Introduction
Urinary bladder cancer, pathologically presented
mainly as transitional cell carcinoma (TCC) (1), is one of the most common malignancies
in the world (2). Once diagnosed as
a muscle-invasive type, the 5-year survival and freedom from
relapse rates under conservative multimodality therapies were 54
and 42%, respectively (3).
Chemotherapy with standard regimen methotrexate, vinblastine,
adriamycin and cisplatin (MVAC) or more tolerable regimen GC
(gemcitabine and cisplatin) for advanced or metastatic bladder
cancer has demonstrated poor response (4). Currently, due to cisplatin-induced
nephrotoxicity, gemcitabine is the chief strategy for bladder
cancer therapy (5). However, fair
prognosis was noted in several trials of gemcitabine plus other
anti-neoplastic drugs (6,7). Gemcitabine resistance in TCC cells has
been noted (8).
Survivin, a member of the inhibitor of apoptosis
protein family, has been shown to be a diagnostic biomarker and
prognostic parameter of bladder cancer (9). Sepantronium bromide, a small molecule
also known as YM155, directly binds to the transcription factor
ILF3/NF110 or disrupts ILF3/p54nrb complex to inhibit
the activity of survivin gene promoter (10,11).
YM155 has been demonstrated to inhibit the progression of certain
neoplasms, such as prostate (10,12)
and lung cancer (13,14). In the present study, we demonstrated
that gemcitabine-resistant human TCC BFTC905 cells were sensitive
to YM155 at very low concentrations, and the anti-neoplastic
effects were more potent than cisplatin and/or gemcitabine.
Notably, YM155 did not elicit cytotoxic effects on primary human
urothelial cells. YM155 may be a suitable candidate for TCC that is
difficult to treat in the future.
Materials and methods
Cell lines and cell culture
BFTC905 (15),
BFTC909 (15), T24 and TSGH8301 TCC
cell lines were maintained as previously described (16,17).
Primary human urothelial cells (HUCs) were also used and cultured
in the special medium as suggested (#4312) (both from ScienCell
Research Laboratories, Carlsbad, CA, USA).
MTT cytotoxicity assay
Procedures were followed as previously described
(16,17). YM155 (Selleck, Houston, TX, USA),
cisplatin (Sigma, St. Louis, MO, USA), sirolimus and everolimus
(both from LC Laboratories, Woburn, MA, USA) were dissolved in DMSO
(J.T. Baker, Phillipsburg, NJ, USA). Gemcitabine and
3-methyladenine (3-MA) (both from Sigma) were dissolved in
distilled water.
Proliferation assay
For proliferation detection, the growth of
1×105 TCC cells or HUCs was determined in a 10 cm dish
with complete medium plus YM155 or DMSO at 0 h. After 24 h, the
medium was removed and the cells were treated as before. Cell
counts were calculated by a hemocytometer 48 h later.
Cell cycle analysis
BFTC905 cells were plated at a density of
2×105 cells/60-mm petri dish. After treatment, the
surfactant and the dead cells were removed. Only the cell cycle
changes in the viable cells were analyzed. Procedures using flow
cytometry (BD Biosciences, Bedford, MA, USA) as previously
described were followed (18).
Immunofluorescent staining
Hoechst 33342 (Enzo Life Sciences, Farmingdale, NY,
USA) staining was used for nuclei labeling. Expression of
phospho-histone H2AX (#9719) and cleaved
poly(ADP-ribose) polymerase [PARP (#9418); both from Cell Signaling
Technology, Inc., Danvers, MA, USA] were measured by the
fluorescent intensity in flow cytometry. Cyto-ID®
Autophagy Detection kit (Enzo Life Sciences) was used for staining
of autophagic vesicles. For semi-quantitation of condensed nuclei,
cells were chosen randomly from 10 fields and photographed by
Olympus fluorescence microscope BX43 (x400) plus Panasonic DMC-G1
digital camera.
Western blot analysis
Conventional procedures were followed as previously
described (16). Primary antibodies
were purchased from Cell Signaling Technology, Inc., with
anti-survivin (#2808), anti-LC3B (#2775), cell cycle regulation
(#9932) and cell cycle/checkpoint antibody sampler kits (#9917),
Abcam (Cambridge, MA, USA) with anti-securin (ab3305), GeneTex,
(Irvine, CA, USA) with anti-bcl2 (GTX127958), anti-TCTP (GTX63597),
anti-cyclin A1 (GXT103042), anti-cyclin B1 (GTX100911), anti-cyclin
D1 (GTX112874), cyclin E1 (GTX103045) Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA) with anti-Ki67 (sc-15402), α-tubulin
(sc-8305) and actin (sc-1616). Expression of α-tubulin or actin was
used as the internal standard.
DNA fragment assay
The Cell Death Detection ELISAplus
(Roche, Mannheim, Germany) assay kit was used to differentiate
apoptotic or necrotic condition of 2×104 BFTC905 cells
after YM155 treatment. Procedures were followed as previously
described (19).
Statistical analysis
Data are presented as means ± SEM (standard error of
mean) and analyzed by Student's t-test or two-way ANOVA based on
individual data. In all cases, p<0.05 was considered to indicate
a statistically significant difference and is labeled by
* in the figures. **p<0.01 and
***p<0.005.
Results
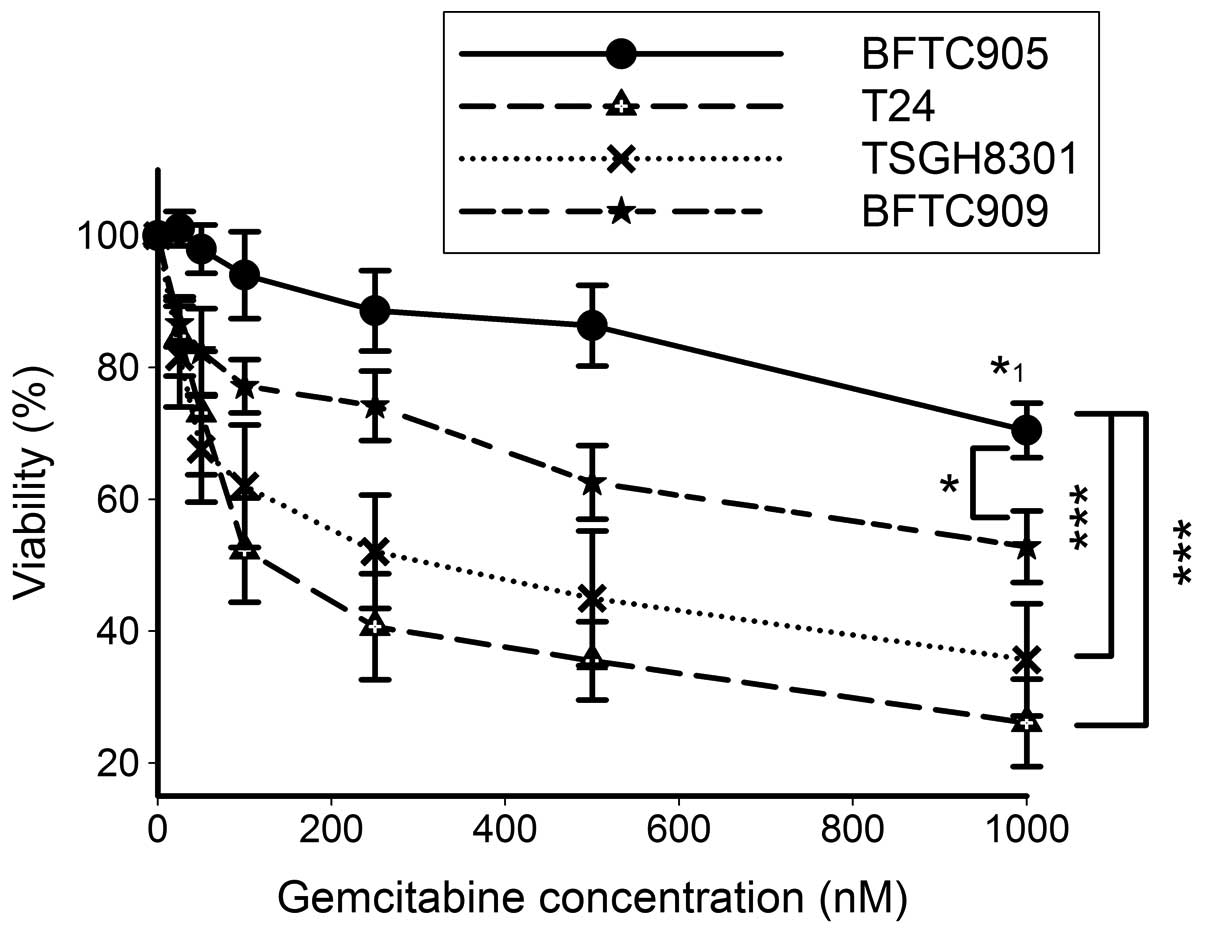
Cytotoxicity of gemcitabine in TCC
cells
Fig. 1 shows that up
to 500 nM of gemcitabine did not exert a significant cytotoxic
effect on the viability of BFTC905 cells after 48-h treatment
(n=5). Even after 1 μM gemcitabine treatment, a moderate but
significant reduction of 29.5% cell viability of BFTC905 cells was
found (p<0.001 vs. vehicle control). BFTC909 cells were also
relatively resistant to gemcitabine, but the viability difference
between BFTC905 and BFTC909 cells still reached statistical
significance (two-way ANOVA, p=0.03). The viability of T24 and
TSGH8301 cells was significantly lower than BFTC905 cells after
YM155 treatment (two-way ANOVA, both p<0.001). Thus, among these
4 cell lines examined, BFTC905 was chosen as a model to further
investigate the new therapy for gemcitabine-resistant bladder
cancer.
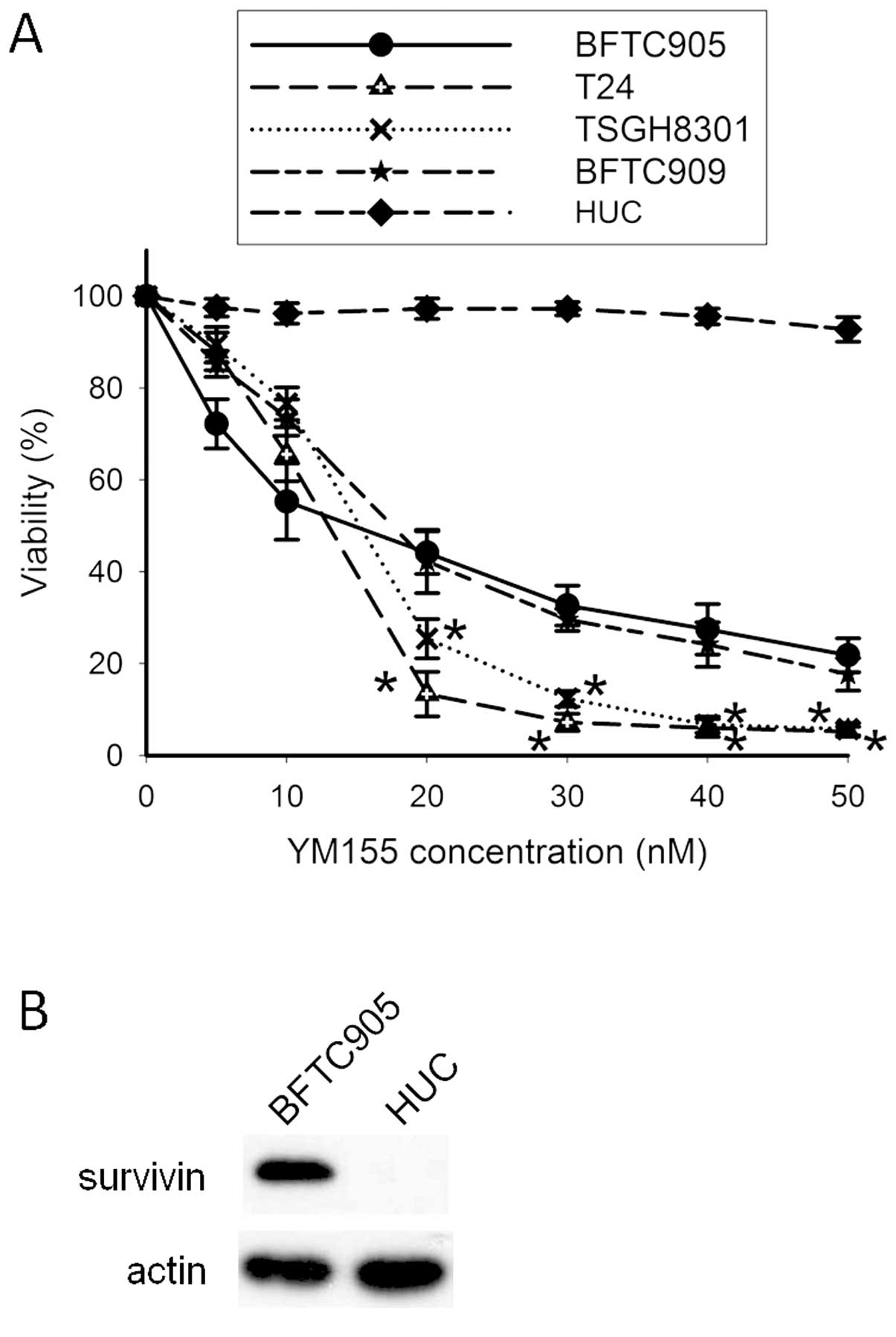
Cytotoxicity of YM155 in TCC and primary
human urothelial cells
After 48-h treatment, YM155 elicited potent
cytotoxicity in a concentration-dependent manner in TCC cells (n=3;
Fig. 2A). The IC50s for
4 TCC cell lines were all within 20 nM. No significant difference
of YM155-induced cytotoxicity between BFTC905 and BFTC909 cells was
noted (two-way ANOVA, p=0.28), but YM155 significantly exhibited
more potent cytotoxic effects on T24 and TSGH8301 than BFTC905
cells (two-way ANOVA, p<0.001). Within 20–50 nM YM155, the
viability between BFTC905 and T24 or TSGH8301 reached statistically
significant difference. The viability of HUCs was not altered after
YM155 treatment. Expression of survivin was not detected in HUCs,
and the expression of survivin in BFTC905 cells served as a
positive control (Fig. 2B).
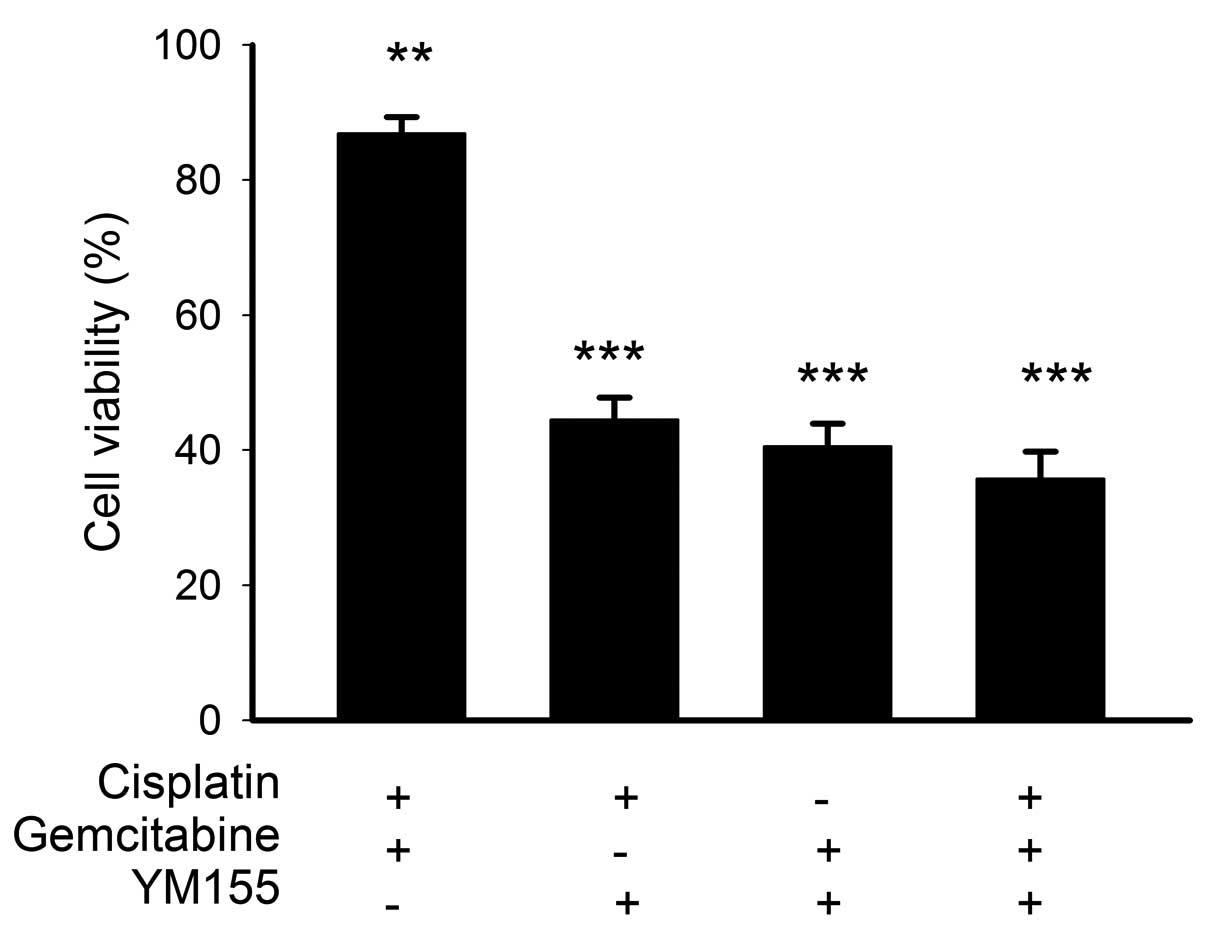
Cytotoxicity of YM155 plus cisplatin
and/or gemcitabine in BFTC905 cells
Next, we evaluated if synergistic or additive
cytotoxic effects may be observed when gemcitabine-resistant
BFTC905 cells are treated with YM155 plus cisplatin and/or
gemcitabine for 48 h (n=3; Fig. 3).
Cisplatin at 2.5 μM plus gemcitabine at 100 nM decreased the
viability of BFTC905 cells by 13.1% (86.9±2.4% of viability when
compared to control; p=0.005). When the corresponding
concentrations of cisplatin and/or gemcitabine were combined with
20 nM YM155, the cytotoxicities of these combinations were mainly
attributed to YM155 (Fig. 3) as 20
nM YM155 alone caused ~45% reduction in the viability of BFTC905
(Fig. 2). Compared to control, any
YM155 based regimen showed very potent cytotoxicity on BFTC905
cells (p<0.001). No synergistic or additive cytotoxicities in
BFTC905 cells were observed when YM155 was combined with cisplatin
and/or gemcitabine. YM155 alone provided marked efficacy in
treating gemcitabine-resistant TCC cells in vitro.
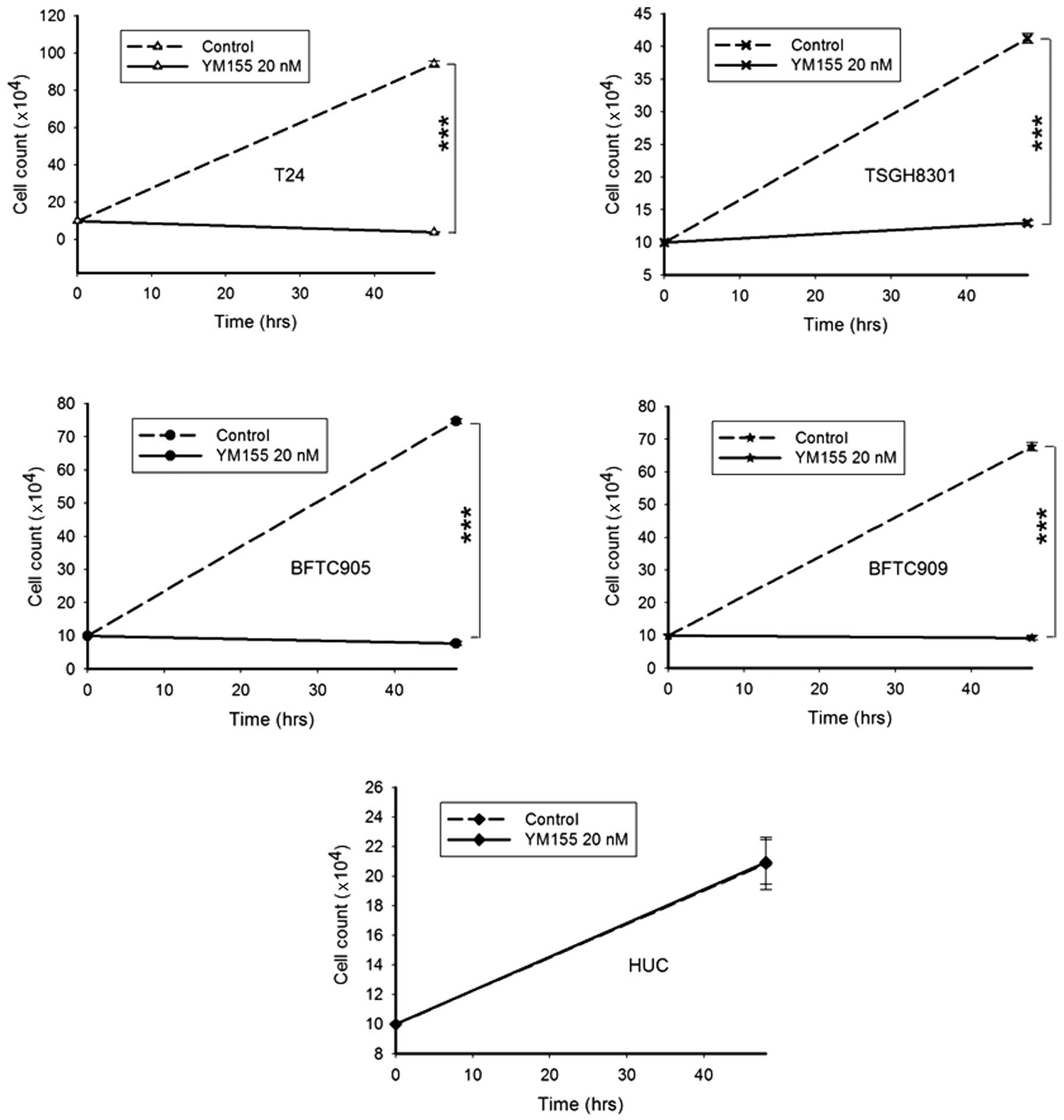
YM155 inhibits the proliferation of TCC
cells
Daily treatment with 20 nM YM155 for 2 days
significantly inhibited the proliferation of all TCC cell lines
(Fig. 4, n=3, all p<0.001). T24
cells were the fastest growing (from 1×105 to
9.4×105), and the most sensitive to inhibition by YM155
(from 9.4×105 to 3.8×104). The slowest growth
among the 4 cell lines was TSGH8301 (from 1×105 to
4.1×105) but also the least suppression of proliferation
by YM155 (from 4.1×105 to 1.3×105). The
proliferation of gemcitabine-resistant BFTC905 and BFTC909 cells
were also clearly inhibited (from 7.4×105 to
7.7×104 and from 6.8×105 to
9.3×104, respectively). The proliferation of HUCs was
not affected following YM155 treatment.
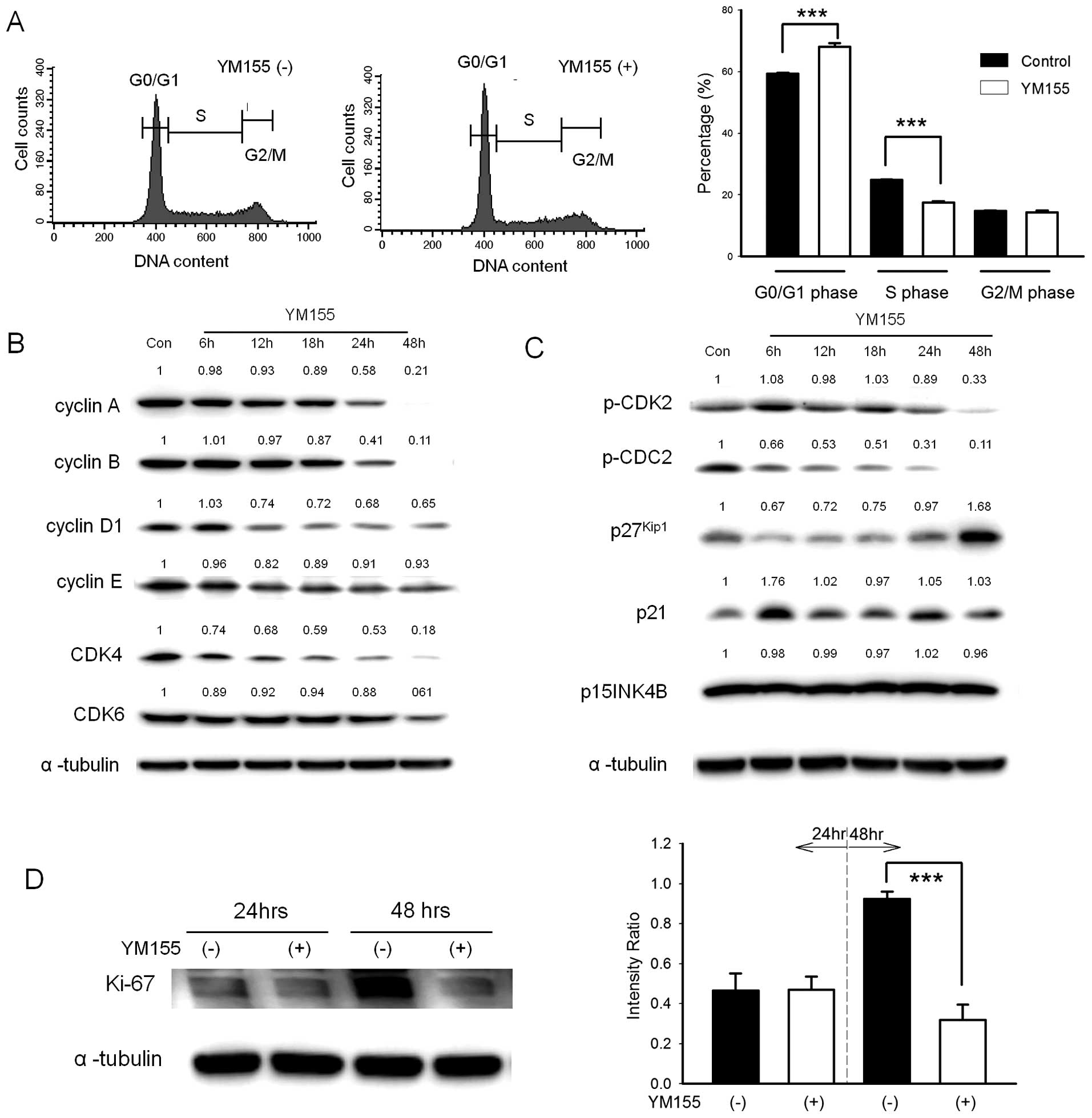
YM155 elicits quiescence in BFTC905 cells
after YM155 treatment
After 48-h treatment, a significant accumulation in
G0/G1 phase (68.1±1.1 vs. 59.4±0.3% after YM155 vs. DMSO treatment;
n=3, p=0.002) was observed (Fig.
5A). The expression of cell cycle related proteins is shown in
Fig. 5B and C. After 48-h
treatment, cyclin A/B/D1, CDK4/6, phospho-CDC2 and phospho-CDK2
were significantly decreased. However, cyclin E, p15INK4B and p21
did not show obvious changes. By contrast, the expression of
p27kip1 was increased at 48 h after treatment. These
results indicated that no stasis or arrest was found in all
proliferation phases of cell cycle, including G1, S and G2/M. We
hypothesized that BFTC905 cells were arrested in the G0 phase after
YM155 treatment. As shown in Fig.
5D, the expression of proliferation marker Ki-67 was
significantly decreased 48 h after YM155 treatment, indicating that
YM155 induced quiescence in BFTC905 cells.
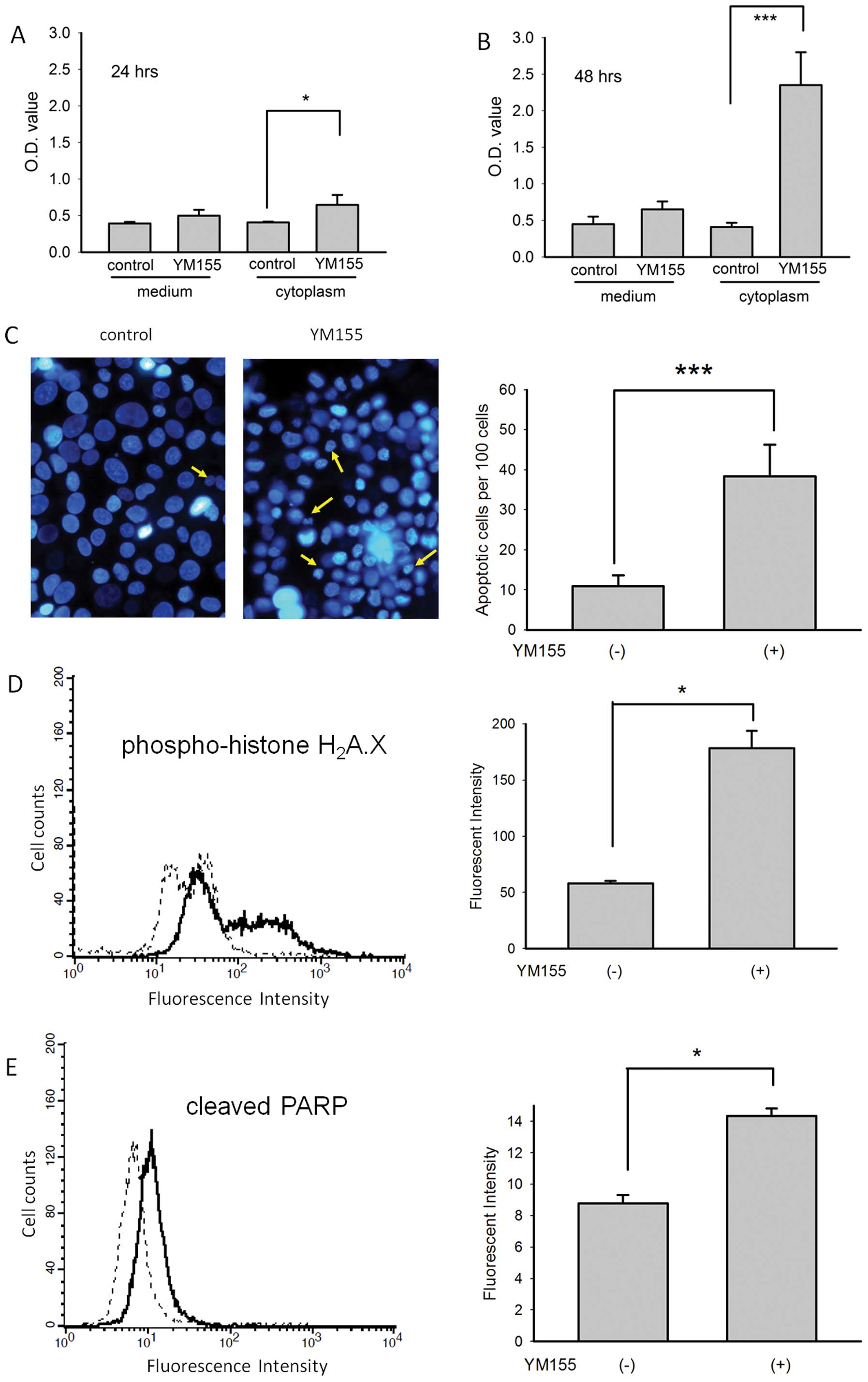
YM155 induces apoptosis in BFTC905
cells
Low basal levels of DNA fragments bound by
anti-histone plus anti-DNA antibodies were detected in the culture
medium of BFTC905 cells 24 and 48 h after 20 nM YM155 treatment. A
significant amount of cytoplasmic DNA fragments was detected 24 h
after 20 nM YM155 treatment when compared to control (n=3, p=0.04;
Fig. 6A). By contrast, a
significant amount of cytoplasmic DNA fragments was detected 48 h
following YM155 treatment (n=3, p=0.002; Fig. 6B). We also examined the morphology
of nuclei 24 and 48 h after 20 nM YM155 treatment using Hoechst
33342 staining. Significantly more nuclear condensation (yellow
arrow) in BFTC905 cells was observed after 48-h treatment (n=10,
p<0.001; Fig. 6C).
Semi-quantitation of the immunofluorescent intensity by flow
cytometry revealed significantly increased expression of DNA
double-strain break marker phospho-histone H2AX (n=3,
p=0.02; Fig. 6D) and cleaved PARP
(n=3, p=0.02; Fig. 6E) 48 h after
treatment with 20 nM YM155. These results indicated that YM155
induced apoptosis of gemcitabine-resistant TCC cells.
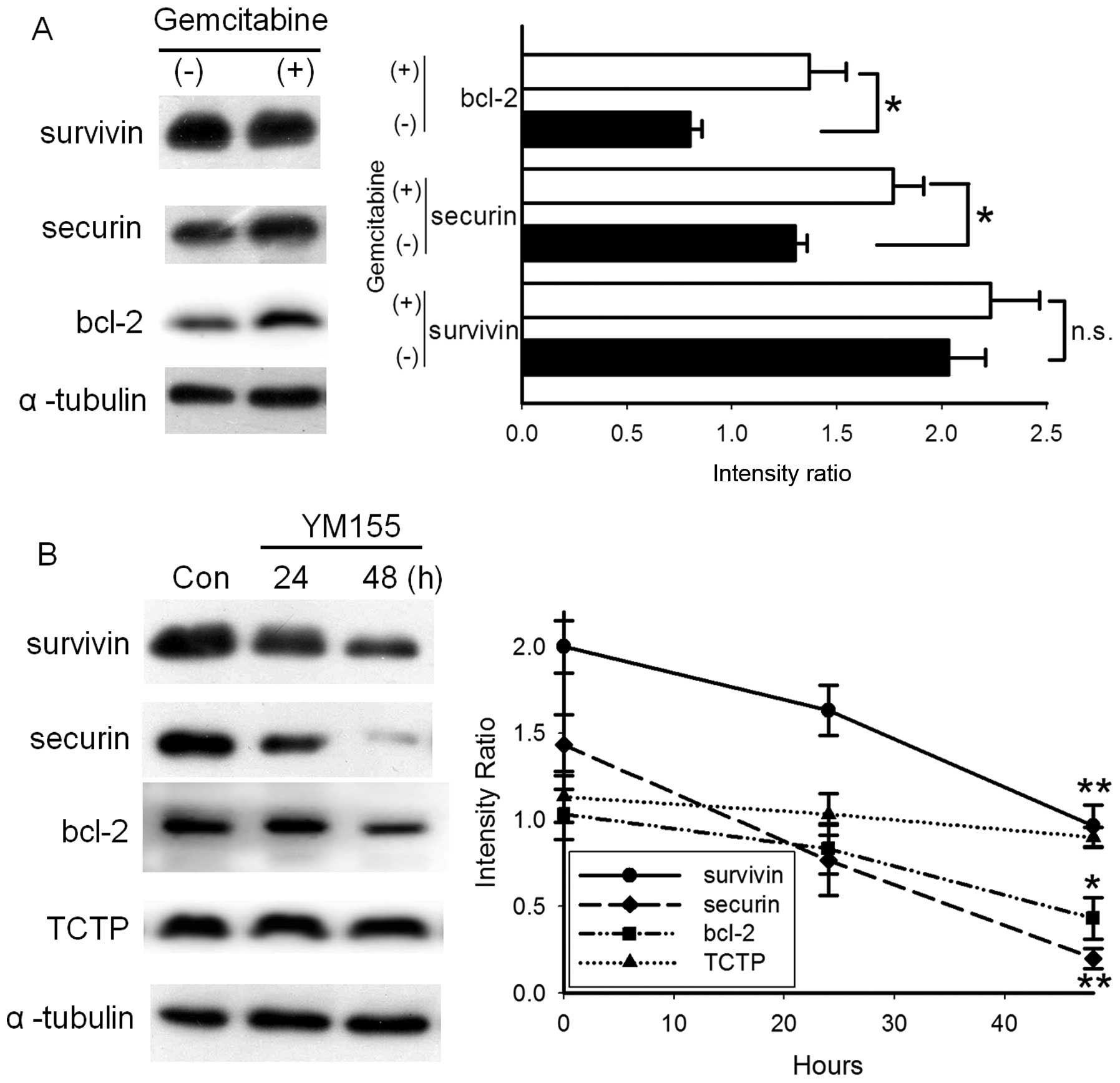
YM155 decreases the expression of
survivin, securin and bcl-2 in BFTC905 cells
Apart from survivin, securin and bcl-2 are also
correlated with the histological or clinical grading of bladder
cancer (20–22). Treatment with 1 μM gemcitabine for
48 h did not significantly alter the expression of survivin but it
increased the expression of securin and bcl-2 in BFTC905 cells
(both p=0.03, n=3; Fig. 7A). These
data may explain the mechanism of gemcitabine resistance in BFTC905
cells. Expressions of survivin, securin and bcl-2 in BFTC905 cells
were not significantly altered after 24-h treatment with 20 nM
YM155. After 48-h treatment with 20 nM YM155, not only survivin but
also securin and bcl-2 were significantly downregulated (p=0.006,
0.003 and 0.03, respectively, n=3; Fig.
7B). Translationally controlled tumor protein (TCTP) can
prevent apoptosis of cells (23,24).
No significant changes of TCTP expression were observed following
YM155 treatment.
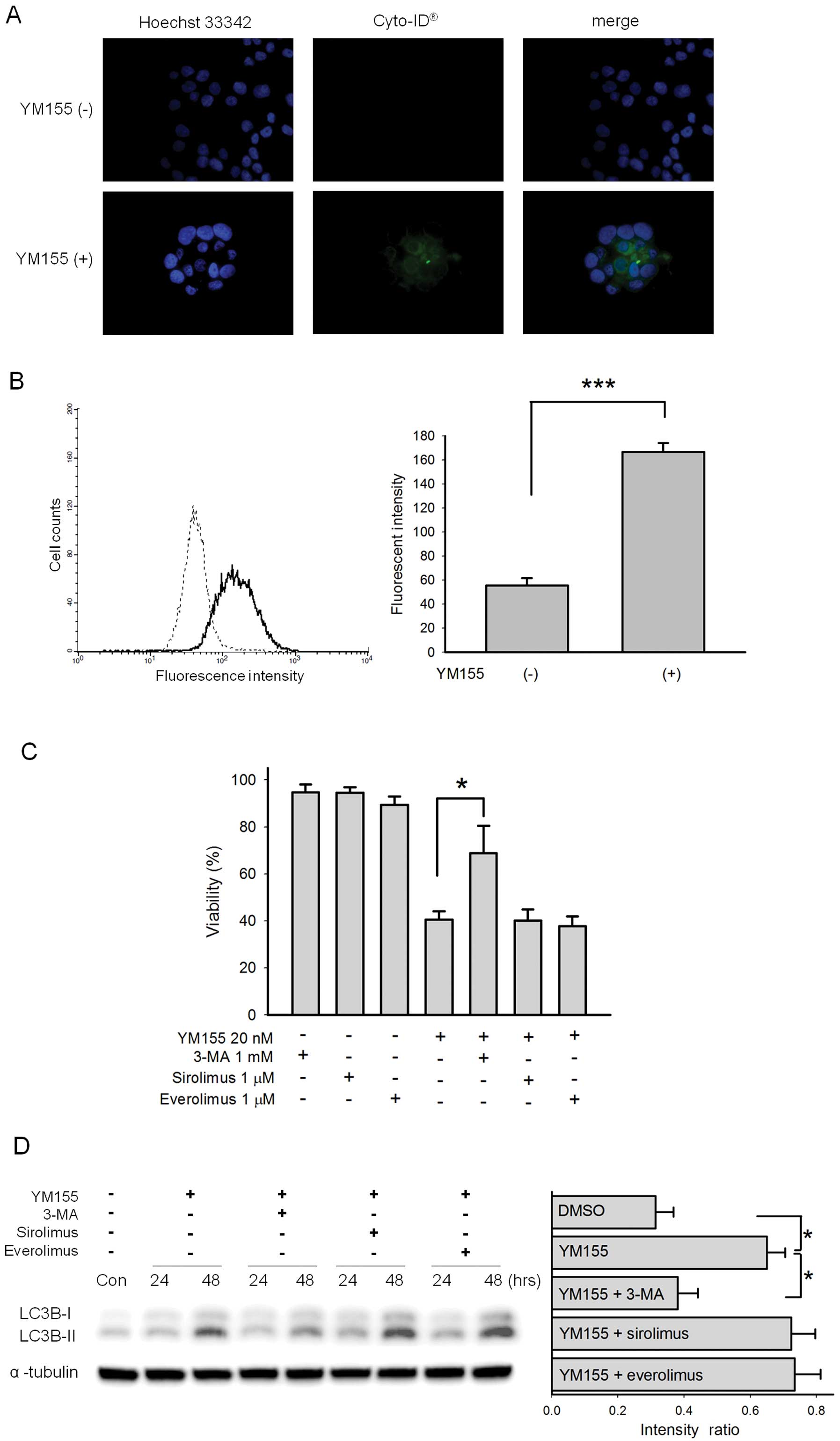
YM155 induces autophagy in BFTC905
cells
After 20 nM YM155 treatment, autophagic vacuoles
detected by Cyto-ID® dye were observed in BFTC905 cells
(Fig. 8A). Further flow cytometric
analysis showed a significant autophagic flux after YM155 treatment
(Fig. 8B). To determine the roles
of autophagy in YM155-induced BFTC905 cell death, autophagy
inhibitor 3-MA and autophagy inducers sirolimus and everolimus,
known as mammalian target of rapamycin (mTOR) inhibitors, were
administered additionally with YM155. 3-MA reversed YM155-induced
cytotoxicity (p=0.04, n=6) while sirolimus and everolimus did not
significantly alter YM155-induced cytotoxicity (Fig. 8C). LC3B-II, a marker of autophagy
converted from LC3B-I, was also significantly more abundant in
BFTC905 cells after 20 nM YM155 treatment (p=0.01, n=3; Fig. 8D). Concomitantly administered with
YM155, 3-MA significantly decreased the expression of LC3B-II
(p=0.03, n=3) while sirolimus and everolimus did not augment its
expression (Fig. 8D).
Discussion
Our results showed that novel survivin inhibitor,
YM155, elicited cytotoxicity in TCC cells including
gemcitabine-resistant cell line BFTC905. Moreover, no cytotoxic
effect was noted in normal human epithelial cells. We demonstrated
that treatment with a single agent YM155 already provided marked
therapeutic effects through multiple mechanisms. Few phase I/II
clinical trials of YM155 for cancer therapies have been reported
and it was well tolerated by patients with minimal adverse effects
such as fatigue and nausea (25).
Thus, YM155 may offer a new hope for refractory bladder cancer
patients.
Very low IC50s of gemcitabine (usually
<100 nM) have been reported for many TCC cell lines (26,27)
except one gemcitabine-resistant TCC cell line UM-UC-3R (8). IC50s of gemcitabine for T24
and TSGH8301 cells in our experiments were >100 nM, and we
suppose the variation may result from different cytotoxic assays
and protocols. However, the viability of T24 and TSGH8301 cells was
indeed much lower than BFTC905 cells after gemcitabine treatment.
Gemcitabine upregulated survivin expression in pancreatic cancer
MiaPaCa-2 cells, but this phenomenon was not observed in BFTC905
cells (28). Securin, the product
of pituitary tumor-transforming gene (PTTG) (29), has been reported to mediate
gefitinib resistance in cancer cells (30). Bcl-2, an anti-apoptotic protein, has
also been reported to mediate cisplatin resistance in urothelial
carcinoma cells (31). In the
present investigation, upregulation of securin and bcl-2 in BFTC905
cells may be a compensatory mechanism for gemcitabine resistance.
We have also reported that only BFTC905 but not T24 and TSGH8301
xenografts successfully grew in SCID mice with less cell loading
(32). Collectively, the above
observations indicate that BFTC905 is the first choice of TCC cell
lines for investigating the new therapy for gemcitabine-resistant
bladder cancer.
Our results showed that YM155 caused potent
cytotoxicity in TCC cells, including gemcitabine-resistant ones. It
may result from survivin similarly expressed in these cell lines
(33). However, other different
characteristics that exist among BFTC905 and other high grade TCC
cell lines should be further investigated. Since survivin is not
expressed in HUCs, it is reasonable that YM155 did not elicit
cytotoxic effects in HUCs. YM155 acting on bladder cancer cells
without affecting normal urothelium would be the best choice for
targeted therapy. Cisplatin plus gemcitabine is the standard
chemotherapy regimen for bladder cancer, but fair cytotoxic effect
was noted in BFTC905 cells. YM155 has been reported to reverse
cisplatin resistance in head and neck squamous cell carcinoma cells
(34). Thus, we also tested the
cytotoxic effects of YM155 combined with cisplatin, gemcitabine, or
both. YM155 has been reported to potentiate the therapeutic effects
of many chemotherapy or targeted-therapy agents (13,14,35–39).
However, our results demonstrated that YM155 alone exerted potent
cytotoxicity in BFTC905 cells, and addition of cisplatin and/or
gemcitabine to YM155 did not elicit synergistic or additive
effects. The present study also confirmed that survivin plays a key
role in the survival of TCC cells. YM155 may be a suitable option
for refractory bladder cancer therapy.
Survivin, a member of the inhibitor of apoptosis
(IAP) family, is abundantly expressed in the G2/M phase of the cell
cycle to regulate mitosis (40).
Downregulation of survivin by siRNA inhibited bladder cancer growth
with G2/M phase arrest (41).
However, survivin inhibitor YM155 caused variable cell cycle arrest
and even a lack of changes in cell cycles were found in previous
reports. In malignant peripheral nerve sheath tumor cells, YM155
caused G2/M cell cycle arrest (42). Cheng et al demonstrated a
slight S phase stasis in PC3 prostate cancer cells after 24- and
48-h YM155 treatment (43). Since
cell cycle analysis using flow cytometry only represents the
proportional changes in each phase after treatment, we further
examined the specific cell cycle related proteins. The roles of
cyclins, cyclin dependent kinases and related molecules in cancer
cell cycles have been well established (44,45).
Downregulation of G2/M phase marker cyclin B and phospho-CDC2 in
the present study indicated no G/M arrest. Downregulation of cyclin
A, which is expressed in S phase, indicated no S phase arrest. CDK2
is expressed in late G1 and S phase, therefore a decrease in CDK2
expression indicated no cell cycle stasis in late G1 and S phase.
Cyclin D1 and CDK4/6 are expressed in G1 phase before R point,
while cyclin E is expressed in late G1 phase after R point.
Expression of these markers was all decreased after YM155
treatment; thus, YM155 did not induce G1 phase arrest. Due to the
increased ratio of G0/G1 phase without G1 arrest, YM155 induced G0
phase stasis, known as quiescence, was substantiated. Our results
are consistent with those of Arora et al who demonstrated
the early inhibition of DNA synthesis in Merkel cell carcinoma by
YM155 (46). After 48-h YM155
treatment, the increased expression of p27kip1, a potent
inhibitor of cyclin-CDK complex formation, supported this idea.
Ki-67 protein is present in the nucleus during all active phases of
the cell cycle (G1, S, G2 and mitotic phase) but absent from
quiescent cells (G0), so Ki-67 is the most referenced marker for
cellular proliferation (47,48).
In our results, expression of Ki-67 after 48-h YM155 treatment was
much lower than control. Thus, we found the novel effect of YM155
in promoting quiescence in gemcitabine-resistant TCC cells.
Survivin plays important roles in anti-apoptosis, so
any method to decrease the expression of survivin in bladder cancer
cells should elicit apoptosis (49,50).
In this regard, survivin inhibitor YM155 promoted apoptosis of
cancer cells (19,51), and we also observed that YM155
induced apoptosis in TCC cells with enhanced expression of cleaved
PARP and phospho-histone γ-H2AX. PARP is involved in the repair of
single-strand DNA nicks, but cleaved PARP facilitates cellular
disassembly and promotes ongoing apoptosis (52). Enhanced expression of cleaved PARP
after YM155 treatment was also noted in SU-DHL-4 large-B cell
lymphoma, triple-negative breast cancer and adult T-cell leukemia
cells (37,39,53).
γ-H2AX is the DNA double-strand break marker and γ-H2AX
dephosphorylation facilitates DNA double-strand break repair
(54,55). Expression of phospho-γ-H2AX varied
after YM155 treatment, for instance, an increase in non-small cell
lung cancer Calu6 cells but no changes in H460 cells were reported
(35). Similarly, expression of
bcl-2 in A375 melanoma cells was not altered after YM155 treatment
(38), but marked inhibition of
bcl-2 expression by survivin siRNA in human renal clear cell
carcinoma 786-O cells, similar to our results, was observed
(56). It may result from the
characteristics of individual cell lines. Securin was found as an
inhibitor of premature sister chromatid separation in nuclei
(57). In our previous report,
securin was overexpressed in human TCC specimens when compared to
normal urothelium (22). Chao and
Liu also demonstrated that survivin siRNA significantly induced
apoptosis and decreased the levels of securin in A549 cells
(58). We have also reported
YM155-induced apoptosis with securin suppression in glioblastoma
multiforme cells (32).
Overexpression of TCTP was observed in many malignancies (12), but whether TCTP is expressed in
bladder cancer has not be determined. Our results demonstrated the
first observation of TCTP expression in TCC cells. Expression of
TCTP in the rat urothelium has been reported (59). No changes in TCTP expression after
YM155 treatment suggest that the function of urothelium may not be
altered to induce adverse effects. The detailed signaling among
survivin, securin and bcl-2 in TCC should be further
investigated.
Survivin is also involved in autophagy. Survivin
controls autophagosome formation and protects cancer cells from
autophagic death, known as type II cell death, by inhibiting the
expression of LC3II (60). In
prostate cancer cells, YM155 induced autophagy and autophagy
inhibitor 3-MA attenuated YM155-induced apoptosis (12). In that study, autophagy induced by
YM155 played a pro-apoptotic role. However, autophagy is a
double-edged sword (61). Autophagy
is also important for survival in tumor cells by overcoming hypoxia
and a shortage of nutrients. Therefore, we evaluated the role of
YM155-induced autophagy in BFTC905 cells. As expected, 3-MA
diminished YM155-induced cytotoxicity by decreasing the expression
of LC3B-II. Sirolimus and everolimus, however, did not elicit
additive cytotoxic effects with YM155 in BFTC905 cells. Compared to
treatment with YM155 alone, expression of LC3B-II was not increased
after YM155 plus mTOR inhibitors, indicating that YM155 per
se is a strong activator of LC3B-II. YM155-induced autophagy
promoted the death of BFTC905 cells.
In conclusion, low concentrations of YM155 induced
quiescence, apoptosis, and autophagy in gemcitabine-resistant human
TCC cells without causing cytotoxicity in normal human urothelium.
YM155 may become a new candidate for advanced or metastatic bladder
cancer therapy.
Acknowledgements
The present study was supported by a grant-in-aid
from Tzu Chi General Hospital, Hualien 970, Taiwan.
References
|
1
|
Tavora F and Epstein JI: Bladder cancer,
pathological classification and staging. BJU Int. 102:1216–1220.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murta-Nascimento C, Schmitz-Dräger,
Zeegers MP, et al: Epidemiology of urinary bladder cancer: from
tumor development to patient's death. World J Urol. 25:285–295.
2007. View Article : Google Scholar
|
|
3
|
George L, Bladou F, Bardou VJ, et al:
Clinical outcome in patients with locally advanced bladder
carcinoma treated with conservative multimodality therapy. Urology.
64:488–493. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
von der Maase H, Hansen SW, Roberts JT, et
al: Gemcitabine and cisplatin versus methotrexate, vinblastine,
doxorubicin, and cisplatin in advanced or metastatic bladder
cancer: results of a large, randomized, multinational, multicenter,
phase III study. J Clin Oncol. 18:3068–3077. 2000.
|
|
5
|
Sternberg CN: Gemcitabine in bladder
cancer. Semin Oncol. 27:31–39. 2000.
|
|
6
|
Chaudhary UB, Verma N, Keane T and Gudena
V: A phase II study of gemcitabine and irinotecan in patients with
locally advanced or metastatic bladder cancer. Am J Clin Oncol. Dec
13–2012.(Epub ahead of print).
|
|
7
|
Culine S, Fléchon A, Guillot A, et al:
Gemcitabine or gemcitabine plus oxaliplatin in the first-line
treatment of patients with advanced transitional cell carcinoma of
the urothelium unfit for cisplatin-based chemotherapy: a randomized
phase 2 study of the French Genitourinary Tumor Group (GETUG V01).
Eur Urol. 60:1251–1257. 2011.
|
|
8
|
Muramaki M, So A, Hayashi N, et al:
Chemosensitization of gemcitabine-resistant human bladder cancer
cell line both in vitro and in vivo using antisense oligonucleotide
targeting the anti-apoptotic gene, clusterin. BJU Int. 103:384–390.
2009. View Article : Google Scholar
|
|
9
|
Margulis V, Lotan Y and Shariat SF:
Survivin: a promising biomarker for detection and prognosis of
bladder cancer. World J Urol. 26:59–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakahara T, Takeuchi M, Kinoyama I, et al:
YM155, a novel small-molecule survivin suppressant, induces
regression of established human hormone-refractory prostate tumor
xenografts. Cancer Res. 67:8014–8021. 2007. View Article : Google Scholar
|
|
11
|
Nakamura N, Yamauchi T, Hiramoto M, et al:
Interleukin enhancer-binding factor 3/NF110 is a target of YM155, a
suppressant of survivin. Mol Cell Proteomics. 11:M111.013243. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Q, Chen Z, Diao X and Huang S:
Induction of autophagy-dependent apoptosis by the survivin
suppressant YM155 in prostate cancer cells. Cancer Lett. 302:29–36.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakahara T, Yamanaka K, Hatakeyama S, et
al: YM155, a novel survivin suppressant, enhances taxane-induced
apoptosis and tumor regression in a human Calu 6 lung cancer
xenograft model. Anticancer Drugs. 22:454–462. 2011. View Article : Google Scholar
|
|
14
|
Iwasa T, Okamoto I, Suzuki M, et al:
Radiosensitizing effect of YM155, a novel small-molecule survivin
suppressant, in non-small cell lung cancer cell lines. Clin Cancer
Res. 14:6496–6504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tzeng CC, Liu HS, Li C, et al:
Characterization of two urothelium cancer cell lines derived from a
blackfoot disease endemic area in Taiwan. Anticancer Res.
16:1797–1804. 1996.PubMed/NCBI
|
|
16
|
Huang YT, Lai PC, Wu CC, et al: BDNF
mediated TrkB activation is a survival signal for transitional cell
carcinoma cells. Int J Oncol. 36:1469–1476. 2010.PubMed/NCBI
|
|
17
|
Lai PC, Yang YC, Cheng CC, Chiu TH and
Huang YT: Brain-derived neurotrophic factor plus vascular
endothelial growth factor additively promotes early growth of the
transitional cell carcinoma cell line BFTC905 in vitro and in vivo.
Tzu Chi Med J. 25:155–160. 2013. View Article : Google Scholar
|
|
18
|
Chao JI, Su WC and Liu HF: Baicalein
induces cancer cell death and proliferation retardation by the
inhibition of CDC2 kinase and survivin associated with opposite
role of p38 mitogen-activated protein kinase and AKT. Mol Cancer
Ther. 6:3039–3048. 2007. View Article : Google Scholar
|
|
19
|
Lai PC, Chen SH, Yang SH, Cheng CC, Chiu
TH and Huang YT: Novel survivin inhibitor YM155 elicits
cytotoxicity in glioblastoma cell lines with normal or deficiency
DNA-dependent protein kinase activity. Pediatr Neonatol.
53:199–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liukkonen TJ, Lipponen PK, Helle M and
Jauhiainen KE: Immunoreactivity of bcl-2, p53 and EGFr is
associated with tumor stage, grade and cell proliferation in
superficial bladder cancer. Finnbladder III Group. Urol Res.
25:1–7. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gazzaniga P, Gradilone A, Silvestri I, et
al: Variable levels of bcl-2, bcl-X and bax
mRNA in bladder cancer progression. Oncol Rep. 5:901–904. 1998.
|
|
22
|
Lai PC, Fang TC, Chiu TH and Huang YT:
Overexpression of securin in human transitional cell carcinoma
specimens. Tzu Chi Med J. 22:171–176. 2010. View Article : Google Scholar
|
|
23
|
Bommer UA and Thiele BJ: The
translationally controlled tumour protein (TCTP). Int J Biochem
Cell Biol. 36:379–385. 2004. View Article : Google Scholar
|
|
24
|
Koziol MJ and Gurdon JB: TCTP in
development and cancer. Biochem Res Int. 2012:1052032012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lewis KD, Samlowski W, Ward J, et al: A
multi-center phase II evaluation of the small molecule survivin
suppressor YM155 in patients with unresectable stage III or IV
melanoma. Invest New Drugs. 29:161–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoon CY, Lee JS, Kim BS, et al: Sunitinib
malate synergistically potentiates anti-tumor effect of gemcitabine
in human bladder cancer cells. Korean J Urol. 52:55–63. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moibi JA, Mak AL, Sun B and Moore RB:
Urothelial cancer cell response to combination therapy of
gemcitabine and TRAIL. Int J Oncol. 39:61–71. 2011.PubMed/NCBI
|
|
28
|
Yoon DH, Shin JS, Jin DH, et al: The
survivin suppressant YM155 potentiates chemosensitivity to
gemcitabine in the human pancreatic cancer cell line MiaPaCa-2.
Anticancer Res. 32:1681–1688. 2012.PubMed/NCBI
|
|
29
|
Zou H, McGarry TJ, Bernal T and Kirschner
MW: Identification of a vertebrate sister-chromatid separation
inhibitor involved in transformation and tumorigenesis. Science.
285:418–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu SY, Liu HF, Wang SP, Chang CC, Tsai CM
and Chao JI: Evidence of securin-mediated resistance to
gefitinib-induced apoptosis in human cancer cells. Chem Biol
Interact. 203:412–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu HM and Wang TC: Mechanism of cisplatin
resistance in human urothelial carcinoma cells. Food Chem Toxicol.
50:1226–1237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lai PC, Chiu TH and Huang YT:
Overexpression of BDNF and TrkB in human bladder cancer specimens.
Oncol Rep. 24:1265–1270. 2010.PubMed/NCBI
|
|
33
|
Huang YT, Lai PC, Wu CC, Cheng CC and Chiu
TH: TrkB antibody elicits cytotoxicity and suppresses
migration/invasion of transitional cell carcinoma cells. Int J
Oncol. 37:943–949. 2010.PubMed/NCBI
|
|
34
|
Kumar B, Yadav A, Lang JC, et al: YM155
reverses cisplatin resistance in head and neck cancer by decreasing
cytoplasmic survivin levels. Mol Cancer Ther. 11:1988–1998. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iwasa T, Okamoto I, Takezawa K, et al:
Marked anti-tumour activity of the combination of YM155, a novel
survivin suppressant, and platinum-based drugs. Br J Cancer.
103:36–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kita A, Mitsuoka K, Kaneko N, et al:
Sepantronium bromide (YM155) enhances response of human B-cell
non-Hodgkin lymphoma to rituximab. J Pharmacol Exp Ther.
343:178–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaneko N, Kita A, Yamanaka K and Mori M:
Combination of YM155, a survivin suppressant with a STAT3
inhibitor: a new strategy to treat diffuse large B-cell lymphoma.
Leuk Res. 37:1156–1161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamanaka K, Nakahara T, Yamauchi T, et al:
Antitumor activity of YM155, a selective small-molecule survivin
suppressant, alone and in combination with docetaxel in human
malignant melanoma models. Clin Cancer Res. 17:5423–5431. 2011.
View Article : Google Scholar
|
|
39
|
Chen J, Pise-Masison CA, Shih JH, et al:
Markedly additive antitumor activity with the combination of a
selective survivin suppressant YM155 and alemtuzumab in adult
T-cell leukemia. Blood. 121:2029–2037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li F, Ambrosini G, Chu EY, et al: Control
of apoptosis and mitotic spindle checkpoint by survivin. Nature.
396:580–584. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ning S, Fuessel S, Kotzsch M, et al:
siRNA-mediated down-regulation of survivin inhibits bladder cancer
cell growth. Int J Oncol. 25:1065–1071. 2004.PubMed/NCBI
|
|
42
|
Ghadimi MP, Young ED, Belousov R, et al:
Survivin is a viable target for the treatment of malignant
peripheral nerve sheath tumors. Clin Cancer Res. 18:2545–2557.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng Q, Ling X, Haller A, et al:
Suppression of survivin promoter activity by YM155 involves
disruption of Sp1-DNA interaction in the survivin core promoter.
Int J Biochem Mol Biol. 3:179–197. 2012.PubMed/NCBI
|
|
44
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hunter T and Pines J: Cyclins and cancer.
II: cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Arora R, Shuda M, Guastafierro A, et al:
Survivin is a therapeutic target in Merkel cell carcinoma. Sci
Transl Med. 4:133ra562012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Scholzen T and Gerdes J: The Ki-67
protein: from the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gerdes J, Li L, Schlueter C, et al:
Immunobiochemical and molecular biologic characterization of the
cell proliferation-associated nuclear antigen that is defined by
monoclonal antibody Ki-67. Am J Pathol. 138:867–873. 1991.
|
|
49
|
Gou X, Yang HA, He WY, Xioa MC and Wang M:
Gene silence-induced downregulation of survivin inhibits bladder
cancer cells. Oncol Res. 19:535–541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ku JH, Seo SY, Kwak C and Kim HH:
Cytotoxicity and apoptosis by survivin small interfering RNA in
bladder cancer cells. BJU Int. 106:1812–1816. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tao YF, Lu J, Du XJ, et al: Survivin
selective inhibitor YM155 induce apoptosis in SK-NEP-1 Wilms tumor
cells. BMC Cancer. 12:6192012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Oliver FJ, de la Rubia G, Rolli V,
Ruiz-Ruiz MC, de Murcia G and Murcia JM: Importance of
poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson
from an uncleavable mutant. J Biol Chem. 273:33533–33539. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yamanaka K, Nakata M, Kaneko N, et al:
YM155, a selective survivin suppressant, inhibits tumor spread and
prolongs survival in a spontaneous metastatic model of human triple
negative breast cancer. Int J Oncol. 39:569–575. 2011.
|
|
54
|
Kuo LJ and Yang LX: γ-H2AX - a novel
biomarker for DNA double-strand breaks. In Vivo. 22:305–309.
2008.
|
|
55
|
Chowdhury D, Keogh MC, Ishii H, Peterson
CL, Buratowski S and Lieberman J: γ-H2AX dephosphorylation by
protein phosphatase 2A facilitates DNA double-strand break repair.
Mol Cell. 20:801–809. 2005.
|
|
56
|
Zhang Y, Chen ZD, Du CJ, Xu G and Luo W:
siRNA targeting survivin inhibits growth and induces apoptosis in
human renal clear cell carcinoma 786-O cells. Pathol Res Pract.
205:823–827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Salehi F, Kovacs K, Scheithauer BW, Lloyd
RV and Cusimano M: Pituitary tumor-transforming gene in endocrine
and other neoplasms: a review and update. Endocr Relat Cancer.
15:721–743. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chao JI and Liu HF: The blockage of
survivin and securin expression increases the cytochalasin
B-induced cell death and growth inhibition in human cancer cells.
Mol Pharmacol. 69:154–164. 2006.
|
|
59
|
Sheverdin V, Bae SY, Shin DH and Lee K:
Expression and localization of translationally controlled tumor
protein in rat urinary organs. Microsc Res Tech. 75:1576–1581.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Roca H, Varsos ZS, Mizutani K and Pienta
KJ: CCL2, survivin and autophagy: new links with implications in
human cancer. Autophagy. 4:969–971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kreuzaler P and Watson CJ: Killing a
cancer: what are the alternatives? Nat Rev Cancer. 12:411–424.
2012. View Article : Google Scholar : PubMed/NCBI
|