Introduction
Retinoblastoma is the most common intraocular
malignant solid tumor in childhood (1). Traditionally, external beam
radiotherapy or enucleation was previously the mainstay of
treatment, but emerging evidence of an increased risk for secondary
cancers with external beam radiation has shifted our management
strategies (2). Recently, systemic
chemotherapy has become the primary approach to salvage eyes
(3). Although chemotherapy regimens
vary between institutions, cisplatin (cis-diammine dichloro
platinum) is widely used for the treatment of retinoblastoma
(4–6). It interacts with DNA to form DNA
adducts leading to intrastrand or interstrand cross-links, which
impair proper DNA replication and activate apoptotic pathways
(7,8). Cisplatin treatment led to initial
success in the treatment of solid neoplasms, but many patients
exhibit intrinsic resistance (9).
Moreover, a significant fraction of initially sensitive cancers
eventually develop chemoresistance (10). Similarly, primary chemotherapy
frequently fails to achieve success on eyes with advanced
retinoblastoma, which finally results in enucleation (11). Thus, eyes with advanced stages are
in need of more effective chemotherapy to reduce the tumor volume
for local therapy, eventually saving the globes.
Clusterin is a sulfated glycoprotein of 75–80 kDa
encoded by a single gene, which undergoes a maturation process
finally resulting in the secreted heterodimeric form consisting of
α- and β-chains (12). Clusterin
has been viewed as a cytoprotective chaperone protein that is
upregulated during many types of stress and exerts a putative role
in the quality control of protein folding (13). Recently, we demonstrated that
clusterin protects against blood-retinal barrier dysfunction in
diabetic retinopathy, ischemia-induced cell death of human retinal
endothelial cells (HRECs) and oxidative stress-induced apoptosis of
retinal cells including retinal pigment epithelial cells,
astrocytes and HRECs (14–17). Notably, these protective roles of
clusterin were also proven in malignant neoplasms by demonstrating
that its expression was correlated with chemoresistance and poor
survival in ovarian and cervical cancer (18,19).
Based on our previous studies concerning its
protective role in the retina and data regarding its association
with chemoresistance, we investigated the role of clusterin in
cisplatin-induced apoptosis of retinoblastoma cells. In the present
study, overexpression of clusterin in human retinoblastoma tissues
and cells was confirmed. We also demonstrated that exogenous
supplement and overexpression of clusterin attenuated
cisplatin-induced apoptosis of retinoblastoma cells.
Materials and methods
Human retinoblastoma tissues
Three patients with unilateral group E
retinoblastoma, the most extensive stage according to the
International Classification of Retinoblastoma (ICRB) (11), and one patient with bilateral
retinoblastoma (group B and E by ICRB) were included in this study.
The patients had no evidence of metastasis and underwent
enucleation of eyes with group E retinoblastoma without prior
systemic chemotherapy or local treatments at 8.3±3.3 months of age.
All human retinoblastoma tissue samples were obtained with informed
consent and approval by the Institutional Review Board for Clinical
Research at the Seoul National University Hospital complying with
the tenets of the Declaration of Helsinki.
Culture of human retinoblastoma
cells
Human retinoblastoma cell line Y79 (American Type
Culture Collection, Rockville, MD, USA) and SNUOT-Rb1, which was
established by our group and is distinguished from Y79 by adherent
growth and rapid proliferation (16), were incubated in RPMI-1640 medium
(WelGENE Inc., Daegu, Korea), supplemented with 10% fetal bovine
serum (Gibco-BRL, Rockville, MD, USA) and 100 μg/ml streptomycin
(Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C in a
moist atmosphere of 95% air and 5% CO2. We replaced the
medium every third day and checked the cultured tumor cells daily
under a phase-contrast microscope (Carl Zeiss, Chester, VA, USA).
If needed, 5 μg/ml of clusterin (provided by B.H. Min, Korea
University, Seoul, Korea) and/or 1 μg/ml of cisplatin
(Sigma-Aldrich, St. Louis, MO, USA) were administered.
Immunohistochemistry
The enucleated eyes were fixed in formalin, embedded
in paraffin, and then sectioned (4 μm). The slides were
de-paraffinized and incubated with proteinase K at 37°C. After
blocking endogenous peroxidase activity with hydrogen peroxide and
blocking non-specific binding with a blocking kit (Zymed
Laboratories Inc., South San Francisco, CA, USA), slides were
incubated overnight with rabbit anti-clusterin antibody (1:100;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C,
followed by a biotinylated goat anti-rabbit antibody (Dako,
Glostrup, Denmark), revealed by the avidin-biotin complex
(Vectastain kit; Vector Laboratories, Burlingame, CA, USA) and the
3-amino-9-ethyl-carbazole chromogen (AEC; Dako). Nuclei were then
counterstained with methyl green. After being mounted with
Faramount aqueous mounting medium (Dako), slides were observed
under a light microscope (Carl Zeiss).
Purification of clusterin from human
serum
Clusterin was purified from fresh normal human
plasma as described in our previous studies, in compliance with the
Declaration of Helsinki (14,17,20).
Human plasma supplemented with 0.5 mM phenylmethylsulfonyl fluoride
(PMSF) was precipitated by adding 12–23% polyethylene glycol (PEG,
MW 3350; Sigma-Aldrich) overnight at 4°C. The precipitate was
dissolved and subjected to dimethylamino ethanol-Sepharose column
chromatography (GE Healthcare Life Sciences, Buckinghamshire, UK).
Fractions containing clusterin were subjected to heparin-Sepharose
column chromatography (GE Healthcare Life Sciences). The obtained
clusterin was finally purified by a clusterin monoclonal antibody
affinity chromatography (cyanogen bromide-activated Sepharose 4B;
Sigma-Aldrich). The eluted protein was dialyzed and lyophilized
before being stored at −80°C.
Transfection of pcDNA expressing
clusterin
For transfection, the Lipofectamine Plus™ reagent
(Invitrogen Life Technologies) was used following the
manufacturer's instructions. Briefly, 8 μl of Plus™ reagent and 5
μg of plasmid DNA (pcDNA-CLU; provided by B.H. Min) were suspended
with 487 μl of RPMI-1640 medium without serum and antibiotics
[RPMI(−)], and incubated for 15 min at room temperature.
Lipofectamine (12 μl) was mixed with 488 μl of RPMI(−). The
Lipofectamine suspension was then mixed with the Plus-pcDNA
mixture, and incubated for 15 min at room temperature. The mixtures
of 1 ml RPMI(−) containing 5 μg of pcDNA were added to
2.5×105 cells and incubated for 3 h at 37°C. Then, the
medium was replaced with RPMI-1640 medium. The time when pcDNA was
added to cells was defined as time 0. After 24 h of induction,
cisplatin treatment was carried out.
Cell viability assay
Cell viability was evaluated by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. SNUOT-Rb1 cells were seeded into each well of 48-well plates
at a concentration of 2×104 cells/well. After incubation
for 24 h, cells were treated with various concentrations of
cisplatin. After incubation for the following 24 h, MTT solution
was added to each well at a final concentration of 0.2 mg/ml. After
incubation at 37°C for 2 h, the medium was carefully removed, and
DMSO was added to solubilize the formazan produced by the viable
cells. Optical density values at 540 nm were measured by a
microplate spectrophotometer (Molecular Devices, Sunnyvale, CA,
USA). Three independent experiments were performed for each
experimental condition.
Trypan blue dye exclusion was also used to assess
the viability of cells following clusterin treatment. Viable cells
were counted on a Luna™ automated cell counter (Logos Biosystems,
Gyunggi-Do, Korea). Three independent experiments were performed
for each experimental condition.
Western blot analysis
Cell lysates were obtained by resuspending cells in
radioimmunoprecipitation assay (RIPA) buffer (Tris 50 mM pH 7.4;
NaCl 150 mM; SDS 0.1%; Na deoxycholate 0.5%; Triton X-100 1%; Cell
Signaling Technology, Inc., Danvers, MA, USA) with a complete
protease inhibitor cocktail (Roche Molecular Biochemicals,
Indianapolis, IN, USA), incubated on ice for 1 h and centrifuged at
20,000 × g for 30 min at 4°C. After measurement of the protein
concentration using a BCA protein assay kit (Pierce Biotechnology
Inc., Rockford, IL, USA), equal amounts of protein from the
supernatant were separated by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) using 7% Tris-Tricine gel (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and transferred to
nitrocellulose membranes (Amersham Hybond ECL; GE Healthcare,
Piscataway, NJ, USA). After being blocked with diluted 5% dry skim
milk for 1 h, the membranes were rinsed and incubated with specific
antibodies against clusterin and β-actin in PBS-T (PBS containing
0.1% Tween-20; Bio-Rad Laboratories, Inc.) overnight at 4°C. The
primary antibody was removed by washing the membranes with PBS-T
and incubation for 1 h with horseradish peroxidase-conjugated
secondary antibodies.
4,6-Diamidino-2-phenolindole
staining
We performed 4,6-diamidino-2-phenolindole (DAPI;
Sigma-Aldrich) staining for cell viability analysis. SNUOT-Rb1
cells (1×106 cells) were plated in 6-well plates and
cultured for 24 h. The cells were treated with cisplatin (1 μg/ml)
and/or clusterin (5 μg/ml). After 24 h of incubation, cells were
fixed and stained with 10 μg/ml of DAPI (Sigma-Aldrich). After
incubation for 5 min in the dark, the cells were washed and
observed under a fluorescence microscope (Leica, Wetzlar,
Germany).
Statistical analysis
Statistical analyses were performed using SPSS
software version 18.0 (SPSS Inc., Chicago, IL, USA). A P-value
<0.05 was considered to indicate a statistically significant
result. Values are expressed as means ± SD.
Results
Expression of clusterin in human
retinoblastoma
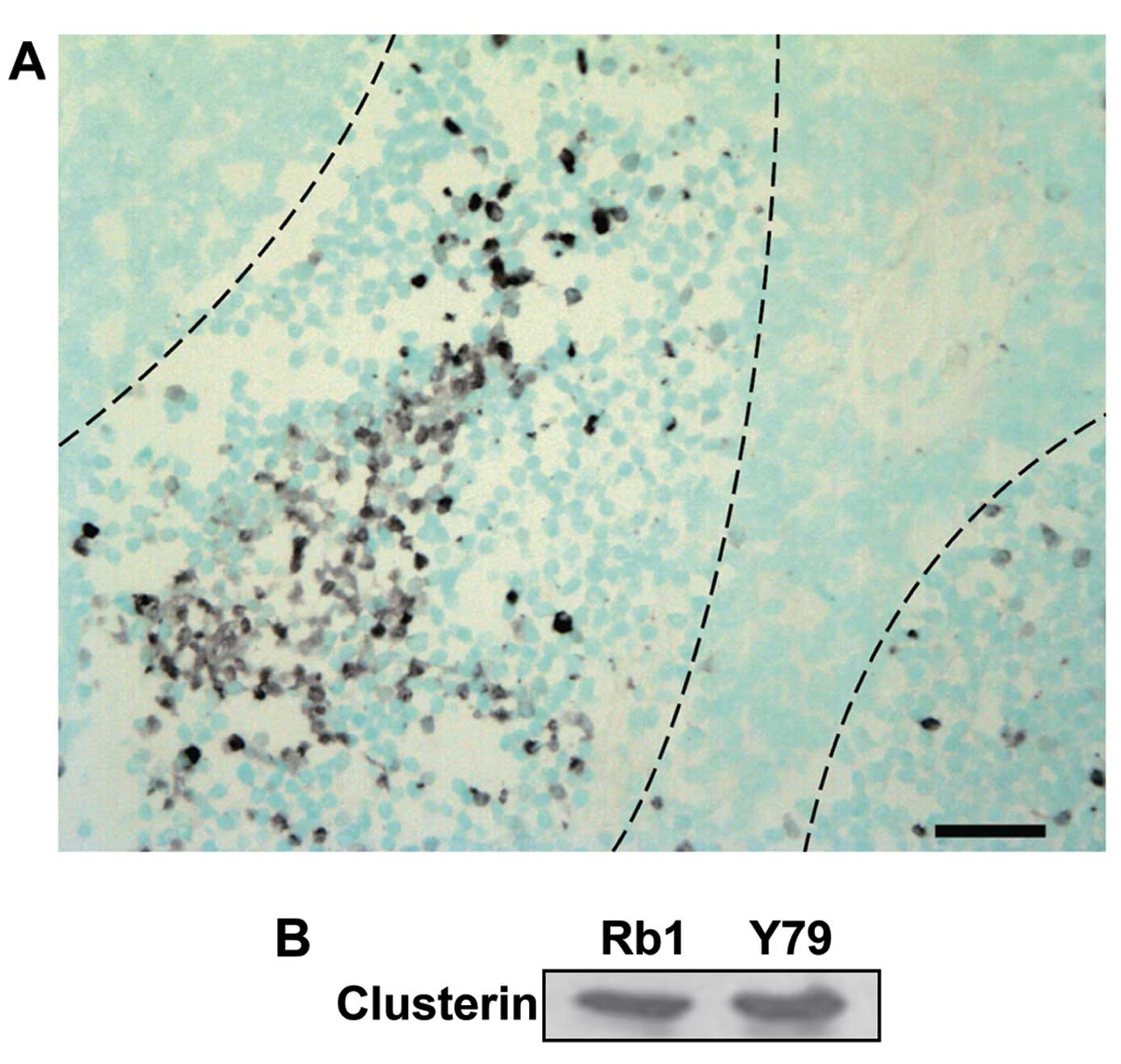
To investigate the expression of clusterin in
clinical samples, we performed immunohistochemical staining with an
anti-clusterin antibody on tissues from enucleated human eyeballs
with retinoblastoma. Microscopic examination showed that all the
retinoblastoma tissues consisted of viable cells around blood
vessels and zones of necrosis relatively far from the vessels.
Clusterin was highly expressed in the retinoblastoma particularly
at the peripheral areas of viable cells, adjacent to the necrotic
zones, while barely detectable in the area with numerous
Flexner-Wintersteiner rosettes marked by central lumen with
surrounding cells showing cytoplasmic extensions (Fig. 1A). Counterstaining with methyl green
revealed that clusterin was mainly stained in the cytoplasm.
Furthermore, we assessed the expression of clusterin
in human retinoblastoma cell lines. As shown in Fig. 1B, clusterin was detected in
SNUOT-Rb1 and Y79 cells by western blot analysis. Following
evidence of clusterin expression in human retinoblastoma tissues
and SNUOT-Rb1 cells, we further evaluated the effect of clusterin
on cisplatin-induced cell death in SNUOT-Rb1 cells.
Induction of apoptotic cell death by
cisplatin treatment on retinoblastoma cells
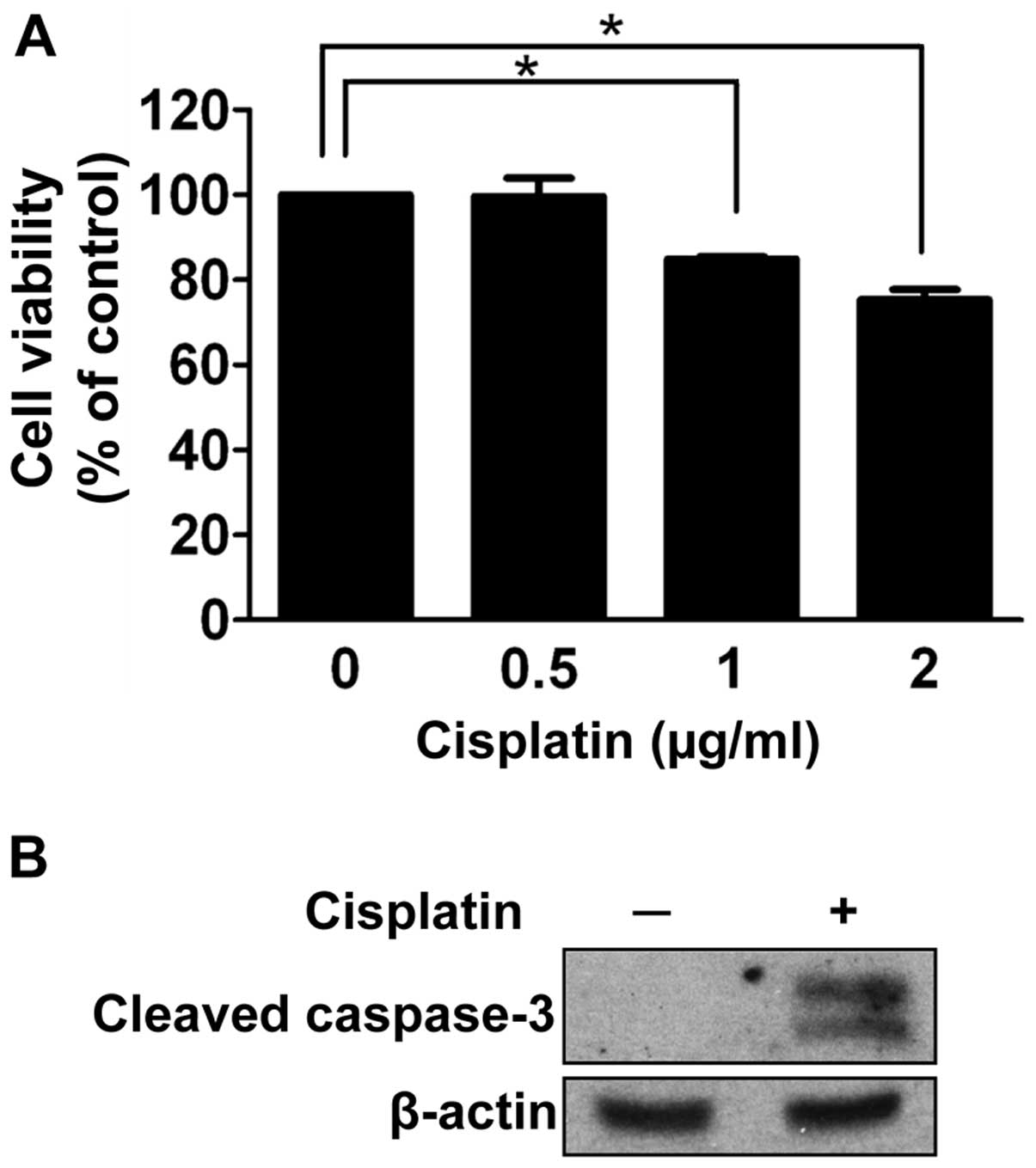
To investigate the effect of cisplatin on
retinoblastoma cell death, we performed a cell viability assay
under a condition of gradually increasing concentrations of
cisplatin for 24 h. As shown in Fig.
2A, at least 1 μg/ml of cisplatin was required to significantly
affect the cell viability of SNUOT-Rb1 cells (P=0.001). We
determined the concentration with enough chemotherapeutic activity
as 1 μg/ml for further experiments.
As cisplatin is known to impair proper DNA
replication and activate apoptotic pathways (7,8),
apoptosis was evaluated in SNOT-Rb1 cells. After 24 h of cisplatin
treatment, cleaved caspase-3 was increased (Fig. 2B). Thus, cisplatin induced the
apoptotic death of retinoblastoma cells.
Effect of exogenous clusterin on
cisplatin-induced apoptotic cell death
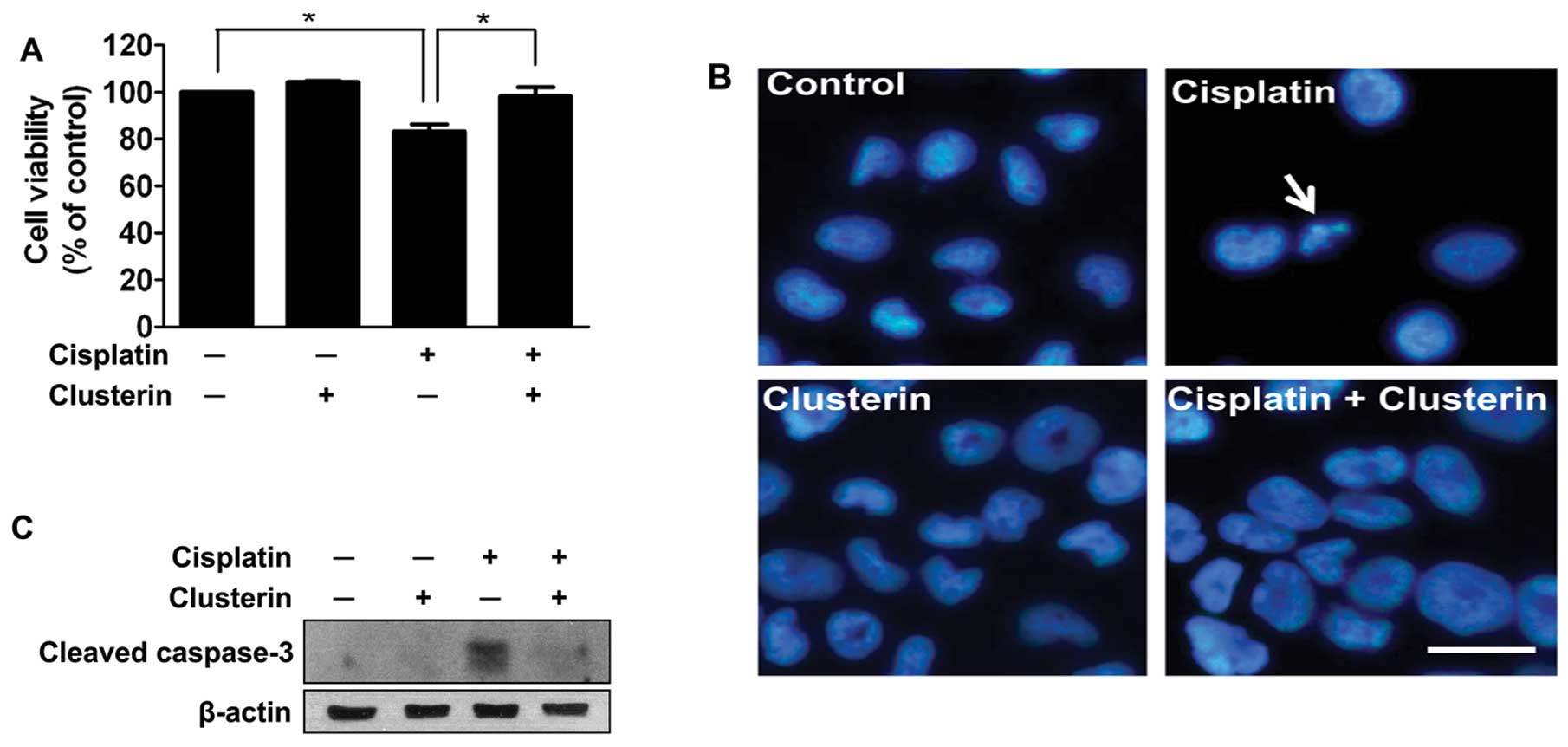
To determine the effect of exogenous clusterin on
cisplatin-induced apoptotic cell death, trypan blue dye exclusion
was carried out following treatment with cisplatin and/or exogenous
clusterin. Clusterin was administered 4 h prior to the cisplatin
treatment. The viability of SNUOT-Rb1 cells was not affected by 5
μg/ml of exogenous clusterin (Fig.
3A). Cisplatin (1 μg/ml) significantly decreased the viability,
but the exogenous supplement of clusterin effectively prevented
cisplatin-induced cell death (Fig.
3A). The results were confirmed by DAPI staining of the culture
plates. Condensated and fragmented nuclei (arrow) with decreased
cell numbers in the cisplatin-treated group reflected cell death by
cisplatin, which was completely abrogated by the supplement of
exogenous clusterin (Fig. 3B).
To evaluate the mechanism by which clusterin
protects retinoblastoma cells from cisplatin-induced cell death,
the inhibitory effect of clusterin on caspase-3 activity was
assessed by western blot analysis. As demonstrated in Fig. 3C, increased activity of caspase-3 by
cisplatin treatment was dramatically attenuated by cotreatment with
5 μg/ml of clusterin. These data suggest that exogenous clusterin
inhibits apoptosis induced by cisplatin in retinoblastoma
cells.
Effect of clusterin overexpression on
cisplatin-induced apoptotic cell death
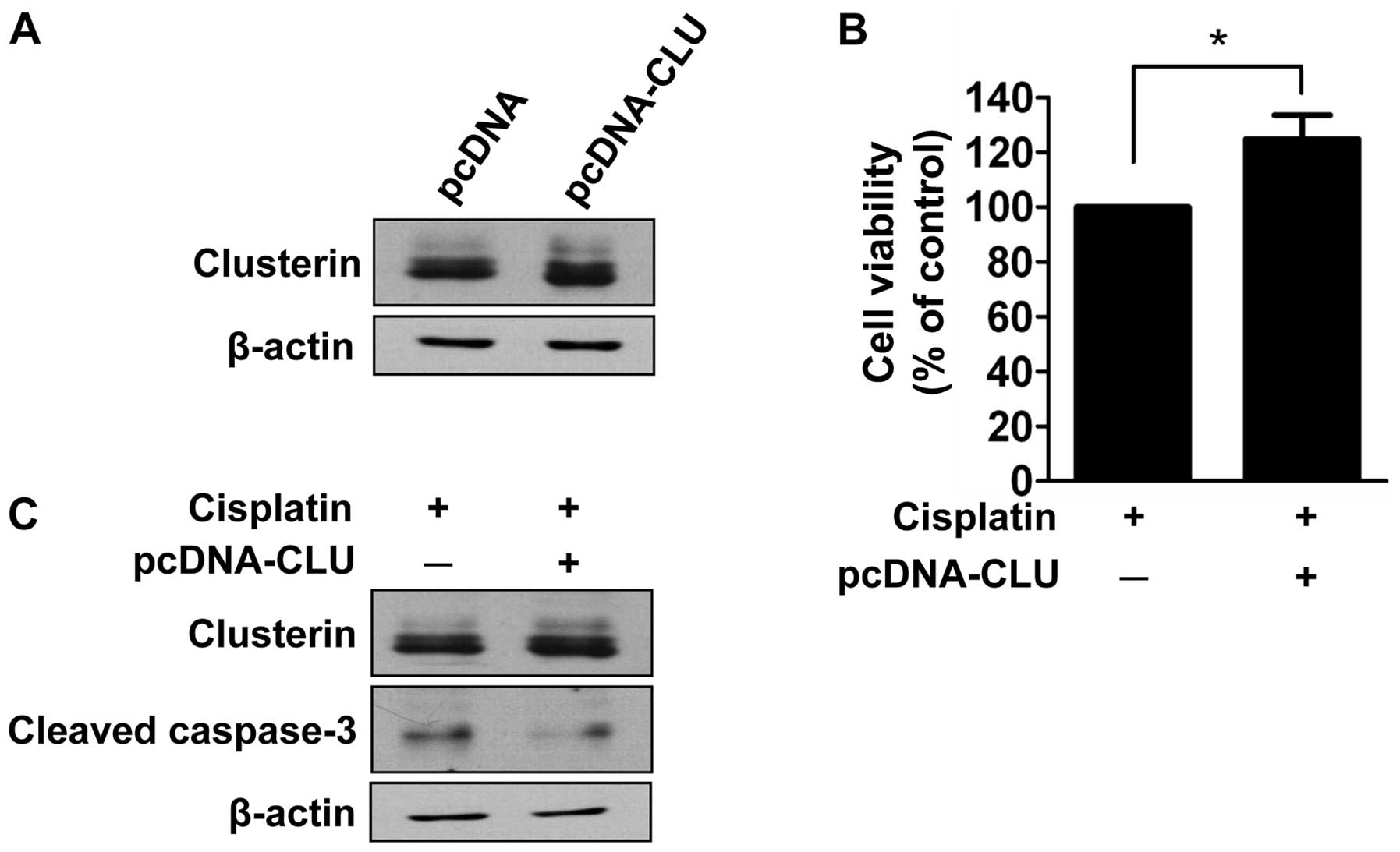
Given that clusterin is overexpressed in human
retinoblastoma tissues and cells as determined in the present
study, we evaluated the effect of clusterin overexpression on
cisplatin-induced cell death. SNUOT-Rb1 cells were transfected with
pcDNA-CLU using empty vectors as a control, and underwent a cell
viability assay. Cells transfected with pcDNA-CLU exhibited
increased expression of clusterin (Fig.
4A). The cell viability assay demonstrated that transfected
cells exhibited significantly less cell death following cisplatin
treatment (Fig. 4B). The effect of
clusterin overexpression on apoptosis was also evaluated by western
blot analysis of cleaved caspase-3. As shown in Fig. 4C, increased activity of cleaved
caspase-3 following cisplatin treatment was attenuated in cells
transfected with pcDNA-CLU. Thus, clusterin overexpression inhibits
cisplatin-induced apoptotic cell death similar to that of exogenous
clusterin.
Discussion
In the present study, we demonstrated the
overexpression of clusterin in human retinoblastoma tissues and
cells. Cisplatin treatment induced retinoblastoma cell death, which
was prevented with the supplement or overexpression of clusterin by
inhibiting cisplatin-induced apoptosis. Therefore, expression of
clusterin in retinoblastoma exerts an anti-apoptotic effect against
cisplatin-induced apoptotic cell death.
Retinoblastoma usually grows so rapidly that oxygen
demands often exceed its blood supplies, which results in extensive
necrosis in relatively avascular areas (21). Notably, clusterin was highly
expressed in the area between viable cells around vessels and
necrotic zones. We previously demonstrated the upregulation of
clusterin in hypoxic condition in HRECs and astrocytes (17) and the protective role of clusterin
against ischemia-induced cell death of HRECs (20). Taken together, clusterin is probably
upregulated to protect retinoblastoma cells from ischemia-induced
cell death.
Clusterin has been reported to be overexpressed in
several types of malignant tumors, whose chemoresistance is related
with clusterin expression (18,19,22).
The mechanism of the enhancement of chemoresistance by clusterin is
known to be associated with its inhibitory effect on apoptosis,
which is consistent with our data indicating changes in cleaved
caspase-3 expression induced by cisplatin treatment. Clusterin
depletion by small interfering RNA was found to result in
disruption of the Ku70-Bax complex, activation of Bax and
translocation of Bax into mitochondria to induce cytochrome
c release and apoptosis (23,24).
Moreover, overexpression of clusterin increased phosphorylation of
Akt and its target protein Bad, and decreased cytochrome c
release and apoptosis (12). On the
other hand, downregulation of Bax and enhanced activity of Akt are
known mechanisms involved in the inhibition of apoptotic signals in
cisplatin-resistant tumor cells (7). Taken together, clusterin
overexpression may exert anti-apoptotic effects resulting in the
change of cisplatin-sensitive tumor cells into cisplatin-resistant
cells.
The association of clusterin and cisplatin
resistance was revealed in previous studies. Transfection of the
clusterin gene into human renal cell carcinoma cells enhanced their
resistance to cisplatin in vitro and in vivo(25), which is consistent with our data on
the effect of clusterin overexpression. The administration of
clusterin-specific antisense oligonucleotides enhanced cisplatin
sensitivity of KoTCC-1 human bladder tumors in vitro and
in vivo(26). Based on these
preclinical data, clinical studies are underway. Phase II trials of
custirsen (OGX-011), a second generation antisense oligonucleotide
targeting clusterin, in combination with conventional chemotherapy
have been carried out in patients with chemotherapy-naive advanced
non-small cell lung cancer and metastatic castration-resistant
prostate cancer progressing after initial docetaxel therapy
(27,28). This type of approach can be applied
to retinoblastoma. Although the toxicity of the combination did not
differ from what is reported for conventional chemotherapy in the
study mentioned above (27), there
are safety issues to consider before applying the treatment to
retinoblastoma, as clusterin is reported to protect against
ischemia-induced cell death of HRECs, oxidative stress-induced
apoptosis of retinal pigment epithelial cells and blood-retinal
barrier dysfunction in diabetic retinopathy (14–16).
In the present study, following evidence of the
expression of clusterin in human retinoblastoma, we demonstrated
that exogenously administered or overexpressed clusterin inhibited
cisplatin-induced apoptosis in human retinoblastoma cells.
Clusterin could play a role in the chemoresistance of
retinoblastoma to cisplatin and could be applied as an adjuvant
treatment modality to conventional chemotherapy for
retinoblastoma.
Acknowledgements
This study was supported by the SNUH research fund
(04-2012-0570), the Bio-Signal Analysis Technology Innovation
Program (2009-0090895), the Pioneer Research Program (2012-0009544)
and the Global Core Research Center (GCRC) grant from NRF/MEST,
Republic of Korea (2012-0001187).
References
|
1
|
Abramson DH: Retinoblastoma in the 20th
century: past success and future challenges the Weisenfeld lecture.
Invest Ophthalmol Vis Sci. 46:2683–2691. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim JW, Abramson DH and Dunkel IJ: Current
management strategies for intraocular retinoblastoma. Drugs.
67:2173–2185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gombos DS and Chevez-Barrios AP: Current
treatment and management of retinoblastoma. Curr Oncol Rep.
9:453–458. 2007. View Article : Google Scholar
|
|
4
|
Varan A, Kiratli H, Aydin B, et al: The
treatment of retinoblastoma with four-drug regimen including
cisplatin, etoposide, vincristine, and cyclophosphamide. Pediatr
Hematol Oncol. 29:529–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim H, Lee JW, Kang HJ, et al: Clinical
results of chemotherapy based treatment in retinoblastoma patients:
a single center experience. Cancer Res Treat. 40:164–171. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Makimoto A: Results of treatment of
retinoblastoma that has infiltrated the optic nerve, is recurrent,
or has metastasized outside the eyeball. Int J Clin Oncol. 9:7–12.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siddik ZH: Cisplatin: mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galluzzi L, Senovilla L, Vitale I, et al:
Molecular mechanisms of cisplatin resistance. Oncogene.
31:1869–1883. 2012. View Article : Google Scholar
|
|
10
|
Koberle B, Tomicic MT, Usanova S and Kaina
B: Cisplatin resistance: preclinical findings and clinical
implications. Biochim Biophys Acta. 1806:172–182. 2010.PubMed/NCBI
|
|
11
|
Shields CL, Mashayekhi A, Au AK, et al:
The International Classification of Retinoblastoma predicts
chemoreduction success. Ophthalmology. 113:2276–2280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ammar H and Closset JL: Clusterin
activates survival through the phosphatidylinositol 3-kinase/Akt
pathway. J Biol Chem. 283:12851–12861. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wyatt A, Yerbury J, Poon S, Dabbs R and
Wilson M: Chapter 6: the chaperone action of clusterin and its
putative role in quality control of extracellular protein folding.
Adv Cancer Res. 104:89–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JH, Kim JH, Yu YS, Min BH and Kim KW:
Protective effect of clusterin on blood-retinal barrier breakdown
in diabetic retinopathy. Invest Ophthalmol Vis Sci. 51:1659–1665.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JH, Kim JH, Jun HO, et al: Protective
effect of clusterin from oxidative stress-induced apoptosis in
human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci.
51:561–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JH, Kim JH, Yu YS, Kim DH, Kim CJ and
Kim KW: Establishment and characterization of a novel,
spontaneously immortalized retinoblastoma cell line with adherent
growth. Int J Oncol. 31:585–592. 2007.PubMed/NCBI
|
|
17
|
Kim JH, Kim JH, Yu YS, Min BH and Kim KW:
The role of clusterin in retinal development and free radical
damage. Br J Ophthalmol. 91:1541–1546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang GF, Li XM and Xie D: Overexpression
of clusterin in ovarian cancer is correlated with impaired
survival. Int J Gynecol Cancer. 19:1342–1346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watari H, Kanuma T, Ohta Y, et al:
Clusterin expression inversely correlates with chemosensitivity and
predicts poor survival in patients with locally advanced cervical
cancer treated with cisplatin-based neoadjuvant chemotherapy and
radical hysterectomy. Pathol Oncol Res. 16:345–352. 2010.
View Article : Google Scholar
|
|
20
|
Kim JH, Yu YS, Kim KW and Min BH: The role
of clusterin in in vitro ischemia of human retinal endothelial
cells. Curr Eye Res. 32:693–698. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burnier MN, McLean IW, Zimmerman LE and
Rosenberg SH: Retinoblastoma. The relationship of proliferating
cells to blood vessels. Invest Ophthalmol Vis Sci. 31:2037–2040.
1990.PubMed/NCBI
|
|
22
|
Lourda M, Trougakos IP and Gonos ES:
Development of resistance to chemotherapeutic drugs in human
osteosarcoma cell lines largely depends on up-regulation of
clusterin/apolipoprotein J. Int J Cancer. 120:611–622. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Kim JK, Edwards CA, Xu Z,
Taichman R and Wang CY: Clusterin inhibits apoptosis by interacting
with activated Bax. Nat Cell Biol. 7:909–915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trougakos IP, Lourda M, Antonelou MH, et
al: Intracellular clusterin inhibits mitochondrial apoptosis by
suppressing p53-activating stress signals and stabilizing the
cytosolic Ku70-Bax protein complex. Clin Cancer Res. 15:48–59.
2009. View Article : Google Scholar
|
|
25
|
Hara I, Miyake H, Gleave ME and Kamidono
S: Introduction of clusterin gene into human renal cell carcinoma
cells enhances their resistance to cytotoxic chemotherapy through
inhibition of apoptosis both in vitro and in vivo. Jpn J Cancer
Res. 92:1220–1224. 2001. View Article : Google Scholar
|
|
26
|
Miyake H, Hara I, Kamidono S and Gleave
ME: Synergistic chemsensitization and inhibition of tumor growth
and metastasis by the antisense oligodeoxynucleotide targeting
clusterin gene in a human bladder cancer model. Clin Cancer Res.
7:4245–4252. 2001.
|
|
27
|
Laskin JJ, Nicholas G, Lee C, et al: Phase
I/II trial of custirsen (OGX-011), an inhibitor of clusterin, in
combination with a gemcitabine and platinum regimen in patients
with previously untreated advanced non-small cell lung cancer. J
Thorac Oncol. 7:579–586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saad F, Hotte S, North S, et al:
Randomized phase II trial of custirsen (OGX-011) in combination
with docetaxel or mitoxantrone as second-line therapy in patients
with metastatic castrate-resistant prostate cancer progressing
after first-line docetaxel: CUOG trial P-06c. Clin Cancer Res.
17:5765–5773. 2011. View Article : Google Scholar : PubMed/NCBI
|