Introduction
Osteosarcoma (OS) is one of the most common primary
malignant bone tumors in children and adolescents. With the advent
of effective chemotherapy, the 5-year survival rate for patients
treated with intensive multidrug chemotherapy and aggressive local
control has been reported to be 55–80% (1–4).
However, chemotherapy fails to eliminate all OS cells due to
intrinsic or acquired drug resistance, which is the most common
cause of tumor recurrence resulting in poor clinical outcomes
(5). Therefore, novel reagents are
urgently needed for the effective chemotherapy of OS.
Human epidermal growth factor receptor 2 (HER2) is a
185-kDa transmembrane receptor tyrosine kinase (RTK), belonging to
the epidermal growth factor receptor (EGFR) family. Aberrant
upregulation of HER2 is found in various types of cancers such as
breast cancer (6), ovarian cancer
(7) and OS (8–10).
Patients with HER2-positive cancer have a high risk for diminished
effectiveness of cancer treatments and poor clinical outcomes due
to increased tumor cell metastasis (11). Ligand stimulation induces
dimerization of the HER2 receptor (homodimer or heterodimer), which
leads to self-phosphorylation on tyrosine residues localized to the
C-terminal domain of HER2 receptors. Furthermore, the
phosphorylated HER2 receptors activate a variety of downstream
signaling pathways, such as the phosphatidylinositol 3-kinase
(PI3K)/Akt (12), which plays an
essential role in cell-extracellular matrix (ECM) and cell-cell
adhesion in cell proliferation and survival. Recently, various
studies have revealed that targeting HER2 is an important
therapeutic strategy for treating OS (13,14).
Lapatinib is a small-molecule kinase inhibitor and a
derivative of 4-anilinoquinoline (15) whose molecular formula is
C29H26ClFN4O4S, and
chemically it is
N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[5-[(2-methylsulfonylethylamino)
methyl]-2-furyl]quinazolin-4-amine (16). Lapatinib potently and reversibly
binds to the intracellular TK domains of EGFR and HER2, which leads
to inhibition of substrate phosphorylation. This inhibition blocks
downstream MAPK and PI3K/AKT proliferation and survival signaling
pathways both in vitro and in vivo(17,18).
Lapatinib was reported to effectively inhibit human tumor cell
proliferation (19). In March 2007,
the US Food and Drug Administration approved the use of lapatinib
for the treatment of advanced breast cancer overexpressing HER2
(HER2+) (20).
However, the effect of lapatinib on the malignant
potential of human OS cells and the potential molecular mechanisms
are still unclear. To explore the possibility of developing
lapatinib as a therapeutic agent and to clarify its potential
molecular mechanisms, we investigated the effect and underlying
molecular mechanisms of lapatinib in human OS cells. The present
study was conducted by evaluating the effect of lapatinib on the
proliferation, migration and invasion abilities of OS cell lines
U2-OS and MG-63 cells and the involvement of the HER2-PI3K/AKT-FASN
signaling pathway in vitro.
Materials and methods
Cell lines and cell culture
The human OS cell lines, U2-OS and MG-63, were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and routinely cultured in Dulbecco’s modified Eagle’s medium
(DMEM) (HyClone, Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS) (Sigma, St. Louis, MO, USA) in a humidified 37°C
incubator containing 5% CO2.
Cell growth assay
The U2-OS and MG-63 cells were cultured in 96-well
tissue culture plates at a cell density of 5,000 cells/well, in
DMEM containing 10% FBS and 2 mM glutamine. Following adherence
overnight, the medium was replaced and the cells were incubated
with different concentrations (5, 10, 20, 30 and 40 μmol/l for
U2-OS cells and 5, 10, 15, 20 and 25 μmol/l for MG-63 cells) of
lapatinib for 24, 48 and 72 h. Viable proliferating cells were
detected by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, using 6-wells/time period. Cell viability was expressed as
optical density (OD), which was detected by an enzyme-linked
immunosorbent assay (ELISA) reader (MK3; Thermo, USA) at a 490-nm
wavelength. The inhibitory rate of cell proliferation was
calculated. Six independent experiments were performed over
multiple days.
Flow cytometry (FCM)
Human OS U2-OS and MG-63 cells were seeded at
5×105 cells/ml into T25 culture flasks for 24 h. The
cells were then treated with 0, 5, 10 and 15 μmol/l lapatinib.
Following incubation, the cells were trypsinized, washed with
phosphate-buffered saline (PBS) and fixed overnight in ice-cold 70%
ethanol. Subsequent to fixation, the cells were washed twice with
1% bovine serum albumin (BSA) in PBS, and resuspended in 1 ml
DNA-binding propidium iodide (PI) solution (10 mg/ml in PBS,
containing 0.05 mg/ml RNase A), incubated at room temperature in
the dark for 15 min and analyzed with an EPICS XL flow cytometer
(Beckman Coulter, Miami, FL, USA). The number of apoptotic cells
was measured with the control software of the flow cytometer. Six
independent experiments were performed over multiple days.
Colony formation assay
Cells (2×106/2 ml/well) were seeded in
tissue culture plastic dishes, and treated with lapatinib (15
μmol/l for U2-OS and 10 μmol/l for MG-63) for 2 weeks to form
colonies. The formed colonies were stained with Giemsa, and the
colonies containing >50 cells were counted under an inverted
microscope. Six independent experiments were performed over
multiple days.
Invasion assay
The invasiveness of the OS cells was measured using
BD BioCoat™ BD Matrigel™ invasion chambers (BD Biosciences,
Franklin Lakes, NJ, USA) according to the manufacturer’s
instructions. The medium in the lower chamber contained 15% FBS as
a source of chemoattractant. The cells were suspended in serum-free
medium containing lapatinib (15 μmol/l for U2-OS and 10 μmol/l for
MG-63) and added to the upper chambers simultaneously
(2×103 cells in 0.1 ml). The cells that passed through
the Matrigel-coated membrane were stained with Diff-Quik (Sysmex,
Kobe, Japan), and images were captured. Cell invasion was
quantified by direct microscopic visualization and counting. The
invaded cells were counted in 5 randomly selected fields under an
inverted microscope. The cells not treated with lapatinib were used
as a normalization control. Six independent experiments were
performed over multiple days.
Migration assay
Cell migration was assessed by determining the
ability of the cells to move into a cellular space in a
two-dimensional wound healing assay in vitro. In brief, the
cells were cultured in a 6-well tissue culture plastic dishes to
5×106 cells/well, and subsequently treated with
lapatinib (15 μmol/l for U2-OS and 10 μmol/l for MG-63) cells for
24 h. The cells were then denuded by dragging a rubber policeman
(Fisher Scientific, Hampton, NH, USA) through the center of the
plate well. The culture plates were rinsed with PBS, and fresh
quiescent medium alone or with 10% BSA was added, in which the
cells were incubated at 37°C for 24 h. The cells were photographed
at 0 and 24 h, and the migrated distance was measured. The rate of
migration was assessed from 5 randomly selected fields under an
inverted microscope. The cells not treated with lapatinib were used
as a normalization control. Six independent experiments were
performed over multiple days.
Western blot analysis
U2-OS and MG-63 cells in the exponential growth
phase were treated with lapatinib (15 μmol/l for U2-OS and 10
μmol/l for MG-63) for 24 h. The cells were then washed with cold
PBS. Total protein from the cells was extracted using
radioimmunoprecipitation assay (RIPA) lysis buffer containing 60
μg/ml phenylmethanesulfonyl fluoride (PMSF), and the protein
concentration was determined using a Bradford assay. Equal amounts
of protein were electrophoresed by 10% SDS-PAGE and transferred
onto pure nitrocellulose blotting membranes (0.22-μm pore size).
The membranes were blocked with 5% Difco skim milk for 1 h at room
temperature (RT), and then blocked with the primary antibody
(rabbit anti-human P-HER2, AKT, P-AKT, FASN, mouse anti-human PI3K,
GAPDH IgG, 1:2,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) overnight at 4°C. The membranes were then washed prior to
incubation with the appropriate peroxidase-conjugated secondary
antibodies (anti-rabbit, anti-mouse, 1:5,000; Santa Cruz
Biotechnology, Inc.). The immune complexes were detected with a
Pro-Light HRP kit (Tiangen, Beijing, China). All experiments were
repeated 6 times over multiple days.
Statistical analysis
Data are expressed as the means ± SD. The
differences in invasion and migration capabilities between the
cells treated with and without lapatinib were evaluated with
independent-sample t-tests. A value of P<0.05 was considered to
indicate a statistically significant difference. All analyses were
performed with SPSS version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Effect of lapatinib on OS cell
proliferation in vitro
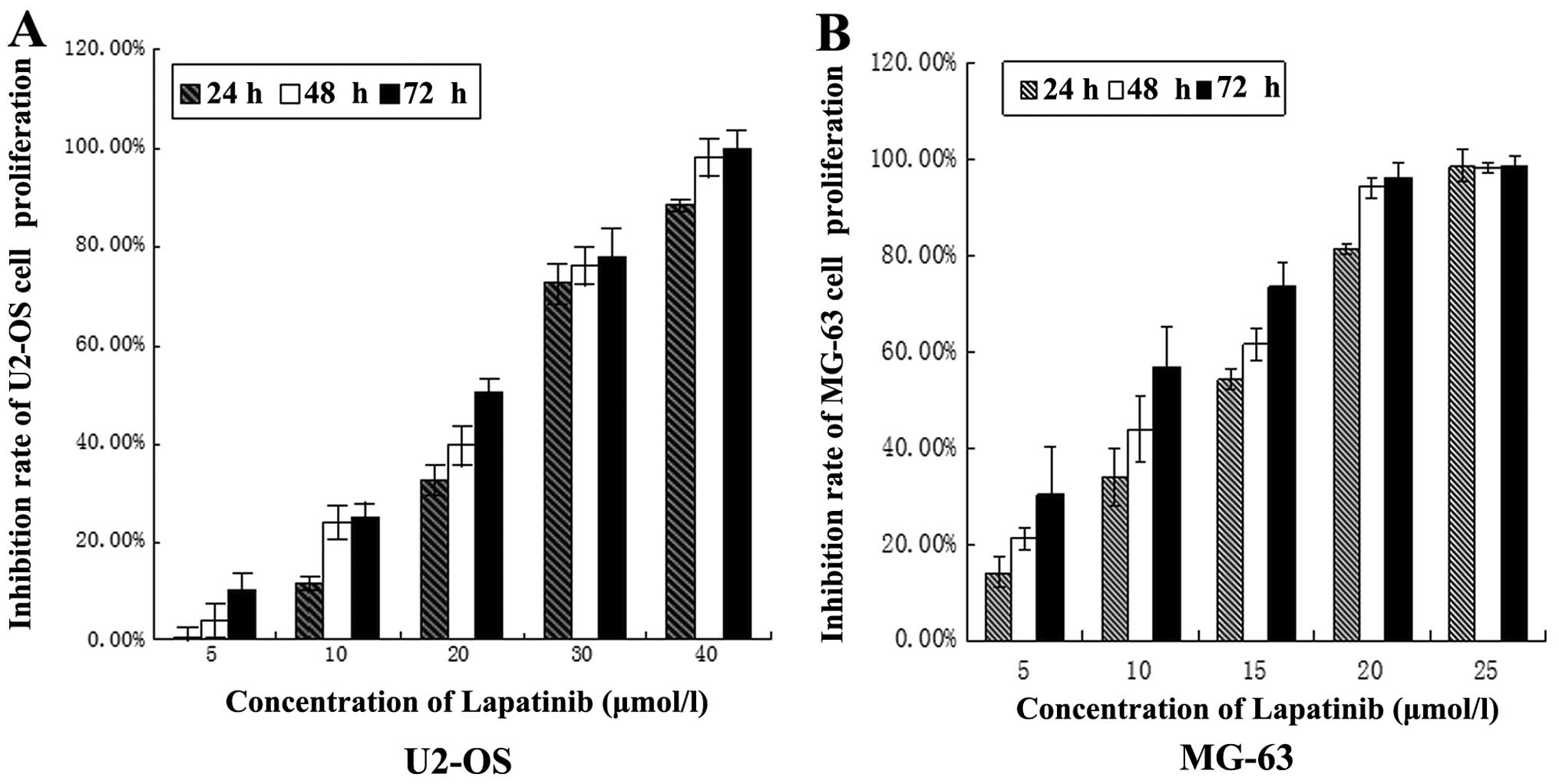
The effect of lapatinib on the growth of the U2-OS
and MG-63 cell lines was investigated using MTT and colony
formation assays. The results showed that the proliferation of
U2-OS and MG-63 cells was inhibited by lapatinib in a dose- and
time-dependent manner (Fig. 1). The
IC50 values for lapatinib in U2-OS and MG-63 cells at 24
h were 22.150 and 11.646 μmol/l, respectively. A concentration of
15 μmol/l for U2-OS and 10 μmol/l for MG-63 was chosen for
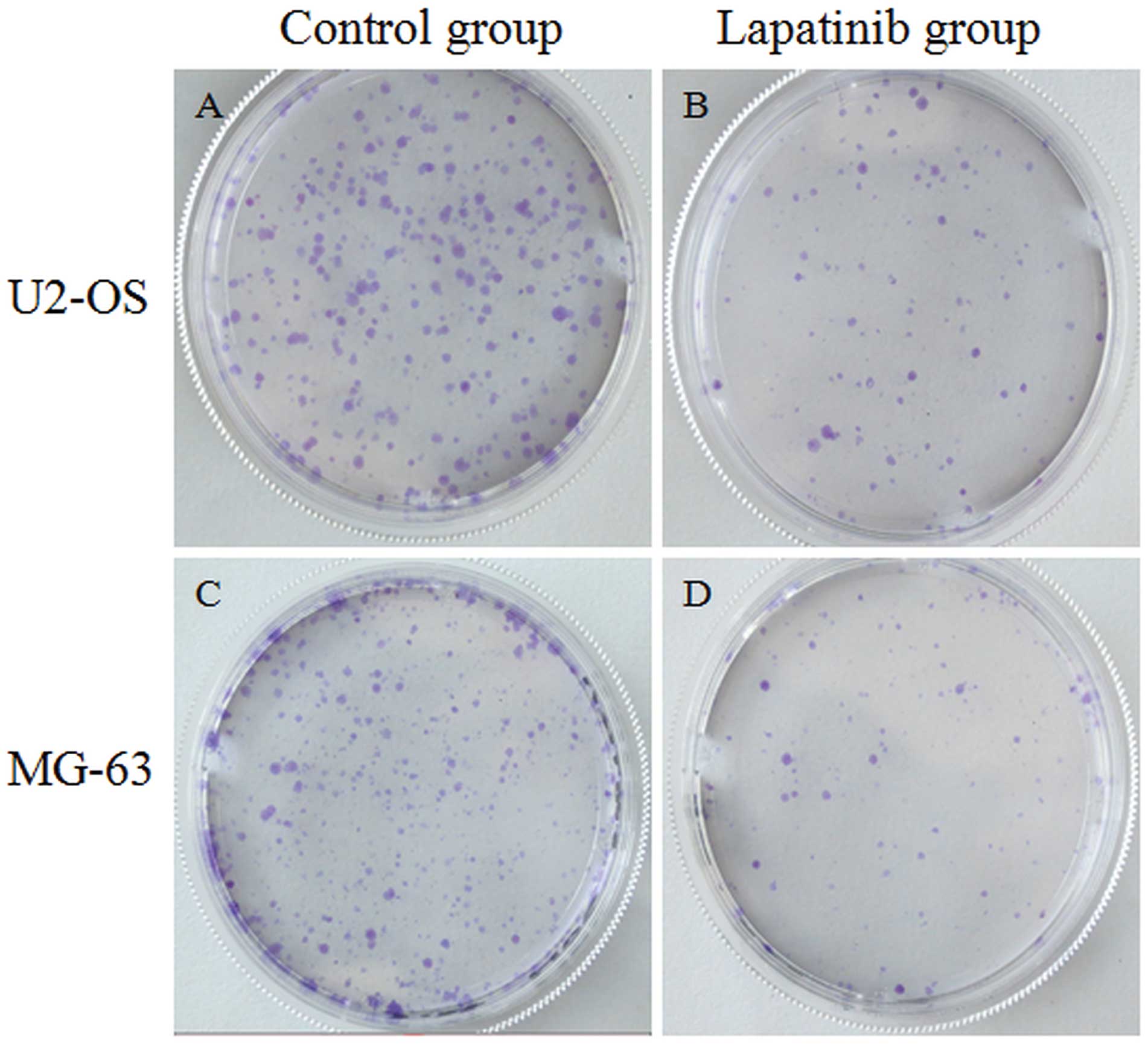
treatment of the OS cells in the following assays. The colony
formation rate of cells treated with lapatinib was obviously lower
than the rate of the cells not treated by lapatinib (Fig. 2). These data indicate that lapatinib
inhibits U2-OS and MG-63 cell proliferation in vitro.
Lapatinib induces OS cell apoptosis
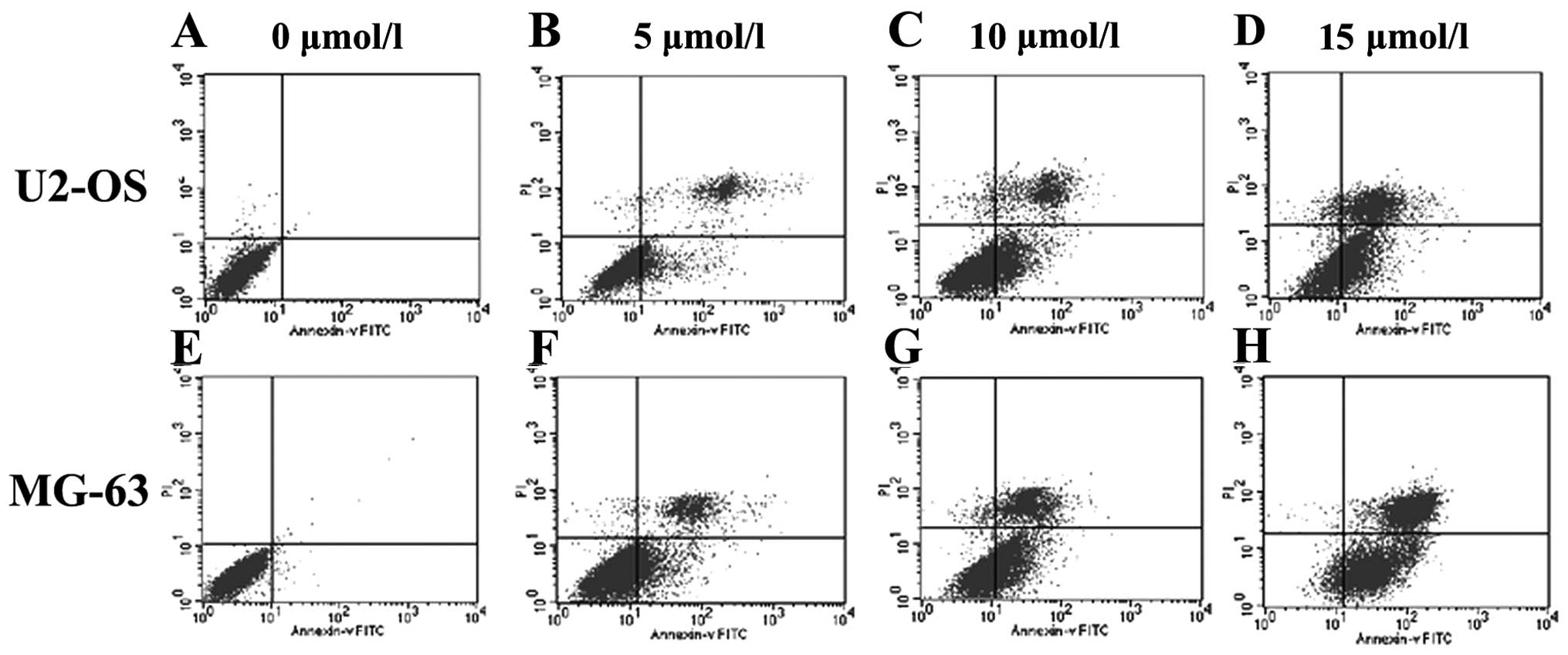
FCM analysis was used to investigate the effect of
lapatinib on the induction of apoptosis of U2-OS and MG-63 cells
in vitro. Lapatinib at various concentrations was added to
the U2-OS and MG-63 cells in the exponential growth phase for 24 h,
and cell samples were obtained and fixed for FCM analysis. The
results revealed that apoptosis was induced by lapatinib in a
dose-dependent manner (Fig. 3).
This indicated that lapatinib induced U2-OS and MG-63 cell
apoptosis in vitro.
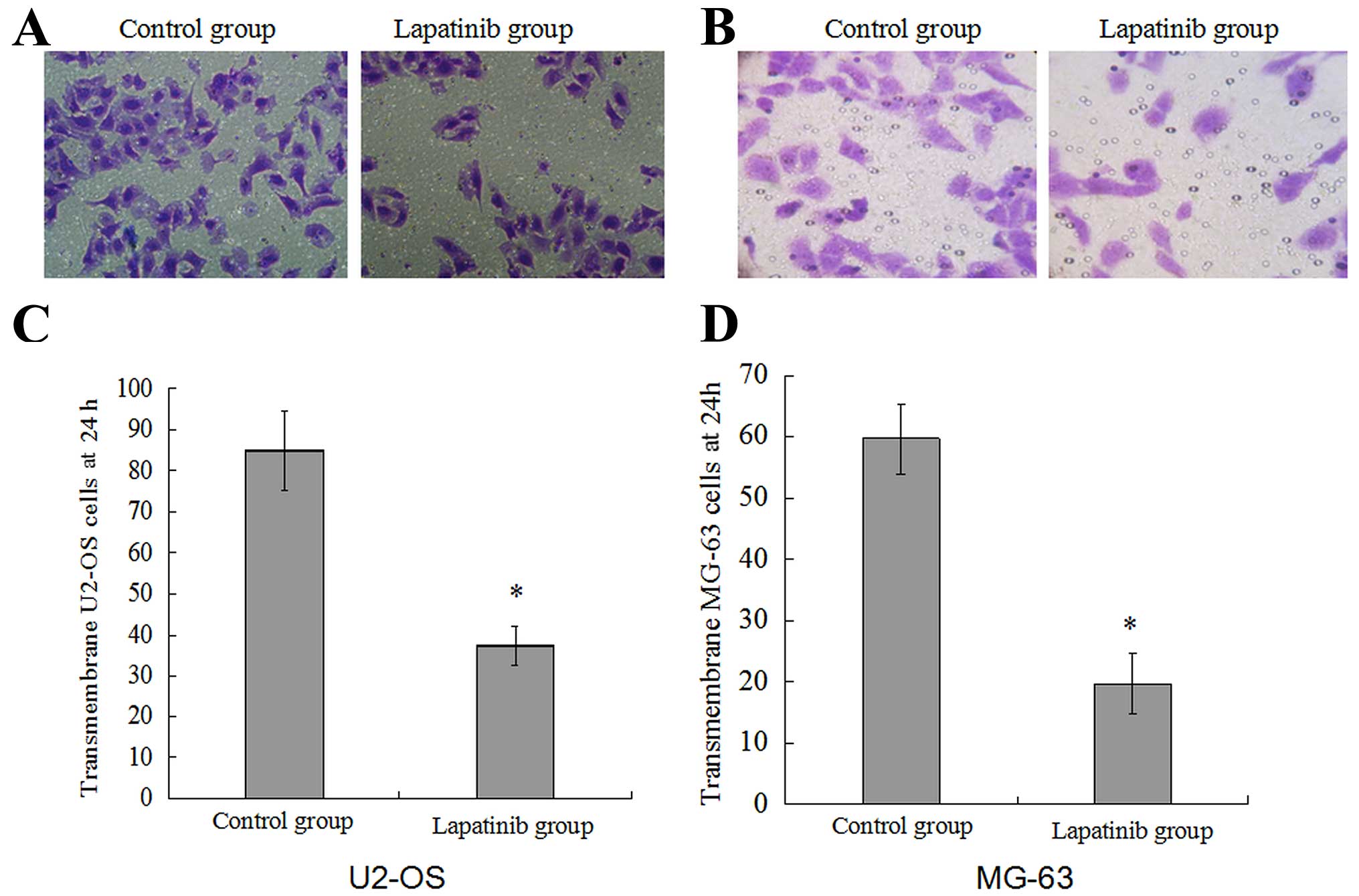
Lapatinib inhibits OS cell invasion and
migration in vitro
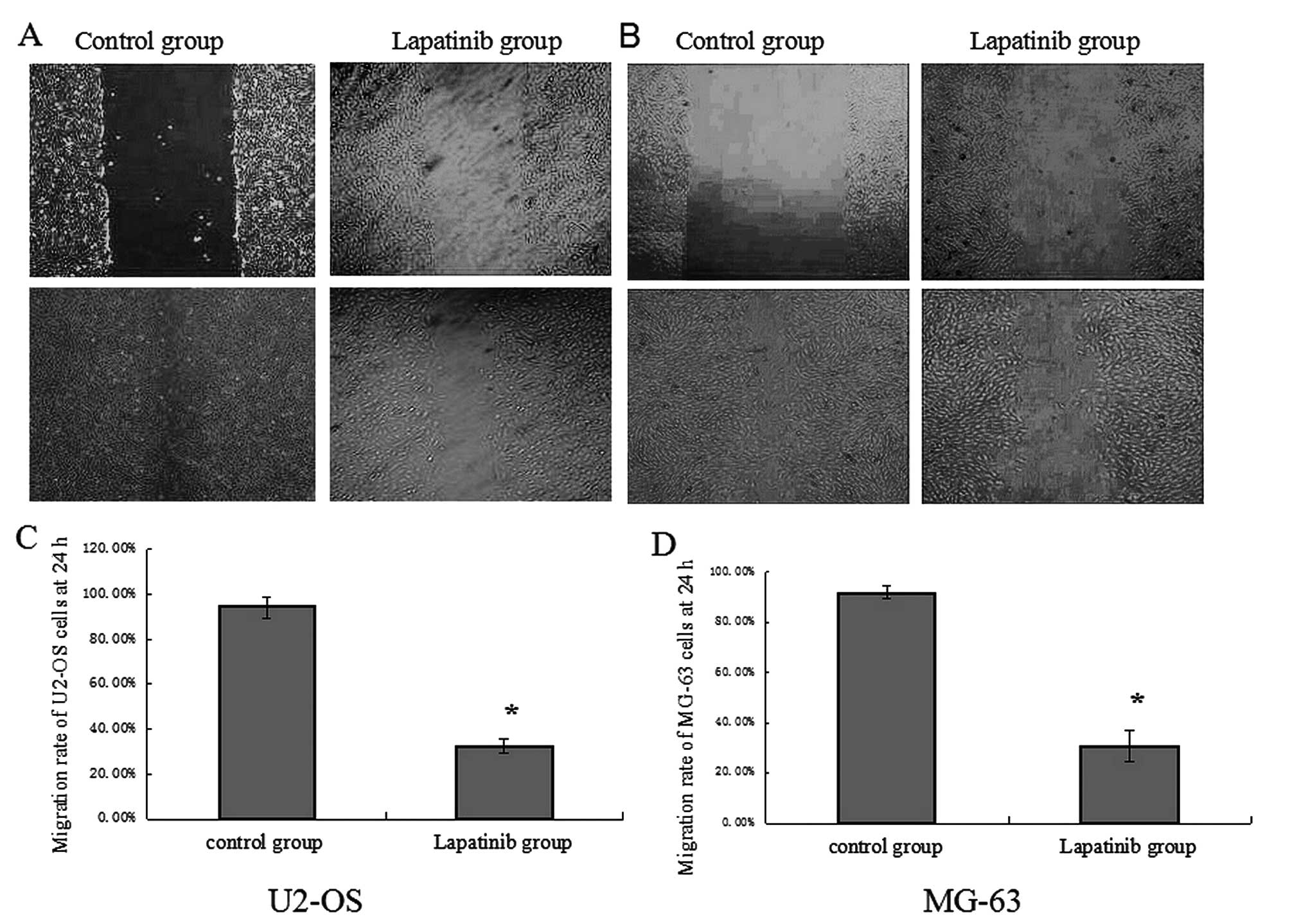
To examine the effect of lapatinib on OS cell
migration and invasion, the migration and invasion capabilities
were assessed with the wound healing and Transwell invasion assays.
As shown in Fig. 5, the migration
rate of cells treated with lapatinib was 32.70±3.00% in the U2-OS
cells and 30.65±6.15% in the MG-63 cells, compared with 94.52±4.76%
and 91.83±2.32% in the cells not treated with lapatinib. In the
Transwell invasion assay (Fig. 4),
the invasion of the cells treated with lapatinib was significantly
inhibited compared with that in the untreated cells (P<0.05).
This suggests that lapatinib suppresses U2-OS and MG-63 cell
migration and invasion in vitro.
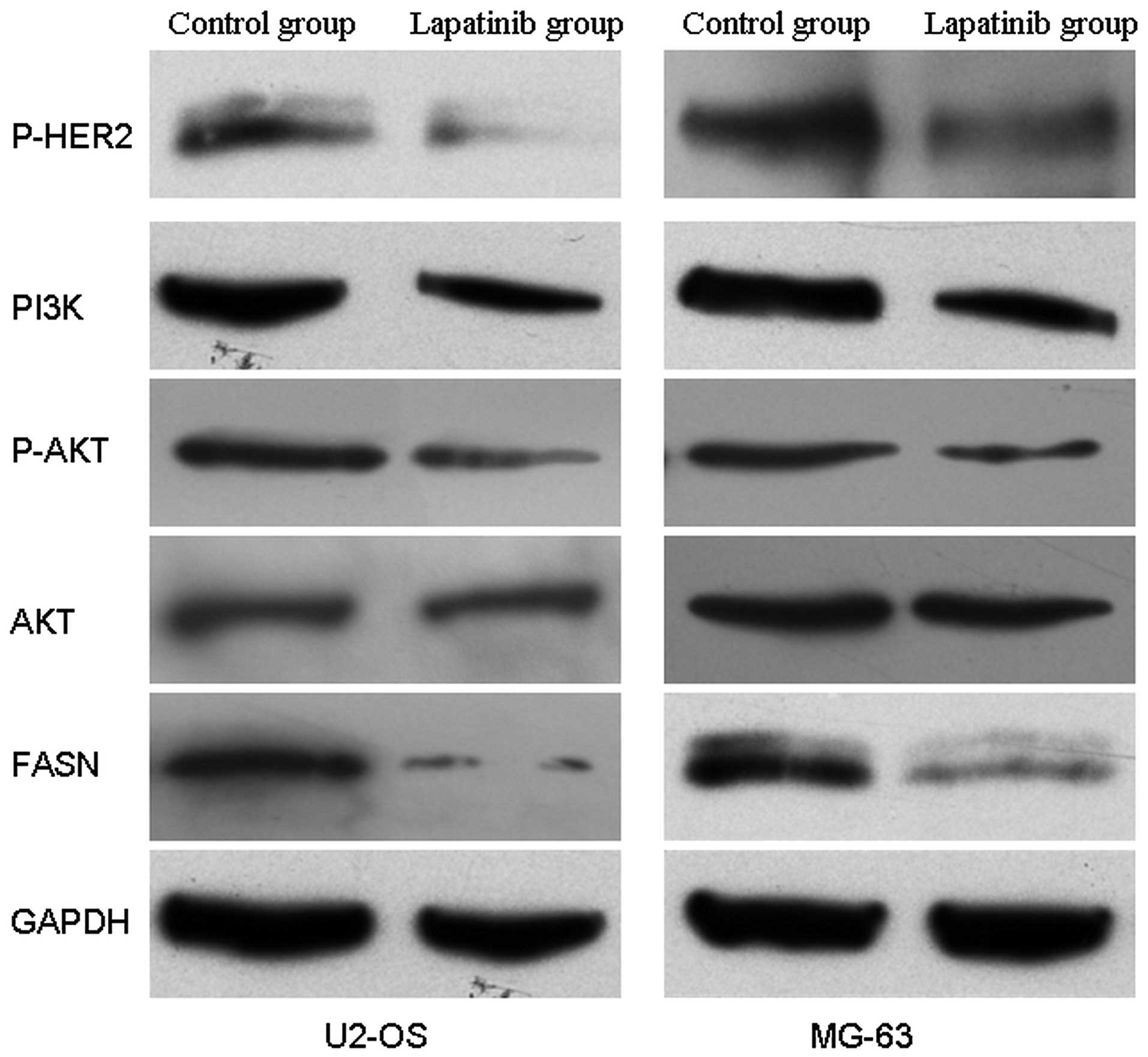
Lapatinib suppresses the activity of the
HER2-PI3K/AKT-FASN signaling pathway
To investigate the effect of lapatinib on the
activity of the HER2-PI3K/AKT-FASN signaling pathway, the protein
expression levels of p-HER2, PI3K, p-AKT, AKT and FASN were
detected. The results showed that the protein expression levels of
p-HER2, PI3K, p-AKT and FASN except for AKT were significantly
decreased in the cells treated with lapatinib when compared with
these levels in the untreated cells (Fig. 6). This suggests that lapatinib
suppresses the activity of HER2-PI3K/AKT-FASN in U2-OS and MG-63
cells in vitro.
Discussion
Lapatinib (originally known as GW572016) is a
4-anilinoquinoline derivative, which was reported to induce cell
apoptosis and inhibit cell proliferation, migration and invasion in
various types of tumors (21,22).
It was widely used for chemotherapeutic treatment alone or in
combination with other anticancer drugs (23). Recently, studies have shown that
lapatinib is not active against EGFR-positive/HER2-negative disease
(24,25). However, the effect of lapatinib on
the malignant phenotype of osteosarcoma (OS) is still uncertain.
Morris et al(13) suggested
that targeting HER2 should be considered for the treatment of
patients with osteogenic sarcoma. Therefore, to examine the effect
of lapatinib on OS cell apoptosis, proliferation, migration and
invasion, OS cell lines U2-OS and MG-63 were selected for study.
The cell proliferation was evaluated with MTT and colony formation
assays, and cell migration and invasion were assessed by wound
healing and Transwell invasion assays. We found that lapatinib
inhibited the proliferation of U2-OS and MG-63 cells in a dose- and
time-dependent manner, and the rate of colony formation of
lapatinib-treated cells was significantly lower than that in cells
not treated with lapatinib. In the wound healing and Transwell
invasion assays, the results revealed that the migratory and
invasive capabilities were inhibited by lapatinib. These results
indicate that the malignant phenotype of OS cells may be inhibited
by lapatinib in vitro. Lapatinib may be an effectively agent
for chemotherapy in the treatment of OS. However, further studies
are necessary to unveil the potential molecular mechanisms of the
inhibition of the malignant phenotype of OS by lapatinib.
Recently, studies have shown that target metabolic
pathways such as glycolysis and lipid metabolism may represent a
promising therapeutic strategy in cancer therapy (26). Fatty acid synthase (FASN), a
lipogenic multi-enzyme complex, is an enzyme crucial for endogenous
lipogenesis in mammals and is responsible for catalyzing the
synthesis of long-chain fatty acids. The metabolic products of the
FASN complex are rapidly consumed by actively dividing cells.
Recent data demonstrate that FASN expression is important for tumor
growth and survival, suggesting that FASN is a metabolic oncogene.
It is more pronounced in OS and correlates with pulmonary
metastasis (27). Importantly, we
demonstrated that inhibition of FASN with pharmacological
inhibitors or siRNA leads to a significant antitumor effect in OS
(28). HER2 overexpression
increases the translation of FASN by altering the activity of the
mTOR and PI3K/AKT signaling pathway in breast cancer cell (29), and inhibition of the HER2/PI3K/AKT
signaling pathway leads to blockade of FASN in colorectal cancer
cells (30). However, various
studies have demonstrated that inhibiting FASN caused a marked
decrease in the active forms of the HER2 protein (31,32).
These findings suggest that the HER2 oncogene possibly established
a positive bidirectional relationship with FASN, strictly ensuring
a hyperactive de novo fatty acid biogenesis.
Lapatinib is a small-molecule, reversible inhibitor
of HER2 TKS (33). In murine
xenograft models, lapatinib inhibited autophosphorylation of HER2,
as well as downstream MAPK/Erk1/2 and PI3K/Akt pathways (18,34).
To investigate the possible involvement of the inhibition of the
phosphorylation of HER2 by the PI3K/Akt/FASN signaling pathway in
OS, the levels of phosphorylated HER2 in the OS cell lines U2-OS
and MG-63 were downregulated by lapatinib. Our results demonstrated
that the inhibition of phosphorylation of HER2 by lapatinib
markedly reduced the expression of PI3K, p-AKT and FASN protein in
U2-OS and MG-63 cells. This implies that HER2 effectively regulates
the activity of the ‘PI3K/Akt/FASN’ signaling pathway in OS
cells.
In conclusion, our findings suggest that lapatinib
alters the cell malignant phenotype of OS cells via downregulation
of the activity of the HER2-PI3K/AKT-FASN signaling pathway in
vitro, and lapatinib may be an effective chemotherapeutic agent
for OS. However, the tumor microenvironment plays an important role
in tumor progression, invasion and cell migration. Thus, further
experiments in vivo are necessary to ascertain whether
lapatinib represents a new chemotherapeutic agent for the treatment
of OS.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81260400) and the
Natural Science Foundation of Jiangxi Province (no.
20114BAB205093).
References
|
1
|
Meyers PA, Schwartz CL, Krailo M,
Kleinerman ES, Betcher D, Bernstein ML, et al: Osteosarcoma: a
randomized, prospective trial of the addition of ifosfamide and/or
muramyl tripeptide to cisplatin, doxorubicin, and high-dose
methotrexate. J Clin Oncol. 23:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacci G, Forni C, Longhi A, Ferrari S,
Mercuri M, Bertoni F, et al: Local recurrence and local control of
non-metastatic osteosarcoma of the extremities: a 27-year
experience in a single institution. J Surg Oncol. 96:118–123.
2007.PubMed/NCBI
|
|
3
|
Jawad MU, Cheung MC, Clarke J, Koniaris LG
and Scully SP: Osteosarcoma: improvement in survival limited to
high-grade patients only. J Cancer Res Clin Oncol. 137:597–607.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brand RA: 50 years ago in CORR: the
present trend in treatment of osteogenic sarcoma. Clin Orthop Relat
Res. 467:3038–3039. 2009.PubMed/NCBI
|
|
5
|
Ferrari S, Smeland S, Mercuri M, Bertoni
F, Longhi A, Ruggieri P, et al: Neoadjuvant chemotherapy with
high-dose Ifosfamide, high-dose methotrexate, cisplatin, and
doxorubicin for patients with localized osteosarcoma of the
extremity: a joint study by the Italian and Scandinavian Sarcoma
Groups. J Clin Oncol. 23:8845–8852. 2005. View Article : Google Scholar
|
|
6
|
Gradishar WJ: Emerging approaches for
treating HER2-positive metastatic breast cancer beyond trastuzumab.
Ann Oncol. 24:2492–2500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shanmughapriya S, Senthilkumar G, Arun S,
Vinodhini K, Sudhakar S and Natarajaseenivasan K: Polymorphism and
overexpression of HER2/neu among ovarian carcinoma women
from Tiruchirapalli, Tamil Nadu, India. Arch Gynecol Obstet. May
31–2013.(Epub ahead of print).
|
|
8
|
Zhou BG, Liu MY, Qiu XC, Xu YM, Fan QY,
Yang AG, et al: A novel recombinant immunocasp-6 fusion gene
specifically and efficiently suppresses HER2-overexpressing
osteosarcoma. Oncol Rep. 29:276–282. 2013.PubMed/NCBI
|
|
9
|
Ma Q, Zhou Y, Ma B, Chen X, Wen Y, Liu Y,
et al: The clinical value of CXCR4, HER2 and CD44 in human
osteosarcoma: a pilot study. Oncol Lett. 3:797–801. 2012.PubMed/NCBI
|
|
10
|
Scotlandi K, Manara MC, Hattinger CM,
Benini S, Perdichizzi S, Pasello M, et al: Prognostic and
therapeutic relevance of HER2 expression in osteosarcoma and
Ewing’s sarcoma. Eur J Cancer. 41:1349–1361. 2005.PubMed/NCBI
|
|
11
|
Sheng WQ, Huang D, Ying JM, Lu N, Wu HM,
Liu YH, et al: HER2 status in gastric cancers: a retrospective
analysis from four Chinese representative clinical centers and
assessment of its prognostic significance. Ann Oncol. 24:2360–1264.
2013. View Article : Google Scholar
|
|
12
|
Baselga J and Swain SM: Novel anticancer
targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer.
9:463–475. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morris CD, Gorlick R, Huvos G, Heller G,
Meyers PA and Healey JH: Human epidermal growth factor receptor 2
as a prognostic indicator in osteogenic sarcoma. Clin Orthop Relat
Res. 382:59–65. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shan LQ, Ma S, Qiu XC, Wang T, Yu SB, Ma
BA, et al: A novel recombinant immuno-tBid with a furin site
effectively suppresses the growth of HER2-positive osteosarcoma
cells in vitro. Oncol Rep. 25:325–331. 2011.PubMed/NCBI
|
|
15
|
Burris HA III, Hurwitz HI, Dees EC,
Dowlati A, Blackwel KL, O’Neil B, et al: Phase I safety,
pharmacokinetics, and clinical activity study of lapatinib
(GW572016), a reversible dual inhibitor of epidermal growth factor
receptor tyrosine kinases, in heavily pretreated patients with
metastatic carcinomas. J Clin Oncol. 23:5305–5313. 2005. View Article : Google Scholar
|
|
16
|
Bence AK, Anderson EB, Halepota MA, Doukas
MA, DeSimone PA, Davis GA, et al: Phase I pharmacokinetic studies
evaluating single and multiple doses of oral GW572016, a dual
EGFR-ErbB2 inhibitor, in healthy subjects. Invest New Drugs.
23:39–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spector NL, Xia W, Burris H III, Hurwitz
H, Dees EC, Dowlati A, et al: Study of the biologic effects of
lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine
kinases, on tumor growth and survival pathways in patients with
advanced malignancies. J Clin Oncol. 23:2502–2512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rusnak DW, Lackey K, Affleck K, Wood ER,
Alligood KJ, Rhodes N, et al: The effects of the novel, reversible
epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor,
GW2016, on the growth of human normal and tumor-derived cell lines
in vitro and in vivo. Mol Cancer Ther. 1:85–94. 2001.PubMed/NCBI
|
|
19
|
Kalous O, Conklin D, Desai AJ, O’Brien NA,
Ginther C, Anderson L, et al: Dacomitinib (PF-00299804), an
irreversible Pan-HER inhibitor, inhibits proliferation of
HER2-amplified breast cancer cell lines resistant to trastuzumab
and lapatinib. Mol Cancer Ther. 11:1978–1987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryan Q, Ibrahim A, Cohen MH, Johnson J, Ko
CW, Sridhara R, et al: FDA drug approval summary: lapatinib in
combination with capecitabine for previously treated metastatic
breast cancer that overexpresses HER-2. Oncologist. 13:1114–1119.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seidel J, Kunc K, Possinger K, Jehn C and
Lüftner D: Effect of the tyrosine kinase inhibitor lapatinib on
CUB-domain containing protein (CDCP1)-mediated breast cancer cell
survival and migration. Biochem Biophys Res Commun. 414:226–232.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu X, Wu L, Qiao H, Han T, Chen S, Liu X,
et al: Autophagy stimulates apoptosis in HER2-overexpressing breast
cancers treated by lapatinib. J Cell Biochem. Jun 21–2013.(Epub
ahead of print). View Article : Google Scholar
|
|
23
|
Bian L, Wang T, Zhang S and Jiang Z:
Trastuzumab plus capecitabine vs. lapatinib plus capecitabine in
patients with trastuzumab resistance and taxane-pretreated
metastatic breast cancer. Tumour Biol. 34:3153–3158. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Di Leo A, Gomez HL, Aziz Z, Zvirbule Z,
Bines J, Arbushites MC, et al: Phase III, double-blind, randomized
study comparing lapatinib plus paclitaxel with placebo plus
paclitaxel as first-line treatment for metastatic breast cancer. J
Clin Oncol. 26:5544–5552. 2008.PubMed/NCBI
|
|
25
|
Johnston S, Trudeau M, Kaufman B, Boussen
H, Blackwell K, LoRusso P, et al: Phase II study of predictive
biomarker profiles for response targeting human epidermal growth
factor receptor 2 (HER-2) in advanced inflammatory breast cancer
with lapatinib monotherapy. J Clin Oncol. 26:1066–1072. 2008.
View Article : Google Scholar
|
|
26
|
Vandhana S, Coral K, Jayanthi U, Deepa PR
and Krishnakumar S: Biochemical changes accompanying apoptotic cell
death in retinoblastoma cancer cells treated with lipogenic enzyme
inhibitors. Biochim Biophys Acta. 1831:1458–1466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu ZL, Wang G, Peng AF, Luo QF, Zhou Y
and Huang SH: Fatty acid synthase expression in osteosarcoma and
its correlation with pulmonary metastasis. Oncol Lett. 4:878–882.
2012.PubMed/NCBI
|
|
28
|
Liu ZL, Mao JH, Peng AF, Yin QS, Zhou Y,
Long XH and Huang SH: Inhibition of fatty acid synthase suppresses
osteosarcoma cell invasion and migration via downregulation of the
PI3K/Akt signaling pathway in vitro. Mol Med Rep. 7:608–612.
2013.PubMed/NCBI
|
|
29
|
Yoon S, Lee MY, Park SW, Moon JS, Koh YK,
Ahn YH, et al: Up-regulation of acetyl-CoA carboxylase α and fatty
acid synthase by human epidermal growth factor receptor 2 at the
translational level in breast cancer cells. J Biol Chem.
282:26122–26131. 2007.
|
|
30
|
Li N, Bu X, Wu P, Wu P and Huang P: The
‘HER2-PI3K/Akt-FASN Axis’ regulated malignant phenotype of
colorectal cancer cells. Lipids. 47:403–411. 2012.
|
|
31
|
Puig T, Turrado C, Benhamú B, Aguilar H,
Relat J, Ortega-Gutiérrez S, et al: Novel inhibitors of fatty acid
synthase with anticancer activity. Clin Cancer Res. 15:7608–7615.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vazquez-Martin A, Colomer R, Brunet J,
Lupu R and Menendez JA: Overexpression of fatty acid synthase gene
activates HER1/HER2 tyrosine kinase receptors in human breast
epithelial cells. Cell Prolif. 41:59–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wood ER, Truesdale AT, McDonald OB, Yuan
D, Hassell A, Dickerson SH, et al: A unique structure for epidermal
growth factor receptor bound to GW572016 (Lapatinib): relationships
among protein conformation, inhibitor off-rate, and receptor
activity in tumor cells. Cancer Res. 64:6652–6659. 2004. View Article : Google Scholar
|
|
34
|
Xia W, Mullin RJ, Keith BR, Liu LH, Ma H,
Rusnak DW, et al: Anti-tumor activity of GW572016: a dual tyrosine
kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream
Erk1/2 and AKT pathways. Oncogene. 21:6255–6263. 2002. View Article : Google Scholar : PubMed/NCBI
|