Introduction
Gastric cancer (GC) is the fourth most common
malignant tumor worldwide (1). GC
patients in China have an extremely high rate of morbidity and
mortality. Patients with early-stage GC account for only 20% of
cases in China, which is much less than that in Japan and Korea.
Therefore, most GC patients in China are diagnosed with advanced GC
and require comprehensive therapy including surgery, radiotherapy
and chemotherapy. Currently, there are many neoadjuvant, adjuvant
and palliative chemotherapies available for GC patients, such as
ECF, DCF, oxaliplatin and 5-fluorouracil (5-Fu). However, the most
crucial drug is 5-Fu. Recently, targeted drugs have become an
intense field in the research and application of GC treatment.
Cetuximab (targeted to EGFR), bevacizumab (targeted to VEGF) and
Herceptin (targeted to Her-2) have been applied in clinical trails
of gastric cancer. Importantly, in October 2010, the Food and Drug
Administration approved the combination of Herceptin and
chemotherapy for the treatment of late-stage or metastatic GC
patients with positive expression of Her-2.
However, to date, no other targeted drugs have
achieved a breakthrough in the treatment of GC apart for Herceptin.
Although targeted drug treatment is the future direction, exploring
new therapeutic targets in GC has become an essential goal. Katoh
(2) suggested that the FGFR family
may be important in clinical cancer diagnostics and therapeutics.
Several literature reports indicate that FGFR4, a member of the
FGFR family, has a crucial role in tissue repair and embryonic
development (3,4). However, the role of FGFR4 in GC has
not been fully clarified.
Our previous research showed that the mRNA
expression of FGFR4 was markedly increased in gastric cancer tissue
when compared with that in corresponding normal tissue as detected
by real-time PCR. The FGFR4 Arg388 genotype, a marker for GC
progression, was suggested to predict prognosis in GC (5). Furthermore, a series of functional
assays in vitro, utilizing small interfering RNA, were
carried out, including proliferation assay, clone assay and
apoptosis detection. The results demonstrated that knockdown of
FGFR4 expression led to a decrease in the proliferative ability and
an increase in the apoptosis rate in the MKN45 and SGC7901 GC cell
lines. Moreover, western blot analysis demonstrated that the
expression of caspase-3 was observably increased while Bcl-xl
expression was markedly decreased in MKN45 and SGC7901 cells
following FGFR4-siRNA transfection. Therefore, FGFR4 may contribute
to GC progression through regulation of proliferation and
anti-apoptosis, indicating that FGFR4 may be used as a novel drug
target against GC (6).
In order to further clarify the clinical value of
FGFR4 expression in GC and explore new targeted drugs, PD173074
(PD), a FGFR inhibitor, was introduced in the present study. In
terms of the application of Herceptin, we first investigated
whether a single agent treatment and the combination of 5-Fu and PD
influence the biological behavior of GC cells using a series of
functional research methods in vitro, including
proliferation assay, apoptosis detection, assessment of cell cycle
distribution as well as determination of the expression of cell
signal pathway and downstream effector molecules by western blot
analysis. Furthermore, to verify whether PD has an impact on GC
cells by inhibiting FGFR4, FGF19, a special agonist of FGFR4, was
applied in the present study. Through a series of functional
assays, we aimed to clarify the mechanism of PD and 5-Fu effects on
GC cells. We suggest that PD has the potential to become a new
targeted drug similar to Herceptin and could be applied for the
treatment of GC patients.
Materials and methods
Antibodies and reagents
The rabbit polyclonal anti-FGFR4 antibody was
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Rabbit monoclonal anti-Bcl-xl, anti-Akt, anti-phospho-Akt,
anti-phospho-ERK, anti-caspase-3 and anti-GAPDH antibodies were all
purchased from Cell Signaling Technology (Beverly, MA, USA).
Secondary horseradish peroxidase-conjugated antibodies included
goat anti-mouse and goat anti-rabbit from Sigma-Aldrich Corp. (St.
Louis, MO, USA). PD173074 (P2499) was purchased from Sigma-Aldrich
Corp. (Shanghai, China), and recombinant human FGF19 was obtained
from PeproTech Inc. (Rocky Hill, NJ, USA). 5-Fu was provided by the
clinical trial group in our research center.
Cell lines and cell culture
Human gastric cancer cell lines, SNU-1 and SNU-16,
were purchased from the American Type Culture Collection (Manassas,
VA, USA). MKN45 and SGC7901 cell lines were obtained from the
Chinese Academy of Sciences, the Sciences Cell Bank of the Type
Culture Collection (CBTCCCAS, Shanghai, China). Cell lines were
cultivated in RPMI-1640 medium (Gibco, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U
ml−1 of penicillin and 100 μg ml−1 of
streptomycin (Caisson Laboratories, Inc., North Logan, UT, USA) at
37°C in a humidified atmosphere containing 5% CO2.
Reverse transcriptase-PCR
According to the protocol supplied by Invitrogen
(San Diego, CA, USA), TRIzol was used to extract total RNA from the
GC cell lines. RevertAid™ First Strand cDNA synthesis kit (MBI,
Fermantas, Burlington, ON, Canada) was used to reverse transcribe 1
μg of each RNA sample into cDNA in a total volume of 20 μl. Primers
consisted of: FGFR-4, 5′-AGATGCTCAAAGACAACGCCT-3′ and
5′-CGCACTCCACGATCACGTA-3′; GAPDH 5′-GAAGAT GGTGATGGGATTTC-3′ and
5′-GAAGGTGAAGGTCGG AGTC-3′. Taq 2X PCR Master Mix (Tiangen
Biotech, Co., Ltd., Beijing, China) was used for PCR amplication.
The annealing temperature of FGFR4 was 57°C. PCR products were
subjected to electrophoresis on a 2% agarose gel and were
visualized by ethidium bromide staining.
Quantitative real-time PCR
Gene specific primers for FGFR4 and GAPDH were the
same as those used for PCR in the present study. Specificity of
real-time PCR primers was checked by melting curve analysis and by
loading PCR products on agarose gel. Four gastric cancer cell line
specimens were used to carry out real-time PCR in a final reaction
volume of 20 μl according to the protocol supplied by Takara
(Shiga, Japan). The experiment was carried out in duplicate. GAPDH
was used as a housekeeping control for possible differences in cDNA
amounts. Relative differences (-fold) were calculated according to
the comparative Ct method.
Protein extraction and western
blotting
Cells were harvested following treatment for 72 h,
and whole-cell lysates were prepared using the Mammalian Protein
Extraction reagent (Merck, Darmstadt, Germany) in accordance with
the manufacturer’s instructions. Protein concentrations of the
samples were determined by the bicinchoninic acid (BCA) protein
assay (Pierce, Rockford, IL, USA). Protein samples (40 μg of each
protein) boiled for 5 min were separated on 10% SDS-polyacrylamide
gels and transferred onto PVDF membranes. The membranes were
blocked for 1 h at room temperature with phosphate-buffered saline
(PBS) containing 0.05% Tween-20 and 5% non-fat dried milk, and
incubated overnight at 4°C with the primary antibodies following
the manufacturer’s recommended conditions. Immunoblots were washed
three times with PBS containing 0.05% Tween-20 and 1% non-fat milk
and incubated with secondary antibodies conjugated with horseradish
peroxidase against mouse IgG or rabbit IgG for 1 h at room
temperature. Immunoreactive proteins were visualized using the ECL
detection system (ImageQuant LAS 3000; General Electric Co.,
Fairfield, CT, USA). Three independent western blot assays were
performed for all samples.
Proliferation assay
The effect of each compound on the proliferation of
GC cells was determined by Cell Counting Kit-8 (CCK-8) (Dojindo
Laboratories, Kumamoto, Japan) according to the instructions
provided by the manufacturer. Cell viability following treatment
with 5-Fu, PD and FGF19 was also determined using the CCK-8. The
concentrations of each compound were as follows in order to choose
a suitable concentration for a series of assays; PD173074 (0, 1.25,
2.5, 5, 10 and 20 μM) and FGF19 (0, 12.5, 25, 50, 100 and 200
ng/ml) were studied; 5-Fu (0, 50, 100, 200, 400 and 800 μM) was
used based on a previous study (7).
Cells, treated with the different compounds at the indicated
concentrations for 48 h, were detached using trypsin, and the
number of cells was counted using a hematocytometer counting
chamber (VWR International, Darmstadt, Germany). Cells
(2×103/well), were then incubated in 96-well culture
plates (Corning Inc., New York, NY, USA) in 100 μl of medium. After
culturing for 1, 2, 3, 4 and 5 days, the supernatant was removed,
and cell growth was detected using CCK-8 according to the
manufacturer’s instructions. Absorbance was measured at 450 nm
using a microplate reader. The percentage of cell viability was
determined as the ratio of the absorbance of the sample vs. the
control. The IC50 of the reagents was determined as the
concentration of each reagent exhibiting 50% cell growth inhibition
as compared with the control cell growth. Six replicate wells were
used for each reagent concentration. All experiments were performed
in triplicate and repeated at least three times.
Assay of apoptosis
Cells were treated with each agent alone or
combinations of PD, 5-Fu and FGF19 at the suitable concentration
for 24 h. Then the cells were harvested. Annexin V and propidium
iodide (PI) for flow cytometry were purchased from Invitrogen
(catalog no. V13241, USA) for detecting apoptosis. A working
solution of 5 μl of Annexin V and 1 μl 100 μg/ml PI was added to
each 100 μl of the cell suspension. The cells were incubated at
room temperature for 15 min. Subsequently, 400 μl 1X
Annexin-binding buffer was added, gently mixed and the sample was
kept on ice in accordance with the manufacturer’s instructions.
Thereafter, all samples were analyzed by a FACSCalibur flow
cytometer with CellQuest software (BD Biosciences, Mountain View,
CA, USA).
Statistical analysis
Statistical analysis was performed with SPSS 13.0
software (SPSS Inc., Chicago, IL, USA). Data are expressed as means
± SD, and three individual experiments were carried out in
triplicate. The Student’s t-test was used to compare data between
two groups. One-way ANOVA and Dunnett’s test were used to compare
data between three or more groups. P<0.05 was considered to
indicate a statistically significant result.
Results
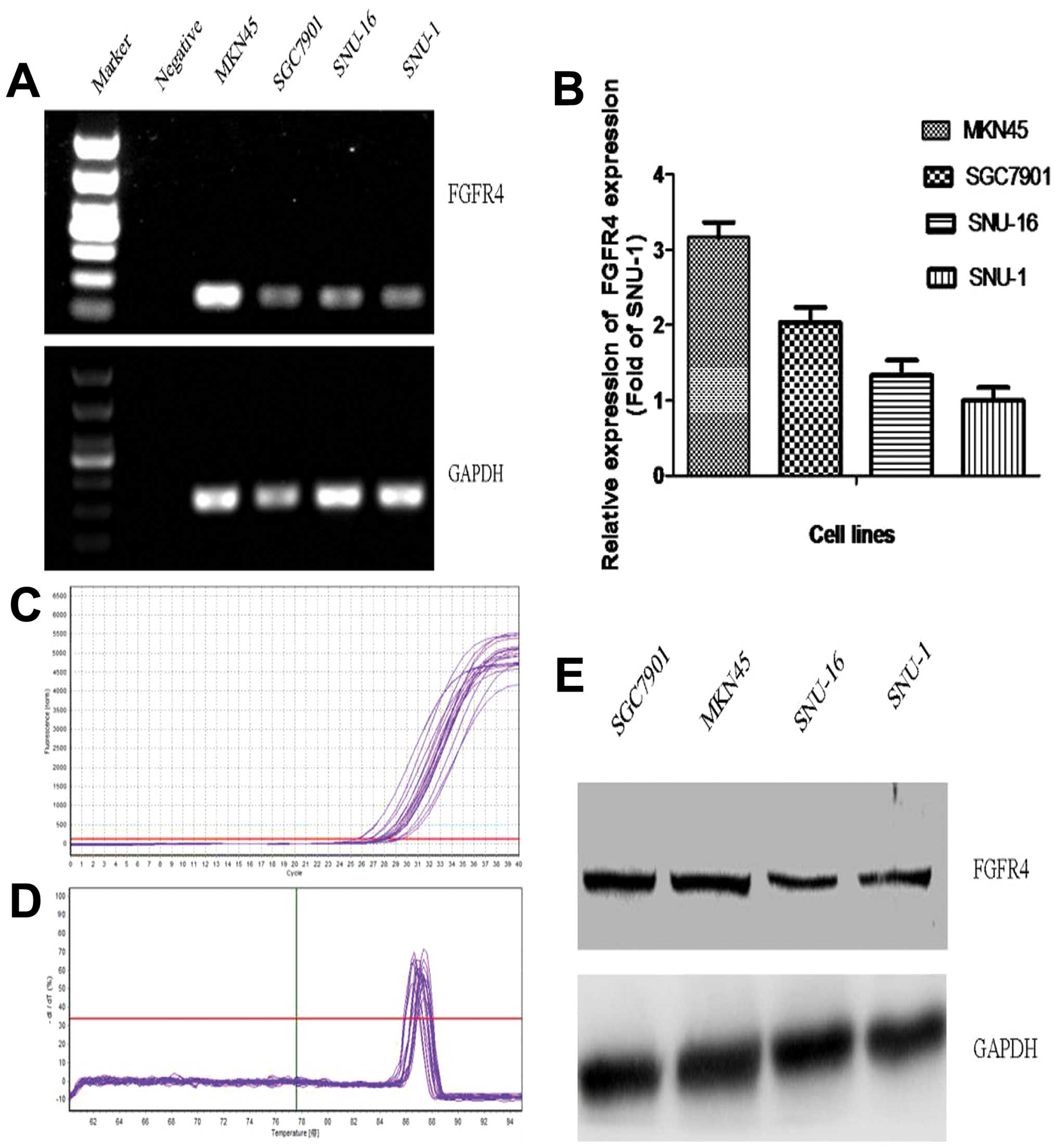
FGFR4 is expressed in the gastric cancer
cell lines
Expression of FGFR4 in the gastric cancer cell
lines, at the mRNA and protein levels, was evaluated using reverse
transcription PCR, quantitative real-time PCR and western blot
analysis. As shown in Fig. 1A,
expression of FGFR4 mRNA in the MKN45 cells was much stronger than
these levels in the other 3 common GC cell lines, SGC7901, SNU-1
and SNU-16. Moreover, the quantitative analysis results by
real-time PCR confirmed that expression of FGFR4 mRNA in the MKN45
cells was highest among the 4 common GC cell lines (Fig. 1B). In addition, expression of FGFR4
protein in the MKN45 and SGC7901 cells was obviously higher than
that in the other 2 cell lines; in particular, high expression of
FGFR4 protein was noted in the MKN45 cells (Fig. 1E). Therefore, the MKN45 cell line
was chosen to undergo subsequent assays.
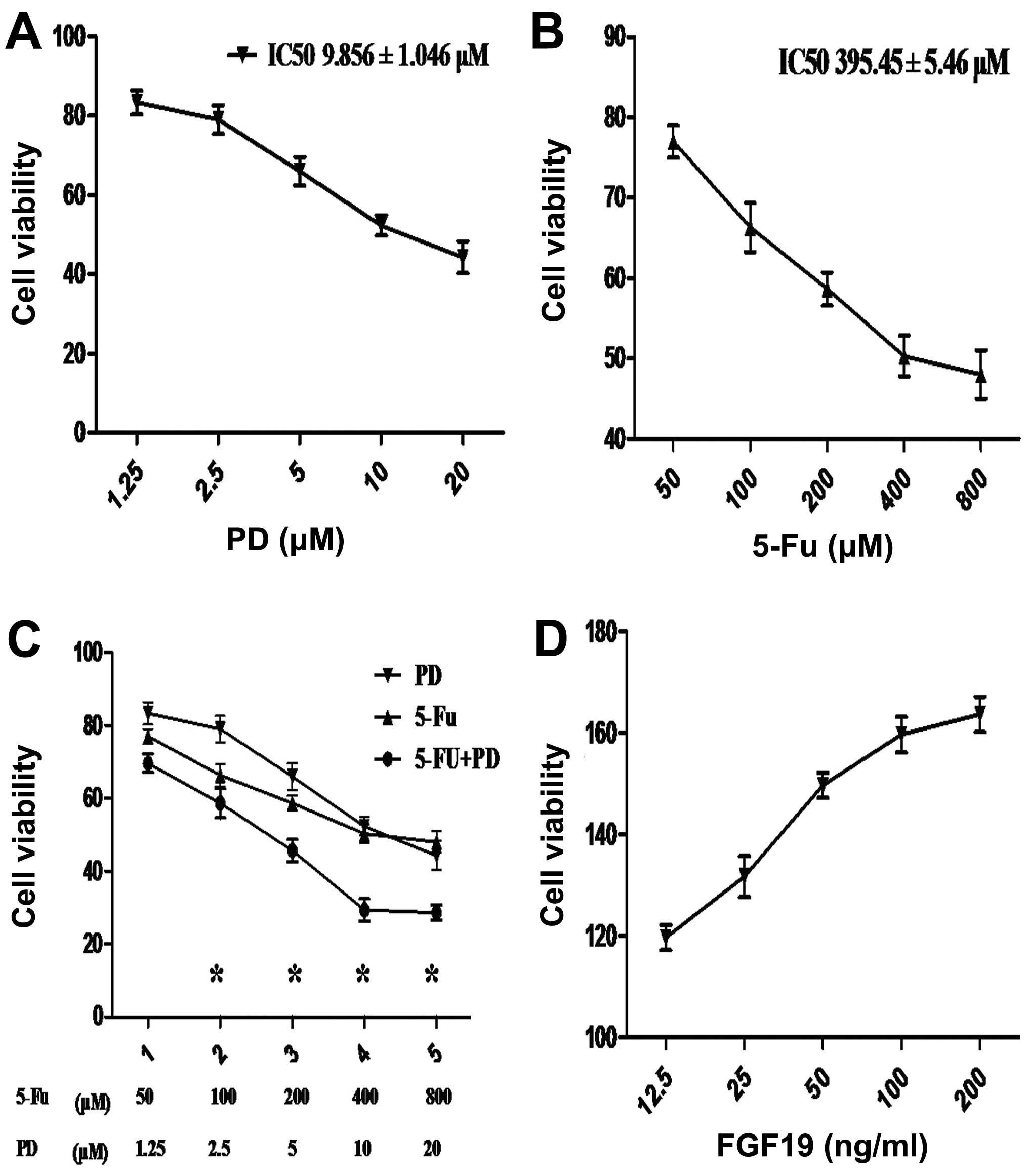
PD173074, 5-Fu and FGF19 have an impact
on the cell viability of MKN45 cells
To evaluate the growth effect of the different
agents on MKN45 cells, CCK-8 was used to detect the cell viability
using a microplate reader. As the concentration of PD increased,
the cell viability progressively decreased; the IC50
value was 9.856±1.046 μM (Fig. 2A).
A similar trend was found when MKN45 cells were treated with
different concentrations of 5-Fu; the IC50 was
395.45±5.46 μM (Fig. 2B). As shown
in Fig. 2C, the inhibitory effect
on the cell viability of MKN45 cells was more significant following
the combination treatment of 5-Fu and PD than that following single
administrations at different concentrations; the differences
achieved statistical significance (one-way ANOVA and Dunnett’s
test; P<0.05). Moreover, as the concentration of FGF19
increased, the cell viability gradually rose and the approximate
linear relation was shown at concentrations of 12.5–100 ng/ml
(Fig. 2D). In order to carry out
subsequent assays, the appropriate concentration of each agent was
chosen in the linear area. According to the above results, the
final suitable concentrations of PD, 5-Fu and FGF19 were 10 μM, 400
μM and 50 ng/ml, respectively.
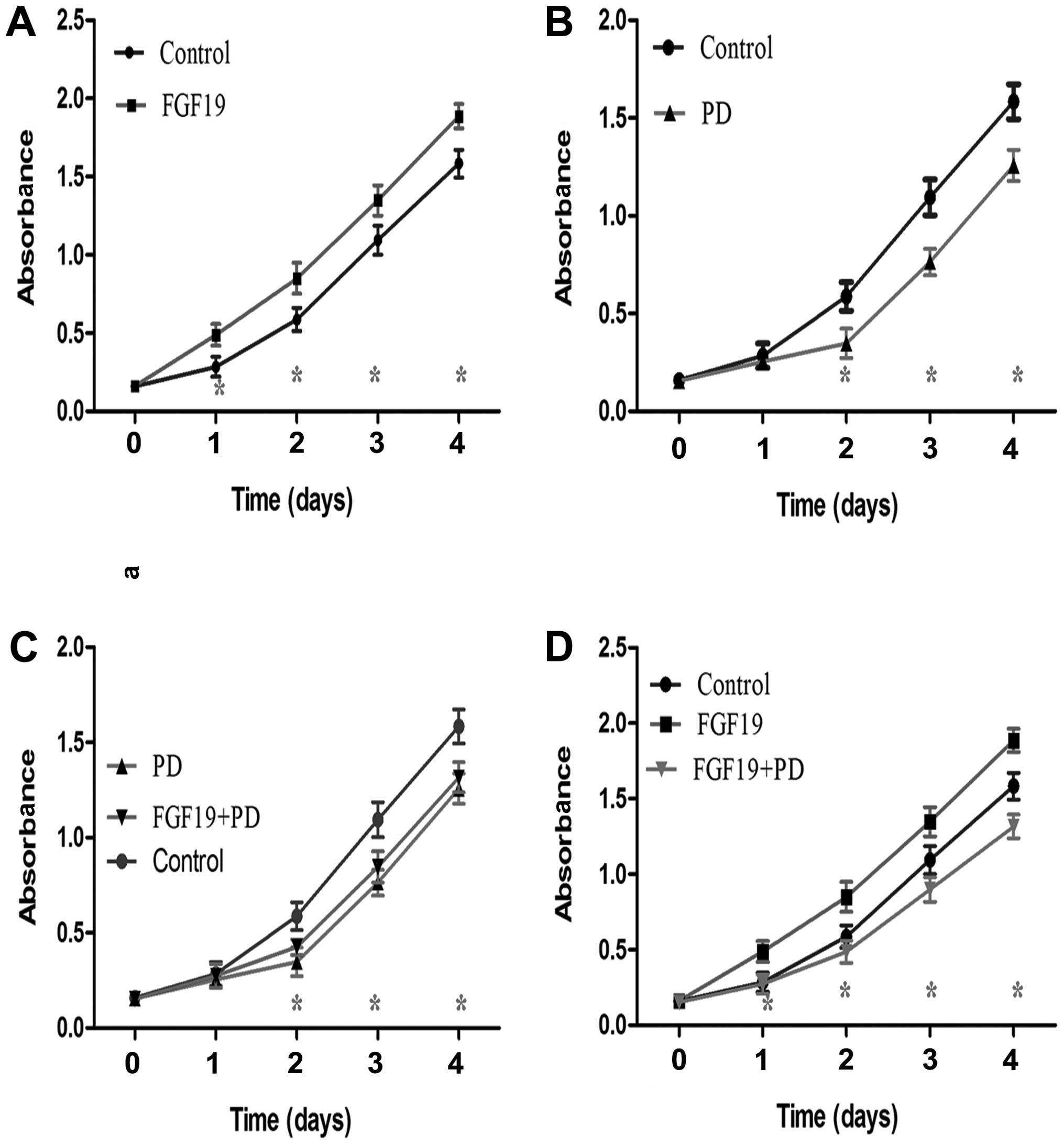
Single agent treatments and the
combination of PD173074 and FGF19 influence the proliferation of
MKN45 cells
To verify the effect of PD on the proliferation of
gastric cancer cells mainly by inhibiting the FGFR4 pathway, FGF19,
a special agonist of FGFR4, was introduced to clarify this issue.
The 4 treatment groups were categorized as follows: group 1 (FGF19,
50 ng/ml); group 2 (PD, 10 μM); group 3 (cells were treated with PD
for 2 h prior to adding FGF19); group 4 (cells were treated with
FGF19 for 2 h prior to adding PD). MKN45 cells without any
treatment served as the control group. CCK-8 was used to detect
cell absorbance to evaluate cell proliferation.
FGF19 markedly increased the proliferative ability
of the MKN45 cells when compared with that of the control group
(Fig. 3A, Student’s t-test;
P<0.05), particularly from day 2 to day 5 after CCK-8 detection.
Compare to the control, PD obviously weakened the proliferative
ability of the MKN45 cells from day 3 to day 5 after CCK-8 assay
(Fig. 3B, Student’s t-test;
P<0.05). As shown in Fig. 3C,
the effect of treatment with PD for 2 h prior to adding FGF19
(group 3) on the proliferation of MKN45 cells was similar to that
of cells treated with 10 μM PD (group 2) (Student’s t-test;
P>0.05), although a significant difference was noted when the
two groups were compared with control from day 3 to day 5 after
CCK-8 detection (Fig. 3C, one-way
ANOVA; P<0.05). However, the proliferation of MKN45 cells was
more significantly increased following single agent FGF19 treatment
than that following treatment of FGF19 for 2 h prior to adding PD
(group 4) and the control group from day 2 to day 5 after CCK-8
assay (Fig. 3D, one-way ANOVA;
P<0.05).
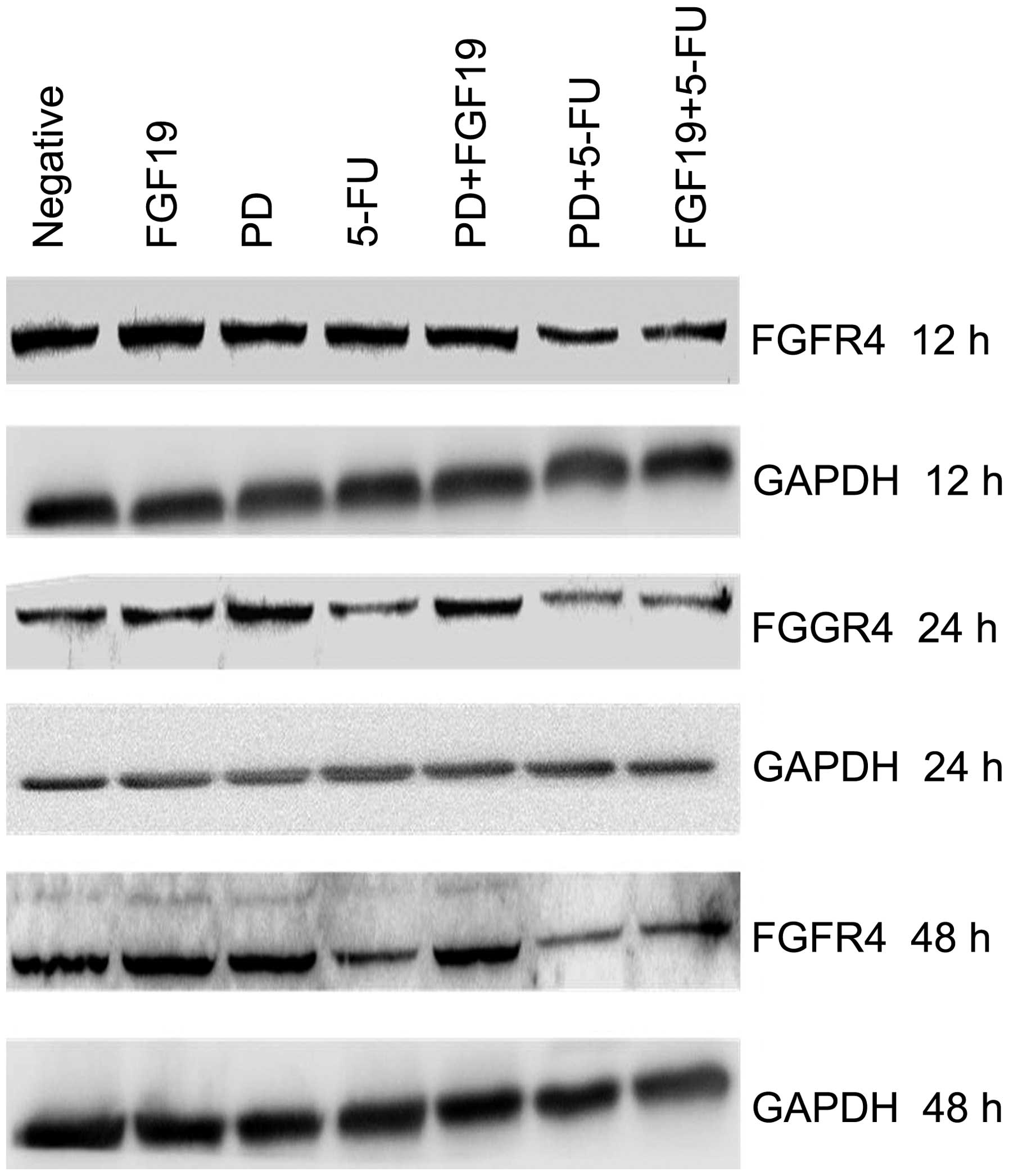
Single agent treatment and combinations
of FGF19, PD173074 and 5-Fu affect the expression of FGFR4 in MKN45
cells
To clarify whether the different reagents affect the
expression of FGFR4 in GC cells, western blot analysis was applied
to detect the expression of FGFR4 in MKN45 cells following
treatment with the single agents and combinations of FGF19, PD and
5-Fu for 12, 24 and 48 h, respectively. As shown in Fig. 4, the single agent treatments and the
combination of FGF19 and PD had no obvious influence on the
expression of FGFR4 in the MKN45 cells when compared with the
negative control. However, FGFR4 expression was markedly weakened
following single agent treatment with 5-Fu, the combination of 5-Fu
and PD as well as the combination of 5-Fu and FGF19, in particular
following treatment for 24 and 48 h (Fig. 4). In other words, 5-Fu reduced the
expression of FGFR4 in MKN45 cells.
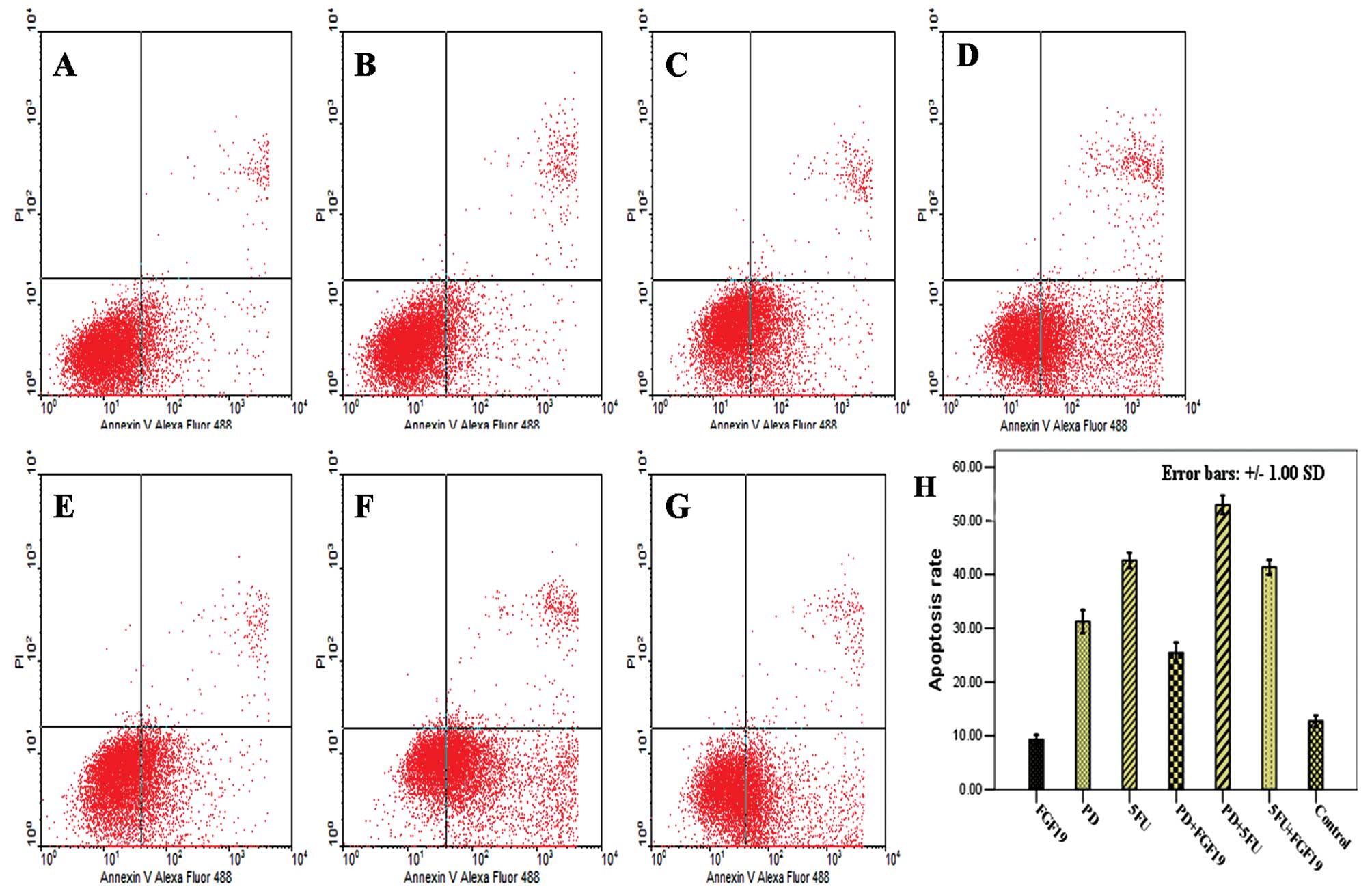
Single agent treatment and combinations
of FGF19, PD173074 and 5-Fu impact the apoptosis rate of MKN45
cells
To explore whether the different reagents impact the
apoptosis of GC cells, Annexin V and PI staining and flow cytometry
were used to detect the apoptosis rate of MKN45 cells following
treatments with the single agents and combinations of FGF19, PD and
5-Fu for 24 h. The apoptosis rate of the control group was
12.70±1.06%. An increased apoptosis rate was noted in all treatment
groups expect for cells treated with the single agent FGF19, and
the differences were significantly (Table I; one-way ANOVA, F-value=327.82;
P<0.000; Dunnett’s test, P<0.05). In comparison of the rates,
the apoptosis rate following the combination of 5-Fu and PD, the
highest following all treatments, was obviously increased when
compared with the apoptosis rate following treatment with the
single agent treatments of 5-Fu and PD (Fig. 5).
 | Table IEffect on the apoptosis rate following
different treatments in the MKN45 cells for 24 h (one-way
ANOVA). |
Table I
Effect on the apoptosis rate following
different treatments in the MKN45 cells for 24 h (one-way
ANOVA).
| Treatment | Apoptosis rate
(%)a | P-valueb |
|---|
| Control | 12.70±1.06 | |
| FGF19 (50 ng/ml) | 9.25±0.89 | 0.069 |
| PD (10 μM) | 31.23±2.09c | 0.000 |
| 5-FU (400 μM) | 42.64±1.86c | 0.000 |
| PD (10 μM) + FGF19
(50 ng/ml) | 25.44±1.86c | 0.000 |
| PD (10 μM) + 5-Fu
(400 μM) | 52.97±1.97c | 0.000 |
| FGF19 (50 ng/ml) +
5-Fu (400 μM) | 41.35±1.35c | 0.000 |
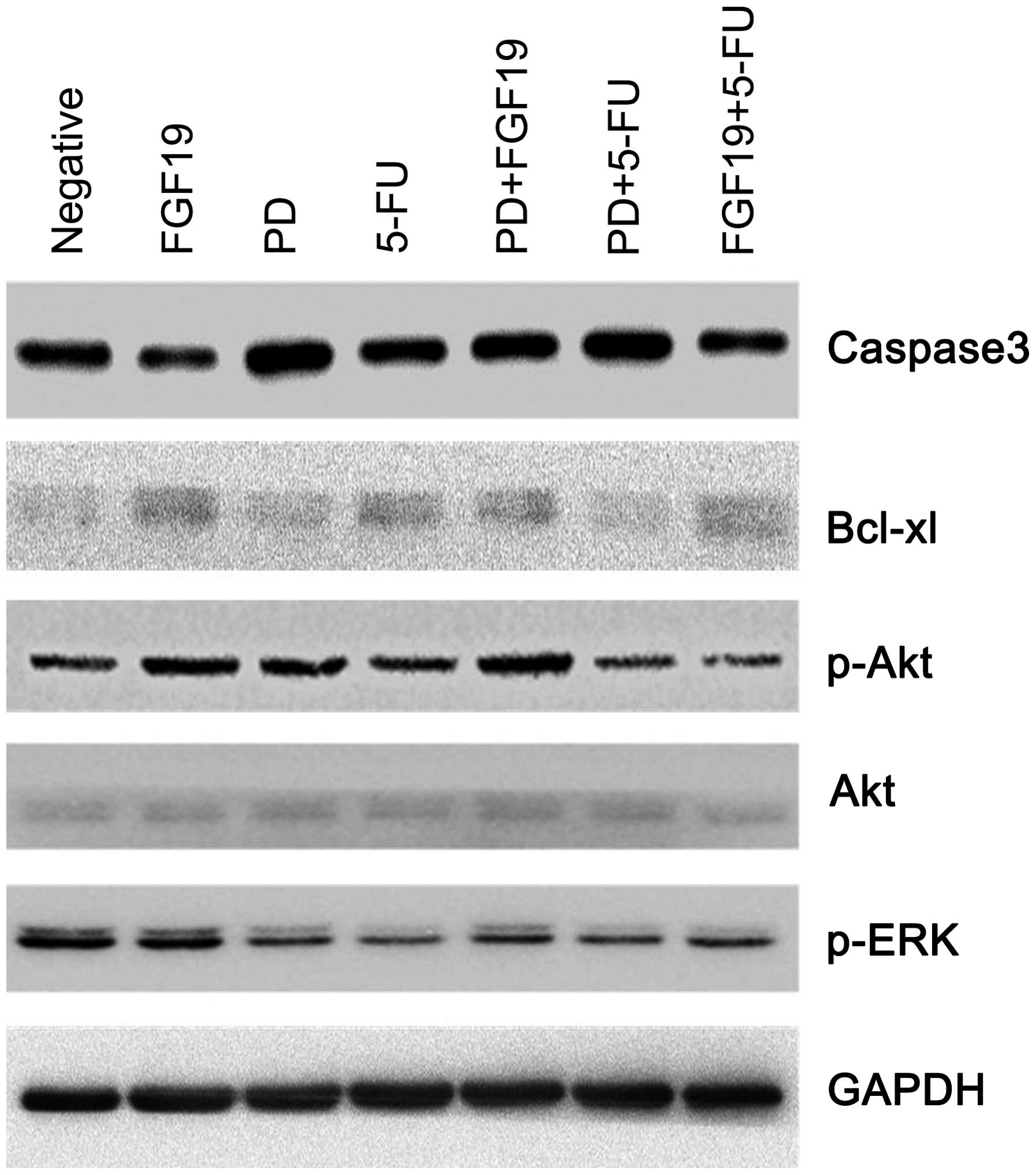
Different treatments affect the
expression of signaling pathway and downstream effector molecules
in MKN45 cells
To explore how different treatments affect the
biological behavior of MKN45 cells, expression of cell signaling
pathway-associated molecules (Akt, p-Akt and p-ERK) and
apoptosis-associated molecules (caspase-3 and Bcl-xl) was
determined to clarify the mechanism.
As shown in Fig. 6,
expression of p-Akt was increased in the single agent PD and single
FGF19 treatment groups while no obviously change was noted in the
5-Fu single agent treatment group when compared with the negative
control. However, the combination of 5-Fu and PD decreased p-Akt
expression. As for Akt, there were no marked changes in the
different treatment groups. Furthermore, compared to the negative
control, p-ERK expression was dramatically decreased in the single
agent and combination treatment groups of 5-Fu and PD.
When compared with the negative control, expression
of caspase-3 was obviously increased in the single agent PD group
while expression of Bcl-xl was prominently weakened. Furthermore,
compared to treatment with the single agent 5-Fu, expression of
caspase-3 was markedly strengthened while expression of Bcl-xl was
notably reduced following the combination treatment of 5-Fu and PD.
In other words, the combination of 5-Fu and PD had a synergistic
effect on promoting apoptosis. As for the FGF19 group, expression
of effector molecules was contrary to that noted in the single
agent PD treatment group. However, in the combination group of
FGF19 and PD, expression of effector molecules was similar to that
in the single agent PD treatment group when compared with that in
the single FGF19 treatment group. Therefore, PD influenced the
effect of FGF19 by inhibiting FGFR4.
Discussion
Recently, molecular-targeted drugs are prevalently
investigated in gastrointestinal tumors. However, no other targeted
drug has achieved a breakthrough in gastric cancer apart from
Herceptin. Therefore, it is urgently necessary that novel molecular
markers and therapeutic targets should be explored and
investigated. In addition to EGFR, VEGF and Her-2, the FGFR family
has currently received increased attention for the treatment of
malignant tumors, attracting the interest of numerous
investigators. Among them, FGFR4, a member of the FGFR family, has
become a popular research molecule in various tumors. However, the
effect of FGFR4 on GC has seldom been studied, and research is
urgently required to fully clarify its role. Our previous research
revealed that FGFR4 leads to GC progression by influencing
proliferation and causing an anti-apoptotic effect, suggesting
FGFR4 as a new drug target for the treatment of GC (6). Based on the clinical application of
Herceptin, the aim of the present study was to initially explore
whether single agent treatments and a combination of 5-Fu and
PD173074 impact the biological behavior of GC cells as well as the
mechanism of the two-drug combination using a series of functional
research methods in vitro.
PD was found to inhibit wild-type and constitutively
activated mutant FGFR3 autophosphorylation in multiple myeloma,
which was associated with decreased viability and tumor cell growth
arrest (8). Selective FGFR
inhibitor PD blocks H-510 and H-69 SCLC proliferation and
clonogenic growth in a dose-dependent fashion; PD was also found to
significantly strengthen the effect of cisplatin and increase
apoptosis (9). PD weakens the
activity of FGFR4 and reduces the phosphorylation FGFR4 in breast
cancer and medullary thyroid cancer (10,11).
In the thyroid cancer cell lines MRO and ARO with high FGFR4
expression, cell proliferation gradually decreased with increasing
PD concentration. SCID mice implanted with aggressively growing MRO
cells that endogenously express significant amounts of FGFR-4
demonstrated a significant decrease in tumor size following PD
treatment, suggesting that PD inhibits the activity of FGFR4 in
thyroid carcinoma (12). Specific
and complete reversal of FGF19-stimulated AFP production by PD
justified that inhibiting the activity of FGFR4 may be the most
important mechanism of PD in hepatocellular carcinoma; PD promoted
apoptosis and increased chemotherapy sensitivity in HCC (7).
Among more than 20 FGF ligands, only FGF19 can
directly and specifically combine to FGFR4 (13,14).
Moreover, no matter whether β-Klotho was present, FGF19 led to
biological effects by combining with FGFR4 (15). FGF19-induced hepatocyte
proliferation was mediated through FGFR4 activation (16). FGF19 reduced the expression of
cyp7A1 mRNA in liver tissue by FGFR4 activation (17). Furthermore, 5-Fu is a cell-cycle
specific chemotherapy drug and mainly arrests cells in the S
phase.
As shown in Fig. 1,
expression of FGFR4 in MKN45 cells, at the mRNA and protein levels,
was highest among the 4 common gastric cancer cell lines. Thus,
MKN45 cells were chosen for the subsequent assays. As the
concentration of 5-Fu and PD increased, following administration as
single agents, the cell viability of MKN45 cells in both groups
gradually declined. Moreover, the growth inhibition of MKN45 cells
following the combination treatment of 5-Fu and PD was more
significant than that in the single agent treatments, suggesting
that the combination of 5-Fu and PD had synergistic effects on the
growth inhibition of GC cells.
In the present study, the effects of the single
agent treatment and the combination of PD and FGF19 on the
proliferation of MKN45 was detected by CCK-8. The cells treated
with PD for 2 h prior to adding FGF19, PD could almost inhibit cell
proliferation as that promoted by FGF19 (Fig. 3C). However, when cells were treated
with FGF19 for 2 h prior to adding PD, the proliferation rate of
the combination group was significantly less than that of the
single agent FGF19 treatment group. Therefore, in MKN45 cells with
high expression of FGFR4, PD decreased the proliferation of GC
cells mainly through inhibiting the activity of FGFR4, which is in
accordance with the research in thyroid cancer and hepatocellular
carcinoma (7,12).
A significant findings was that FGFR4 expression was
obviously weakened in the single agent 5-Fu treatment group and in
the combination 5-Fu and PD/FGF19 treatment groups, particularly
after treatments for 24 and 48 h (Fig.
4). This indicated that 5-Fu reduced FGFR4 expression in the GC
cells, beyond the effect of FGF19 and PD. This finding clarifies
the mechanism of the synergistic effect in the combination group of
5-Fu and PD, through decreasing FGFR4 expression and inhibiting the
activity of FGFR4, respectively.
Compared with the negative control group, single
agent treatments of PD and 5-Fu increased the apoptosis rate of
MKN45 cells. Furthermore, the apoptosis rate following treatment
with the combination of 5-Fu and PD was much higher than that in
the single administration groups, suggesting that PD strengthens
the activity of 5-Fu in regulating apoptosis (Fig. 5). Western blot analysis showed that
PD increased caspase-3 expression and reduced Bcl-xl expression
when compared with the negative control, implying that PD promoted
MKN45 cell apoptosis. Roidl et al(18) reported that an FGFR4 inhibitor
reduced Bcl-xl expression in breast cancer cell lines, dramatically
increased the apoptosis rate of breast cancer cells and
strengthened the sensitivity to doxorubicin, which was in line with
our results. The apoptosis rate in the combination group of PD and
FGF19, similar to the single agent PD treatment group, was between
the values for the single agent treatment groups of the two drugs.
This indicates that PD may increase the apoptosis of GC by
inhibiting the FGF19/FGFR4 pathway. Furthermore, Drafahl et
al(19) found that activation
of FGFR4 may lead to a negative effect on the NF-κB pathway,
suggesting that NF-κB may be one of the signal pathways influencing
apoptosis.
Our research also investigated the effects of
different treatments on the expression of signaling pathway
molecules in MKN45 cells. Except for FGF19, p-ERK expression was
decreased with different levels in all other groups compared to the
negative control (Fig. 6). Roidl
et al(18) showed that FGFR4
inhibitor reduced p-ERK expression in breast cancer cells and
inhibited p-ERK activated by FGF19, which was in accordance with
our results. Akt expression had no obvious difference. However,
p-Akt expression was markedly increased following the single agent
treatments and the combination of FGF19 and PD while p-Akt
expression was slightly decreased following treatment with the
combinations of 5-Fu and FGF19/PD when compared to the negative
group, implying that p-Akt may have an effect on the FGF19/FGFR4
signaling pathway, which needs to be further clarified.
In conclusion, the present study initially explored
the effect and the mechanism of single agent treatments and
combinations of PD173074, FGF19 and 5-Fu on the biological
characteristics of gastric cancer cells. Inhibiting the activity of
FGFR4 may be one of the vital important mechanisms by which PD
inhibits GC cell proliferation and increases the rate of apoptosis.
5-Fu reduces the expression of the FGFR4 protein, which may provide
a basis for the treatment of gastric cancer by a combination of
5-Fu and PD. Furthermore, the combination of 5-Fu and PD had a
synergistic effect in reducing proliferation and increasing the
apoptosis rate in gastric cancer cells. The results of the present
study may contribute to the development of the new target drug
PD173074 in combination with 5-Fu, similar to Heceptin, for the
clinical treatment of gastric cancer. Obviously, our results should
be verified by further research in vitro and in
vivo.
Acknowledgements
The present study was supported by the Department of
Gastrointestinal Surgery, The First Affiliated Hospital, Zhengzhou
University and the National Natural Science Foundation of China
(grant no. 81201955).
Abbreviations:
|
FGFR4
|
fibroblast growth factor receptor
4
|
|
PD
|
PD173074
|
|
5-Fu
|
5-fluorouracil
|
|
CCK-8
|
Cell Counting Kit-8
|
|
GC
|
gastric cancer
|
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Katoh M: Genetic alterations of FGF
receptors: an emerging field in clinical cancer diagnostics and
therapeutics. Expert Rev Anticancer Ther. 10:1375–1379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Stockton DW and Ittmann M: The
fibroblast growth factor receptor-4 Arg388 allele is associated
with prostate cancer initiation and progression. Clin Cancer Res.
10:6169–6178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eswarakumar VP, Lax I and Schlessinger J:
Cellular signaling by fibroblast growth factor receptors. Cytokine
Growth Factor Rev. 16:139–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ye Y, Shi Y, Zhou Y, et al: The fibroblast
growth factor receptor-4 Arg388 allele is associated with gastric
cancer progression. Ann Surg Oncol. 12:3354–3361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye YW, Zhou Y, Yuan L, et al: Fibroblast
growth factor receptor 4 regulates proliferation and antiapoptosis
during gastric cancer progression. Cancer. 117:5304–5313. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ho HK, Pok S, Streit S, et al: Fibroblast
growth factor receptor 4 regulates proliferation, anti-apoptosis
and alpha-fetoprotein secretion during hepatocellular carcinoma
progression and represents a potential target for therapeutic
intervention. J Hepatol. 50:118–127. 2009. View Article : Google Scholar
|
|
8
|
Trudel S, Ely S, Farooqi Y, et al:
Inhibition of fibroblast growth factor receptor 3 induces
differentiation and apoptosis in t(4;14) myeloma. Blood.
103:3521–3528. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pardo OE, Latigo J, Jeffery RE, et al: The
fibroblast growth factor receptor inhibitor PD173074 blocks small
cell lung cancer growth in vitro and in vivo. Cancer
Res. 69:8645–8651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koziczak M and Hynes NE: Cooperation
between fibroblast growth factor receptor-4 and ErbB2 in regulation
of cyclin D1 translation. J Biol Chem. 279:50004–50011. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ezzat S, Huang P, Dackiw A, et al: Dual
inhibition of RET and FGFR4 restrains medullary thyroid cancer cell
growth. Clin Cancer Res. 11:1336–1341. 2005.PubMed/NCBI
|
|
12
|
St Bernard R, Zheng L, Liu W, et al:
Fibroblast growth factor receptors as molecular targets in thyroid
carcinoma. Endocrinology. 146:1145–1153. 2005.PubMed/NCBI
|
|
13
|
Xie MH, Holcomb I, Deuel B, et al: FGF-19,
a novel fibroblast growth factor with unique specificity for FGFR4.
Cytokine. 11:729–735. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harmer NJ, Pellegrini L, Chirgadze D, et
al: The crystal structure of fibroblast growth factor (FGF) 19
reveals novel features of the FGF family and offers a structural
basis for its unusual receptor affinity. Biochemistry. 43:629–640.
2004. View Article : Google Scholar
|
|
15
|
Wu X, Ge H, Lemon B, et al: Selective
activation of FGFR4 by an FGF19 variant does not improve glucose
metabolism in ob/ob mice. Proc Natl Acad Sci USA. 106:14379–14384.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu X, Ge H, Lemon B, et al: FGF19-induced
hepatocyte proliferation is mediated through FGFR4 activation. J
Biol Chem. 285:5165–5170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inagaki T, Choi M, Moschetta A, et al:
Fibroblast growth factor 15 functions as an enterohepatic signal to
regulate bile acid homeostasis. Cell Metab. 2:217–225. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roidl A, Berger HJ, Kumar S, et al:
Resistance to chemotherapy is associated with fibroblast growth
factor receptor 4 up-regulation. Clin Cancer Res. 15:2058–2066.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Drafahl KA, McAndrew CW, Meyer AN, et al:
The receptor tyrosine kinase FGFR4 negatively regulates NF-kappaB
signaling. PLoS One. 5:e144122010. View Article : Google Scholar : PubMed/NCBI
|