Introduction
The development of cancer is often associated with
chronic inflammation, which suggests a strong relationship between
inflammation and tumorigenesis (1).
Tumor necrosis factor α (TNFα) is one of the key inflammatory
mediators involved in inflammation-associated cancer (2). Although over the last few decades, a
high dose of TNFα has been used as a cytotoxic agent, recent
reports support the link between chronic low-level TNFα exposure
and the acquisition of certain malignant phenotypes, such as
increased growth, invasion and metastasis (2). In addition, TNFα is an important
activator of the canonical NF-κB pathway. Upon stimulation,
activated IKK-β phosphorylates the NF-κB inhibitor, IκBα, and
triggers its rapid degradation resulting in the liberation of
NF-κB. As a consequence, the NF-κB heterodimer translocates to the
nucleus, binds to its cognate DNA motifs in the promoters, and
induces expression of a myriad of genes implicated in the immune
response, cell proliferation, angiogenesis, cell survival and
invasion (3,4).
Epithelial-mesenchymal transition (EMT), which is a
complex reprogramming process of epithelial cells, plays an
important role in tumor invasion and metastasis (5). EMT is characterized as the morphologic
alteration from an epithelial to a mesenchymal phenotype, including
loss of epithelial cell markers, such as E-cadherin, α-catenin and
γ-catenin, and a gain in mesenchymal components, such as vimentin,
N-cadherin and fibronectin (6,7). In
addition, studies show that a group of transcriptional factors
regulate EMT, such as TWIST, Snail and Slug. Previous studies
demonstrate that TWIST is implicated in metastasis by regulating
EMT in HNSCC (8,9). Recent reports indicate that TNFα
induces EMT via AKT/GSK or NF-κB-mediated expression of Snail and
TWIST in breast, renal and colon cancer (10–13).
Collectively, the evidence suggests that TNFα may regulate the
critical processes of tumor promotion and progression, including
angiogenesis, oncogene activation and EMT.
Hypopharyngeal cancer is one of the most common head
and neck squamous cell carcinomas (HNSCCs). More than 75% of
patients with hypopharyngeal cancer are at an advanced stage at the
time of diagnosis (14). Lymph node
metastasis is present in 60–80% of patients and it directly affects
the prognosis of this disease (15). Metastasis of tumors is a complex
process, and various factors are involved in each step of
metastasis (16). Thus, in the
present study, we investigated whether TNFα induces EMT via an
increase in TWIST expression in human hypopharyngeal cancer FaDu
cells and thereby promotes FaDu cell metastasis. Next, we aimed to
ascertain whether the NF-κB signaling pathway is activated and
regulates TNFα-induced TWIST expression.
Materials and methods
Materials
Commercially available antibodies used were as
follows: NFκbp65, TWIST, E-cadherin and N-cadherin (all from Abcam,
UK); vimentin, p-IKK and p-IκBα (all from Cell Signaling
Technology, Inc., USA); lamin B and actin (both from Santa Cruz
Biotechnology, Inc., USA) and TNFα (Cell Signaling Technology,
Inc.). p65siRNA(h) and Bay 11-7082 were both from Santa Cruz
Biotechnology, Inc.
Cell culture and transfection
The human hypopharyngeal cancer FaDu cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing
10% fetal calf serum, 100 U/ml penicillin and 100 mg streptomycin
at 37°C in a humidified atmosphere composed of 95% air and 5%
CO2. Detailed experimental procedures of the cell
transfection were previously described (9).
Observation of morphological changes
The morphological changes in the FaDu cells were
observed using an inverted microscope. Images were captured using a
Leica microscope image system (Leica, Germany).
Immunofluorescence
The cells were cultured on chamber slides, serum
starved for 12 h, then exposed to TNFα (10 ng/ml) for the indicated
times. Cells were washed 3 times with PBS, fixed with 4%
paraformaldehyde for 20 min and permeabilized with 0.3% Triton
X-100 for 10 min. After blocking with bovine serum albumin for 2 h
at room temperature, cells were incubated with antibodies against
E-cadherin, N-cadherin, p65 or TWIST (1:100 dilution) at 4°C
overnight. Slides were washed 3 times with PBS and incubated with
fluorescein isothiocyanate (FITC) or tetramethylrhodamine
isothiocyanate (TRITC) secondary antibodies for 1 h at room
temperature. The nuclei were stained with
4′,6-diamidine-2′-phenylindole (DAPI) for 2 min. Samples were
examined using confocal microscopy (Leica, Germany) to analyze
expression of E-cadherin, N-cadherin, TWIST and p65.
Western blotting
Detailed experimental procedures of western blot
analysis of gene expression were previously described (17). Western blot analysis was performed
with antibodies against TWIST (1:100), E-cadherin (1:200),
N-cadherin (1:200), p65 (1:400), vimentin (1:1,000), p-IKK
(1:1,000), p-IκBα (1:1,000), lamin B (1:1,000) and β-actin
(1:3,000).
Wound healing assay
The FaDu cells were plated onto 6-well plates at a
concentration of 5×105 cells/well, and were serum
starved for 12 h. Tumor cells were then treated with or without
TNFα (10 ng/ml). Cell monolayers were carefully wounded by
scratching with a sterile plastic pipette tip. The cells were then
washed twice with cooled PBS before observation. For each wound,
the images were captured at 0 and 24 h in the same fields after
treatment.
Transwell chamber assay
FaDu cells were pretreated with or without TNFα (10
ng/ml) for 24 h, and 3×104 cells were plated in the
upper chamber. Detailed experimental procedures of the invasion and
migration assays were previously described (9).
Statistical analysis
Data are expressed as means ± standard deviation
(SD), and statistical significance was assessed by the analysis of
variance test. All statistical tests employed in the present study
were two-sided. P-values <0.05 were considered to indicate
statistically significant results. Statistical calculations were
performed using SPSS software package, version 13.0 (SPSS Inc.,
USA).
Results
TNFα induces EMT
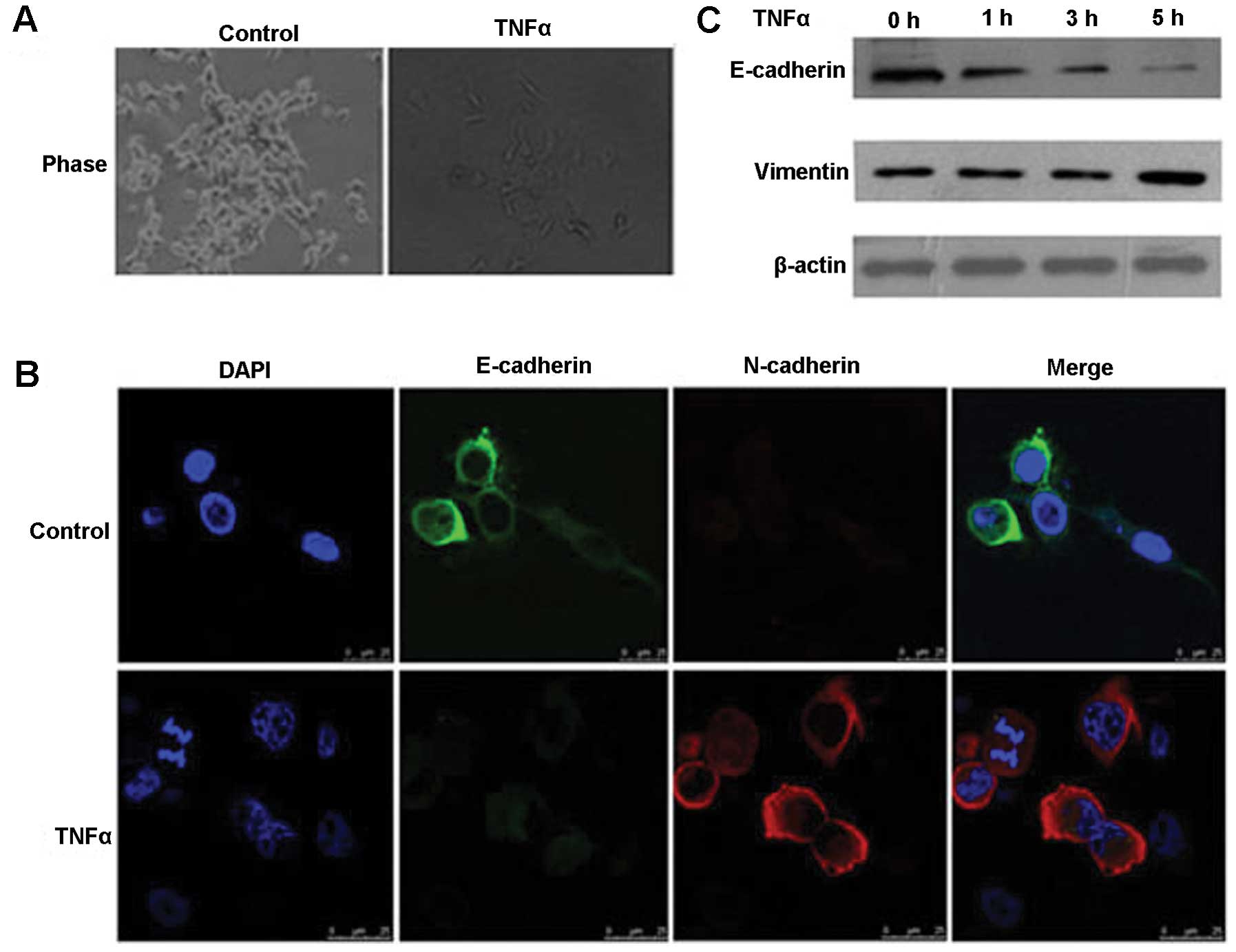
To explore whether TNFα has an effect on the
morphology of FaDu cells, FaDu cells were treated with TNFα (10
ng/ml). We found that the morphology of the FaDu cells following
treatment with TNFα was altered from well organized cell-cell
adhesion and cell polarity to loss of cell-cell contacts and cell
scattering (Fig. 1A). Cells
underwent a significant change in morphology from a cobblestone
morphology to exhibiting mesenchymal spindle-like features. Next,
we observed the expression of EMT molecular markers using
immunofluorescence and western blotting, and found that the
expression of the epithelial marker E-cadherin was downregulated,
whereas the mesenchymal markers, vimentin and N-cadherin, were
significantly upregulated in the FaDu cells (Fig. 1B and C). This phenomenon indicates
that TNFα induces EMT in human hypopharyngeal cancer FaDu
cells.
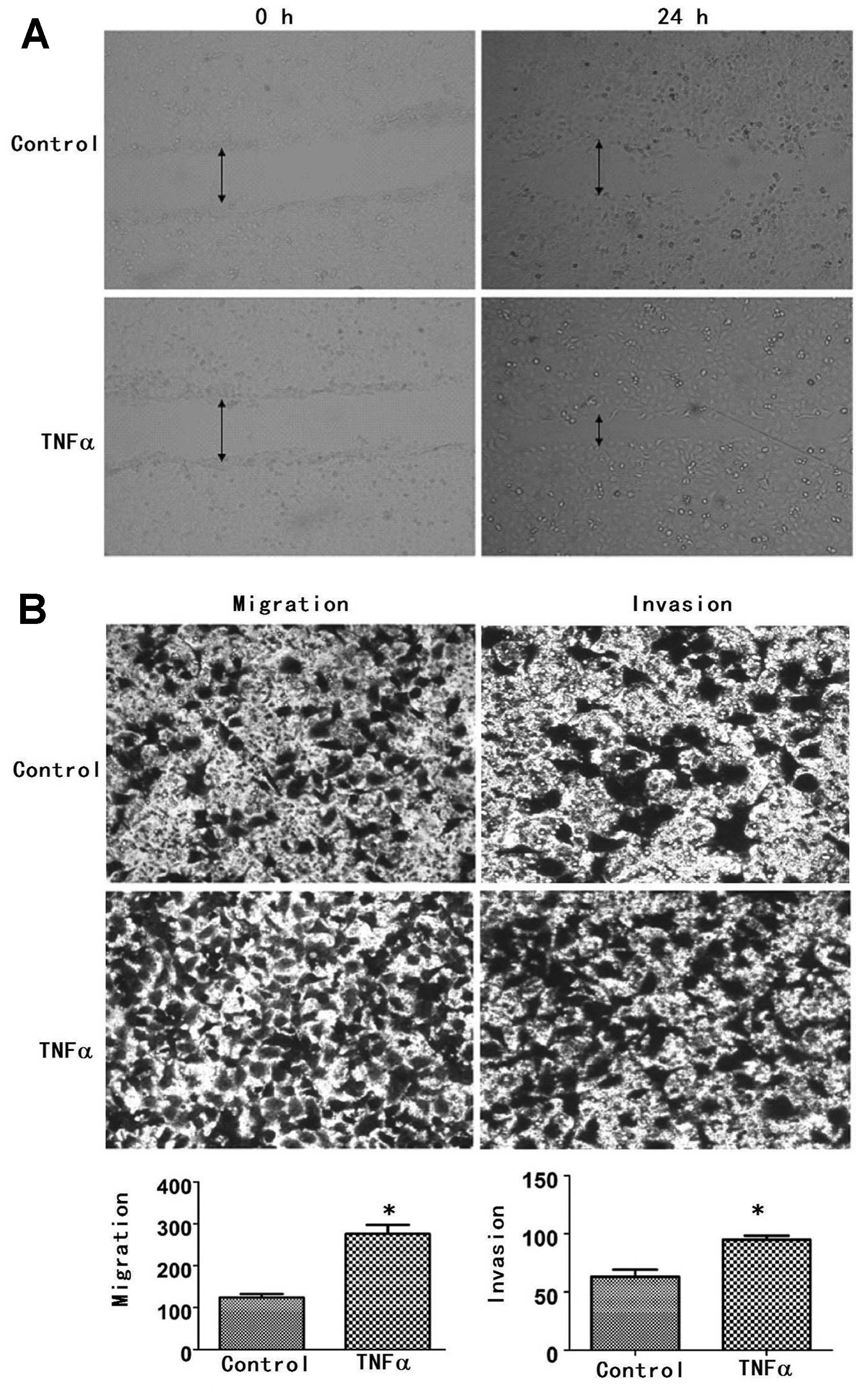
TNFα increases cancer cell motility,
migratory and invasive abilities
To confirm that the change in morphology has an
effect on the function of the cells, wound healing and Transwell
chamber assays were used to measure cellular motility, migratory
and invasive abilities. After 24 h following exposure to TNFα, the
speed of motility of the FaDu cells was found to be more rapid than
that of the control group; the former was closer to the center of
the wound area than the control group (Fig. 2A). Promotion of migration and
invasion in the FaDu cells by TNFα was also confirmed by the
Transwell chamber assay. The numbers of cells that had migrated in
the control and TNFα treatment groups were 124±15 and 276±38,
respectively (P<0.05) (Fig. 2B).
In the in vitro invasion assay, we found that the number of
invasive cells in the TNFα treatment group was 95±3, significantly
more than the number in the control group (63±6, P<0.05)
(Fig. 2B). These results suggest
that TNFα increased cellular motility, migratory and invasive
abilities in the FaDu cells.
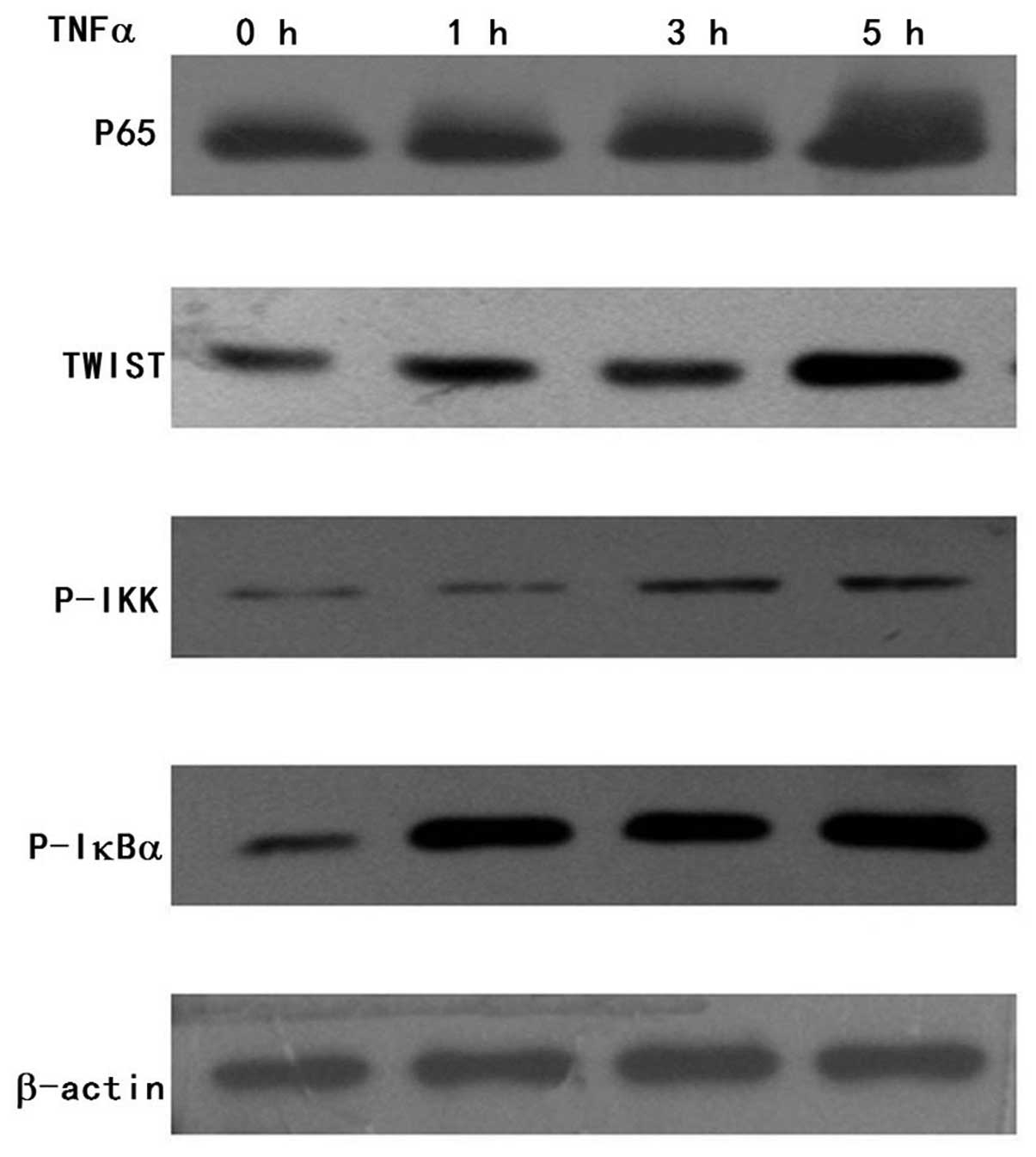
TNFα induces TWIST expression in the FaDu
cells
Given that TWIST plays an important role in
promoting the migration and invasion of HNSCC cells by regulating
EMT, we aimed to ascertain whether expression of TWIST is altered
in TNFα-induced EMT by western blotting. We found that TWIST
expression was increased in a time-dependent manner in the FaDu
cells following exposure to TNFα (Fig.
3). The results indicate that TNFα induces TWIST expression in
the FaDu cells.
P65 expression is upregulated and
translocated into the nucleus along with TNFα-induced TWIST
expression
Given that TNFα induces p65 activation in the
canonical NF-κB pathway, we investigated whether p65 is activated
upon TNFα-induced TWIST expression in the FaDu cells. We measured
the p65 expression by western blotting, and the results showed that
p65 expression following exposure to TNFα was increased in a
time-dependent manner (Fig. 3).
This suggests that P65 was upregulated together with TNFα-induced
TWIST expression in the FaDu cells. To further elucidate how TNFα
induces p65 activation upon TNFα-induced TWIST expression, we
examined p-IKK and p-IκBα expression. Western blot analysis showed
that p-IKK and p-IκBα expression was increased in the
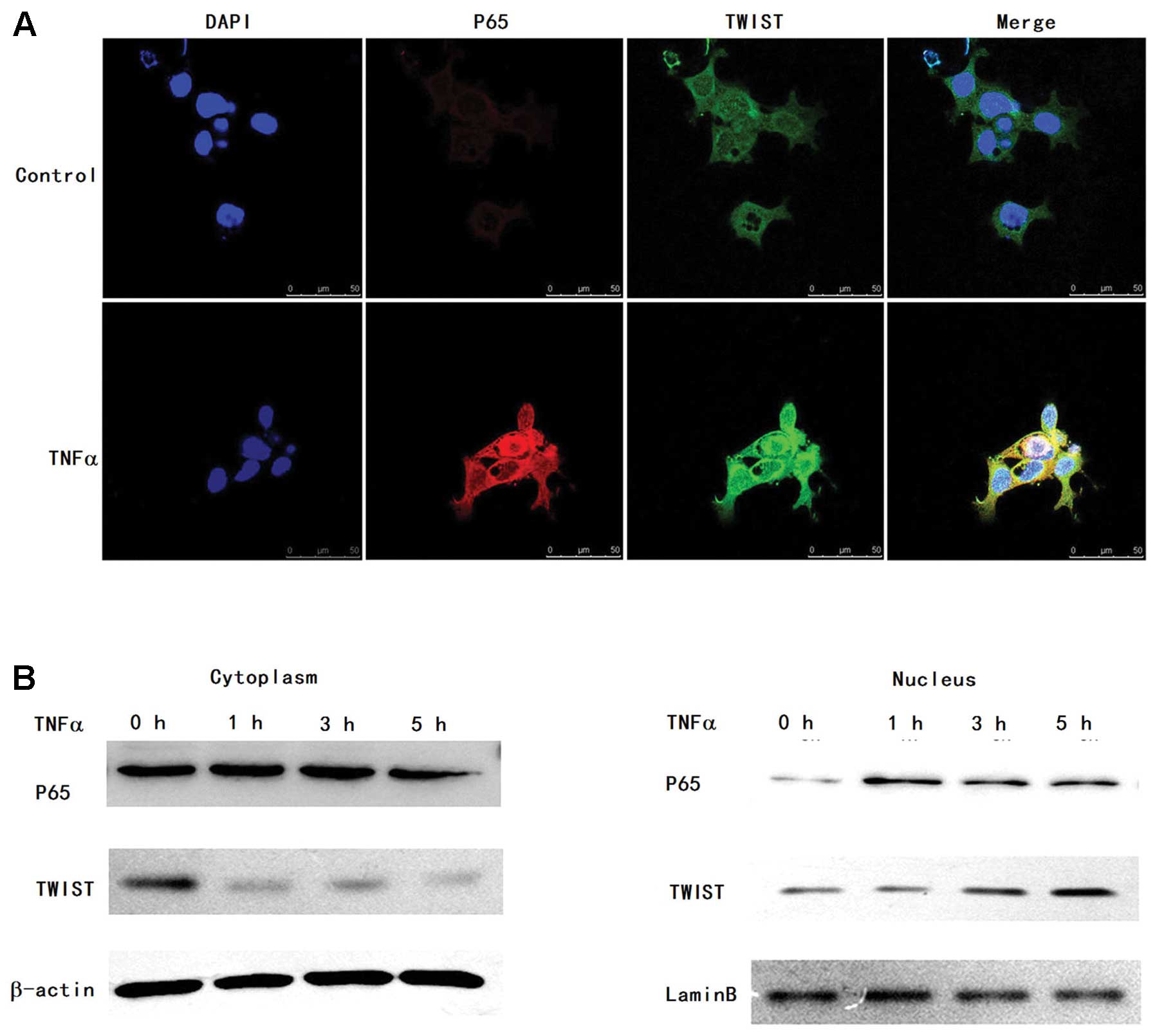
TNFα-activated p65-expressing cells, respectively (Fig. 3). Next, we tested the nuclear p65
and TWIST expression by immunofluorescence. Compared to the control
group, we observed that the levels of nuclear p65 and TWIST were
increased in the TNFα treatment group (Fig. 4A). To further confirm the
upregulation of nuclear TWIST and p65 expression, cytoplasmic and
nuclear fractions in the FaDu cells were isolated upon treatment
with TNFα. We also observed that TNFα-induced nuclear expression of
p65 and TWIST was significantly increased by western blotting
(Fig. 4B). These results suggest
that TNFα activates P65 expression and triggers a dynamic
interaction between nuclear translocation of p65 and nuclear
expression of TWIST.
Downregulation of p65 expression inhibits
TNFα-induced TWIST expression in FaDu cells
To further explore whether the alteration of p65
expression has any effect on TNFα-induced TWIST expression,
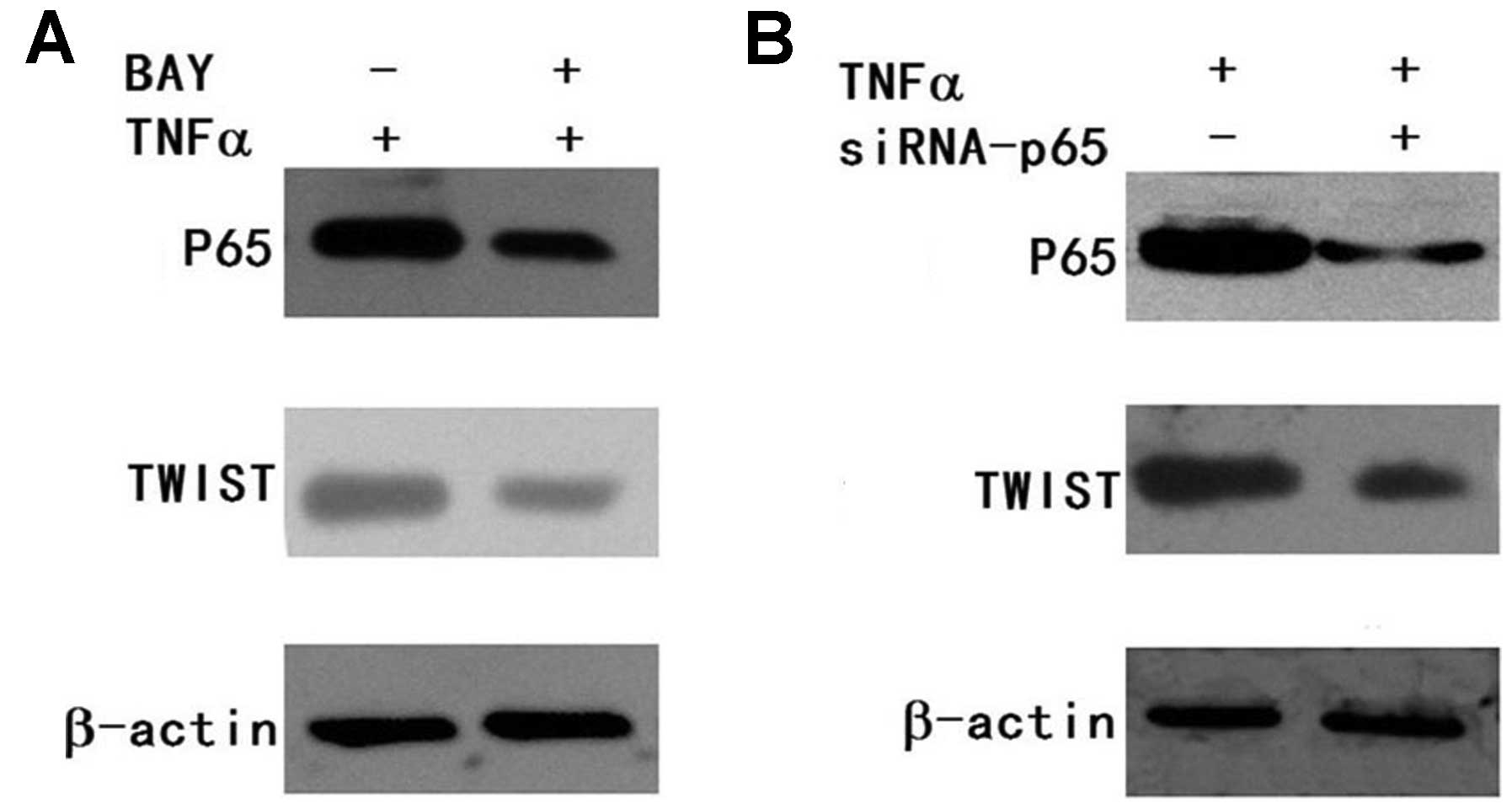
BAY11-7082 (inhibitor of NF-κB) was used. FaDu cells were
pretreated with BAY11-7082 for 2 h before TNFα stimulation, and
FaDu cells were then treated with TNFα for 5 h. p65 and TWIST
expression was determined by western blotting. We found that
BAY11-7082 inhibited p65 expression and blocked TNFα-induced TWIST
expression (Fig. 5A). To diminish
the off-target effect of the chemical inhibitor in TNFα-induced
TWIST expression, we introduced siRNA-p65 into FaDu cells. The
transfected cells were incubated for 24 h and used for analysis by
western blotting. We found that p65 expression was decreased in the
siRNA-p65 group. At the same time, TWIST expression was also
decreased in the siRNA-p65 group (Fig.
5B). These results showed that silencing of p65 expression also
attenuated TNFα-induced TWIST expression.
Discussion
TNFα, a pro-inflammatory cytokine predominantly
produced by macrophages, is a key molecule in the regulation of the
inflammatory processes in tumor promotion. Clinically, increased
expression of TNFα is present in various preneoplastic and
malignant diseases. TNFα has been reported to be elevated in the
blood serum of patients diagnosed with advanced stage breast tumors
and to be correlated with an increased number and size of
metastatic sites (18,19). In addition, TNFα is frequently
detected in human cancers with poor prognosis, such as ovarian,
renal and breast cancers (20).
Furthermore, TNFα has been shown to promote the growth and
invasiveness of colon and prostate cancer epithelial cells in
vitro and in vivo(18,21).
It is known that TNFα is involved in EMT and
enhances transforming growth factor-β1 (TGFβ-1)-induced EMT in
multiple cancer cell types (18).
EMT is an important step during primary tumor metastasis. Although
increasing evidence indicates that TNFα induces EMT and promotes
cancer migration and invasion (19,22),
the effect of TNFα on HNSCC remains undetermined.
In the present study, we found that TNFα induced
morphological alterations in FaDu cells, and their morphology
switched from a tightly packed growth pattern to scattered and
fibroblast-like colonies. At the same time, we found that
expression of the epithelial marker E-cadherin was downregulated,
whereas that of mesenchymal markers vimentin and N-cadherin was
significantly upregulated. These results indicate that TNFα induces
EMT.
The most distinguished characteristic of EMT is the
morphologic alteration from an epithelial to a mesenchymal
phenotype, which is often accompanied by the dissolution of
epithelial tight junctions, loss of cellular adhesion,
downregulation of the expression of various epithelial markers, but
acquired expression of mesenchymal components, resulting in loss of
cell polarity, cell-basement adhesion, and cell-cell contact and
the acquisition of migratory and invasive abilities (7). When epithelial cells undergo EMT,
which is thought to contribute to the invasive ability of cancer
cells (5), EMT and its accompanying
reduction in E-cadherin expression have been shown to be essential
for the extravasation of cancer cells into secondary organs
(23). Thus, we aimed to ascertain
whether TNFα-induced EMT has an effect on HNSCC cell motility,
migratory and invasive abilities. We found that TNFα increased
cellular motility, migratory and invasive abilities.
TWIST, is known as an essential regulator of the
aggressive phenotype of EMT (24).
Once TWIST is activated, it recruits histone deacetylases to the
E-box elements within the E-cadherin promoter, resulting in
transcriptional silencing of E-cadherin expression and increased
motility, migration and invasion (24,25).
Our previous research confirmed that alteration of TWIST affects
EMT in HNSCC cells, and knockdown of TWIST inhibits cell migration,
invasion and formation of the EMT phenotype in HNSCC and breast
cancer cells (8,9,26).
Thus, we hypothesized that TWIST may be implicated in TNFα-induced
EMT in HNSCC cells. Indeed, the present study showed that
expression of TWIST was significantly elevated in TNFα-induced EMT.
The results indicate that TNFα induced EMT by mediating TWIST
expression consequently increasing cell motility, migration and
invasion.
TNFα is one of the most important pro-inflammatory
cytokines produced in the tumor microenvironment. Several lines of
evidence demonstrate that TNFα and/or the NF-κB signaling pathway
plays a key role in the regulation of EMT (26). The contribution of NF-κB signaling
to the initiation and progression of cancer is clearly documented
(26–28). NF-κB is composed of 5 subunits,
including RELA (p65), RELB, c-REL, NF-κB1 (p105/p50) and NF-κB2
(p100/p52). P65 expression activation reflects activation of the
NF-κB signaling pathway. In the canonical pathway of NF-κB
activation, NF-κB activation is induced by various inflammatory
stimuli, including TNFα, interleukin-1 (IL-1) and
lipopolysaccharide (LPS). Upon stimulation, activated IKKb
phosphorylates IκBα (NF-κB inhibitor) and results in dissociation
of NF-κB from IκBα. As a consequence, the NF-κB heterodimer
translocates to the nucleus and activates expression of a myriad of
target genes involved in cell proliferation, angiogenesis, cell
survival, invasion and EMT (29).
Given that TNFα can activate the NF-κB signaling
pathway and provides a mechanistic link between inflammation and
cancer, we hypothesized that the NF-κB signaling pathway may be
involved in TNFα-induced TWIST expression. In the present study, we
found that p-IKK, p-IκBα and p65 expression was increased upon
TNFα-induced TWIST expression. The results indicate that the
canonical NF-κB signaling pathway was activated. Furthermore, p65
translocated to the nucleus after TNFα stimulation increased the
nuclear expression of TWIST. The change in p65 expression was
consistent with TWIST.
In order to further determine whether the alteration
in p65 expression has an effect on TNFα-induced TWIST expression,
we analyzed and found that when p65 was inhibited by siRNA-65 or
BAY11-7082, TWIST expression was also attenuated. The results
indicated that the alteration in p65 expression affected
TNFα-induced TWIST expression. Taken together, these data revealed
that the NF-κB signaling pathway is involved in TNF-induced EMT,
and p65 activation regulates TWIST expression in TNFα-induced EMT.
A recent study showed that TNFα induces EMT via upregulation of
TWIST in breast cancer cells (26).
TNFα can also upregulate Slug, which imparts breast cancer cells
with a stem cell-like phenotype (30). Further research demonstrated that
inflammation induces invasion and metastasis via NF-κB-mediated
stabilization of Snail (12).
In contrast, several studies reported that EMT
induced by TNFα requires the AKT/GSK-3β signaling pathway and
revealed that AKT/GSK-3β-mediated stabilization of Snail is
required for TNFα-induced EMT in colorectal cancer (10,11).
Thus, further investigation is warranted to determine whether TWIST
cooperates with other transcriptional factors, such as Snail and
Slug regarding the regulation of TNFα-mediated EMT in FaDu
cells.
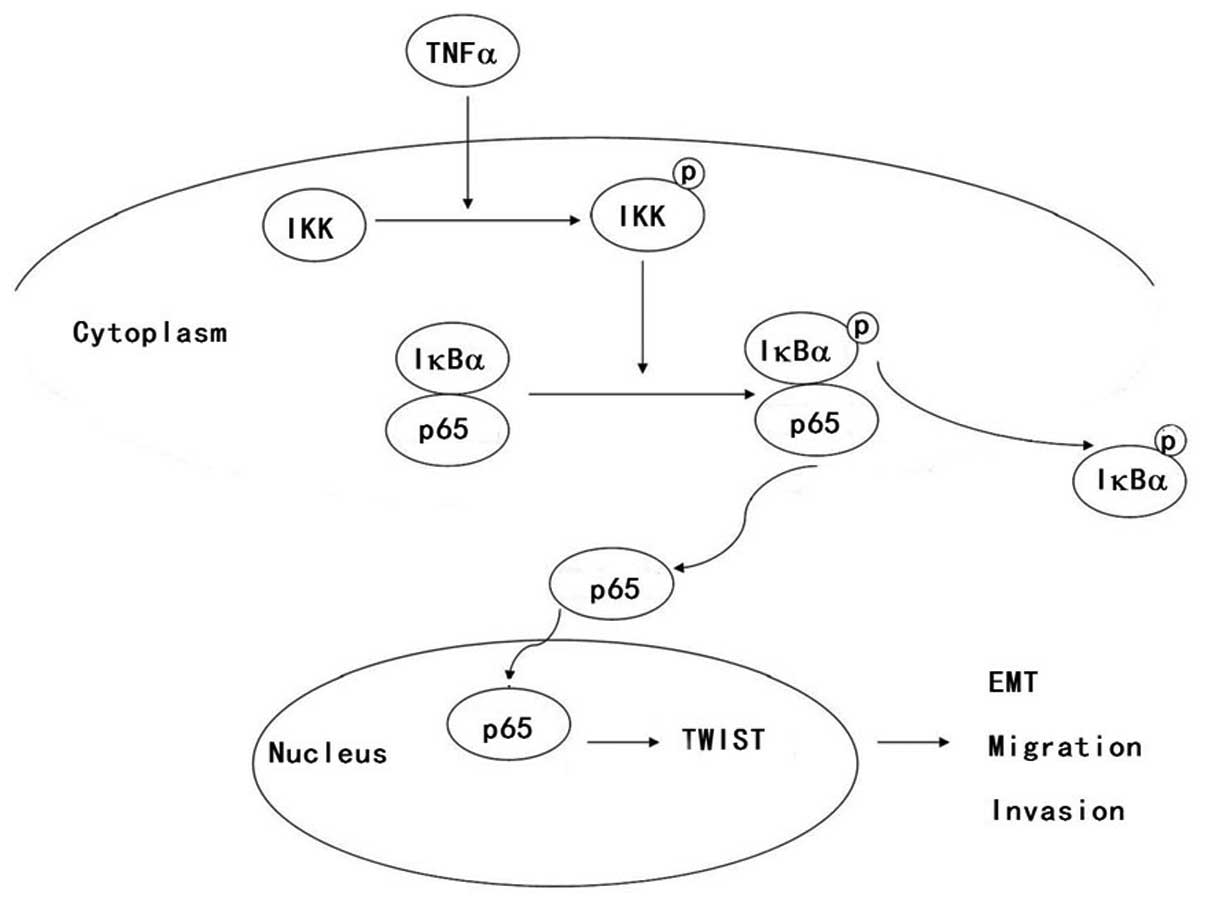
In summary, we demonstrated that TNFα induces EMT
via increased TWIST expression in human FaDu cells and promotes
hypopharyngeal cancer migration and invasion. Furthermore, we
elucidated that the NF-κB signaling pathway was activated in FaDu
cells, which regulated TNFα-induced TWIST expression. The detailed
mechanism is illustrated in Fig. 6.
We conclude that TNFα induced EMT and promoted metastasis via NF-κB
signaling pathway-mediated TWIST expression in hypopharyngeal
cancer.
Acknowledgements
The present study was supported by the Shandong
Provincial International Science and Technology Cooperation Project
of China (no. 2010GHZ20202) and the Graduate Independent Innovation
Foundation of Shandong University (GIIFSDU) (no. yzc12157).
References
|
1
|
Shacter E and Weitzman SA: Chronic
inflammation and cancer. Oncology. 16:217–226. 2292002.
|
|
2
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar
|
|
3
|
Karin M and Greten FR: NF-κB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005.
|
|
4
|
Luo JL, Kamata H and Karin M: IKK/NF-κB
signaling: balancing life and death - a new approach to cancer
therapy. J Clin Invest. 115:2625–2632. 2005.
|
|
5
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu L, Lu S, Tian J, et al: TWIST
expression in hypopharyngeal cancer and the mechanism of
TWIST-induced promotion of metastasis. Oncol Rep. 27:416–422.
2012.PubMed/NCBI
|
|
9
|
Yu L, Li HZ, Lu SM, et al: Down-regulation
of TWIST decreases migration and invasion of laryngeal carcinoma
Hep-2 cells by regulating the E-cadherin, N-cadherin expression. J
Cancer Res Clin Oncol. 137:1487–1493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Wang HS, Zhou BH, et al:
Epithelial-mesenchymal transition (EMT) induced by TNF-α requires
AKT/GSK-3β-mediated stabilization of Snail in colorectal cancer.
PLoS One. 8:e566642013.
|
|
11
|
Techasen A, Namwat N, Loilome W, et al:
Tumor necrosis factor-α (TNF-α) stimulates the
epithelial-mesenchymal transition regulator Snail in
cholangiocarcinoma. Med Oncol. 29:3083–3091. 2012.
|
|
12
|
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM
and Zhou BP: Stabilization of Snail by NF-κB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009.
|
|
13
|
Wu ST, Sun GH, Hsu CY, et al: Tumor
necrosis factor-α induces epithelial-mesenchymal transition of
renal cell carcinoma cells via a nuclear factor κB-independent
mechanism. Exp Biol Med. 236:1022–1029. 2011.
|
|
14
|
Smith RB, Apostolakis LW, Karnell LH, et
al: National Cancer Data Base report on osteosarcoma of the head
and neck. Cancer. 98:1670–1680. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lefebvre JL, Castelain B, De la Torre JC,
Delobelle-Deroide A and Vankemmel B: Lymph node invasion in
hypopharynx and lateral epilarynx carcinoma: a prognostic factor.
Head Neck Surg. 10:14–18. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eccles SA and Welch DR: Metastasis: recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu L, Li HZ, Lu SM, et al: Alteration in
TWIST expression: possible role in paclitaxel-induced apoptosis in
human laryngeal carcinoma Hep-2 cell line. Croat Med J. 50:536–542.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bates RC and Mercurio AM: Tumor necrosis
factor-α stimulates the epithelial-to-mesenchymal transition of
human colonic organoids. Mol Biol Cell. 14:1790–1800. 2003.
|
|
19
|
Takahashi E, Nagano O, Ishimoto T, et al:
Tumor necrosis factor-α regulates transforming growth
factor-β-dependent epithelial-mesenchymal transition by promoting
hyaluronan-CD44-moesin interaction. J Biol Chem. 285:4060–4073.
2010.
|
|
20
|
Balkwill F: Tumor necrosis factor or tumor
promoting factor? Cytokine Growth Factor Rev. 13:135–141. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SO, Lou W, Hou M, de Miguel F, Gerber
L and Gao AC: Interleukin-6 promotes androgen-independent growth in
LNCaP human prostate cancer cells. Clin Cancer Res. 9:370–376.
2003.PubMed/NCBI
|
|
22
|
Yamauchi Y, Kohyama T, Takizawa H, et al:
Tumor necrosis factor-α enhances both epithelial-mesenchymal
transition and cell contraction induced in A549 human alveolar
epithelial cells by transforming growth factor-β1. Exp Lung Res.
36:12–24. 2010.
|
|
23
|
Drake JM, Strohbehn G, Bair TB, Moreland
JG and Henry MD: ZEB1 enhances transendothelial migration and
represses the epithelial phenotype of prostate cancer cells. Mol
Biol Cell. 20:2207–2217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li CW, Xia W, Huo L, et al:
Epithelial-mesenchymal transition induced by TNF-α requires
NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res.
72:1290–1300. 2012.
|
|
27
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-κB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007.
|
|
28
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-κB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008.
|
|
29
|
Kaisho T, Takeda K, Tsujimura T, et al:
IκB kinase α is essential for mature B cell development and
function. J Exp Med. 193:417–426. 2001.
|
|
30
|
Storci G, Sansone P, Mari S, et al:
TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which
imparts breast cancer cells with a stem cell-like phenotype. J Cell
Physiol. 225:682–691. 2010. View Article : Google Scholar : PubMed/NCBI
|