Introduction
Liposarcoma (LPS) is one the most common sarcomas in
adulthood. It is divided into different histotypes with different
biological characteristics and clinical behavior. Thus, the correct
classification is required for the prognostic stratification of
patients and proper therapeutic approach. Well-differentiated LPSs
(WDLPSs) (40–45% of all LPSs) tend to recur locally but do not
metastasize, while the myxoid LPSs (MLPSs), if associated with
higher hypercellularity, have a poor prognosis (1,2). LPSs
are associated with a variety of molecular and genetic alterations
that focus primarily on the short arm of chromosome 12 (3).
These alterations include translocations t(12;16)
(q13;p11) and t(12;22) (q13;q12), which were essentially found in
MLPS, and amplification of chromosomal region 12q13-15, associated
with atypical lipomatous tumors\WDLPSs and dedifferentiated LPSs
(DDLPS) (2,3).
The chromosomal region 12q13-15 contains ~164 genes.
As shown by immunohistochemistry and quantitative RT-PCR analyses,
some of these genes are systematically overexpressed in WDLPSs and
DDLPSs. In particular, MDM2 (12q15) and CDK4 (12q14.1) are
consistently amplified and overexpressed in WDLPS/DDLPS (4,7).
In this region, there are also other genes whose
function has been associated with carcinogenesis, including CHOP
(DDIT3), SAS and HMGA2, frequently rearranged/overexpressed in
human sarcomas (3,5–7), an
entire cluster of basic cytokeratins and the HOX C locus genes
(8).
Homeobox genes are transcription factors that
function during normal development (9) and contain the homeobox, a 183-bp DNA
sequence coding for a 61-amino acid homeodomain. In mice (hox
genes) and humans (HOX genes) there are 39 genes organized into
four genomic clusters of ~100 kb in length, defined as HOX loci,
each localized on a different chromosome (HOX A at 7p15.3, HOX B at
17p21.3, HOX C at 12q13.3 and HOX D at 2q31) (10).
Numerous studies associate abnormal expression of
HOX genes to the development of various types of human cancer
(11–21). In particular, several genes of the
HOX C locus, are frequently overexpressed in several neoplasia
(22–27).
Preliminary data, carried out on a multitumor tissue
array to investigate HOXC13 distribution on several types of human
cancer (unpublished data), showed the aberrant expression of HOXC13
protein in a small series of LPSs. In the present study, we
evaluated HOXC13 expression in a whole spectrum of adipocytic
tumors, including lipomas, WDLPSs, DDLPSs, pleomorphic (PLPS) and
MLPSs, associating this analysis to the evaluation of
amplification/translocation status of chromosomal region
12q13-15.
Materials and methods
Patients and specimens
Histological blocks and fresh cryostored tissues of
57 patients with WDLPSs, DDLPSs, MLPSs, PLPSs and lipomas, were
selected from the files of the Pathology Unit of the National
Cancer Institute Fondazione ‘G. Pascale’ of Naples. All patients
were Caucasians and all provided written informed consent according
to the institutional regulations.
This study was approved by the Ethics Committee of
the National Cancer Institute ‘G. Pascale’. All diagnoses were
established according to the World Health Organization
Classification of Tumors (28).
Medical records were reviewed for clinical
information. In addition, all cases were reviewed by expert
pathologists (A.D.C. and G.B.), in order to confirm the
diagnosis.
TMA building
Fifty-seven tissue samples were used for a tissue
microarray (TMA) building, using the most representative areas from
each single case. Discrepancies between two pathologists for the
same case were resolved with a joint analysis. Tissue cylinders
with a diameter of 0.6 mm were punched from morphologically
representative tissue areas of each donor tissue block and brought
into one recipient paraffin block (3×2.5 cm) using a semiautomatic
tissue arrayer (Galileo TMA).
Immunohistochemistry
Immunohistochemical staining was carried out on TMA
slides to evaluate the expression of HOXC13 marker. Paraffin slides
were then deparaffinized in xylene and rehydrated through graded
alcohols. Antigen retrieval was performed by microwave pretreatment
in 0.01 M citrate buffer for 10 min. After protein block (BSA 5% in
1X PBS), the slides were incubated with primary antibody to human
HOXC13 (cod. ab55251, dilution 1:1,200; Abcam, Cambridge, UK)
overnight. Sections were incubated with mouse anti-rabbit or goat
anti-mouse secondary IgG biotinylated secondary antibody for 30
min. Immunoreactivity was visualized by means of
avidin-biotin-peroxydase complex kit reagents (Novocastra,
Newcastle, UK) as the chromogenic substrate. Finally, sections were
weakly counterstained with hematoxylin and mounted. Human hair
follicles were used as positive controls. Irrelevant rabbit or
mouse IgG antibodies were applied to negative control. Results were
interpreted using a light microscope by 2 investigators (R.F. and
A.D.C.).
For HOXC13, cytoplasmic and membrane staining were
considered. Tissues were scored semi-quantitatively by evaluating
the proportion of positive tumor cells over the total number of
tumor cells (percentage of positive tumor cells per tissue
microarray punch). Negative (score 0), low expression cases, and
high expression cases were recorded when neoplastic cells
expressing HOXC13 were comprised between 0 and 10% (score 1+),
<30% (score 2+) and >30% (score 3+), respectively.
RNA extraction from fresh and
paraffin-embedded tissues
The sections obtained from paraffin-embedded samples
were incubated at 37°C in the presence of xylene for ~20 min. Total
RNA was purified using High Pure FFPE RNA Micro kit (Roche)
following the manufacturer’s instructions. Total RNA was isolated
from fresh tissues, using RNeasy Mini kit (Qiagen GmbH, Hilden,
Germany) following the manufacturer’s instructions. All samples
were treated with RNase-free DNase (Qiagen GmbH) to prevent
amplification of genomic DNA. A total of 1 μg RNA was subjected to
cDNA synthesis for 1 h at 37°C using the Ready-To-Go You-Prime
First-Strand Beads kit (cod. 27-9264-01; Amersham Biosciences
Europe Gmbh, Freiburg, Germany) in a reaction mixture containing
0.5 μg random hexamers (GeneAmp RNA PCR Random Hexamers Set
N808-0127; Applied Biosystems, Foster City, CA, USA).
Real-time PCR
Quantitative RT-PCR was performed in a LightCycler
system (Roche Molecular Biochemicals, Mannheim, Germany) using
TaqMan® analysis. All reactions were run in glass
capillaries with the LightCycler TaqMan Master Mix (cod.
04735536001; Roche Molecular Biochemicals), 10 μl, in a volume of
20 μl containing 2 μl of cDNA and 1 μl of specific TaqMan Gene
Expression Assays for human HOXC13 (Real-Time Designer Assay cod.
04162498001; Roche Molecular Biochemicals), according to the
manufacturer’s instructions. All reactions were performed in
triplicate. The thermal cycling conditions included a step of 20
sec at 95°C followed by 40 cycles of 95°C for 1 sec and 60°C for 20
sec. The comparative Ct method was employed to determine
the human HOXC13 gene variation, using as reference gene TaqMan
Endogenous Controls Human ACTB (β-actin) Endogenous Control
(Real-Time Designer Assay cod. 05532957001; Roche Molecular
Biochemicals). We identified a calibrator cell line that represents
the unitary amount of the target of interest and, consequently, the
samples express n-fold mRNA relative to the calibrator. Final
amounts of target were determined as follows: Target amount = 2-Ct,
where Ct = [Ct (HOXC13) − Ct (ACTB)]sample − [Ct
(HOXC13) − Ct (ACTB)]calibrator.
FISH analysis
TMA paraffin block sections cut at 4 μm were mounted
on Superfrost/Plus microscope slides (Fisher Scientific,
Pittsburgh, PA, USA). Slides were deparaffinized in xylene,
dehydrated in 100% ethanol and then allowed to dry. They were
placed in pre-treatment solution (Vysis) at 80°C for 10 min,
followed by a rinse in purified water for 3 min. The slides were
digested at 37°C for 15 min in 62.5 ml of 0.2 N HCl containing 250
mg protease (2,500–3,000 U/mg; Vysis) and rinsed in purified water
for 3 min. The slides were then dehydrated through a series of
graded ethanol solutions for 1 min each and allowed to air dry. For
the cytogenetic investigation, we used the LSI CHOP Dual
Color Break Apart Rearrangement Probe that contains a Spectrum
Orange-labeled probe that spans a 700-kb region just centromeric of
the CHOP (DDIT3) gene, and a Spectrum Green-labeled
probe that spans a 660-kb region just telomeric of the CHOP
(DDIT3) gene. Hybridization was performed by placing the
slides in a humidified chamber at 37°C for overnight incubation.
Following hybridization, rubber cement and coverslips were removed.
Slides were treated in a posthybridization wash of 2X SSC
containing 0.3% Nonidet P-40 at 73°C for 2 min and then transferred
to ambient temperature 2X SSC/0.3% Nonidet P-40 for 5–60 sec.
Slides were air dried and coverslip mounted with
4′-6-diamidino-2-phenylindole (DAPI, Vector Laboratories,
Burlingame, CA, USA) nuclear counterstain. The sections were viewed
using an Olympus BX41 (Melville, NY, USA) fluorescent microscope
with a dual orange/green filter and were interpreted by 2
investigators (R.F. and G.A.).
The presence of 2 fusion signals/nucleus indicated
an intact CHOP (DDIT3) gene. The presence of a single
orange and single green signal indicated a rearranged CHOP
(DDIT3) gene. Break apart with translocation of the
CHOP (DDIT3) gene was observed in all evaluable
MLPSs.
Statistical analysis
The association between HOXC13 expression with other
clinicopathological parameters was conducted using the
χ2 and Student’s t-test.
Pearson’s χ2 test was used to determine
whether a relationship exists between the variables included in the
study. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were carried out
using the Statistical Package for Social Sciences 8.0 software
(SPSS Inc., Chicago, IL, USA).
Results
Clinicopathological characteristics of
LPS tumors
The main clinical and pathological data are reported
in Table I. In our histological
samples, there were 18 WDLPSs, 9 DDLPSs, 11 MLPSs, 6 PLPSs and 13
lipomas. Twenty five of the 57 patients (43%) were female. The age
of the patients ranged from 17 to 91 years, with an average of 57
years. Three samples are represented by recurrences from the same
patients. Of the 57 samples, 12 (21%) were retroperitoneal, 33
(57%) were thigh location, 2 (3%) were shoulder location and 2 (3%)
were subclavicular location, while of the remaining samples, one
was hypogastric region, one gluteus, one forearm, one arm, one
dorsum hand, one dorsum foot, one vulva region and one neck
location.
 | Table IClinicopathological characteristics
of liposarcoma patients and tumors with respect to 12q13-15
chromosomal rearrangement and HOXC13 expression. |
Table I
Clinicopathological characteristics
of liposarcoma patients and tumors with respect to 12q13-15
chromosomal rearrangement and HOXC13 expression.
| Case no. | Gender/Age | Histological
subtype | Primary
(P)/Recurrence (R) | Location | Chr.12q13-15
rearrangement | HOXC13 score |
|---|
| 1 | M/44 | WDLPS | P | Left thigh | Green ampl | 3+ |
| 2 | F/66 | WDLPS | P | Right thigh | Green ampl | 3+ |
| 3 | M/65 | WDLPS | P |
Retroperitoneal | Green/orange
ampl | 3+ |
| 4 | F/35 | WDLPS | P | Right thigh | Green ampl. | 2+ |
| 5 | M/49 | WDLPS | P | Right thigh | Green/orange
ampl | Not evaluable |
| 6 | M/61 | WDLPS | P |
Retroperitoneal | Green ampl | 3+ |
| 7a | M/82 | WDLPS | P | Left thigh | Green ampl | 2+ |
| 7b | M/82 | WDLPS | R | Left thigh | Green ampl | 2+ |
| 8 | M/75 | WDLPS | P |
Retroperitoneal | Green ampl | 2+ |
| 9 | M/39 | WDLPS | P | Left thigh | Green/orange
ampl | 3+ |
| 10a | M/91 | WDLPS | P | Left thigh | Green ampl | 3+ |
| 10b | M/91 | WDLPS | R | Left thigh | Green/orange
ampl | 1+ |
| 11 | M/43 | WDLPS | P | Left thigh | Green/orange
ampl | 3+ |
| 12 | F/39 | WDLPS | P | Right thigh | Green ampl | 2+ |
| 13 | M/69 | WDLPS | P | Right thigh | Green ampl | 3+ |
| 14 | F/46 | WDLPS | P | Left thigh | Green ampl | 3+ |
| 15 | F/41 | WDLPS | P | Left thigh | Green ampl | 3+ |
| 16 | F/65 | WDLPS | P |
Retroperitoneal | Green ampl | 3+ |
| 17 | F/65 | DDLPS | P |
Retroperitoneal | Not evaluable | 1+ |
| 18 | M/75 | DDLPS | P |
Retroperitoneal | Green/orange
ampl | 2+ |
| 19 | M/62 | DDLPS | P |
Retroperitoneal | Green/orange
ampl | 3+ |
| 20 | M/53 | DDLPS | P |
Retroperitoneal | Green ampl | 1+ |
| 21 | F/90 | DDLPS | P | Left thigh | Green ampl | 3+ |
| 22a | F/78 | DDLPS | P |
Retroperitoneal | Green ampl | 3+ |
| 22b | F/78 | DDLPS | R |
Retroperitoneal | Green ampl | 2+ |
| 23 | M/60 | DDLPS | P |
Retroperitoneal | Green ampl | 2+ |
| 24 | M/69 | DDLPS | P | Hypogastric
region | Green ampl | 3+ |
| 25 | F/51 | MLPS | P | Right thigh | Translocation | 0 |
| 26 | M/39 | MLPS | P | Left thigh | Translocation | 0 |
| 27 | F/37 | MLPS | P | Right thigh | Translocation | 0 |
| 28 | M/66 | MLPS | P | Right thigh | Translocation | 1+ |
| 29 | M/48 | MLPS | P | Left thigh | Translocation | 0 |
| 30 | F/42 | MLPS | P | Right thigh | Translocation | 0 |
| 31 | F/51 | MLPS | P | Right thigh | Translocation | 0 |
| 32 | M/34 | MLPS | P | Right thigh | Translocation | 0 |
| 33 | M/62 | MLPS | P | Left gluteus | Translocation | 0 |
| 34 | F/49 | MLPS | P | Right thigh | Translocation | 0 |
| 35 | F/43 | MLPS | P | Left thigh | Translocation | 0 |
| 36 | M/61 | PLPS | P | Right thigh | Green/orange
ampl | 1+ |
| 37 | M/62 | PLPS | P | Left thigh | No | 0 |
| 38 | M/48 | PLPS | P | Right thigh | No | 0 |
| 39 | F/52 | PLPS | P | Left forearm | No | 1+ |
| 40 | M/51 | PLPS | P |
Retroperitoneal | No | 0 |
| 41 | M/76 | PLPS | P | Left thigh | No | 0 |
| 42 | F/57 | Lipoma | | Left shoulder | No | 0 |
| 43 | M/45 | Lipoma | | Right thigh | Not evaluable | 0 |
| 44 | F/66 | Lipoma | | Right arm | No | 0 |
| 45 | M/62 | Lipoma | | Left thigh | No | 0 |
| 46 | F/60 | Lipoma | | Left dorsum
hand | No | 0 |
| 47 | M/47 | Lipoma | | Right
subclavicular | No | 0 |
| 48 | F/46 | Lipoma | | Left
subclavicular | No | 0 |
| 49 | F/60 | Lipoma | | Left vulva | No | 0 |
| 50 | M/66 | Lipoma | | Neck | No | 0 |
| 51 | F/17 | Lipoma | | Left thigh | No | 0 |
| 52 | F/50 | Lipoma | | Right shoulder | No | 0 |
| 53 | M/33 | Lipoma | | Left thigh | No | 0 |
| 54 | F/66 | Lipoma | | Right dorsum
foot | No | 0 |
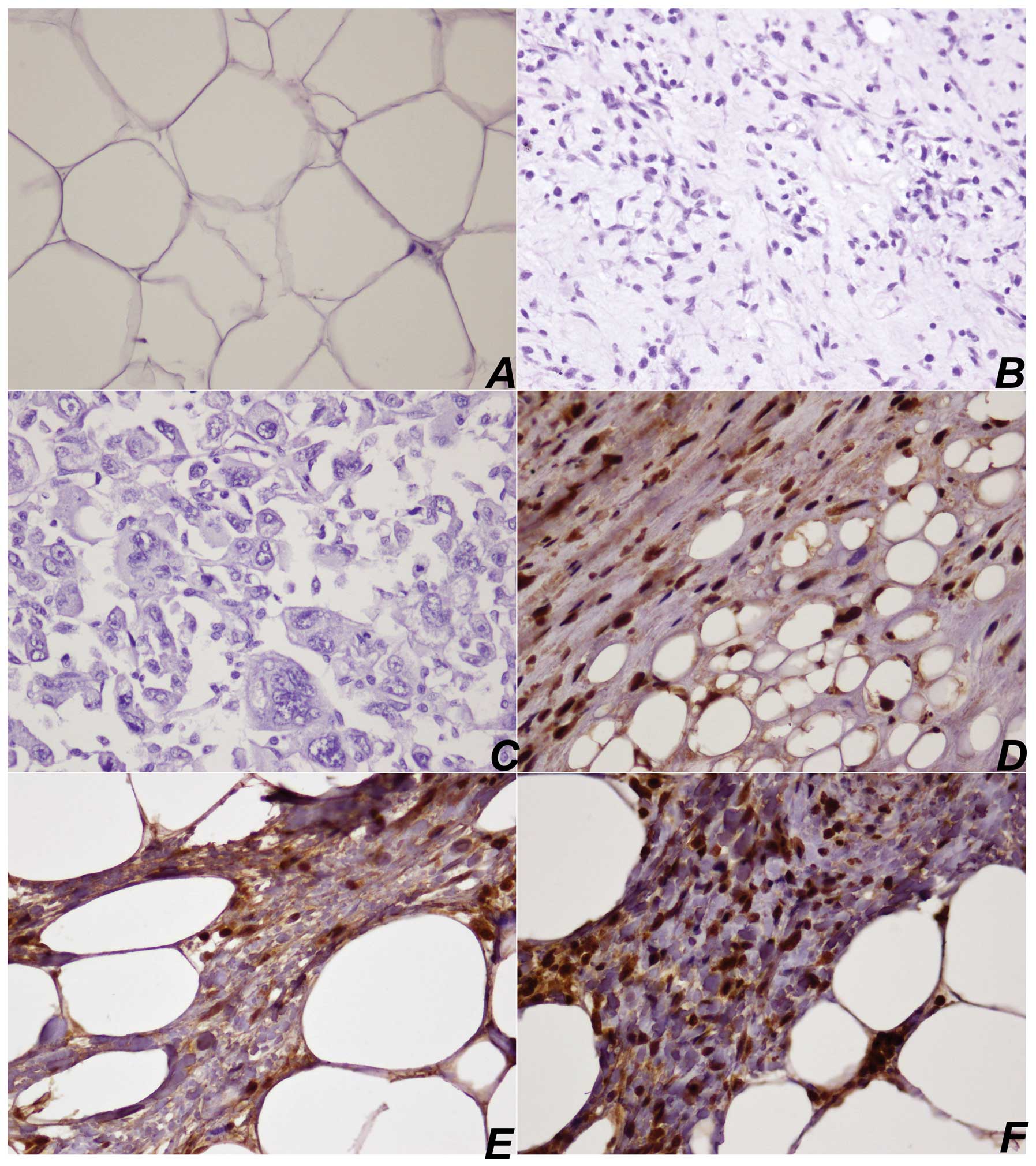
Expression of HOXC13 protein in LPS
tissue microarray
Immunohistochemical detection of HOXC13 protein in
11/18 (61%) WDLPSs was scored as 3+, in 5/18 (27%) WDLPSs as 2+,
while in only 1 case as 1+. In DDLPSs, HOXC13 was scored as 3+ in
4/9 (44%) samples, 2+ in 3/9 (33%) samples and 1+ in 2/9 (22%)
tissues. In these cases, nuclear expression was observed in the
nucleus of both neoplastic adipocyte-like cells and lipoblast. In
MLPSs, there was only 1 sample with score 1+, while in PLPSs, 2
samples scored 1+. In all lipoma HOXC13 samples expression was
absent (Fig. 1, Table I).
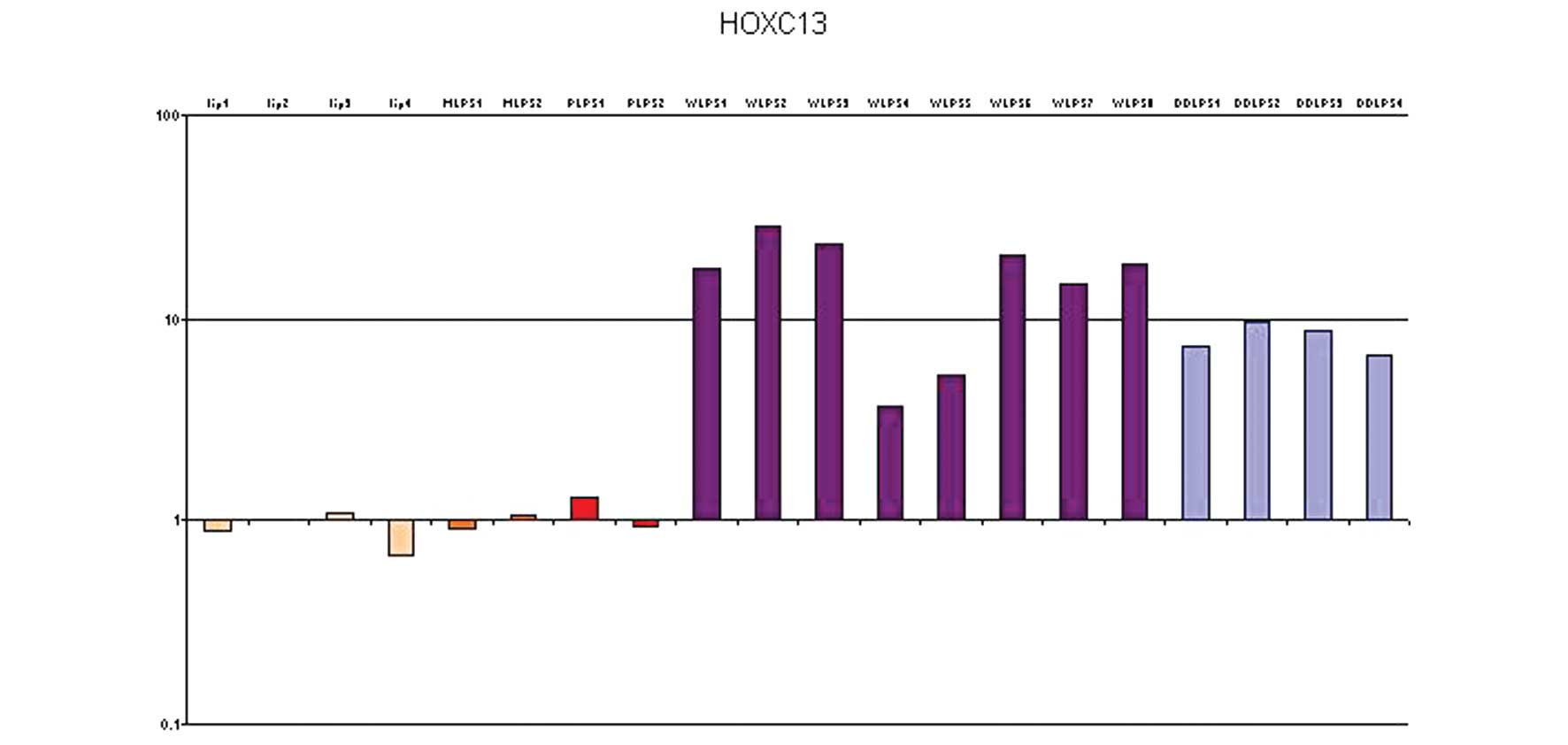
HOXC13 mRNA quantification in LPSs
HOXC13 gene expression was evaluated in 20 selected
fresh and paraffin-embedded tissue samples by real-time PCR
quantification.
In 4 lipoma samples, in 2 MLPSs and in 1 PLPS,
HOXC13 gene expression was absent, while it was very low in the
other PLPS. Moreover, in 6/8 WDLPSs, a significant increase in
HOXC13 mRNA expression (between 10 and 100-fold increase) was
observed, while in the other 2 cases, the increase of expression
was moderate (between 8 and 10-fold). In all 4 DDLPSs, there was a
moderate increase of expression (~10-fold) (Fig. 2).
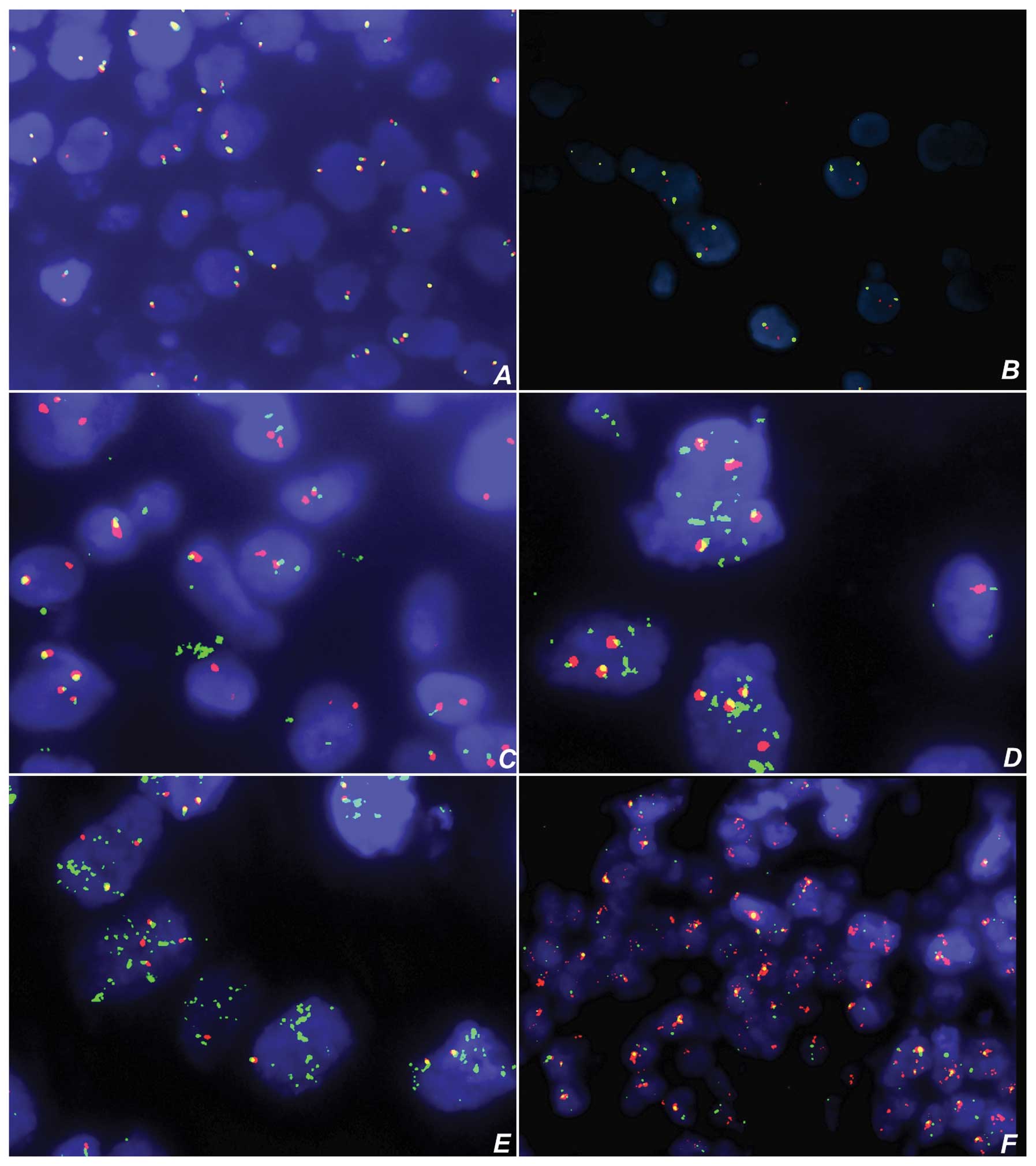
Cytogenetic analysis
Break apart with translocation of the CHOP
(DDIT3) gene was seen in all evaluable MLPSs (Fig. 3, Table
I). Amplification of 660-kb green fluorophore-labelled probe
was seen in 13/18 (72%) WDLPSs, while 660-kb green and 700-kb
orange amplifications were present in 5/18 (27%) WDLPSs.
Amplification of 660-kb green fluorophore-labelled probe was seen
in 6/9 (66%) samples of DDLPS, and 660-kb green and 700-kb orange
amplifications were present in 2/9 (22%) DDLPS. In only 1 case of
PLPS, 660-kb green and 700-kb orange amplifications were present,
while the remaining LPSs and lipomas showed no evidence of
translocation and amplification of 660-kb or 700-kb probe (Fig. 3, Table
I).
Statistical investigations
Square analyses (χ2) showed no
significant association between HOXC13 expression and clinical
characteristics of LPS patients (Table
II).
 | Table IIRelationship between HOXC13 protein
expression and clinical characteristics, histological subtypes and
chromosomal 12q13-15 rearrangement in liposarcoma patients. |
Table II
Relationship between HOXC13 protein
expression and clinical characteristics, histological subtypes and
chromosomal 12q13-15 rearrangement in liposarcoma patients.
| HOXC13 score, n
(%) | | |
|---|
|
| | |
|---|
| Characteristic | 0 | 1 | 2 | 3 | Total, n | P-value |
|---|
| Gender/Age
(years) |
| Female | 14 (58.33) | 2 (8.33) | 2 (8.33) | 6 (25) | 24 | 0.812 |
| Male | 13 (44.83) | 3 (10.34) | 4 (13.79) | 9 (31.03) | 29 | |
| Age (years; mean,
57) |
| ≤57 | 18 (66.67) | 2 (7.41) | 2 (7.41) | 5 (18.52) | 27 | 0.142 |
| >58 | 9 (64.62) | 3 (11.54) | 4 (15.38) | 10 (38.46) | 26 | |
| Histological
subtype |
| Lipoma | 13 (100) | 0 | 0 | 0 | 13 | <0.001 |
| WDLPS | 0 | 0 | 4 (26.67) | 11 (73.33) | 15 | |
| DDLPS | 0 | 2 (25) | 2 (25) | 4 (50) | 8 | |
| MLPS | 10 (90.91) | 1 (9.09) | 0 | 0 | 11 | |
| PLPS | 4 (66.67) | 2 (33.33) | 0 | 0 | 6 | |
| 12q13-15
rearrangement |
| Green ampl | 0 | 2 (8.69) | 6 (26.09) | 15 (65.22) | 17 | <0.001 |
| Translocation | 10 (90.91) | 1 (9.09) | 0 | 0 | 11 | |
| No
rearrangement | 16 (94.12) | 1 (5.88) | 0 | 0 | 17 | |
HOXC13 overexpression was strongly associated with
WDLPS and DDLPS histotypes (P-value <0.001) and with
amplification of chromosomal area detected by FISH probe (P-value
<0.001) (Table II).
Discussion
Liposarcoma (LPS) is the most common neoplasm of
soft tissues and, although it rarely metastasizes, this tumor may
reach considerable size, infiltrating adjacent anatomical
structures. Similar to other sarcomas, this tumor is genetically
characterized by a series of well-studied and highly specific
chromosomal alterations (1–3).
The identification of these cytogenetic
abnormalities, along with the morphological characterization, has
assumed an increasingly important role, not only for a correct
diagnostic definition, but also for prognostic stratification of
patients, with marked therapeutic implications (2).
Most of the molecular abnormalities that
characterize some tumors, including LPSs, involve the short arm of
chromosome 12, in particular the q13–15 region. This region, in
addition to the known oncogenes CHOP, MDM2, CDK4, SAS, GLI, HMGA2,
also co-localizes an entire gene locus, HOX C, belonging to the HOX
genes network (29–36); it includes 9 genes that were
thoroughly studied in the evolution and neoplastic progression of
various human organs and tissues (22–27).
Furthermore, in other types of human cancer, such as bladder
cancer, the amplification of this chromosomal area was associated
not only with amplification of these genes, but also with
overexpression of some genes of the HOX C locus (25).
Preliminary results, obtained by
immunohistochemistry on a Multi-Tumor Array, in which a large
spectrum of human cancer types was included, showed an increased
HOXC13 expression particularly in LPS samples (data not shown).
Based on these data, in the current study, we
analyzed a series of adipocytic tumors, including
well-differentiated LPSs (WDLPSs), dedifferentiated LPSs (DDLPS),
myxoid LPSs (MLPSs), pleomorphic LPSs (PLPSs) and lipomas, in order
to evaluate HOXC13 expression and 12q13-15 chromosomal locus
status.
Immunohistochemical analyses showed HOXC13
overexpression in most WDLPSs and DDLPSs. In addition, the data
were confirmed by real-time PCR analysis, and are higher in WDLPSs
and DDLPSs compared to other histological subtypes and lipomas.
The entire HOX C locus is localized in the same
chromosomal region detected by LSI CHOP Dual Color Break
Apart Rearrangement Probe. All samples of WDLPSs and DDLPS always
show a higher number of green signals and, in some cases, of both
orange and green signals.
It has been clearly demonstrated that the
pathogenesis of LPSs could be directly connected to the block of
adipocyte differentiation processes. In particular, it has been
reported that the overexpression of CHOP protein in LPSs suppresses
adipogenic conversion of preadipocytes through inhibition of C/EBP
α gene expression (37). Moreover,
the molecular mechanism underlying the activity of the anticancer
drug trabectedin in LPS cells has been investigated. This molecule
targeted selectively a specific FUS-CHOP chimeric transcript,
promoting adipocyte differentiation, blocking the proliferation of
neoplastic cells (38).
Numerous observations have linked genes regulating
embryonal development to adipogenesis and lipidic metabolism
(39). The HOX gene network plays a
primary role in transcriptional regulation of human adipogenesis.
Thus, these genes show a highly marked expression in adipose tissue
and, moreover, their expression appears to vary in the different
bodily deposits of white and brown adipose tissue (40). Therefore, there may be a role of HOX
genes in the evolution of neoplastic tumors linked to the processes
of adipocyte differentiation.
Based on our data, we hypothesized that the
overexpression of HOXC13 in WDLPS and DDLPSs, with amplification of
12q13-15 region, may be involved in the pathogenesis of these
tumors.
Since the amplification of the 12q13-15 region
appears to be present in almost all WDLPSs and DDLPSs,
identification of all genes within this area, which are altered in
their expression and thus directly implicated in the pathogenesis
of LPSs, represents an important aim of the clinic research for
this malignancy. Moreover, the specific expression in WDLPS
compared to lipomas may also be a significant tool for differential
diagnosis between these two entities with overlapping
characteristics.
The possibility of modifying, with a high
efficiency, the expression and consequently the activity of HOX
genes strictly associated with tumor development has previously
been reported (41–44). Therefore, the possibility of
interfering with HOXC13 gene expression could provide significant
insight into a better understanding of the pathogenesis of this
disease, and may aid in identifying new potential therapeutic
targets.
Acknowledgements
We wish to thank ASMO (Association of
Multidisciplinary Studies in Oncology), for its contribution.
References
|
1
|
Coindre JM, Pédeutour F and Aurias A:
Well-differentiated and dedifferentiated liposarcomas. Virchows
Arch. 456:167–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dalal KM, Antonescu CR and Singer S:
Diagnosis and management of lipomatous tumors. J Surg Oncol.
97:298–313. 2008. View Article : Google Scholar
|
|
3
|
Dei Tos AP: Liposarcoma: new entities and
evolving concepts. Ann Diagn Pathol. 4:252–266. 2000.PubMed/NCBI
|
|
4
|
Italiano A, Bianchini L, Keslair F,
Bonnafous S, Cardot-Leccia N, Coindre JM, Dumollard JM, Hofman P,
Leroux A, Mainguené C, Peyrottes I, Ranchere-Vince D, Terrier P,
Tran A, Gual P and Pedeutour F: HMGA2 is the partner of MDM2 in
well-differentiated and dedifferentiated liposarcomas whereas CDK4
belongs to a distinct inconsistent amplicon. Int J Cancer.
122:2233–2241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antonescu CR, Elahi A, Humphrey M, Lui MY,
Healey JH, Brennan MF, Woodruff JM, Jhanwar SC and Ladanyi M:
Specificity of TLS-CHOP rearrangement for classic myxoid/round cell
liposarcoma: absence in predominantly myxoid well-differentiated
liposarcomas. J Mol Diagn. 2:132–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pilotti S, Della Torre G, Lavarino C,
Sozzi G, Minoletti F, Vergani B, Azzarelli A, Rilke F and Pierotti
MA: Molecular abnormalities in liposarcoma: role of MDM2 and
CDK4-containing amplicons at 12q13–22. J Pathol. 185:188–190.
1998.PubMed/NCBI
|
|
7
|
Berner JM, Forus A, Elkahloun A, Meltzer
PS, Fodstad O and Myklebost O: Separate amplified regions
encompassing CDK4 and MDM2 in human sarcomas. Genes Chromosomes
Cancer. 17:254–259. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cannizzaro LA, Croce CM, Griffin CA,
Simeone A, Boncinelli E and Huebner K: Human homeo box-containing
genes located at chromosome regions 2q31----2q37 and
12q12----12q13. Am J Hum Genet. 41:1–15. 1987.PubMed/NCBI
|
|
9
|
Gehring WJ and Hiromi Y: Homeotic genes
and the homeobox. Annu Rev Genet. 20:147–173. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Apiou F, Flagiello D, Cillo C, Malfoy B,
Poupon MF and Dutrillaux B: Fine mapping of human HOX gene
clusters. Cytogenet Cell Genet. 73:114–115. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cillo C: HOX genes in human cancers.
Invasion Metastasis. 14:38–49. 1994–1995.PubMed/NCBI
|
|
12
|
Cillo C, Faiella A, Cantile M and
Boncinelli E: Homeobox genes and cancer. Exp Cell Res. 248:1–9.
1999. View Article : Google Scholar
|
|
13
|
Nunes FD, de Almeida FC, Tucci R and de
Sousa SC: Homeobox genes: a molecular link between development and
cancer. Pesqui Odontol Bras. 17:94–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grier DG, Thompson A, Kwasniewska A,
McGonigle GJ, Halliday HL and Lappin TR: The pathophysiology of HOX
genes and their role in cancer. J Pathol. 205:154–171. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Argiropoulos B and Humphries RK: Hox genes
in hematopoiesis and leukemogenesis. Oncogene. 26:6766–6776. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shah N and Sukumar S: The Hox genes and
their roles in oncogenesis. Nat Rev Cancer. 10:361–371. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cantile M, Pettinato G, Procino A,
Feliciello I, Cindolo L and Cillo C: In vivo expression of the
whole HOX gene network in human breast cancer. Eur J Cancer.
39:257–264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cantile M, Kisslinger A, Cindolo L,
Schiavo G, D’Antò V, Franco R, Altieri V, Gallo A, Villacci A,
Tramontano D and Cillo C: cAMP induced modifications of HOX D gene
expression in prostate cells allow the identification of a
chromosomal area involved in vivo with neuroendocrine
differentiation of human advanced prostate cancers. J Cell Physiol.
205:202–210. 2005. View Article : Google Scholar
|
|
19
|
Cantile M, Franco R, Tschan A, Baumhoer D,
Zlobec I, Schiavo G, Forte I, Bihl M, Liguori G, Botti G, Tornillo
L, Karamitopoulou-Diamantis E, Terracciano L and Cillo C: HOX D13
expression across 79 tumor tissue types. Int J Cancer.
125:1532–1541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cantile M, Schiavo G, Franco R, Cindolo L,
Procino A, D’Armiento M, Facchini G, Terracciano L, Botti G and
Cillo C: Expression of lumbosacral HOX genes, crucial in kidney
organogenesis, is systematically deregulated in clear cell kidney
cancers. Anticancer Drugs. 22:392–401. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cillo C, Schiavo G, Cantile M, Bihl MP,
Sorrentino P, Carafa V, D’ Armiento M, Roncalli M, Sansano S,
Vecchione R, Tornillo L, Mori L, De Libero G, Zucman-Rossi J and
Terracciano L: The HOX gene network in hepatocellular carcinoma.
Int J Cancer. 129:2577–2587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lawrence HJ, Stage KM, Mathews CH, Detmer
K, Scibienski R, MacKenzie M, Migliaccio E, Boncinelli E and
Largman C: Expression of HOX C homeobox genes in lymphoid cells.
Cell Growth Differ. 4:665–669. 1993.PubMed/NCBI
|
|
23
|
Bijl J, van Oostveen JW, Kreike M, Rieger
E, van der Raaij-Helmer LM, Walboomers JM, Corte G, Boncinelli E,
van den Brule AJ and Meijer CJ: Expression of HOXC4, HOXC5, and
HOXC6 in human lymphoid cell lines, leukemias, and benign and
malignant lymphoid tissue. Blood. 87:1737–1745. 1996.PubMed/NCBI
|
|
24
|
Cillo C, Cantile M, Mortarini R, Barba P,
Parmiani G and Anichini A: Differential patterns of HOX gene
expression are associated with specific integrin and ICAM profiles
in clonal populations isolated from a single human melanoma
metastasis. Int J Cancer. 66:692–697. 1996. View Article : Google Scholar
|
|
25
|
Cantile M, Cindolo L, Napodano G, Altieri
V and Cillo C: Hyperexpression of locus C genes in the HOX network
is strongly associated in vivo with human bladder transitional cell
carcinomas. Oncogene. 22:6462–6468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miller GJ, Miller HL, van Bokhoven A,
Lambert JR, Werahera PN, Schirripa O, Lucia MS and Nordeen SK:
Aberrant HOXC expression accompanies the malignant phenotype in
human prostate. Cancer Res. 63:5879–5888. 2003.PubMed/NCBI
|
|
27
|
Schiavo G, D’Antò V, Cantile M, Procino A,
Di Giovanni S, Valletta R, Terracciano L, Baumhoer D, Jundt G and
Cillo C: Deregulated HOX genes in ameloblastomas are located in
physical contiguity to keratin genes. J Cell Biochem.
112:3206–3215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fletcher C, Unni K and Mertens F: World
Health Organization Classification of Tumors. Pathology and
Genetics of Tumours of Soft Tissue and Bone. IARC Press; Lyon:
2002
|
|
29
|
Simon R, Struckmann K, Schraml P, Wagner
U, Forster T, Moch H, Fijan A, Bruderer J, Wilber K, Mihatsch MJ,
Gasser T and Sauter G: Amplification pattern of 12q13-q15 genes
(MDM2, CDK4, GLI) in urinary bladder cancer. Oncogene.
21:2476–2483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wikman H, Nymark P, Väyrynen A, Jarmalaite
S, Kallioniemi A, Salmenkivi K, Vainio-Siukola K,
Husgafvel-Pursiainen K, Knuutila S, Wolf M and Anttila S: CDK4 is a
probable target gene in a novel amplicon at 12q13.3-q14.1 in lung
cancer. Genes Chromosomes Cancer. 42:193–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muthusamy V, Hobbs C, Nogueira C,
Cordon-Cardo C, McKee PH, Chin L and Bosenberg MW: Amplification of
CDK4 and MDM2 in malignant melanoma. Genes Chromosomes Cancer.
45:447–454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Willmore-Payne C, Holden J, Turner KC,
Proia A and Layfield LJ: Translocations and amplifications of
chromosome 12 in liposarcoma demonstrated by the LSI CHOP
breakapart rearrangement probe. Arch Pathol Lab Med. 132:952–957.
2008.PubMed/NCBI
|
|
33
|
Barr FG, Duan F, Smith LM, Gustafson D,
Pitts M, Hammond S and Gastier-Foster JM: Genomic and clinical
analyses of 2p24 and 12q13-q14 amplification in alveolar
rhabdomyosarcoma: a report from the Children’s Oncology Group.
Genes Chromosomes Cancer. 48:661–672. 2009.PubMed/NCBI
|
|
34
|
Or YY, Chung GT, To KF, Chow C, Choy KW,
Tong CY, Leung AW, Hui AB, Tsao SW, Ng HK, Yip TT, Busson P and Lo
KW: Identification of a novel 12p13.3 amplicon in nasopharyngeal
carcinoma. J Pathol. 220:97–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fischer U, Leidinger P, Keller A, Folarin
A, Ketter R, Graf N, Lenhof HP and Meese E: Amplicons on chromosome
12q13-21 in glioblastoma recurrences. Int J Cancer. 126:2594–2602.
2010.PubMed/NCBI
|
|
36
|
Mejia-Guerrero S, Quejada M, Gokgoz N,
Gill M, Parkes RK, Wunder JS and Andrulis IL: Characterization of
the 12q15 MDM2 and 12q13-14 CDK4 amplicons and clinical
correlations in osteosarcoma. Genes Chromosomes Cancer. 49:518–525.
2010.PubMed/NCBI
|
|
37
|
Batchvarova N, Wang XZ and Ron D:
Inhibition of adipogenesis by the stress-induced protein CHOP
(Gadd153). EMBO J. 14:4654–4661. 1995.PubMed/NCBI
|
|
38
|
Forni C, Minuzzo M, Virdis E, et al:
Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma
tumors. Mol Cancer Ther. 8:449–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kiess W, Petzold S, Töpfer M, Garten A,
Blüher S, Kapellen T, Körner A and Kratzsch J: Adipocytes and
adipose tissue. Best Pract Res Clin Endocrinol Metab. 22:135–153.
2008. View Article : Google Scholar
|
|
40
|
Cantile M, Procino A, D’Armiento M,
Cindolo L and Cillo C: HOX gene network is involved in the
transcriptional regulation of in vivo human adipogenesis. J Cell
Physiol. 194:225–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morgan R, Pirard PM, Shears L, Sohal J,
Pettengell R and Pandha HS: Antagonism of HOX/PBX dimer formation
blocks the in vivo proliferation of melanoma. Cancer Res.
67:5806–5813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Laurent A, Bihan R, Omilli F, Deschamps S
and Pellerin I: PBX proteins: much more than Hox cofactors. Int J
Dev Biol. 52:9–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morgan R, Plowright L, Harrington KJ,
Michael A and Pandha HS: Targeting HOX and PBX transcription
factors in ovarian cancer. BMC Cancer. 10:892010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seyhan AA: RNAi: a potential new class of
therapeutic for human genetic disease. Hum Genet. 30:583–605. 2011.
View Article : Google Scholar : PubMed/NCBI
|