Introduction
Lung cancer is one of the leading causes of
cancer-related mortality worldwide and is the most frequently
diagnosed cancer in men (1). The
two main types of lung cancer are small cell lung cancer (SCLC) and
non-small cell lung cancer (NSCLC). NSCLC accounts for 80% of lung
cancer patients and has a 5-year survival rate of <20% despite
improvements in diagnosis and treatment (2). Most NSCLC patients are diagnosed at an
advanced stage, in a poor performance status, and are often
unresectable (3). It is crucial for
improving the outcome of NSCLC to develop better molecular
biomarkers for the prediction of disease progression and
therapeutic resistance.
Mitochondrial dysfunction has been postulated to
render cancer cells resistant to apoptosis based on the Warburg
hypothesis (4). The mean copy
number of mitochondrial DNA (mtDNA) in lung carcinoma tissue
samples was statistically lower than that in adjacent
histologically normal lung tissue samples (P<0.001) (5). Low copy number and low oxidative
damage of mtDNA in lung cancer tissues are associated with tumor
progression after neoadjuvant chemotherapy (6). In recent years, somatic mtDNA
mutations including point mutation, deletion and insertion as well
as decreased mtDNA copy number have been usually found in primary
human cancer, including NSCLC (7).
Also, 8701 and 10398 that code for ATPase6 and NADH dehydrogenase 3
were reported to be mutational hotspots in the mitochondrial genome
of lung cancer (8).
African-American women carrying 10398A had higher risk of invasive
breast cancer (9). It has been
proposed that these somatic mtDNA alterations in cancer can
contribute to the switch of energy supply from mitochondrial
oxidative phosphorylation to aerobic glycolysis and cancer
progression (10). However, few
studies have examined the prognostic value of mtDNA content and
G10398A polymorphism in NSCLC patients.
In the present study, the mtDNA copy number and
10398 genotype were assessed in tumor tissues of 128 NSCLC
patients, and their prognostic values were analyzed. Furthermore,
A549 cells were cultured in EB to get ϱ0 cells. The
radiosensitivity of these two cell lines (ϱ0 and
ϱ+) were investigated. The results showed that mtDNA
content and G10398A polymorphism may play a role in patient
prognosis by affecting cancer cell growth. Our studies suggest that
the mtDNA content and G10398A polymorphism may provide new
biomarkers to predict outcome of NSCLC. Intervention of the mtDNA
content and G10398A polymorphism may also represent a novel
approach for cancer treatment.
Materials and methods
Samples and clinical data
A total of 128 NSCLC tissue samples were obtained
from patients who underwent surgical resection (including lymph
node biopsy operation) at Zhongnan Hospital of Wuhan University
between July 2006 and July 2011. Clinical staging was assessed
according to the American Joint Committee on Cancer (AJCC, seventh
edition). None of the patients had received radiotherapy or
chemotherapy prior to surgery. Formalin-fixed and paraffin-embedded
surgical tissue samples were collected from the Department of
Pathology. Pathological diagnosis was made according to the World
Health Organization 2004 scheme. The study protocol was reviewed
and approved by the Institutional Review Board of Zhongnan Hospital
of Wuhan University. All participants (including 95 male and 33
female patients; age range, 20–80 years) provided written informed
consent to participate in this study. Clinical information,
including age, gender, pathological type, tumor node metastasis
(TNM) stage, smoking status, EGFR mutation status and clinical
follow-up data, was recorded prospectively (Table I). In addition to the scheduled
follow-up examination within 6 months, patients were followed-up at
3-month intervals for up to 2 years, then every 6 months for 5
years, and annually thereafter. For the whole group, the median
follow-up time and overall survival (OS) time were 22.5 and 23.4
months, respectively; 58 patients (45.3%) died during this
period.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variable | Group | n (%) |
|---|
| Gender | Male | 95 (74.2) |
| Female | 33 (25.8) |
| Age (years) | Mean | 59.4 |
| Range | 20–80 |
| Cigarette
smoking | No | 48 (37.5) |
| Yes | 80 (62.5) |
| Histological
status | AC | 58 (45.3) |
| SCC | 52 (40.6) |
| ASC | 15 (11.7) |
| LCC | 3 (2.4) |
| EGFR mutation | No | 114 (89.1) |
| Yes | 14 (10.9) |
| Stage | I | 24 (18.8) |
| II | 26 (20.3) |
| III | 62 (48.4) |
| IV | 16 (12.5) |
| Follow-up
status | Survival | 70 (54.7) |
| Mortality | 58 (45.3) |
Tissue genomic DNA acquisition
Total DNA from formalin-fixed, paraffin-embedded
tissues was extracted using the E.Z.N.A.® FFPE DNA kit
(Omega), according to the manufacturer’s instructions. The
extracted DNA was eluted in 100 μl TE buffer and stored at
−20°C.
Determination of mtDNA copy number
mtDNA content was assessed by quantification of a
unique fragment in human mitochondrial genome NC_012920 region
relative to a single copy region of the nuclear gene β2M
using a real-time PCR (RT-PCR) assay. NC_012920 forward,
5′-CTTCTGGCCACAGCACTT AAAC-3′ and reverse, 5′-GCTGGTGTTAGGGTTCTTTGT
TTT-3′ (64 bp product); β2M forward, 5′-GCTGGGTAGCT
CTAAACAATGTATTCA-3′ and reverse, 5′-CCATGTACTA
ACAAATGTCTAAAATGGT-3′ (93 bp product). RT-PCR was carried out using
an iQ™5 Multicolor Real-Time PCR Detection System (Bio-Rad) with a
total volume of 25 μl reaction mixture containing 100 ng DNA
template (2 μl), 12.5 μl QuantiTect SYBR-Green PCR Master mix
(Takara), and 0.5 μl of each primer, 0.5 μl ROX Reference Dye II, 9
μl ddH2O. The run protocol: initial ‘Hot Start’
activation step for 5 min at 94°C followed by 40 cycles of 30 sec
at 94°C, 30 sec at 60°C, and 30 sec at 72°C.
Genotyping of mtDNA 10398
DNA samples were PCR-amplified using primers located
between 10284–10484 np, forward, 5′-CAAACAACTAACCTGCCAC-3′ and
reverse primer, 5′-ATGAGGGGCATTTGGTA-3′ (201 bp product), followed
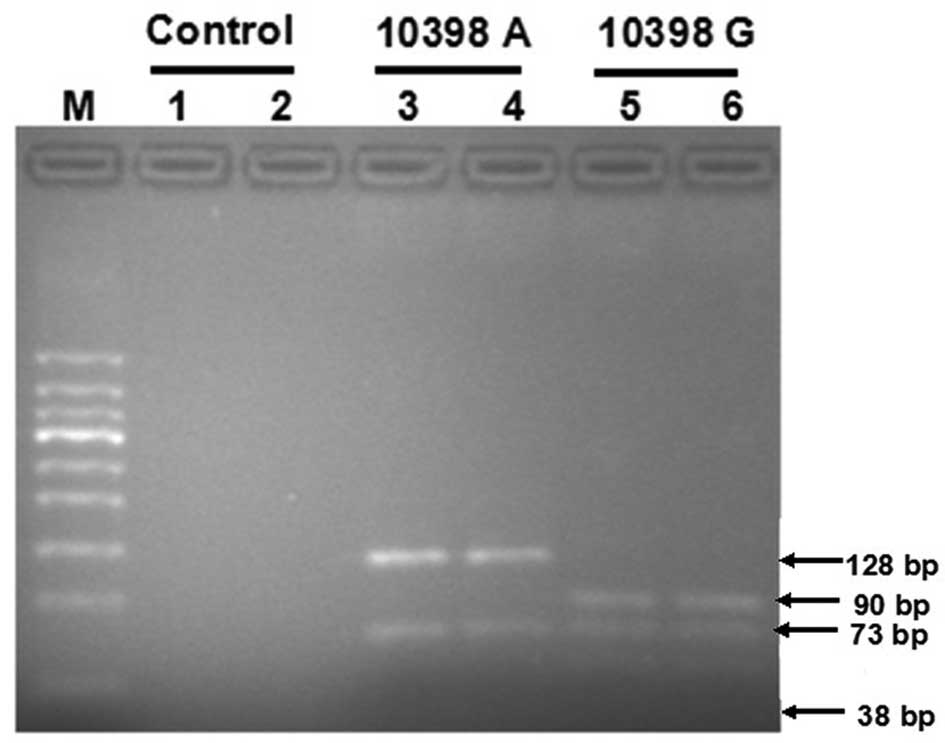
by digestion with DdeI at 37°C. On resolving the
DdeI-digested products in a 2% agarose gel, the 10398A
allele gave rise to bands of 128 and 73 bp, whereas the 10398 G
allele demonstrated bands of 90, 73 and 38 bp.
Detection of EGFR mutations
PCR-RFLP was performed for mutation analysis of EGFR
exons 18, 19 and 21. The mutant allele of exon 18 was not digested
by the restriction enzyme ApaI due to the base substitution
of G to X at the second base of GGGCCC. Since the range of exon 19
deletions containing commonly deleted codons 746 to 751,
differences in the sizes of the PCR products enabled us to
distinguish mutant-type from wild-type. The restriction enzyme
MscI was used to digest the TGGCCA sequence in the amplicon
of the wild-type allele of exon 21. By contrast, mutant type
(L858R) was not digested due to the base substitution of T to G of
TGGCCA. The digested-products were run in 2% agarose gel.
Cell line and treatment
A human NSCLC cell line A549 was used in this study.
ϱ0 cells depleted of mtDNA were generated by incubating
wild-type cells for 18 weeks in complete medium that was
additionally supplemented with 100 ng/ml ethidium bromide (Sigma),
100 μg/ml pyruvate and 50 μg/ml uridine (11). Following selection, ϱ0
cells were cultured in the medium specified above at 37°C in an
incubator with 95% air and 5% CO2 with 110 mg/l sodium
pyruvate and 50 μg/ml uridine. To verify mtDNA depletion in
ϱ0 cells, total cellular DNA from ϱ+ and
ϱ0 cells was extracted and subjected to PCR
amplification using two pairs of human mtDNA specific primers: i)
COX-I F, 5′-ACACGAGCATATTTCACCTCCG-3′ and COX-I R,
5′-GGATTTTGGCGTAGGTTTGGTC-3′, which gave a 337 bp product; ii)
mtDNA-P1 F, 5′-AACATACCCAT GGCCAACCT-3′ and mtDNA-P1 R,
5′-GGCAGGAGTAAT CAGAGGTG-3′, which gave a 533 bp product. As a
control, we measured the expression of β-actin F, 5′-TGGAAGGAC
TCATGACCACA-3′ and R, 5′-TTCAGCTCAGGGATGAC CTT-3′, 283 bp, which is
coded by nuclear DNA.
Clonogenic survival
Cells were plated in triplicate into 60-mm tissue
culture dishes at limiting dilutions. After irradiation (0, 1, 2,
4, 6, 8 and 10 Gy), the cells were incubated for 2 weeks to allow
colony formation. The colonies were then fixed in 70% ethanol and
stained with 1% crystal violet. A colony population should contain
>50 cells. Plating efficiencies (PEs) for untreated control
cultures were calculated using the following formula: PE = (number
of colonies counted/number of cells seeded) ×100. Surviving
fractions (SFs) were calculated using the following formula: SF =
(number of colonies counted)/(number of cells seeded xPE).
Cell cycle analysis
ϱ+ and ϱ0 cells in exponential
growth were irradiated with 4 Gy and collected at 4, 8, 12, 16 and
24 h. Cell cycle phase distributions were measured by flow
cytometry using propidium iodide (PI). Briefly, cells were
collected and fixed in suspension in 70% ethanol on ice and then
stored at 4°C. Cells were centrifuged at 500 g, washed with 1 ml
PBS, centrifuged again, and resuspended in PBS containing 20 μg/ml
PI and 10 μg/ml RNase A. After 30 min incubation in the dark at
room temperature, PI-stained cells were analyzed for DNA content by
flow cytometry, and the percentage of cells in G1, S and G2/M were
calculated using MODFIT software (12).
Reactive oxygen species assay
ϱ+ and ϱ0 cells in exponential
growth were plated in 96-well cell culture plates. Prior to 4 Gy
radiation, DCFH-DA was added and then incubated for 20 min; cells
were washed three times with serum-free cell culture in order to
sufficiently remove DCFH-DA not up-taken by the cells. Modulus™ II
Microplate was used to detect fluorescence immediately, and the
value at this time was recorded as ft0, every 30 min
afterwards, fluorescent values were denoted as ft30,
ft60, ft90 and ft120. The
fluorescent growth rate formula is:
[(ftn-ft0)/ft0] ×100% (13).
Statistical analyses
The relationships between mtDNA content, G10398A
polymorphism and survival time were analyzed by the χ2
test. Kaplan-Meier survival curves and the log-rank test were used
to analyze OS. Multivariate analysis was performed using the Cox
Proportional Hazards model.
Results
mtDNA content and G10398A
polymorphism
The mean Ct values for β2M sequence
(representing total nDNA) and NC_012920 gene sequence (representing
total mtDNA) in cancerous tissues ranged from 20.78 to 28.32 and
from 12.37 to 23.15, respectively. The mtDNA content of NSCLC
tissue samples ranged from 23.75 to 1833.01 copy number. The
analysis of G10398A polymorphism showed that 49.2% (63/128) was A
and 50.8% (65/128) was G (Fig.
1).
Prognostic significance of mtDNA content
and G10398A polymorphism
Low mtDNA content was more common in stage III and
IV than in stage I and II (71.9 vs. 28.1%, respectively;
χ2=6.433; P=0.018). There was no relationship between
mtDNA content and gender, age, smoking status, histology, EGFR
mutation status or G10398A polymorphism (Table II). mtDNA content and G10398A
polymorphism were subjected to survival analysis alone and in
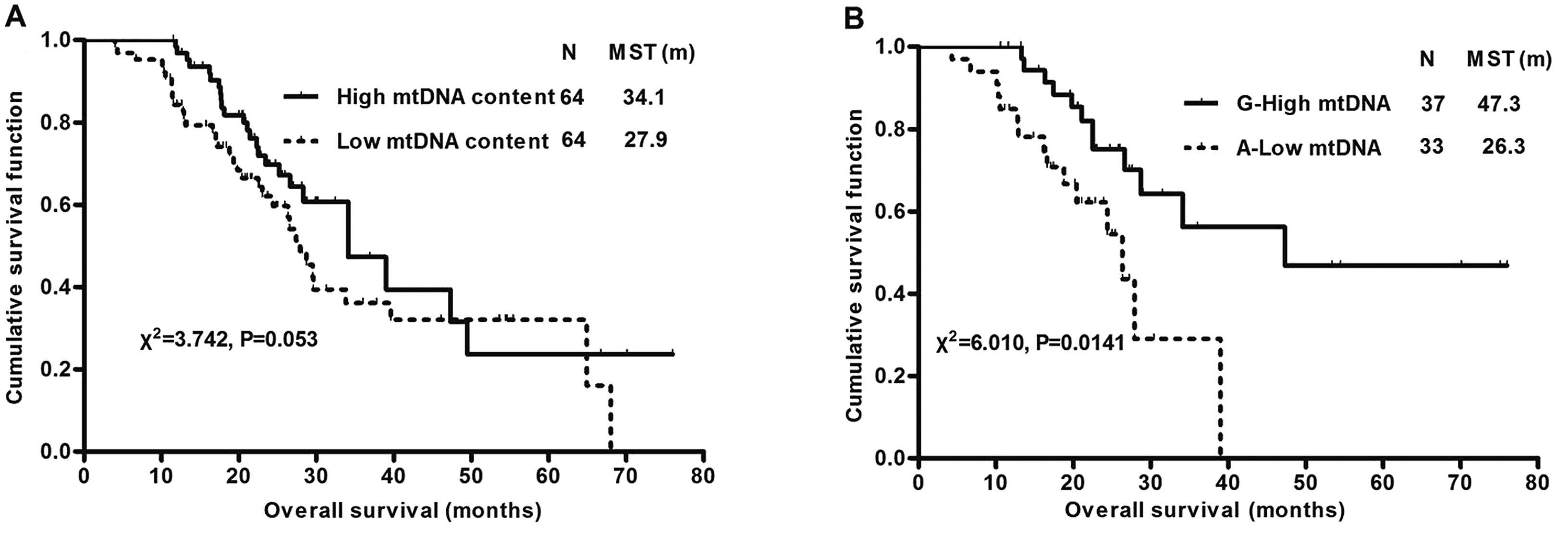
combination (Fig. 2). Low mtDNA
content patients had a marginally shorter survival time than high
mtDNA content patients (median survival time 27.9 vs. 34.1 months;
χ2=3.742; P=0.053). There was no survival difference
when G10398A polymorphism was analyzed alone. However, when
combining mtDNA content with G10398A polymorphism, OS in NSCLC
patients with low mtDNA content plus 10398A was significantly
shorter than in patients with high mtDNA content plus 10398G
(median survival time 26.3 vs. 47.3 months; χ2=6.010;
P=0.0141). All the analyzed clinical parameters (gender, age,
smoking status, histology and stage, EGFR mutation status) and
mtDNA (mtDNA content, G10398A polymorphism, mtDNA content plus
G10398A polymorphism) were entered into a multivariate analysis.
Cox regression analysis showed that stage, low mtDNA content plus
10398A were the two most independent prognostic factors in patients
with NSCLC (χ2=6.235, P=0.013; χ2=18.515,
P<0.0005, respectively, forward: Wald; P=0.05, entry; P=0.10,
removal) (Table III).
 | Table IIRelationships between mtDNA content
and clinical parameters (n=128). |
Table II
Relationships between mtDNA content
and clinical parameters (n=128).
| Variable | Low mtDNA content,
n (%) | High mtDNA content,
n (%) | χ2 | P-value | OR (95% CI) |
|---|
| Gender | | | 0.641 | 0.549 | 0.725
(0.329–1.595) |
| Male | 45 (47.4) | 50 (52.6) | | | |
| Female | 19 (57.6) | 14 (42.4) | | | |
| Age | | | 1.846 | 0.257 | 1.871
(0.752–4.655) |
| ≤50 | 15 (62.5) | 9 (37.5) | | | |
| >50 | 49 (47.1) | 55 (52.9) | | | |
| Smoking status | | | 0.533 | 0.584 | 1.306
(0.637–2.677) |
| No | 26 (54.2) | 22 (45.8) | | | |
| Yes | 38 (47.5) | 42 (52.5) | | | |
| Histology | | | 0.504 | 0.594 | 0.777
(0.387–1.560) |
| AC | 27 (46.6) | 31 (53.4) | | | |
| Others | 37 (52.9) | 33 (47.1) | | | |
| EGFR mutation | | | 0.321 | 0.571 | 1.381
(0.450–4.234) |
| No | 56 (87.5) | 58 (90.6) | | | |
| Yes | 8 (12.5) | 6 (9.4) | | | |
| Stage | | | 6.433 | 0.018 | 0.391
(0.188–0.814) |
| I+II | 18 (28.1) | 32 (50.0) | | | |
| III+IV | 46 (71.9) | 32 (50.0) | | | |
| 10398 | | | 0.781 | 0.480 | 1.368
(0.683–2.741) |
| A | 34 (54.0) | 29 (46.0) | | | |
| G | 30 (46.2) | 35 (53.8) | | | |
 | Table IIIMultivariate analysis of overall
survival. |
Table III
Multivariate analysis of overall
survival.
| Variable | Wald | P-value | OR | 95% CI |
|---|
| Stage | 6.235 | 0.013 | 2.186 | 1.152–4.147 |
| mtDNA plus AG | 18.515 | 0.000 | 0.392 | 0.256–0.601 |
Generation and verification of
ϱ0 cell model
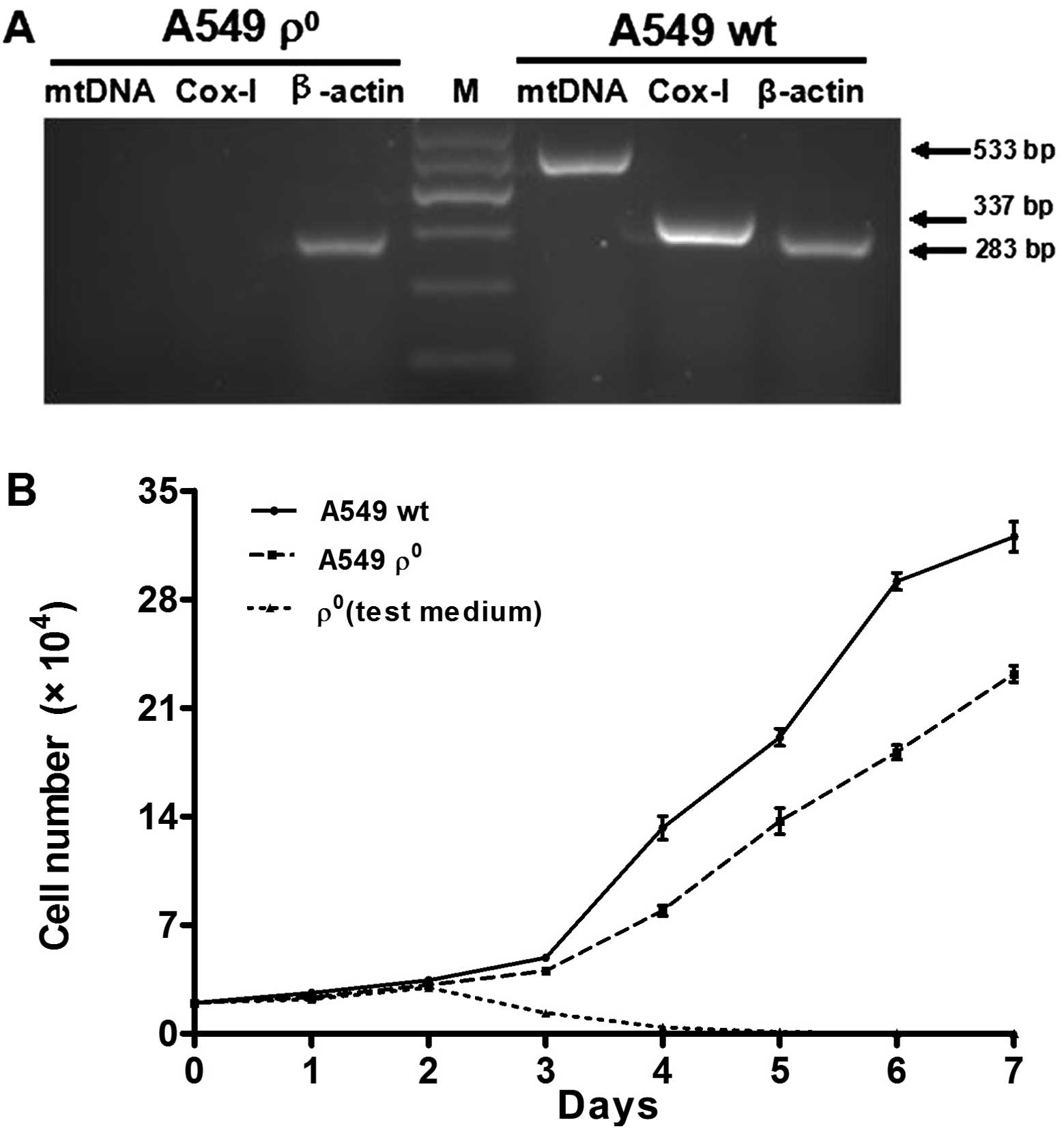
To verify the mtDNA depletion, total cellular DNA
was extracted and subjected to PCR using two pairs of human mtDNA
specific primers, as previously described. ϱ+ and
ϱ0 cells contained equivalent amounts of GAPDH,
indicating that nuclear DNA was similar between the cell lines.
However, ϱ+ cells contained mtDNA, while ϱ0
cells contained no mtDNA (Fig. 3A).
The growth defects experiment found growth inhibition in
ϱ0 cells when the culture medium lacked sodium pyruvate
and uridine. ϱ0 cells continued to proliferate at a
slower speed in the complete medium containing sodium pyruvate and
uridine (Fig. 3B).
Mitochondrial dysfunction results in
radioresistance in human NSCLC cells
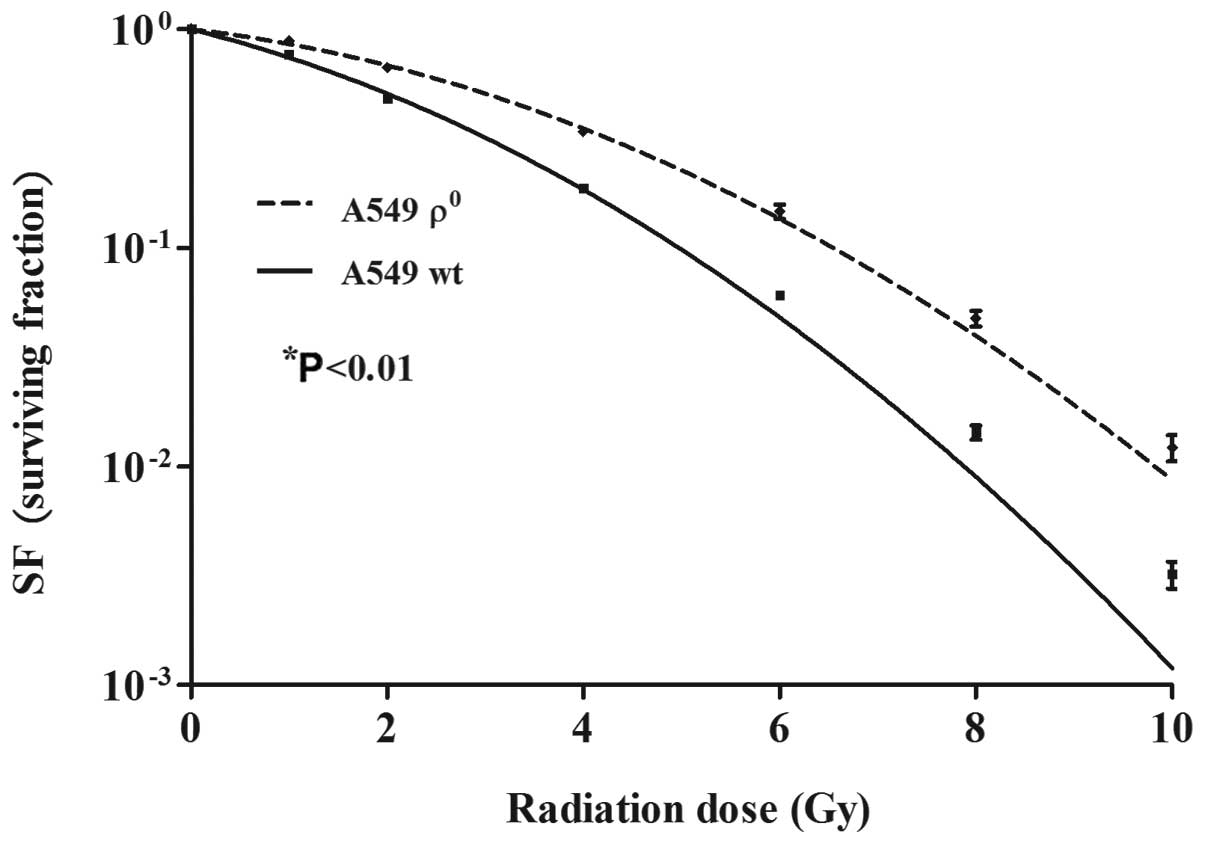
Colony formation assay was used to evaluate the
radiosensitivity of A549 ϱ0 and ϱ+ cells.
A549 ϱ0 and ϱ+ cells were treated with
different doses of X-ray irradiation, and the cloning efficiency
(PE) and survival fraction (SF) were calculated. The
linear-quadratic model was used to fit the survival curves
(Fig. 4). Surviving fraction of
cells after irradiation in 2 Gy (SF2) was 0.675±0.013
and 0.492±0.022 (P<0.01), suggesting that mtDNA deletion induced
radioresistance of A549 cells.
mtDNA depletion suppresses
radiation-induced G2 checkpoint activation in human NSCLC
cells
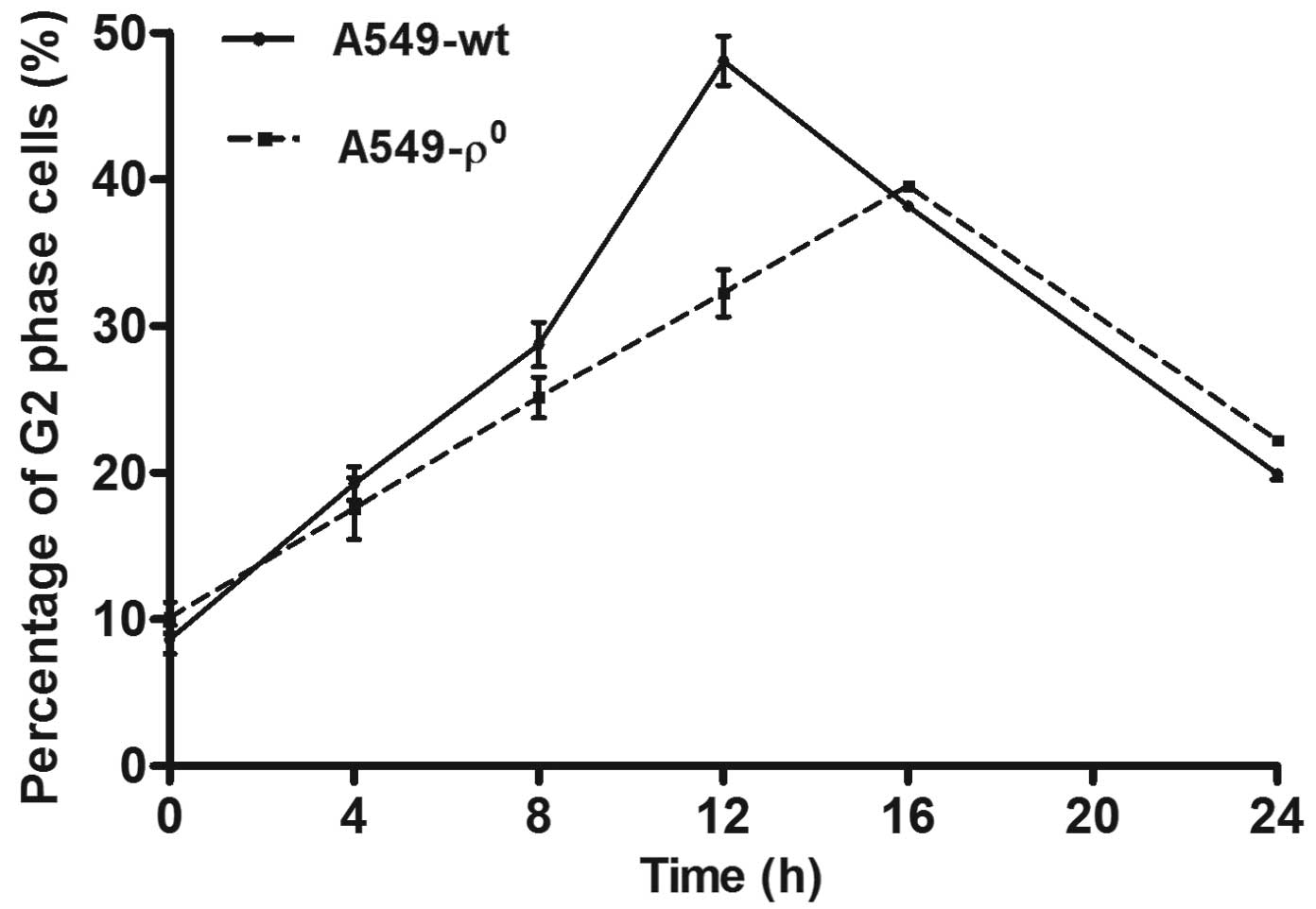
Cell cycle analysis showed that both ϱ0
and ϱ+ cells showed G2 arrest after 4 Gy radiation, but
the G2 arrest in ϱ0 cells was inferior to ϱ+
cells (39.55±0.50% vs. 48.08±2.92%, P<0.01). The A549
ϱ+ cell G2 arrest peak appeared at 12 h after
irradiation, while the A549 ϱ0 cell appeared at 16 h
(Fig. 5); S and G1 phase changes
had no significant difference.
mtDNA depletion suppresses
radiation-induced reactive oxygen species (ROS) production in human
NSCLC cells
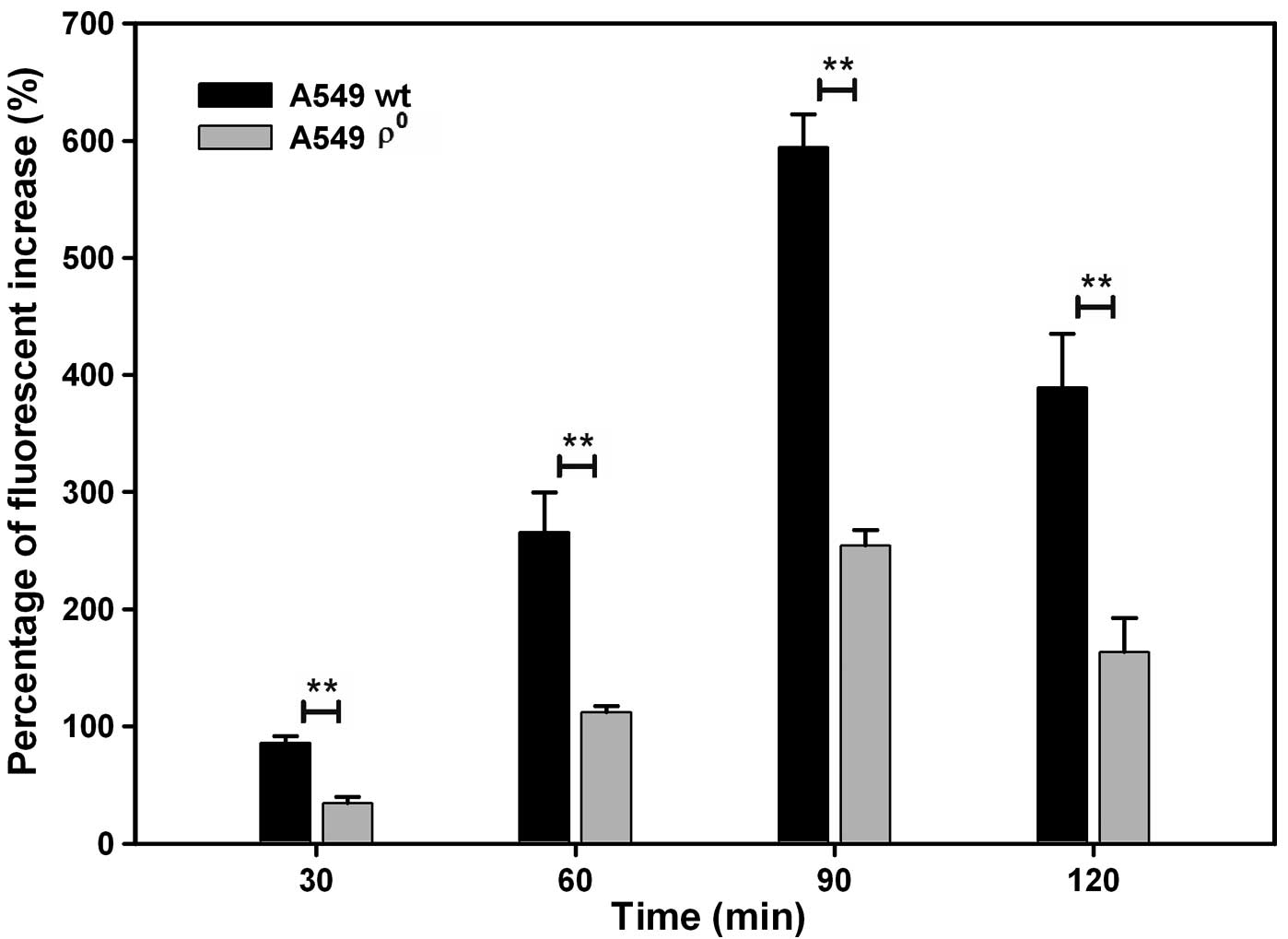
The ROS levels of ϱ0 cells were
significantly less than ϱ+ cells. The rate of
fluorescence increase (%) improved after the 4 Gy radiation
exposure and peaked at 90 min both in ϱ0 and
ϱ+ cells (254.17±13.65% vs. 594.28±38.22%, P<0.01)
(Fig. 6).
Discussion
Mitochondria are important and semi-autonomous
organelles in eukaryotic cells. Each mitochondrion contains 2–10
copies of its genome. The copy number of mtDNA in each cell varies
with cell type. In addition to plenty of somatic mutations in
mtDNA, the increase or reduction of mtDNA copy number has been
increasingly reported in a variety of primary human cancers,
underscoring that accumulation of mtDNA alterations may be a
pivotal factor in cancer pathogenesis and progression (14).
Previous studies indicated the potential involvement
of both mutations and alteration of mtDNA content in the
tumorigenesis of several malignancies (15). For instance, mtDNA content in
patient tissues has been found to be increased in head and neck as
well as in ovary cancer (8), but
decreased in hepatocellular carcinoma (HCC) (16), renal cell carcinoma (RCC) (17) and advanced gastric cancer (18). These results highly suggested that
the role of mtDNA in human cancer was cancer site-specific. A
case-control study including 260 RCC patients and 281 matched
control subjects showed that low mtDNA content was associated with
a significantly increased risk of RCC (OR, 1.53; 95% CI, 1.07–2.19)
(19).
Furthermore, mtDNA content was also found to be
related to patient prognosis. After an anthracycline-based regimen,
the disease-free survival time of breast cancer patients with
higher mtDNA content was significantly shorter than that of
patients with lower mtDNA content (P=0.03) (20). Patients with HCC harboring lower
mtDNA quantity reportedly tended to have poorer prognosis and
shorter 5-year overall survival (OS) rates in comparison with the
cases with higher mtDNA quantity (13). Similarly, the decline in mtDNA
levels was more prevalently identified in the ulcerated and in
filtrating type (Borrmann’s type III) and diffusely thick type
(Borrmann’s type IV) of gastric carcinoma, both of which are more
likely to have adverse post-operational outcome (21).
For NSCLC, Wang et al(17) found that the mean copy number of
mtDNA in lung carcinoma tissue samples was statistically lower than
that in adjacent histologically normal lung tissue samples. Lin
et al(7) reported that
decreased relative value of mtDNA copy number was linked to the
advancement of tumor progression. Similarly, our results revealed
that patients with low mtDNA content had a marginally shorter
survival time than those with high mtDNA content. The reason may be
the small sample size of our study.
mtDNA content as well as mtDNA mutations have been
reported to be potentially involved in cancer; 8701 and 10398 that
code for ATPase6 and NADH dehydrogenase 3 were reported to be
mutational hotspots in the mitochondrial genome of lung cancer. The
mtDNA G10398A polymorphism alters the structure of Complex I in the
mitochondrial electron transport chain, an important site of free
radical production. This polymorphism is associated with several
neurodegenerative disorders. American women with the 10398A allele
had a significantly increased risk of invasive breast cancer
(22). In addition, mtDNA G10398A
variant in African-American women with breast cancer provides
resistance to apoptosis and promotes metastasis in mice (23). However, the relationship between
G10398A polymorphism and NSCLC prognosis has not been reported. In
this study, in NSCLC patients with high mtDNA content plus 10398G,
OS increased by 79.8% and death risk decreased compared to patients
with low mtDNA content plus 10398A (median survival time 47.3 vs.
26.3 months; χ2=6.010; P=0.0141; OR, 0.392, 95% CI,
0.256–0.601).
To explore the possible mechanism of mtDNA influence
on the prognosis of NSCLC, ϱ0 cell model was established
by long-term exposure to low concentration of ethidium bromide. Our
results showed that the A549 ϱ0 cells showed more
resistance to radiation than ϱ+ cells. Upon irradiation,
ϱ0 cells showed delayed G2 arrest and decreased ability
to recover from the G2 checkpoint compared to ϱ+ cells.
Moreover, loss of mtDNA inhibited cell growth and reduced the level
of ROS (24). Recently, a growing
body of functional experiments suggested that mtDNA content
variations have enough capability to affect several aspects of
malignant cell behaviors, such as anticancer drug sensitivity, cell
growth, apoptosis as well as their invasive and metastatic
potentials (14). Disruption of
mtDNA integrity has been demonstrated to significantly influence
cancer cell proliferation both in vitro and in vivo.
For instance, mtDNA-depleted leukemia MOLT-4 ϱ0 cells
grew markedly slower than their respective parental cells, probably
due to reduced ROS generation (25). Similarly, researchers have shown
that mtDNA loss considerably decreased proliferative rate and
inhibited anchorage-independent growth and in vivo
tumorigenicity of T47D breast cancer cells (26).
mtDNA copy number alterations have been revealed to
facilitate cancer cells in acquiring resistance to a number of
antitumor chemotherapeutic agents and radiation. SK-Hep1 hepatoma
cells lacking mtDNA exhibited markedly reduced apoptotic death when
exposed to doxorubicin and two other oxidative stressors, menadione
and paraquat (27). mtDNA depletion
of human pancreatic tumor cells (MiaPaCa-2) suppressed
radiation-induced G2 checkpoint activation, which was accompanied
by increases in both cyclin B1 and CDK1, resulted in
radioresistance (28). However,
Kawamura (29) reported that
ϱ+ cells were more resistant to irradiation than
ϱ0 cells. The p53 status of the cell lines used were
different, Kulawiec et al(30) reported that p53 could regulate mtDNA
copy number and mitocheckpoint pathway, which may explain the
different results.
In conclusion, our results highlight the complex
relationships between mtDNA copy number and G10398A mutation in the
prognosis of patients with NSCLC. There was no relationship between
mtDNA content and gender, age, smoking status, histology, EGFR
mutation status or G10398A polymorphism. Thus, other interfering
prognostic factors in this paper have been eliminated. Low mtDNA
content plus 10398A could be a marker of poor prognosis in NSCLC.
mtDNA copy number decrease and 10398 mutation may lead to
mitochondrial dysfunction, thereby influencing biological behaviors
and the sensitivity to anticancer treatment so as to result in the
poor prognosis. The pitfall of this study is its small sample size.
Therefore, further research is required to verify the prognostic
value of mtDNA copy number and G10398A polymorphism.
Acknowledgements
This study was sponsored by the National Natural
Science Foundation of China (no. 81172129). The authors thank Dr
Gui-Fang Yang and You Wang for their support in pathology.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2010. View Article : Google Scholar
|
|
2
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng J, Yang LX, Zhao XY, Gao ZQ, Yang J,
et al: VCP gene variation predicts outcome of advanced
non-small-cell lung cancer platinum-based chemotherapy. Tumour
Biol. 34:953–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sánchez-Aragó M, Chamorro M and Cuezva JM:
Selection of cancer cells with repressed mitochondria triggers
colon cancer progression. Carcinogenesis. 31:567–576.
2010.PubMed/NCBI
|
|
5
|
Chatterjee A, Dasgupta S and Sidransky D:
Mitochondrial subversion in cancer. Cancer Prev Res (Phila).
4:638–654. 2011. View Article : Google Scholar
|
|
6
|
Wang H and Dai J: Changes on mitochondrial
DNA content in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi.
14:141–145. 2011.(In Chinese).
|
|
7
|
Lin CS, Wang LS, Tsai CM and Wei YH: Low
copy number and low oxidative damage of mitochondrial DNA are
associated with tumor progression in lung cancer tissues after
neoadjuvant chemotherapy. Interact Cardiovasc Thorac Surg.
7:954–958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi SJ, Kim SH, Kang HY, Lee J, Bhak JH,
et al: Mutational hotspots in the mitochondrial genome of lung
cancer. Biochem Biophys Res Commun. 407:23–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pezzotti A, Kraft P, Hankinson SE, Hunter
DJ, Buring J and Cox DG: The mitochondrial A10398G polymorphism,
interaction with alcohol consumption, and breast cancer risk. PloS
One. 4:e53562009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin X, Zhang J, Gao Y, Ding K, Wang N, et
al: Relationship between mitochondrial DNA mutations and clinical
characteristics in human lung cancer. Mitochondrion. 7:347–353.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malik AN, Shahni R, Rodriguez-de-Ledesma
A, Laftah A and Cunningham P: Mitochondrial DNA as a non-invasive
biomarker: accurate quantification using real time quantitative PCR
without co-amplification of pseudogenes and dilution bias. Biochem
Biophys Res Commun. 412:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawada I, Soejima K, Watanabe H, Nakachi
I, Yasuda H, et al: An alternative method for screening EGFR
mutation using RFLP in non-small cell lung cancer patients. J
Thorac Oncol. 3:1096–1103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Datta S, Majumder M, Biswas NK, Sikdar N
and Roy B: Increased risk of oral cancer in relation to common
Indian mitochondrial polymorphisms and Autosomal GSTP1 locus.
Cancer. 110:1991–1999. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu M: Generation, function and diagnostic
value of mitochondrial DNA copy number alterations in human
cancers. Life Sci. 89:65–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu M: Somatic mitochondrial DNA mutations
in human cancers. Adv Clin Chem. 57:99–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim MM, Clinger JD, Masayesva BG, Ha PK,
Zahurak ML, et al: Mitochondrial DNA quantity increases with
histopathologic grade in premalignant and malignant head and neck
lesions. Clin Cancer Res. 10:8512–8515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Liu VW, Xue WC, Cheung AN and Ngan
HY: Association of decreased mitochondrial DNA content with ovarian
cancer progression. Br J Cancer. 95:1087–1091. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamada S, Nomoto S, Fujii T, Kaneko T,
Takeda S, et al: Correlation between copy number of mitochondrial
DNA and clinico-pathologic parameters of hepatocellular carcinoma.
Eur J Surg Oncol. 32:303–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xing J, Chen M, Wood CG, Lin J, Spitz MR,
et al: Mitochondrial DNA content: its genetic heritability and
association with renal cell carcinoma. J Natl Cancer Inst.
100:1104–1112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu CW, Yin PH, Lee HC, Chi CW and Tseng
LM: Mitochondrial DNA content as a potential marker to predict
response to anthracycline in breast cancer patients. Breast J.
16:264–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu CW, Yin PH, Hung WY, Li AF, Li SH, et
al: Mitochondrial DNA mutations and mitochondrial DNA depletion in
gastric cancer. Genes Chromosomes Cancer. 44:19–28. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Canter JA, Kallianpur AR, Parl FF and
Millikan RC: Mitochondrial DNA G10398A polymorphism and invasive
breast cancer in African-American women. Cancer Res. 65:8028–8033.
2005.
|
|
23
|
Kulawiec M, Owens KM and Singh KK: mtDNA
G10398A variant in African-American women with breast cancer
provides resistance to apoptosis and promotes metastasis in mice. J
Hum Genet. 54:647–654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anoopkumar-Dukie S, Conere T, Sisk GD and
Allshire A: Mitochondrial modulation of oxygen-dependent
radiosensitivity in some human tumour cell lines. Br J Radiol.
82:847–854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Armand R, Channon JY, Kintner J, White KA,
Miselis KA, et al: The effects of ethidium bromide induced loss of
mitochondrial DNA on mitochondrial phenotype and ultrastructure in
a human leukemia T-cell line (MOLT-4 cells). Toxicol Appl
Pharmacol. 196:68–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu M, Shi Y, Wei X, Yang Y, Zhou Y, et al:
Depletion of mitochondrial DNA by ethidium bromide treatment
inhibits the proliferation and tumorigenesis of T47D human breast
cancer cells. Toxicol Lett. 170:83–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park SY, Chang I, Kim JY, Kang SW, Park
SH, et al: Resistance of mitochondrial DNA-depleted cells against
cell death: role of mitochondrial superoxide dismutase. J Biol
Chem. 279:7512–7520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cloos CR, Daniels DH, Kalen A, Matthews K,
Du J, et al: Mitochondrial DNA depletion induces radioresistance by
suppressing G2 checkpoint activation in human pancreatic cancer
cells. Radiat Res. 171:581–587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawamura SD, Takai K, Watanabe J, Hayashi
J, Hayakawa K and Akashi M: Role of mitochondrial DNA in cells
exposed to irradiation: generation of reactive oxygen species (ROS)
is required for G2 checkpoint upon irradiation. J Health Sci.
51:385–393. 2005. View Article : Google Scholar
|
|
30
|
Kulawiec M, Ayyasamy V and Singh KK: p53
regulates mtDNA copy number and mitocheckpoint pathway. J Carcinog.
8:82009. View Article : Google Scholar : PubMed/NCBI
|