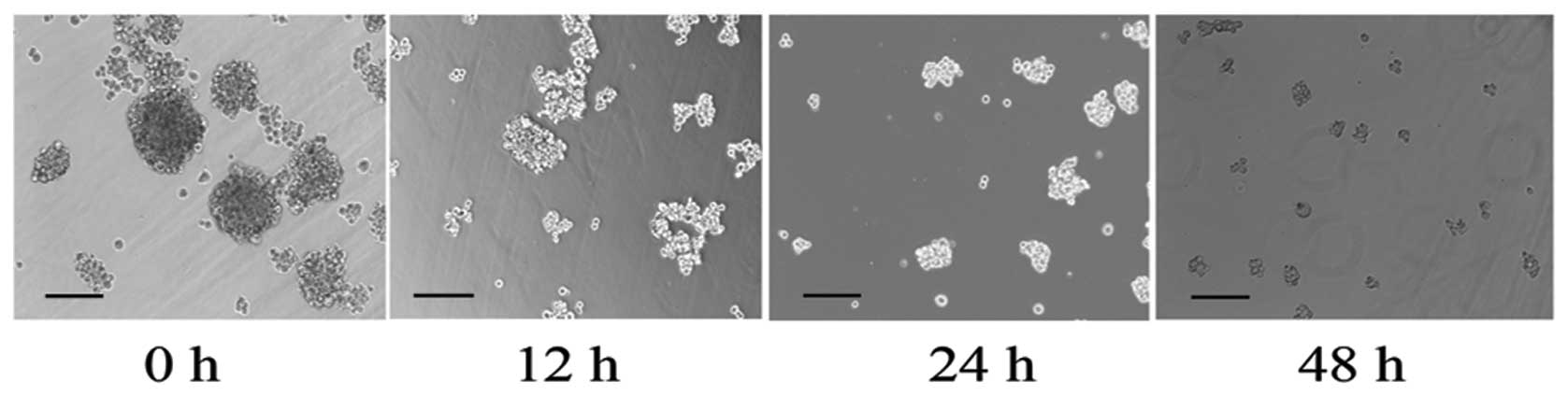
Growth factors induce MKN45 suspension
cells to propagate as mammospheres
Non-CSCs failed to proliferate under the growth
factors rich, low attachment and serum-free culture conditions.
Only CSCs could clonally proliferate to form suspension
mammospheres which was considered to be one of the most important
characteristic of CSCs (12).
Mammospheres formed in significant numbers in cells with growth
factor treatment for 15 days, while a few MKN45 cells formed sphere
bodies without growth factor treatment. Notably, those sphere
bodies could be gradually detached into monolayer culture cells in
2 days after RegIV knockdown, which indicated the loss of the
stemness capacity (Fig. 1).
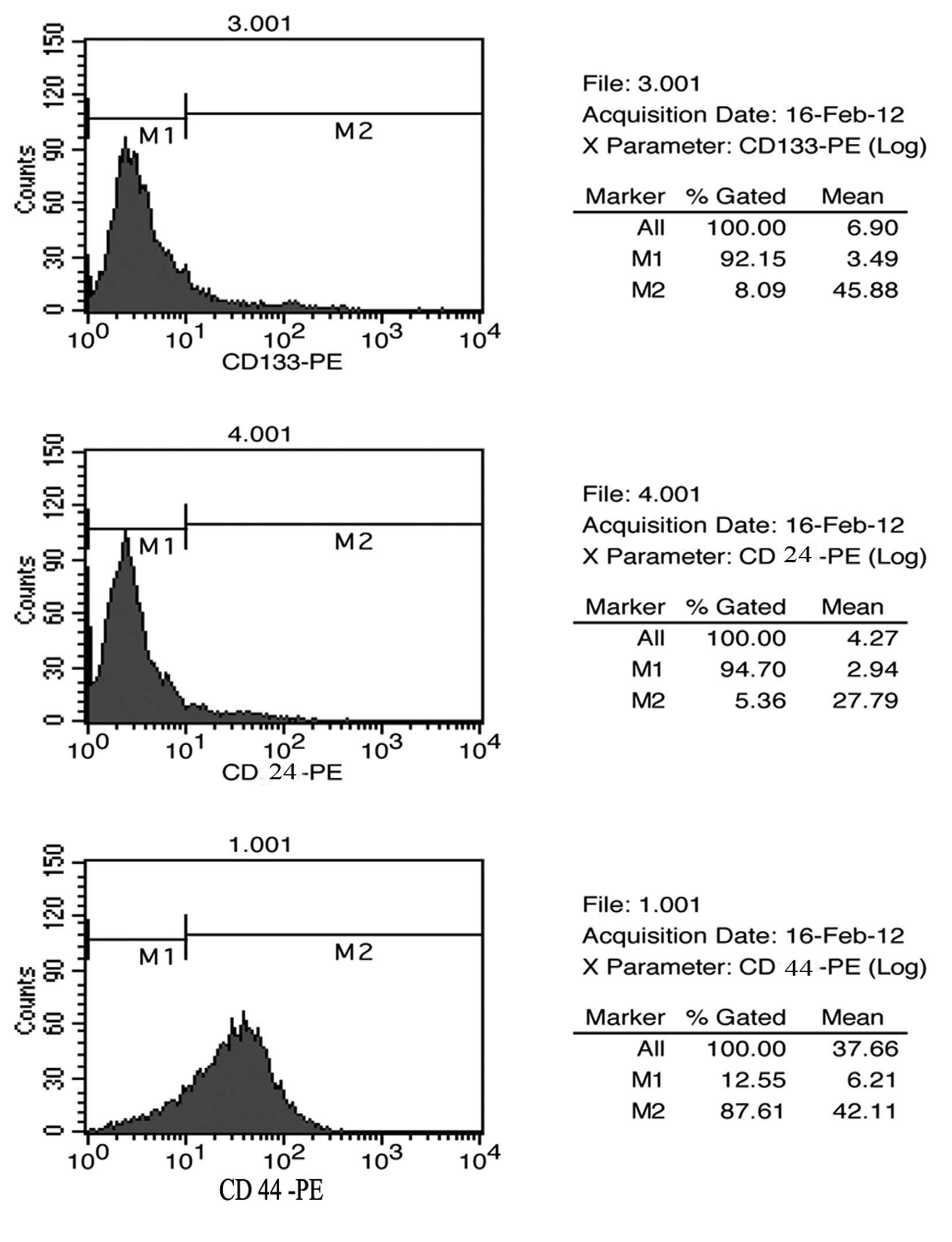
Surface marker expression profile in
mammospheres of MKN45
To further verify whether those mammospheres were
CSCs, we analysed the expression patterns of cell surface markers
for CSCs by using FACS for the mammospheres of human GC cell line
MKN45. Based on previous published reports regarding CSCs in solid
tumours, the following markers were studied: CD44, CD24 and CD133.
The results of the FACS studies for CD44, CD24 and CD133 are shown
in Fig. 2. Mammospheres of MKN45
showed a high level of expression of CD44 with up to 87% of cells
expressing CD44, while they showed as little as 5% expression of
CD24 and CD133, which is consistent with other verified reports
(13). Thus, we interpreted these
data by the fact that those mammospheres are suitable candidates
for CSCs of MKN45 cells.
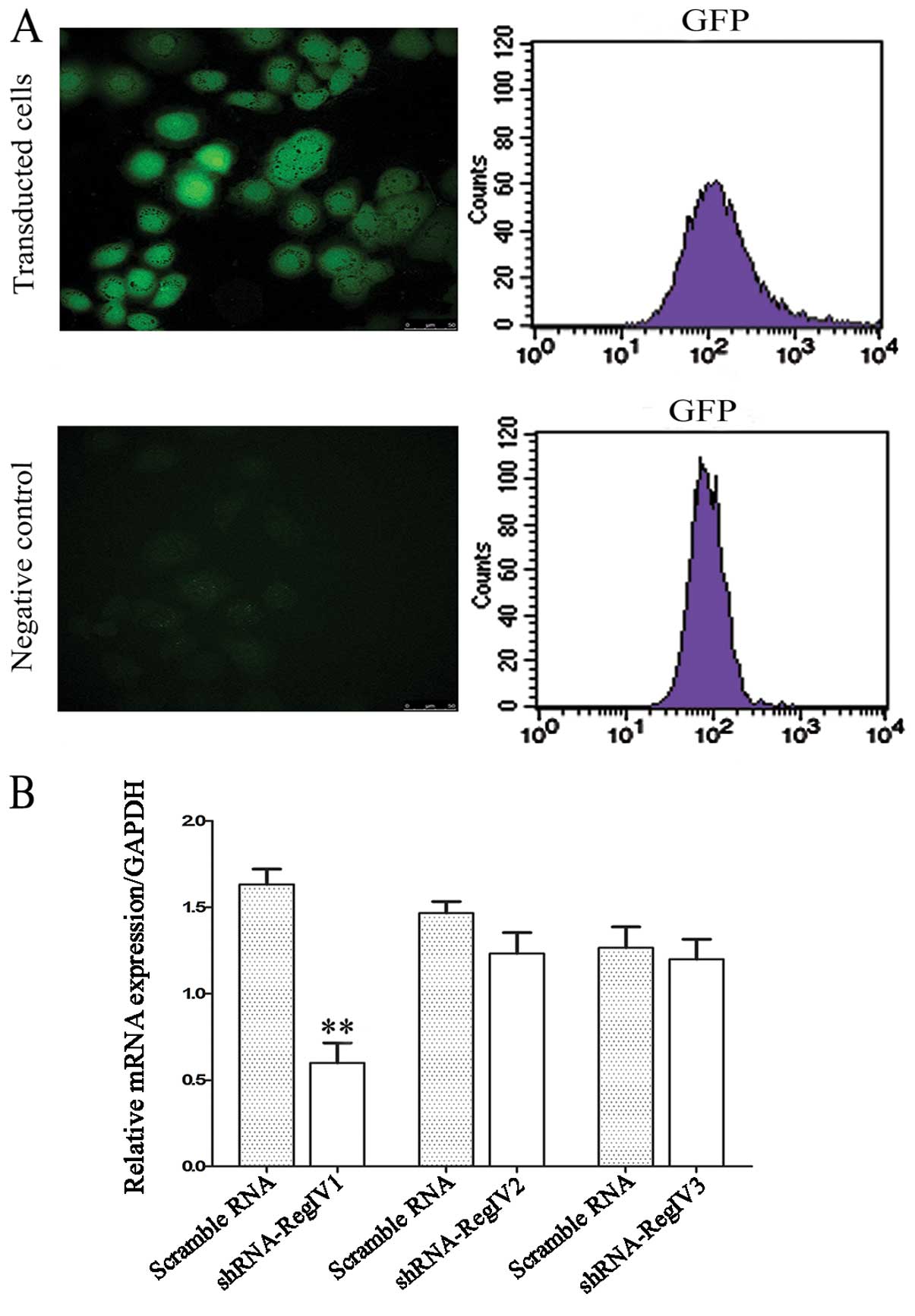
Validation of lentiviral shRNA constructs
for RegIV knockdown
Lentiviral constructs shRNA-RegIV (nos. 1–3) and
controls (scrambled sequence) were first examined in submerged
MKN45 mammosphere cultures. The vector also contains a human EF1-α
promoter driving the GFP marker gene for tracking transduced cells
(Fig. 3). Therefore, cells that
receive silencing constructs can be detected by fluorescence of GFP
at the single cell level. We confirmed the detection of GFP in the
mammospheres of MKN45 in 2 days of post-transfection under laser
confocal microscopy (Fig. 4A). The
real-time quantitative PCR analysis revealed a strong reduction of
RegIV at the mRNA level for designed shRNA (no. 1) when compared to
the scrambled control. The other two shRNA-RegIV constructs (nos.
2–3) showed no differences in mRNA expression levels when compared
to the scrambled control (Fig. 4B).
These data indicate that shRNA-RegIV1 plasmid is the most effective
construct in knockdown RegIV expression. Hence, the stable
transfected clone was used for further studies.
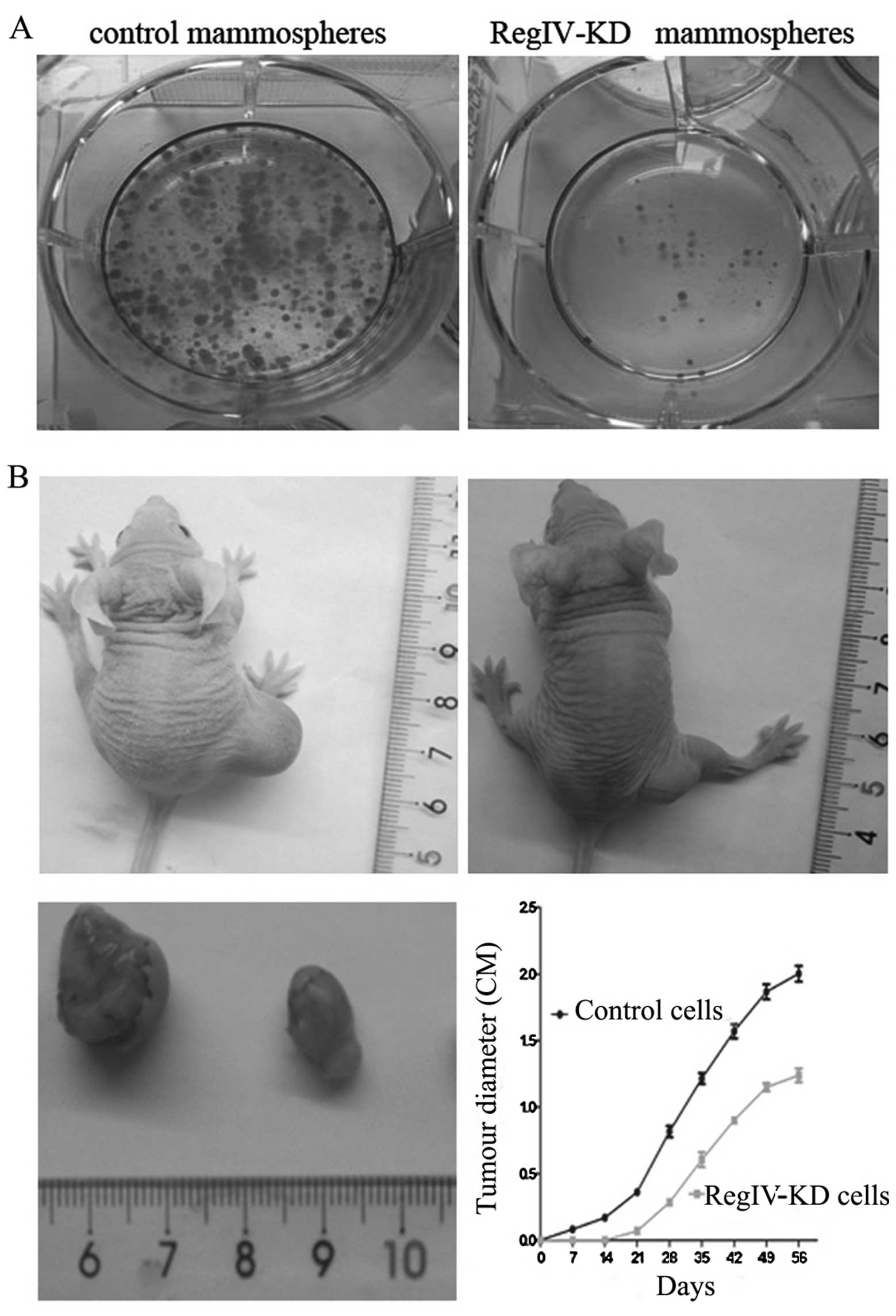
RegIV knockdown reduces tumourigenicity
and clone formation of MKN45 mammospheres in vivo and in vitro
With serum stimulation, cells from control
mammospheres showed higher clone formation ability than RegIV-KD
cells and significantly increased rate of clone efficiency
(Fig. 5A).
To verify RegIV-KD in MKN45 CSCs may have a
significant role in supporting tumourigenicity in vivo, we
injected these RegIV-KD cells subcutaneously in nude mice. We
observed that RegIV-KD cells produced fewer (4/15) and much smaller
tumours than those from control mammospheres (13/15) (Fig. 5B). The tumour diameter was monitored
every week up to 8 weeks. In the experiment, control mammospheres
generated tumours of greater volume, formed measurable tumour
masses in animals in the first week of post-injections (Fig. 5B).
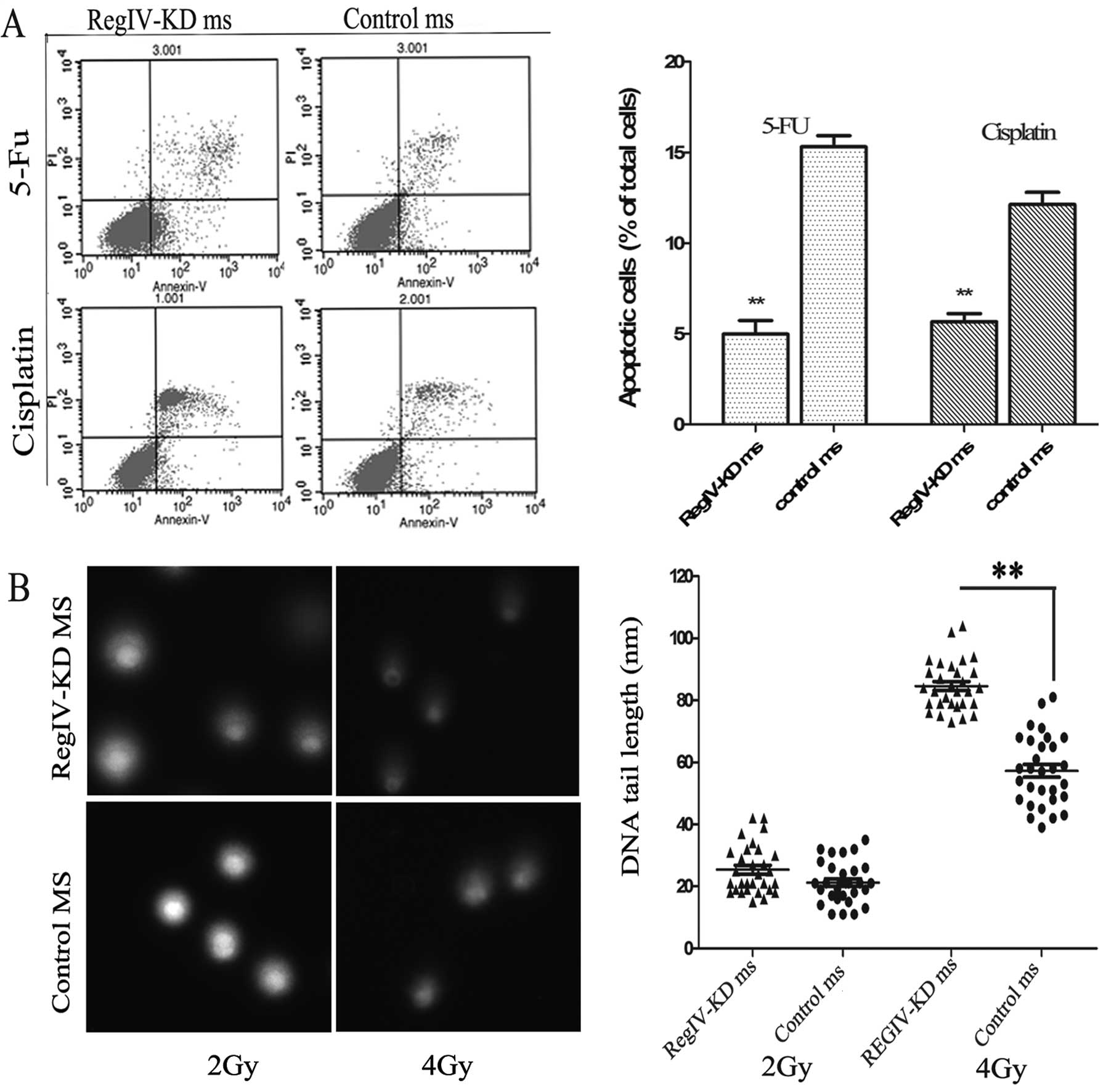
RegIV knockdown enhances
chemoradiosensitivity and apoptosis in MKN45 mammospheres
The ability of chemo-reagent to inhibit the growth
of RegIV-KD and control mammospheres was assessed by cell viability
and apoptosis assay. It was observed that RegIV-KD mammospheres
significantly decreased cell viability to 33% (5-FU) and 44%
(cisplatin) as compared to control mammospheres. Similar results
were obtained in the Annexin V cell death assay wherein RegIV-KD
results in significantly higher cell death as compared to control
(Fig. 6A).
To assess the radiosensitivity of the RegIV-KD
cells, the comet assay was performed on the irradiated tumour
cells. RegIV-KD cells showed a higher percentage of DNA in the tail
when compared to the control, suggesting that the DNA damage was
higher in radiosensitive RegIV knockdown MKN45 cells (Fig. 6B).
Discussion
Although the biological function of RegIV is poorly
understood, it has been reported that RegIV may function as a
growth and antiapoptotic factor in colon and gastric cancer
(6,14,15).
According to previous literature, RegIV expression in different
cell types is associated with regeneration, survival and migration
(6,16,17).
RegIV is systematically overexpressed in colon, pancreatic and
gastric cancer (GC) and in diseases that predispose to colon cancer
such as ulcerative colitis (5,18,19).
However, the role RegIV plays in CSCs has not been fully
elucidated. We demonstrated for the first time that the knockdown
expression of RegIV deprived CSCs of their stemness properties by a
series of experiments, including chemoradioresistance in
vitro and xenograft assay in vivo. Other studies
confirmed RegIV expression in gastric, colorectal and pancreatic
carcinoma, and that RegIV has a potential role in diagnosing
digestive tract neuroendocrine tumours (20–22).
Gastric cancer stem cells with overexpressing RegIV protein grew
more rapidly and were more resistant to 5-FU and cisplatin
treatment. Furthermore, previous studies had shown that RegIV
overexpression was thought to be chemoresistant in GC patients
(7,23). Furthermore, RegIV was recently
reported to be an important target gene of GLI1 (24). Thus, we concluded that the RegIV
plays a key role for maintaining the stemness properties of CSCs
and the SHH-GLI1-RegIV signal cascade may be involved.
CSCs use multiple signalling pathways to control
self-renewal and differentiation (25,26).
Misregulation of these pathways may lead to the loss of CSC
properties. Numerous signalling pathways have been implicated in
this process including Wnt, Notch, EGF, PTEN and SHH (27–31).
Furthermore, frequent misregulation of crucial embryonic signalling
pathways (i.e., the Hedgehog signalling pathway) contribute to the
process of gastric carcinogenesis (32). The Hedgehog signal is transmitted by
transmembrane protein Smoothened (SMO) through binding to a second
receptor Patched (Ptc) in extracellular and terminated in
intracellular Hedgehog signal transduction via Hedgehog
transcription factors that are glioblastoma factors GLI1, GLI2 and
GLI3.
In conclusion, this is the first report to
demonstrate RegIV was able to manipulate the stemness properties of
CSCs in GC cells. Our study contributes to the body of research on
gastric carcinogenesis and provides insight into the possible
network of signalling pathways through the GLI1/RegIV axis. It may
also help to provide new insight into treatment strategies for GC.
Further studies are required to determine whether the biological
behaviour of GC patients may be achieved by regulating RegIV.
Acknowledgements
The authors thank Jian-Ye Xu for his technical
assistance and valuable discussion of the experiments.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Bianchi A and Espin F: Where are we in the
treatment of gastric cancer? Experiences and evidences 30 years
later. Med Clin (Barc). 140:307–309. 2012.(In Spanish).
|
|
3
|
Zhang CJ, Li CW, Yang H and He FT: Cancer
stem cells in gastric cancer. Zhongguo Shengwu Huaxue Yu Fenzi
Shengwu Xuebao. 25:889–895. 2009.(In Chinese).
|
|
4
|
Kelleher FC: Hedgehog signaling and
therapeutics in pancreatic cancer. Carcinogenesis. 32:445–451.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Granlund AvB, Beisvag V, Torp SH, et al:
Activation of REG family proteins in colitis. Scand J
Gastroenterol. 46:1316–1323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo Q, Bishnupuri KS, Houchen C, Anant S
and Dieckgraefe BK: Silencing of the regenerating IV (Reg IV) gene
by siRNA inhibits the growth and proliferation of human colon
adenocarcinomas. Gastroenterology. 130:A5332006.
|
|
7
|
Ying LS, Yu JL, Lu XX and Ling ZQ:
Enhanced RegIV expression predicts the intrinsic
5-fluorouracil (5-FU) resistance in advanced gastric cancer. Dig
Dis Sci. 58:414–422. 2012.
|
|
8
|
Moon J-H, Fujiwara Y, Nakamura Y, et al:
REGIV as a potential biomarker for peritoneal dissemination in
gastric adenocarcinoma. J Surg Oncol. 105:189–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyagawa K, Sakakura C, Nakashima S, et
al: Overexpression of RegIV in peritoneal dissemination of
gastric cancer and its potential as a novel marker for the
detection of peritoneal micrometastasis. Anticancer Res.
28:1169–1179. 2008.
|
|
10
|
Kuniyasu H, Oue N, Sasahira T, et al:
Reg IV enhances peritoneal metastasis in gastric carcinomas.
Cell Prolif. 42:110–121. 2009. View Article : Google Scholar
|
|
11
|
Olive PL, Banath JP and Durand RE:
Heterogeneity in radiation-induced DNA damage and repair in tumor
and normal cells measured using the ‘comet’ assay. Radiat Res.
178:AV35–AV42. 2012.
|
|
12
|
Gilbert CA and Ross AH: Cancer stem cells:
cell culture, markers, and targets for new therapies. J Cell
Biochem. 108:1031–1038. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takaishi S, Okumura T, Tu S, et al:
Isolation of gastric cancer-initiating cells using cell surface
marker CD44. Gastroenterology. 132:A632–A633. 2007.
|
|
14
|
Yasui W, Sentani K, Motoshita J and
Nakayama H: Molecular pathobiology of gastric cancer. Scand J Surg.
95:225–231. 2006.
|
|
15
|
Kadowaki Y, Ishihara S, Miyaoka Y, et al:
Reg protein is overexpressed in gastric cancer cells, where it
activates a signal transduction pathway that converges on ERK1/2 to
stimulate growth. FEBS Lett. 530:59–64. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo Y, Xu J, Li N, Gao F and Huang P:
RegIV potentiates colorectal carcinoma cell migration and
invasion via its CRD domain. Cancer Genet Cytogenet. 199:38–44.
2010. View Article : Google Scholar
|
|
17
|
Kuniyasu H, Kitadai Y, Oue N, Sasahira T,
Ohmori H and Yasui W: Increased peritoneal metastasis of reg
IV-transfected MKN28 gastric carcinoma cells. Proceedings of the
American Association for Cancer Research Annual Meeting.
48:11092007.
|
|
18
|
Numata M, Oshima T, Yoshihara K, et al:
Relationship between RegIV gene expression to outcomes in
colorectal cancer. J Surg Oncol. 104:205–209. 2011.
|
|
19
|
Oue N, Hamai Y, Mitani Y, et al: Gene
expression profile of gastric carcinoma: identification of genes
and tags potentially involved in invasion, metastasis, and
carcinogenesis by serial analysis of gene expression. Cancer Res.
64:2397–2405. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li FY, Ren XB, Xu EP, et al: RegIV
expression showing specificity to gastrointestinal tract and its
potential role in diagnosing digestive tract neuroendocrine tumor.
J Zhejiang Univ Sci B. 11:258–266. 2010. View Article : Google Scholar
|
|
21
|
Heiskala K, Arola J and Andersson L:
Expression of RegIV and Hath1 in neuroendocrine tumors. Virchows
Archiv. 452:S75–S76. 2008.
|
|
22
|
Lee JY, Eom EM, Kim DS, Ha-Lee YM and Lee
DH: Analysis of gene expression profiles of gastric normal and
cancer tissues by SAGE. Genomics. 82:78–85. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vanderlaag K, Wang W, Fayadat-Dilman L, et
al: Regenerating islet-derived family member, 4 modulates multiple
receptor tyrosine kinases and mediators of drug resistance in
cancer. Int J Cancer. 130:1251–1263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang F, Xu L, Guo C, et al: Identification
of RegIV as a novel GLI1 target gene in human pancreatic cancer.
PLoS One. 6:e184342011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hambardzumyan D, Becher OJ and Holland EC:
Cancer stem cells and survival pathways. Cell Cycle. 7:1371–1378.
2008. View Article : Google Scholar
|
|
26
|
Suda T, Arai F and Hirao A: Hematopoietic
stem cells and their niche. Trends Immunol. 26:426–433. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spillane JB and Henderson MA: Cancer stem
cells: a review. ANZ J Surg. 77:464–468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moon SH, Kim DK, Cha Y, Jeon I, Song J and
Park KS: PI3K/Akt and Stat3 signaling regulated by PTEN
control of the cancer stem cell population, proliferation and
senescence in a glioblastoma cell line. Int J Oncol. 42:921–928.
2013.PubMed/NCBI
|
|
29
|
Lee SH, Hong HS, Liu ZX, et al: TNFα
enhances cancer stem cell-like phenotype via Notch-Hes1 activation
in oral squamous cell carcinoma cells. Biochem Biophys Res Commun.
424:58–64. 2012.
|
|
30
|
Rodova M, Fu J, Watkins DN, Srivastava RK
and Shankar S: Sonic hedgehog signaling inhibition provides
opportunities for targeted therapy by sulforaphane in regulating
pancreatic cancer stem cell self-renewal. PLoS One. 7:e460832012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Merchant AA and Matsui W: Targeting
Hedgehog - a cancer stem cell pathway. Clin Cancer Res.
16:3130–3140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin J, Donnelly JM, Houghton J and
Zavros Y: The role of sonic hedgehog reemergence during gastric
cancer. Dig Dis Sci. 55:1516–1524. 2010. View Article : Google Scholar : PubMed/NCBI
|