Introduction
Mutations in autosomal dominant genes are
responsible for ~5–10% of all breast cancers and ~7–10% of all
ovarian cancers (1). Specifically,
inherited mutations in the BRCA1 and BRCA2 genes predispose women
to both breast and ovarian cancers, often at young ages. Women who
carry these BRCA1 or BRCA2 mutations have an estimated lifetime
risk of 60–85% and of 26–54% for breast and ovarian cancer,
respectively (2–5). An increased cancer risk is also
recognized in men with BRCA2 mutations, conferring a 6% lifetime
risk of breast cancer and a 3- to 7-fold increased risk of
developing prostate cancer (6,7).
Moreover, having one or more close relatives with
breast cancer is an important and well-established risk factor for
that disease, with the magnitude of risk varying depending on the
number of affected relatives and the ages at which the relatives
were diagnosed (8).
Several authors have looked at BRCA alterations not
only as a biomarker of risk for breast cancer but also as a marker
of clinical pathological aggressiveness (9); however, previous studies have provided
results which are frequently controversial, with some studies
demonstrating that BRCA1 mutation carriers develop tumors with a
higher proliferative capability and low estrogen receptor levels
(10,11) while others have reported the lack of
a difference in histological tumor features among BRCA2-positive
familial and sporadic cases (12).
A large population study (13) found that the 10-year survival rate
did not differ for BRCA1/2 mutation carriers and non-carriers,
leading to the general opinion that woment with a BRCA1 or BRCA2
gene mutation diagnosed with breast cancer have similar survival
with respect to that of non-carriers.
Moreover, breast cancer-specific mortality rates
have been found to be similar for BRCA mutation carriers and
non-carriers in a Jewish population (13). Analogously, in Sardinia, whose
population is genetically homogeneous, investigators did not find
any significant difference in outcome between patients carrying
BRCA2 mutations and those negative for BRCA2
mutations (most prevalent BRCA sequence variations), as well as no
difference in survival among familial and sporadic BRCA2 mutatation
cases (14).
Following a diagnosis of breast cancer in one
breast, a woman with a BRCA1 or BRCA2 mutation experiences a high
risk for contralateral breast cancer (CBC). The 10-year risk of CBC
has been estimated to be between 20 and 30%. Factors that predict
the risk of CBC include early age at diagnosis of the first breast
cancer and a family history of early-onset breast cancers in
first-degree relatives (15–18).
Notably, these data were frequently obtained from a
non-consecutive series of patients, pooling molecular information
from a multitude of laboratories, with patients receiving primary
surgery and histopathological diagnosis from different
professionals, undergoing treatment with extremely different
systemic approaches and being followed up using various modalities.
This was carried out in spite of the fact that each of these
factors has been repeatedly and clearly demonstrated to be able to
deeply influence the outcome of breast cancer women (19–21).
The Outpatient Clinic for Hereditary Breast/Ovarian
Cancer has been active at the National Cancer Research Centre
(NCRC), Bari, Italy since 2003 (22). It includes a dedicated genetics
laboratory with extensive experience in BRCA analysis (24) and is actively involved in national
and international QC programs (23). Moreover, the Outpatients Clinic is
staffed with skilled professionals, and extensive experience in
breast/ovarian patients follow-up was established and was
specifically dedicated to the present study. This set-up allowed
all patients treated for primary surgery in the Women’s Department
of NCRC, Bari to be enrolled in the present study.
The aim of the study was to verify the mutation
spectra and clinical outcome of patients and relatives with
familial features carrying or not carrying BRCA mutations in a
consecutive monoinstitutional and sufficiently large series of
patients from Southern Italy. Data from a median follow-up of 78
months was available.
Materials and methods
Subjects
Between June, 2003 and June, 2010, a series of
~5,000 women with breast cancer were consecutively treated for a
first diagnosis of breast cancer at the Breast Unit of the Women’s
Department of NCRC, Bari, Italy. Among these, all patients with
familial characteristics (at least one first-degree relative) were
referred to the Genetic Counseling Outpatient Clinic of the
Institute for further data collection. After a family tree
reconstruction and calculation of the BRCA mutation probability
according to BRCAPRO, 136 women with breast cancer and a BRCAPRO
risk >10% were considered to be candidates and underwent a BRCA
mutation test. Among these, 79 patients in the period 2003–2010 and
57 patients before 2003 were identified and underwent surgical
resection for primary breast cancer. All women who underwent
genetic testing for BRCA1 and BRCA2 mutations and
provided informed consent for participation in this study were
considered for inclusion.
The median follow-up time for 132 available affected
patients was 6.5 years (range, 1–42) with a total of 1,195
person-years of follow-up.
Furthermore, during the same period, 66 relatives
from 45 families underwent BRCA mutation testing if belonging to
BRCA mutation carrier families. Recruitment of living parents and
adult siblings of the probands was carried out wherever possible.
Documented verification of reported cancers (through pathology
reports, cancer registries and medical records) was also carried
out.
For each patient and relative involved in this study
the following information was available: i) exact mutation present
in the family, ii) date of genetic testing, iii) age at development
of the cancer, iv) cancer type, v) surgical, radiation therapy and
systemic adjuvant treatment, vi) pathological information such as
tumor size, lymph node status, histological type, estrogen (ER) and
progesterone receptors (PgR), histological grade, proliferation
index and HER2 receptor expression, vii) follow-up information on
the following endpoints: ipsilateral breast tumor recurrence
(IBTR), CBC, distant metastasis (M), disease-free survival and new
cases of cancer.
BRCA1 and BRCA2 mutation testing
The genomic DNA samples were screened for mutations
in the BRCA1 and BRCA2 genes by a sequential
combination of denaturing high performance liquid chromatography
(DHPLC) analysis and direct sequencing (AB 3130xl). All relatives
were screened only for the mutation detected in the index case of
the family.
Statistical analysis
Disease-free survival (DFS) was defined as the time
from cancer diagnosis to documented evidence of disease recurrence
in locoregional and/or distant sites, the manifestation of a CBC, a
second primary cancer in a non-breast site. The DFS probability
[95% confidence interval (CI)] was computed using the Kaplan-Meier
product-limit method. The null hypothesis concerning the
differential effect of carrier status in the univariate analysis
was tested by log-rank test (24).
Estimated hazard ratios (the ratio of the carrier
group to the control group) were calculated from proportional
hazard regression models stratified according to lymph node status,
tumor size and surgery. The multivariate model was used to
investigate potential confounding factors (25). For unaffected subjects at the time
of genetic testing, the age-specific cumulative cancer incidence
was estimated by Kaplan-Meier survival functions in which failure
time was age at cancer diagnosis. All statistical analyses were
carried out using SPSS statistical software (26).
Results
Among the 136 probands analyzed for the molecular
status of the BRCA1/BRCA2 genes, 49 cases had a mutation in the
BRCA1 (n=33) or BRCA2 (n=17) gene, with 1 case carrying a mutation
in both genes; conversely, 87 women did not show any mutations in
either BRCA1 or the two genes. The spectrum of mutations in both
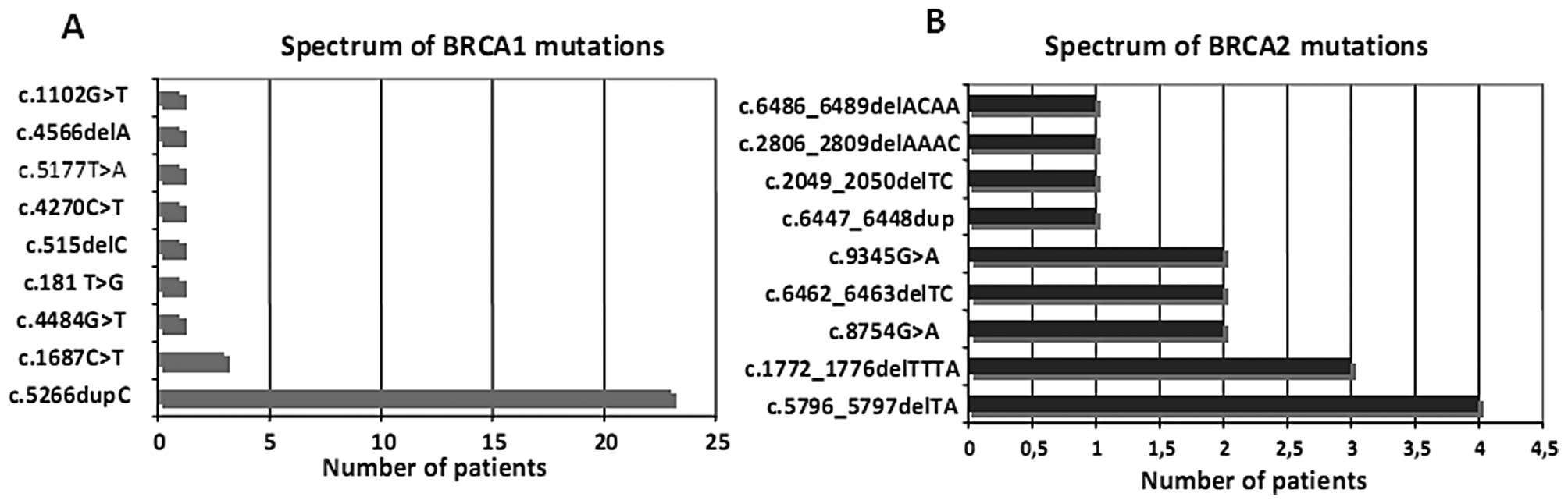
genes is documented in Fig. 1.
Most mutations censored in BRCA1 were c.5266dupC and
c.181T>G, while in BRCA2 they were c.6462_6463delTC,
c.5796_5797delTA and c.1772_1776delTTTA.
In Table I, all
first disease-related events at a median follow-up of 6.5 years are
reported. A total of 44/136 (33.3%) women presenting with a
disease-related event were noted: 4 (2.9%) patients had a relapse
in the ipsilateral breast and 5 (3.7%) in locoregional areas; 18
(13.2%) women presented with a new cancer site in the contralateral
breast; 12 (8.8%) presented metastases in distant sites; finally, 4
(2.9%) women had a second primary cancer in the ovaries and 1
(0.7%) case had a multiple myeloma.
 | Table IFirst event profile in probands at
follow-up of 6.5 years. |
Table I
First event profile in probands at
follow-up of 6.5 years.
| Molecular status
C=49 | | |
|---|
|
| | |
|---|
| Site of disease | BRCA1 (32) | BRCA2 (16) | BRCA1/2 (1) | NC=87 | Overall |
|---|
| Ipsilateral | 1 (3%) | 0 | 1 | 2 | 4 |
| Contralateral | 6 (19%) | 1 | - | 11 | 18 |
| Locoregional | 2 | 1 | - | 2 | 5 |
| Distant
metastasis | 2a | 2b | - | 8c | 12 |
| Ovarian | 3 | 0 | - | 1 | 4 |
| Other | 1d | 0 | - | 0 | 1 |
| 15/32 (46.8%) | 4/16 (25%) | 1 | - | - |
|
| | |
| Total patients with
relapse | 20 | 24 | 44 |
When the disease-related events were analyzed with
respect to the BRCA1/2 status, the probability of recurrence was
slightly higher (P>0.05 by Chi-square test) in mutation carriers
(20/49; 40.8%) than in non-carriers (24/87; 27.5%). Notably, the
probability reached 47% (15/32) when considering only the BRCA1
mutation.
Regarding analysis of all CBCs observed during the
follow-up period, a total of 9 out of 49 carriers and 13 out of 87
non-carrier events were observed (P=0.848, Chi-square test)
(Table II). Notably, CBC
principally occurred as a first event (80% of all contralateral
relapses).
 | Table IIOverall ipsilateral and contralateral
breast cancer relapses. |
Table II
Overall ipsilateral and contralateral
breast cancer relapses.
| Molecular status
C=49 | | |
|---|
|
| | |
|---|
| Site of disease | BRCA1 (32) | BRCA2 (16) | BRCA1/2 (1) | NC=87 | χ2 |
|---|
| Ipsilateral | 3 | - | 1 | 6 | P=0.785 |
| Contralateral | 8 (25%) | 1 (6%) | - | 13 | P=0.848 |
In particular, 8 patients with BRCA1 mutations (5
cases with c.5266dupC, 1 case with c.1687C>T, 1 case with
c.515delC and 1 case with c.5177T>A), and 1 patient with a BRCA2
mutation (c.6486_6489delACAA) presented with CBCs. Ovarian cancers
were observed in 3 cases with the BRCA1 mutation (2 cases with
c.5266dupC and 1 case with c.1687C>T).
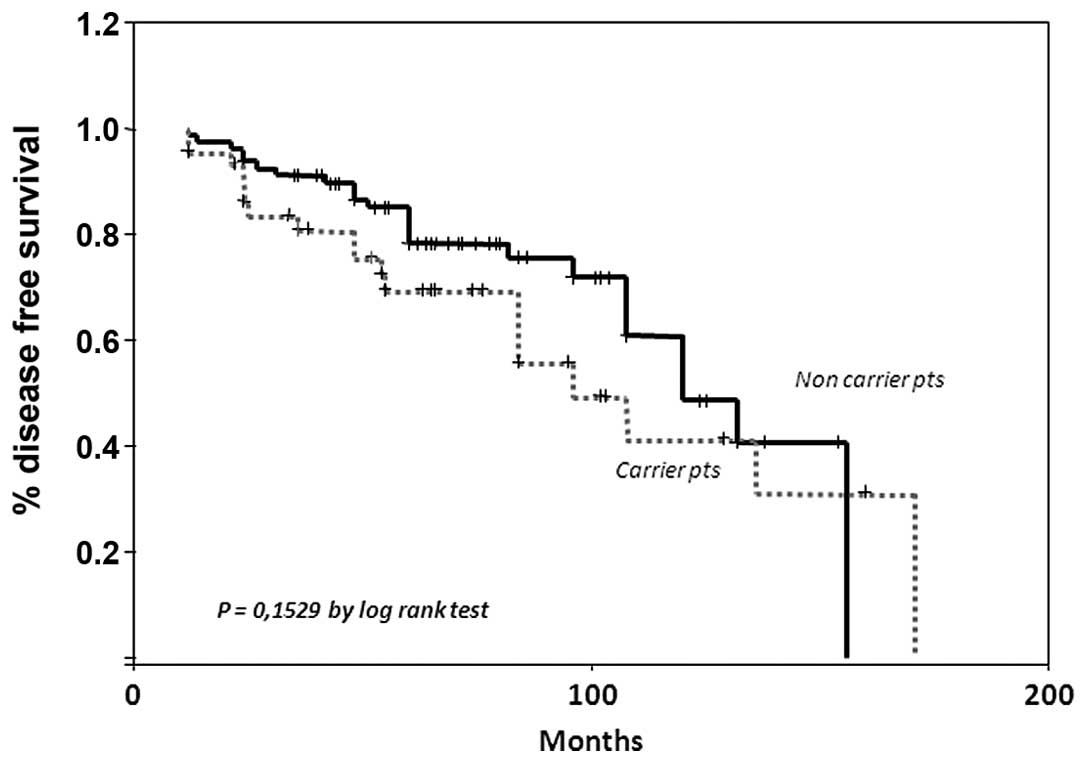
Regarding DFS curves, the 10-year DFS was 57% for
the subgroup of women not carrying a mutation with respect to 50%
in mutation carriers (Fig. 2)
(P=0.1529 by log-rank test). In order to investigate whether other
clinicopathological confounding variables could mask the clinical
impact of a genetic alteration on clinical outcome, a multivariate
analysis was performed with DFS as a dependent variable and genetic
status, tumor size, nodal status and type of primary surgery
included in the model.
The Cox analysis demonstrated that the time of
relapse was significantly associated (HR 0.27; P<0.49; CI,
0.07–0.99) solely with tumor size (Table III).
 | Table IIICox’s proportional hazard regression
models for disease-free survival. |
Table III
Cox’s proportional hazard regression
models for disease-free survival.
| | HR | P-value | (95% CI) |
|---|
| Variable | n (%) | | | |
| All cases | 112 (82) | | | |
| BRCA−
vs. BRCA+ | | 1.49 | 0.240 | 0.76–2.91 |
| T1 vs. T2–T4 | | 0.27 | 0.049 | 0.07–0.99 |
| N− vs.
N+ | | 2.61 | 0.126 | 0.76–8.96 |
| M vs. BCS | | 0.55 | 0.112 | 0.27–1.14 |
When overall survival was analyzed, we observed 15
deaths; 7 in the mutation carrier group and 8 in the group without
mutations, with a 10-year overall survival of 81 vs. 92% (P=0.6091
by log-rank test).
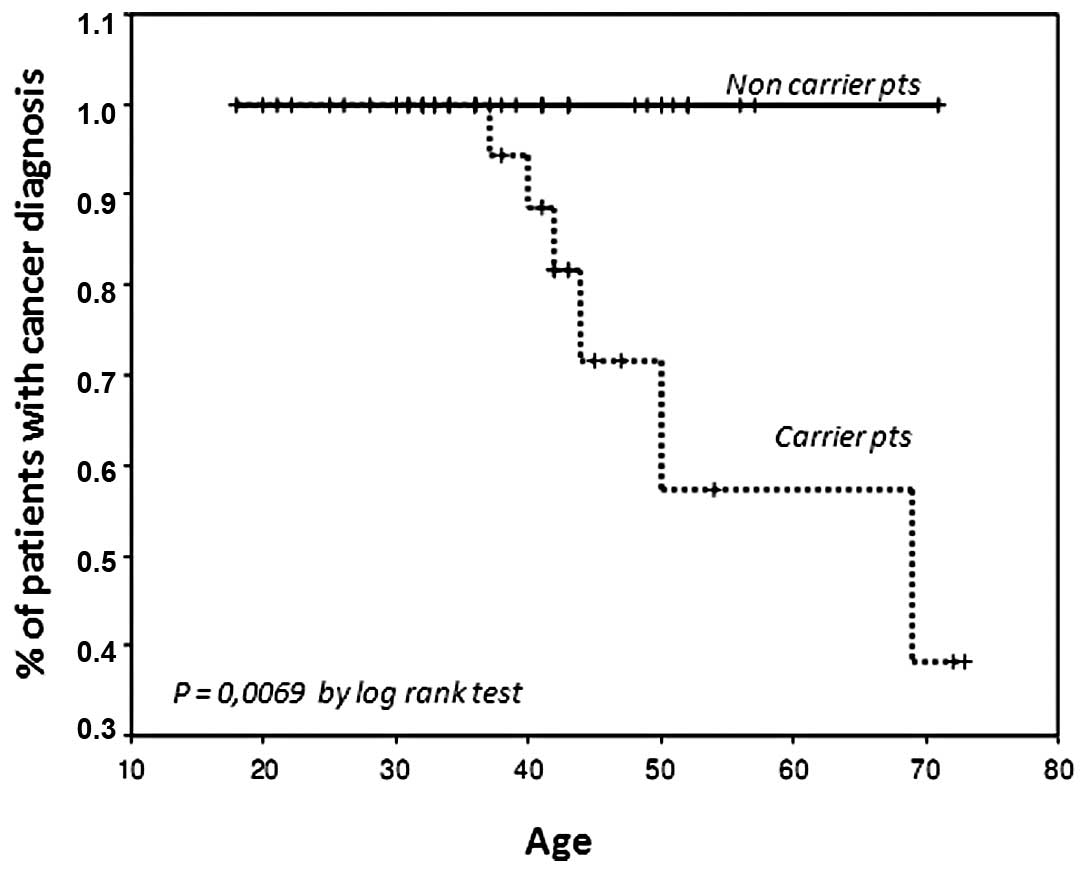
Finally, we examined outcome in the group of 66
healthy relatives carrying (n=32) or not carrying (n=34) a BRCA
gene mutation. In these subgroups, 6 new cancers (5 cases of breast
cancer and 1 case of ovarian cancer) were observed in women
carrying a mutation vs. no new cases in the healthy relatives not
carrying a gene mutation (P=0.02, Chi-square test) (Table IV). Notably, the median age of the
two subgroups was not significantly different at the time of the
last follow-up (median age, 40.5 vs. 41, for carriers and control
groups, respectively) with a 40% risk of breast/ovarian cancer at
the age of 50 years in women carrying a BRCA mutation (Fig. 3) (P=0.0069 by log-rank test).
 | Table IVNew cancer diagnoses in relatives
unaffected at the time of genetic testing (n=66). |
Table IV
New cancer diagnoses in relatives
unaffected at the time of genetic testing (n=66).
| Molecular
status | |
|---|
|
| |
|---|
| Site of
disease | BRCA1 (13) | BRCA2 (19) | C=32 | NC=34 |
|---|
| Breast cancer | 3 | 2 | 5 | 0 |
| Ovarian cancer | 1 | - | 1 | 0 |
| Total | | | 6 | 0 |
Discussion
Our study allowed us to obtain information on two
main aspects concerning the clinical-biological role of the BRCA
mutation: i) to compare the clinical outcome of breast cancer women
carrying or not carrying a BRCA mutation; ii) the risk for
breast/ovarian cancer in healthy relatives carrying or not carrying
a BRCA mutation.
The unique characteristics of our study must be
stressed. We enrolled a monoinstitutional consecutive series of
patients, molecularly characterized at one laboratory who received
primary surgery/radiotherapy/systemic treatment/follow-up at the
same institute and according to standardized guidelines.
We demonstrated that for patients belonging to a
hereditary breast cancer group, the presence or not of a BRCA
mutation does not alter the clinical outcome of women with breast
cancer. In fact, the probability of a relapse is higher but not
statistically significant in carriers of a mutation with respect to
those with no mutation.
The lack of statistical power for this comparison
could be supported by the absence of a difference between the two
groups, but also the possibility that the study concerned consisted
of a limited number of women. A larger series of women should be
studied using the same methodology as applied in the present
study.
The evidence of the absence of a relationship with
clinical outcome is supported by several previous studies,
including that of Rennert et al(13) who analyzed the largest series ever
considered (n=1794). However, our analysis referred to familial
high-risk women eventually presupposing the presence of a genetic
alteration other than BRCA1/2 mutations.
The lack of difference also concerns the
contralateral appearance of the disease. This is in contrast with
the results of Robson et al (27), Kirova et al (28)
and Brekelmans et al (29) who demonstrated a higher risk for
contralateral disease in BRCA carriers with respect to
non-carriers.
It has been shown that age remains a significant
predictor of ipsilateral breast cancer recurrence and, in
particular, that young age, more than the BRCA status, is a strong
predictive factor for local relapse among hereditary breast cancer
patients (28,30).
Thus, to ascertain how young age is important in
CBC, following adjustment for age, we detected all (9/9)
contralateral recurrences out of 49 in mutation carriers compared
with 70% (10/13) of cases out of 89 in non-carriers in a population
with a diagnosis of first breast cancer at an age ranging from 20
to 49 years (P=0.394, Chi-square test). Due to the small number of
patients, this difference was not significant.
However, a more extended follow-up is needed to
ensure that the rate of CBC in young age BRCA+ patients
does not increase in the long-term.
Finally, we investigated the risk of breast cancer
in healthy relatives carrying or not carrying a BRCA gene mutation.
Notably, in this further analysis, we demonstrated that the risk
for breast and ovarian cancers in relatives was significantly
different in carriers with respect to non-carriers, with a 43% risk
at an age of 50 years in women carrying a BRCA mutation.
In conclusion, our data indicate that in women with
a deleterious mutation in BRCA1 or BRCA2, clinical outcome is no
worse than in BRCA-negative patients, while it results in a
significant difference from the outcome of sporadic breast cancer
patients. Therefore, we are currently recruiting a large series of
sporadic breast cancer patients in order to compare the results
with the present findings. Identification of the BRCA status in
relatives of breast cancer patients carrying the mutation is
crucial for the prediction of risk.
Acknowledgements
The present study was supported, in part, by
Progetto Regione Puglia ‘Familial Breast Cancer Screening’ (DIEF
2007).
References
|
1
|
Emery J, Lucassen A and Murphy M: Common
hereditary cancers and implications for primary care. Lancet.
358:56–63. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brose MS, Rebbeck TR, Calzone KA, et al:
Cancer risk estimates for BRCA1 mutation carriers identified in a
risk evaluation program. J Natl Cancer Inst. 94:1365–1372. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Easton DF, Bishop DT, Ford D and Crockford
GP: Genetic linkage analysis in familial breast and ovarian cancer:
results from 214 families. The Breast Cancer Linkage Consortium. Am
J Hum Genet. 52:678–701. 1993.PubMed/NCBI
|
|
4
|
King MC, Marks JH and Mandell JB; New York
Breast Cancer Study Group. Breast and ovarian cancer risks due to
inherited mutations in BRCA1 and BRCA2. Science. 302:643–646. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antoniou A, Pharoah PD, Narod S, et al:
Average risks of breast and ovarian cancer associated with BRCA1 or
BRCA2 mutations detected in case series unselected for family
history: a combined analysis of 22 studies. Am J Hum Genet.
72:1117–1130. 2003. View
Article : Google Scholar
|
|
6
|
Cancer risks in BRCA2 mutation carriers.
The Breast Cancer Linkage Consortium. J Natl Cancer Inst.
91:1310–1316. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liede A, Karlan BY and Narod SA: Cancer
risks for male carriers of germline mutations in BRCA1 or BRCA2: a
review of the literature. J Clin Oncol. 22:735–742. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Metcalfe K, Lubinski J, Lynch HT, et al:
Family history of cancer and cancer risk in women with BRCA1 or
BRCA2 mutations. J Natl Cancer Inst. 102:1874–1878. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee EH, Park SK, Park B, et al: Effect of
BRCA1/2 mutation on short-term and long-term breast cancer
survival: a systematic review and meta-analysis. Breast Cancer Res
Treat. 122:11–25. 2010.
|
|
10
|
Adem C, Reynolds C, Soderberg CL, et al:
Pathologic characteristics of breast parenchyma in patients with
hereditary breast carcinoma, including BRCA1 and BRCA2 mutation
carriers. Cancer. 97:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chappuis PO, Nethercot V and Foules WD:
Clinico-pathological characteristics of BRCA1 and BRCA2-related
breast cancer. Semin Surg Oncol. 18:287–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pathology of familial breast cancer:
differences between breast cancers in carriers of BRCA1 or BRCA2
mutations and sporadic cases. The Breast Cancer Linkage Consortium.
Lancet. 349:1505–1510. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rennert G, Bisland-Naggan S,
Barnett-Griness O, et al: Clinical outcomes of breast cancer in
carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 357:115–123.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Budroni M, Cesaraccio R, Coviello V, et
al: Role of BRCA2 mutation status on overall survival among breast
cancer patients from Sardinia. BMC Cancer. 9:622009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verhoog LC, Brekelmans CT, Seynaejjve, et
al: Contralateral breast cancer risk is influenced by the age at
onset in BRCA1-associated breast cancer. Br J Cancer. 83:384–386.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Metcalfe K, Lynch HT, Ghadirian P, et al:
Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J
Clin Oncol. 22:2328–2335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pierce LJ, Levin AM, Rebbeck TR, et al:
Ten-year multi-institutional results of breast-conserving surgery
and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J
Clin Oncol. 24:2437–2443. 2006.PubMed/NCBI
|
|
18
|
Sariego J: The impact of facility
volume/size on breast cancer treatment and outcome. Am Surg.
76:1333–1337. 2010.PubMed/NCBI
|
|
19
|
Paradiso A, Giardina C and Meyer JS:
Statewide study of diagnostic agreement in breast pathology. J Natl
Cancer Inst. 91:1076–1077. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTCG). Peto R, Davies C, Godwin J, et al:
Comparisons between different polychemotherapy regimens for early
breast cancer: meta-analyses of long-term outcome among 100,000
women in 123 randomised trials. Lancet. 379:432–444. 2012.
View Article : Google Scholar
|
|
21
|
Tommasi S, Crapolicchio A, Lacalamita R,
et al: BRCA1 mutations and polymorphisms in a hospital-based
consecutive series of breast cancer patients from Apulia, Italy.
Mutat Res. 578:395–405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tommasi S, Pilato B, Pinto R, et al:
Molecular and in silico analysis of BRCA1 and BRCA2 variants. Mutat
Res. 644:64–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mueller CR, Kristoffersson U and
Stoppa-Lyonnet D: External quality assessment for mutation
detection in the BRCA1 and BRCA2 genes: EMQN’s experience of 3
years. Ann Oncol. 15(Suppl 1): i14–i17. 2004.PubMed/NCBI
|
|
24
|
Lawless JS: Statistical Models and Methods
for Lifetime Data. John Wiley and Sons; New York, NY: 1982
|
|
25
|
Cox DR: Regression models and life tables.
JR Stat Soc. 34:187–220. 1972.
|
|
26
|
Sweet SA: Data Analysis with SPSS. Allyn
and Bacon; Boston, MA: 1998
|
|
27
|
Robson M, Chappuis PO, Satagopan J, et al:
A combined analysis of outcome following breast cancer: differences
in survival based on BRCA1/BRCA2 mutation status and administration
of adjuvant treatment. Breast Cancer Res. 6:R8–R17. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kirova YM, Stoppa-Lyonnet D, Savignoni A,
et al: Risk of breast cancer recurrence and contralateral breast
cancer in relation to BRCA1 and BRCA2 mutation status following
breast-conserving surgery and radiotherapy. Eur J Cancer.
41:2304–2311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brekelmans CTM, Tilanus-Linthorst MMA,
Seynaeve C, et al: Tumor characteristics, survival and prognostic
factors of hereditary breast cancer from BRCA2-, BRCA1- and
non-BRCA1/2 families as compared to sporadic breast cancer cases.
Eur J Cancer. 43:867–876. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Delaloge S, Kloos I, Ariane D, et al:
Young age is the major predictor of local relapse among
conservatively treated BRCA1-, BRCA2-, or non BRCA-linked
hereditary breast cancer (BC). Proc Am Soc Clin Oncol.
22:412003.
|